Electrochemical Corrosion Behavior of Pure Mg Processed by Powder Metallurgy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
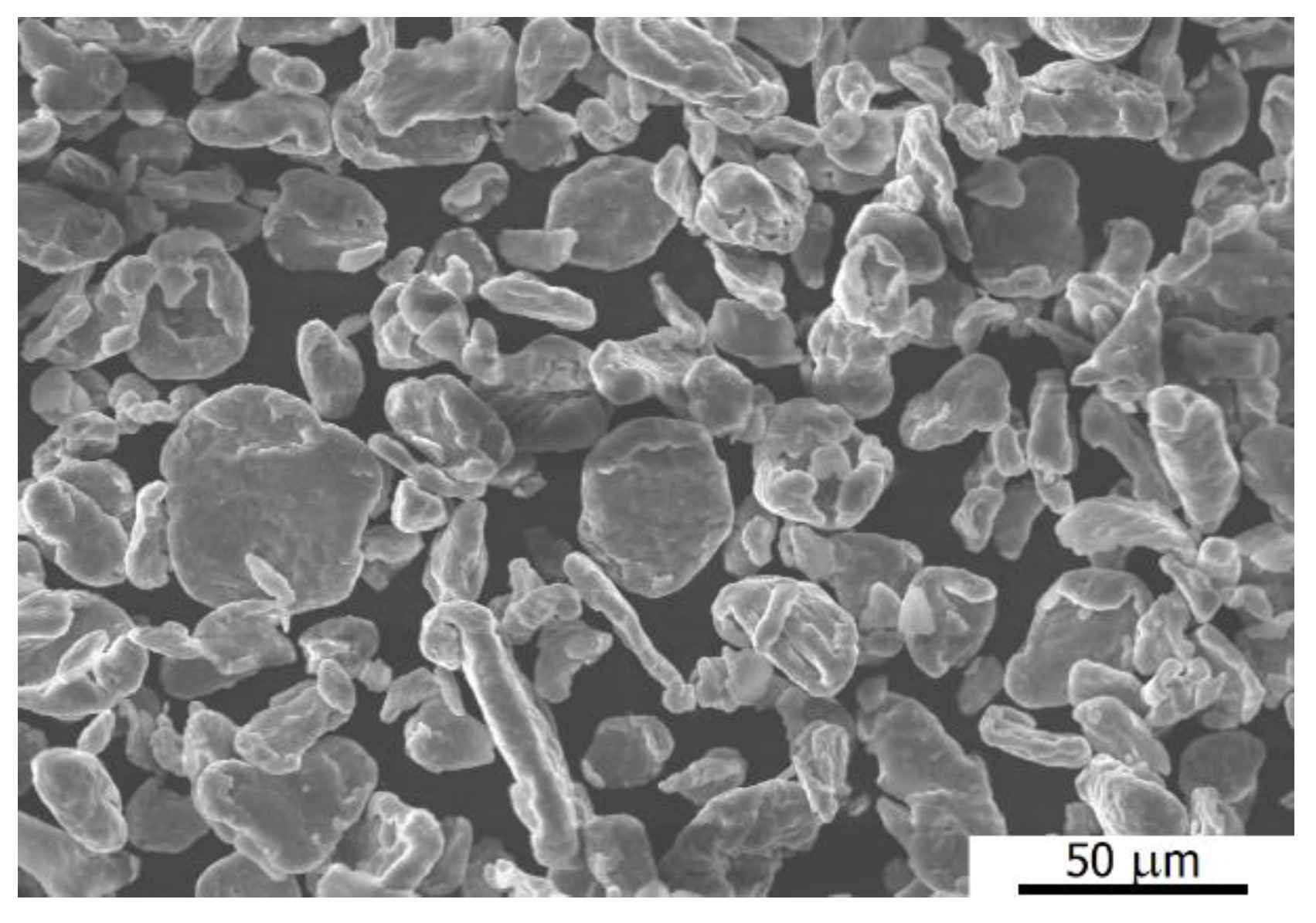
3.1. Microstructural Observation
3.2. Electrochemical Corrosion Characteristics
4. Discussion
5. Conclusions
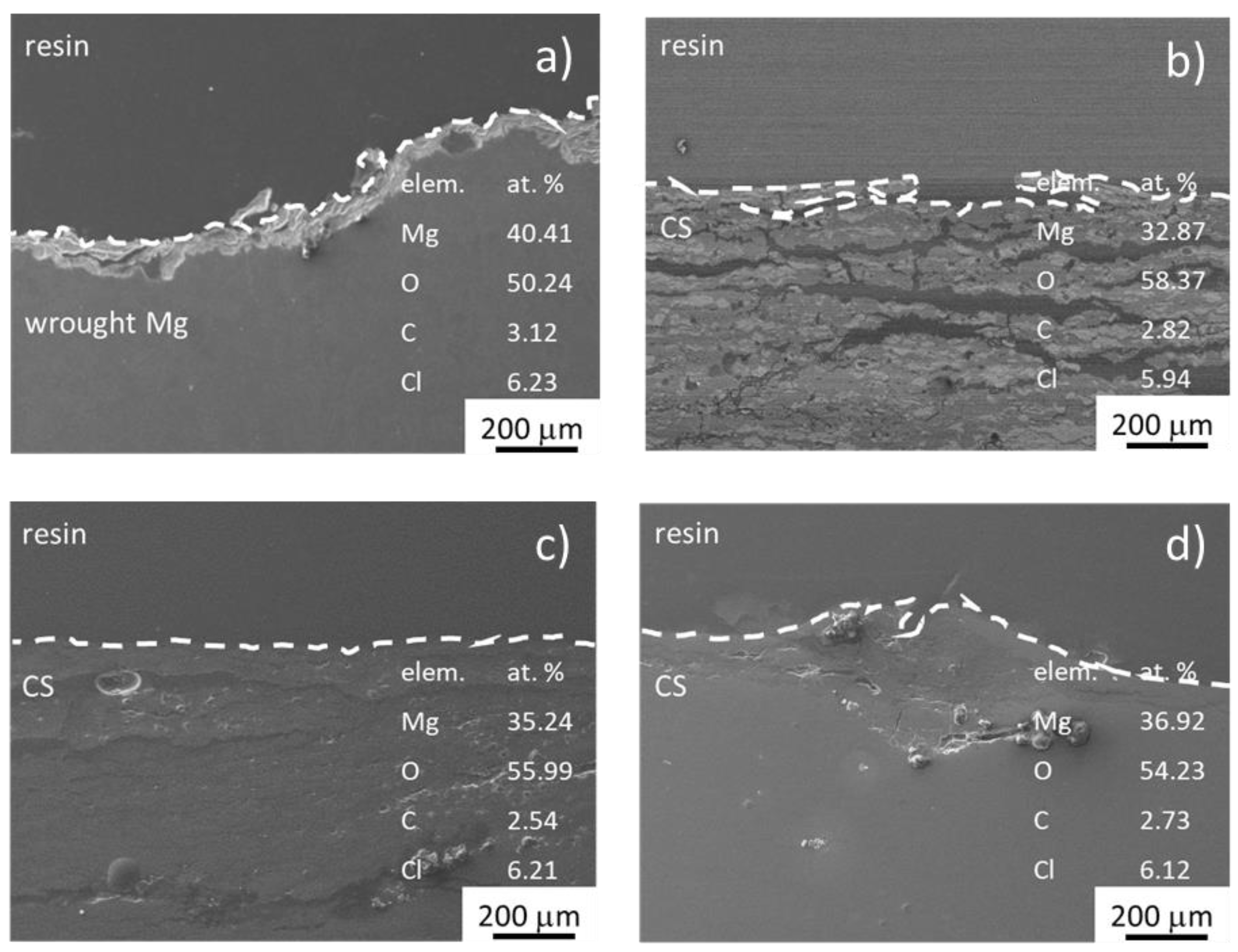
- Only mechanical bonding between Mg powder particles was observed for cold compacted Mg (RT), while a combination of mechanical bonding and diffusion bonding was observed for hot compacted Mg samples.
- EIS measurements revealed similar electrochemical corrosion characteristics for samples compacted at 500 MPa/RT and 100 MPa/400 °C, while material compacted at 500 MPa/400 °C achieved higher values of polarization resistance and was characterized by longer resistance to the corrosion environment of 0.9% NaCl (96 h of exposure).
- The corrosion resistance of all CS was lower compared to wrought pure Mg.
