Non-Linear Evaluation of Coatings Performance: Evaluation of Polyester/Melamine Coil Coating Hydrolysis in NSS Test
Abstract
1. Introduction
- Percent of recurrence (%R): It is the percentage of points whose distance is equal to or less than the established threshold. It can be considered as the probability that the reconstructed path will revisit an area of the phase space determined by a threshold.
- Percent of determinism (%D): It is the percentage of recurrence points that form diagonal lines. It is indicative of the deterministic behavior of the established system.
- Maximum line (LM): It is the longest diagonal line segment, and is inversely proportional to the largest Lyapunov exponent and therefore an indicator of the sensitivity to initial conditions.
- Shannon entropy (Ent): It indicates the probability of the distribution of diagonal lines. This reflects the complexity of the system.
2. Materials and Methods
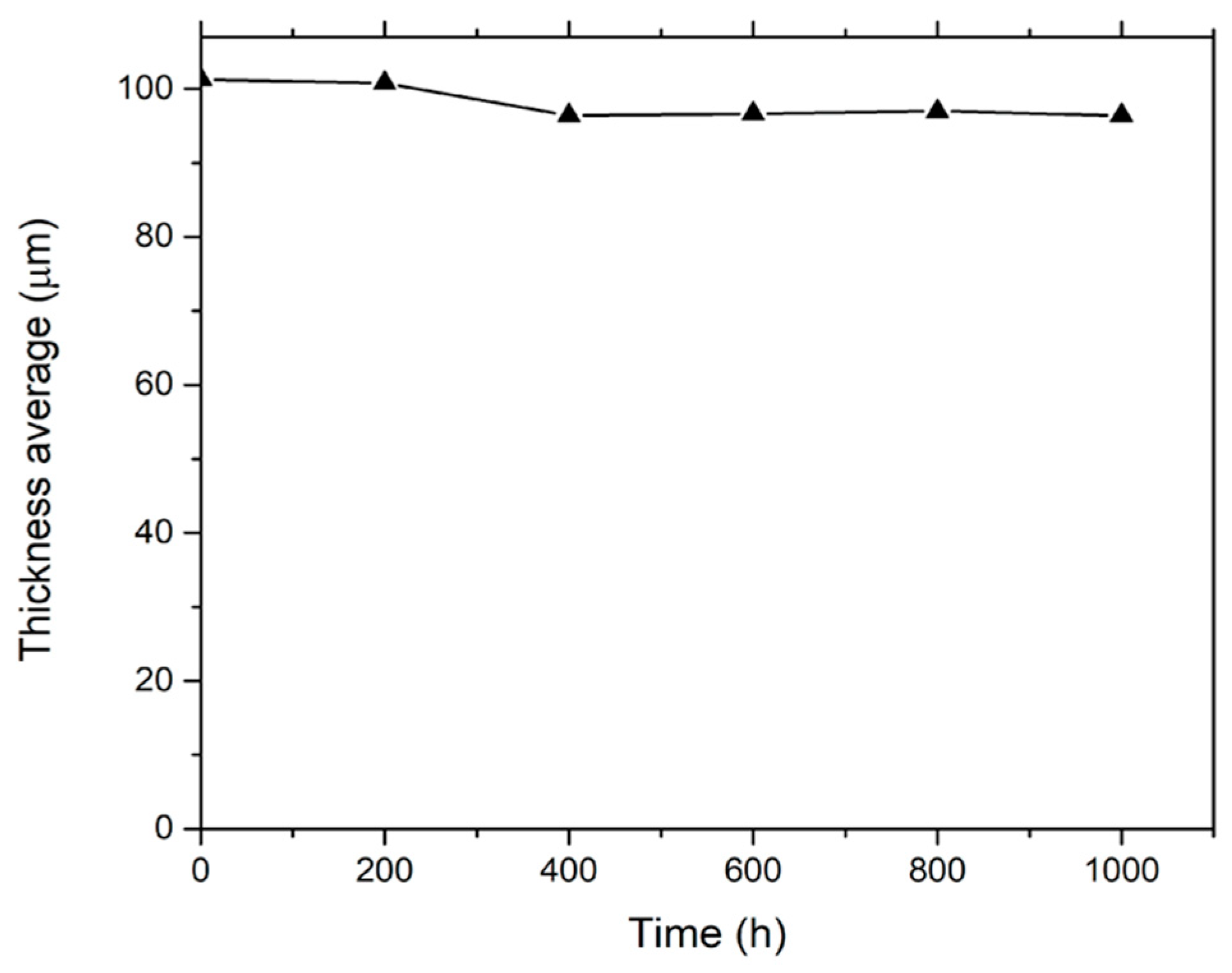
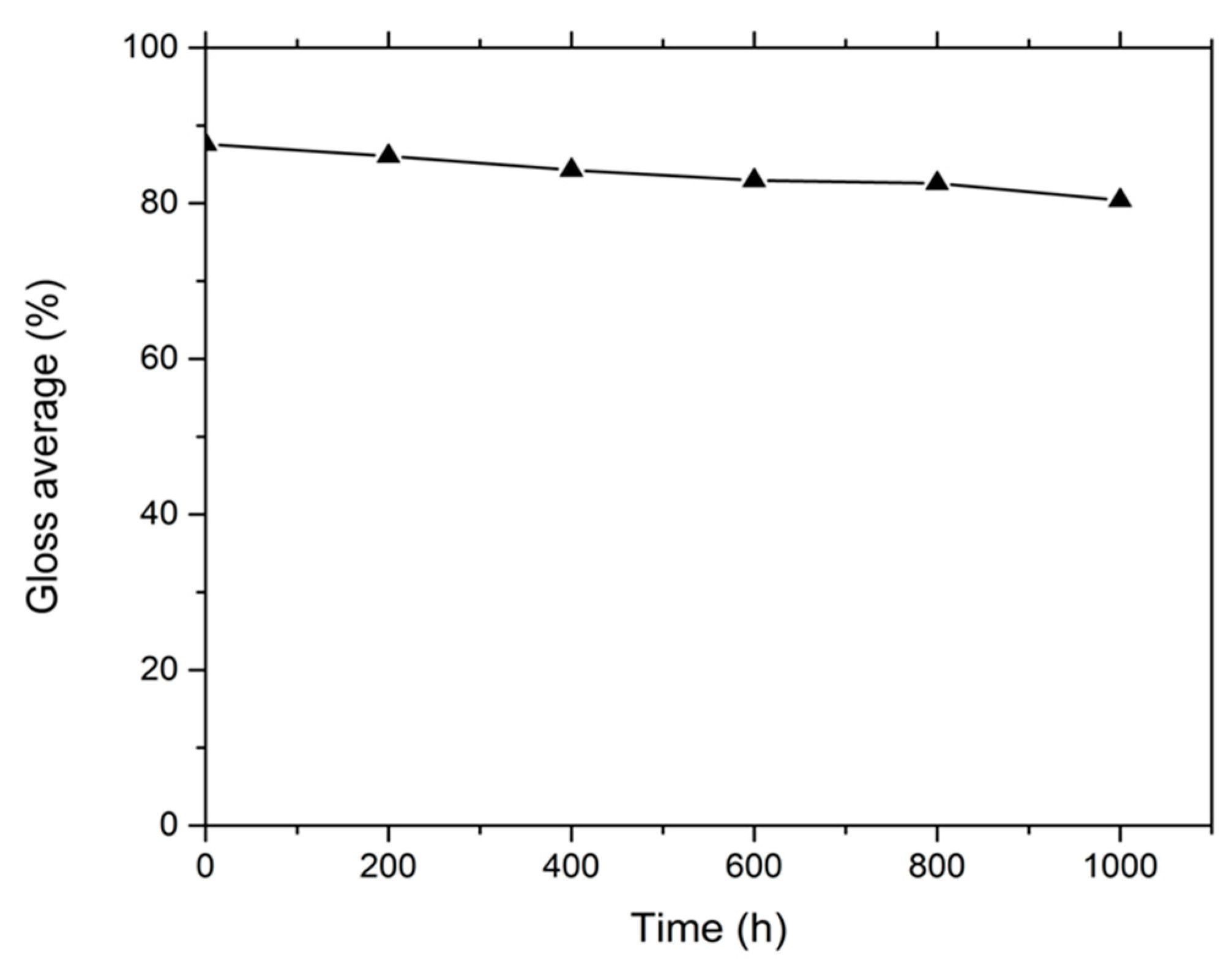
2.1. Thickness and Gloss Tests