- In the case of samples processed at 500 MPa/RT and 100 MPa/400 °C, a different corrosion process was observed relating to the powder particle bonding mechanism. An interparticle corrosion structure was created due to the corrosion attack following powder particle boundaries and pores for both samples. Uniform corrosion attack, comparable to that in wrought pure Mg, was observed in the case of hot CS, where diffusion bonding mechanism plays the main role in particle compaction (500 MPa/400 °C).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salvetr, P.; Novák, P.; Vojtech, D. Porous magnesium alloys prepared by powder metallurgy. Mater. Tehnol. 2016, 50, 917–922. [Google Scholar] [CrossRef]
- Chen, S.; Tan, L.; Zhang, B.; Xia, Y.; Xu, K.; Yang, K. In vivo study on degradation behavior and histologic response of pure magnesium in muscles. J. Mater. Sci. Technol. 2017, 33, 469–474. [Google Scholar] [CrossRef]
- Avedesian, M.M.; Baker, H. (Eds.) Magnesium and Magnesium Alloys; ASM International: Materials Park, OH, USA, 1999. [Google Scholar]
- Nassif, N.; Ghayad, I. Corrosion protection and surface treatment of magnesium alloys used for orthopedic applications. Adv. Mater. Sci. Eng. 2013, 2013, 532896. [Google Scholar] [CrossRef] [Green Version]
- Adolf, B. Magnesium Und Seine Legierungen; Springer: Berlin/Heidelberg, Germany, 1939. [Google Scholar]
- Čapek, J.; Vojtěch, D. Properties of porous magnesium prepared by powder metallurgy. Mater. Sci. Eng. C 2013, 33, 564–569. [Google Scholar] [CrossRef]
- Alias, J.; Harun, W.S.W.; Ayu, H.M. A review on the preparation of magnesium-based alloys prepared by powder metallurgy and the evolution of microstructure and mechanical properties. In Key Engineering Materials; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2019; Volume 796, pp. 3–10. [Google Scholar]
- Bettles, C.J. Magnesium powder metallurgy: Process and materials opportunities. J. Mater. Eng. Perform. 2008, 17, 297–301. [Google Scholar] [CrossRef]
- Perumal, G.; Ramasamy, B.; Dhanasekaran, S.; Ramasamy, S.; Doble, M. Bilayer nanostructure coated AZ31 magnesium alloy implants: In vivo reconstruction of critical-sized rabbit femoral segmental bone defect. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102232. [Google Scholar] [CrossRef] [PubMed]
- Garcés, G.; Domínguez, F.; Pérez, P.; Caruana, G.; Adeva, P. Effect of extrusion temperature on the microstructure and plastic deformation of PM-AZ92. J. Alloys Compd. 2006, 422, 293–298. [Google Scholar] [CrossRef]
- Osorio-García, M.; Suárez-Alcántara, K.; Todaka, Y.; Tejeda-Ochoa, A.; Herrera-Ramírez, M.; Hernández-Silva, O.; Cabañas-Moreno, J.G. Low-temperature hydrogenation of Mg-Ni-Nb2O5 alloy processed by high-pressure torsion. J. Alloys Compd. 2021, 878, 160309. [Google Scholar] [CrossRef]
- Garcia-Casas, A.; Toirac, B.; Jiménez-Morales, A. The electrochemical corrosion behavior of metals processed by powder metallurgy: Future perspectives. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Dvorský, D.; Kubásek, J.; Vojtěch, D. Microstructure, mechanical and corrosion properties of extruded milled magnesium powder. Manuf. Technol. 2020, 20, 708–713. [Google Scholar]
- Zhuang, H.; Han, Y.; Feng, A. Preparation, mechanical properties and in vitro biodegradation of porous magnesium scaffolds. Mater. Sci. Eng. C 2008, 28, 1462–1466. [Google Scholar] [CrossRef]
- Lietaert, K.; Weber, L.; Van Humbeeck, J.; Mortensen, A.; Luyten, J.; Schrooten, J. Open cellular magnesium alloys for biodegradable orthopaedic implants. J. Magnes. Alloys 2013, 1, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Březina, M.; Minda, J.; Doležal, P.; Krystýnová, M.; Fintová, S.; Zapletal, J.; Wasserbauer, J.; Ptáček, P. Characterization of powder metallurgy processed pure magnesium materials for biomedical applications. Metals 2017, 7, 461. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.; Yamada, Y.; Shimojima, K.; Chino, Y.; Hosokawa, H.; Mabuchi, M. Compressibility of porous magnesium foam: Dependency on porosity and pore size. Mater. Lett. 2004, 58, 357–360. [Google Scholar] [CrossRef]
- Bram, M.; Ebel, T.; Wolff, M.; Barbosa, A.C.; Tuncer, N. Applications of powder metallurgy in biomaterials. Adv. Powder Metall. 2013, 520–554. [Google Scholar] [CrossRef]
- Song, G.-L.; Unocic, K.A. The anodic surface film and hydrogen evolution on Mg. Corros. Sci. 2015, 98, 758–765. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.F.; Gu, X.N.; Xi, Y.L.; Chai, D.L. In vitro degradation and cytotoxicity of Mg/Ca composites produced by powder metallurgy. Acta Biomater. 2010, 6, 1783–1791. [Google Scholar] [CrossRef]
- Krystýnová, M.; Doležal, P.; Fintová, S.; Březina, M.; Zapletal, J.; Wasserbauer, J. Preparation and characterization of zinc materials prepared by powder metallurgy. Metals 2017, 7, 396. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, M.; Gao, J. Improve corrosion resistance of magnesium in simulated body fluid by dicalcium phosphate dihydrate coating. Mater. Sci. Eng. C 2009, 29, 1311–1316. [Google Scholar] [CrossRef]
- Gray, J.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Annur, D.; Lestari, F.P.; Erryani, A.; Kartika, I. Study of sintering on Mg-Zn-Ca alloy system. In Proceedings of the AIP Conference Proceedings, Maharashtra, India, 5–6 July 2018; Volume 1964, No. 1. p. 020029. [Google Scholar]
- Drábiková, J.; Pastorek, F.; Fintová, S.; Dolezal, P.; Wasserbauer, J. Improvement of bio-compatible AZ61 magnesium alloy corrosion resistance by fluoride conversion coating. Koroze A Ochr. Mater. 2016, 60, 132. [Google Scholar] [CrossRef] [Green Version]
- Drábiková, J.; Fintová, S.; Ptáček, P.; Kuběna, I.; Březina, M.; Wasserbauer, J.; Doležal, P.; Pastorek, F. Structure and growth kinetic of unconventional fluoride conversion coating prepared on wrought AZ61 magnesium alloy. Surf. Coat. Technol. 2020, 399, 126101. [Google Scholar] [CrossRef]
- Fintova, S.; Drabikova, J.; Hadzima, B.; Trško, L.; Březina, M.; Doležal, P.; Wasserbauer, J. Degradation of unconventional fluoride conversion coating on AZ61 magnesium alloy in SBF solution. Surf. Coat. Technol. 2019, 380, 125012. [Google Scholar] [CrossRef]
- Fintová, S.; Drábiková, J.; Pastorek, F.; Tkacz, J.; Kuběna, I.; Trško, L.; Hadzima, B.; Minda, J.; Doležal, P.; Wasserbauer, J.; et al. Improvement of electrochemical corrosion characteristics of AZ61 magnesium alloy with unconventional fluoride conversion coatings. Surf. Coat. Technol. 2019, 357, 638–650. [Google Scholar] [CrossRef]
- Drábiková, J.; Fintová, S.; Tkacz, J.; Doležal, P.; Wasserbauer, J. Unconventional fluoride conversion coating preparation and characterization. Anti-Corros. Methods Mater. 2017, 64, 613–619. [Google Scholar] [CrossRef]
- Dvorsky, D.; Kubasek, J.; Vojtech, D. A new approach in the preparation of biodegradable Mg-MgF2 composites with tailored corrosion and mechanical properties by powder metallurgy. Mater. Lett. 2018, 227, 78–81. [Google Scholar] [CrossRef]
- Jayasathyakawin, S.; Ravichandran, M.; Baskar, N.; Chairman, C.A.; Balasundaram, R. Magnesium matrix composite for biomedical applications through powder metallurgy—Review. Mater. Today. Proc. 2020, 27, 736–741. [Google Scholar] [CrossRef]
- King, A.D.; Birbilis, N.; Scully, J.R. Accurate electrochemical measurement of magnesium corrosion rates; a combined impedance, mass-loss and hydrogen collection study. Electrochim. Acta 2014, 121, 394–406. [Google Scholar] [CrossRef]
- Atrens, A.; Dietzel, W. The negative difference effect and unipositive Mg+. Adv. Eng. Mater. 2007, 9, 292–297. [Google Scholar] [CrossRef] [Green Version]
- Weber, C.R.; Knörnschild, G.; Dick, L.F.P. The negative-difference effect during the localized corrosion of magnesium and of the AZ91HP alloy. J. Braz. Chem. Soc. 2003, 14, 584–593. [Google Scholar] [CrossRef] [Green Version]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Březina, M.; Doležal, P.; Krystýnová, M.; Minda, J.; Zapletal, J.; Fintová, S.; Wasserbauer, J. Evolution of microstructure and electrochemical corrosion characteristics of cold compacted magnesium. Koroze A Ochr. Mater. 2017, 61, 123. [Google Scholar] [CrossRef] [Green Version]


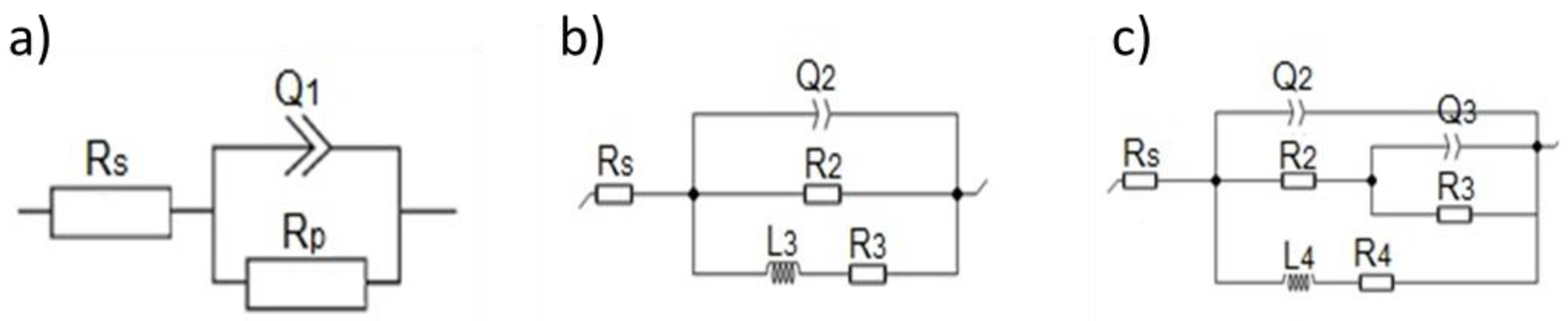
| Time | Rs [Ω·cm2] | R2 [Ω·cm2] | R3 [Ω·cm2] | R4 [Ω·cm2] | Rp [Ω·cm2] | Q2 [µF·sn–1] | Q3 [µF·sn–1] | n2 | n3 | L [H] |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 82 | 394 | 223 | NA | 142 | 18.4 | NA | 0.88 | NA | 1068 |
| 1 | 88 | 49 | 1 | 38 | 35 | 145.1 | 11.26 | 0.99 | 0.99 | 87 |
| 2 | 84 | 81 | NA | NA | 81 | 366.8 | NA | 0.93 | NA | NA |
| 4 | 86 | 150 | NA | NA | 140 | 219.1 | NA | 0.95 | NA | NA |
| 8 | 89 | 240 | NA | NA | 276 | 167.6 | NA | 0.94 | NA | NA |
| 12 | 91 | 282 | 122 | NA | 404 | 157.3 | NA | 0.92 | NA | 1708 |
| 24 | 84 | 401 | 54 | NA | 252 | 123.7 | NA | 0.91 | NA | 2001 |
| 48 | 86 | 223 | 315 | NA | 402 | 84.9 | NA | 0.91 | NA | 6926 |
| 72 | 91 | 438 | 270 | NA | 443 | 83.3 | NA | 0.86 | NA | 2501 |
| 96 | 95 | 739 | NA | NA | 730 | 78.3 | NA | 0.83 | NA | 1068 |
| Time | Rs [Ω·cm2] | R2 [Ω·cm2] | R3 [Ω·cm2] | R4 [Ω·cm2] | Rp [Ω·cm2] | Q2 [µF·sn–1] | Q3 [µF·sn–1] | n2 | n3 | L [H] |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 59 | 58 | 21 | 96 | 43 | 0.0 | 0.0 | 1.00 | 0.66 | 343 |
| 1 | 60 | 284 | 88 | NA | 45 | 439.0 | NA | 0.65 | NA | 922 |
| 2 | 61 | 81 | 87 | NA | 42 | 514.0 | NA | 0.68 | NA | 1107 |
| 4 | 64 | 931 | 116 | NA | 49 | 677.0 | NA | 0.62 | NA | 1888 |
| 8 | 66 | 62 | 204 | 212 | 118 | 327.0 | 0.5 | 0.24 | 0.97 | 727 |
| 12 | 74 | 132 | 849 | 178 | 151 | 49.4 | 204.0 | 0.55 | 0.67 | 0 |
| Time | Rs [Ω·cm2] | R2 [Ω·cm2] | R3 [Ω·cm2] | Rp [Ω·cm2] | Q2 [µF·sn–1] | n2 | L3 [H] |
|---|---|---|---|---|---|---|---|
| 0 | 117 | 298 | 107 | 79 | 36.11 | 0.83 | 2026 |
| 1 | 120 | 247 | 126 | 84 | 61.99 | 0.86 | 2201 |
| 2 | 121 | 227 | 165 | 96 | 90.66 | 0.86 | 3001 |
| 4 | 118 | 187 | 213 | 99 | 180.1 | 0.78 | 3856 |
| 8 | 116 | 141 | 317 | 98 | 252.0 | 0.73 | 5957 |
| 12 | 116 | 141 | 523 | 111 | 236.6 | 0.73 | 8440 |
| Time | Rs [Ω·cm2] | R2 [Ω·cm2] | R3 [Ω·cm2] | R4 [Ω·cm2] | Rp [Ω·cm2] | Q2 [µF·sn–1] | Q3 [µF·sn–1] | n2 | n3 | L [H] |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 61 | 6 | 40 | 306 | 40 | 0.0 | 22.2 | 1.00 | 0.88 | 1801 |
| 1 | 64 | 399 | 1617 | 174 | 160 | 28.9 | 1230.0 | 0.89 | 1.00 | 2242 |
| 2 | 64 | 375 | 167 | NA | 115 | 33.0 | NA | 0.89 | NA | 5244 |
| 4 | 63 | 325 | 154 | NA | 105 | 36.1 | NA | 0.90 | NA | 3950 |
| 8 | 60 | 290 | 157 | NA | 102 | 34.3 | NA | 0.92 | NA | 2590 |
| 12 | 59 | 1877 | 367 | NA | 307 | 33.6 | NA | 0.92 | NA | 0 |
| 24 | 65 | 323 | 182 | NA | 116 | 37.3 | NA | 0.91 | NA | 5233 |
| 48 | 63 | 272 | 203 | NA | 116 | 41.7 | NA | 0.88 | NA | 4774 |
| 72 | 68 | 250 | 239 | NA | 122 | 59.8 | NA | 0.84 | NA | 4854 |
| 96 | 68 | 204 | 163 | NA | 91 | 82.9 | NA | 0.81 | NA | 3781 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minda, J.; Fintová, S.; Hadzima, B.; Doležal, P.; Hasoňová, M.; Doskočil, L.; Wasserbauer, J. Electrochemical Corrosion Behavior of Pure Mg Processed by Powder Metallurgy. Coatings 2021, 11, 986. https://doi.org/10.3390/coatings11080986
Minda J, Fintová S, Hadzima B, Doležal P, Hasoňová M, Doskočil L, Wasserbauer J. Electrochemical Corrosion Behavior of Pure Mg Processed by Powder Metallurgy. Coatings. 2021; 11(8):986. https://doi.org/10.3390/coatings11080986
Chicago/Turabian StyleMinda, Jozef, Stanislava Fintová, Branislav Hadzima, Pavel Doležal, Michaela Hasoňová, Leoš Doskočil, and Jaromír Wasserbauer. 2021. "Electrochemical Corrosion Behavior of Pure Mg Processed by Powder Metallurgy" Coatings 11, no. 8: 986. https://doi.org/10.3390/coatings11080986
APA StyleMinda, J., Fintová, S., Hadzima, B., Doležal, P., Hasoňová, M., Doskočil, L., & Wasserbauer, J. (2021). Electrochemical Corrosion Behavior of Pure Mg Processed by Powder Metallurgy. Coatings, 11(8), 986. https://doi.org/10.3390/coatings11080986






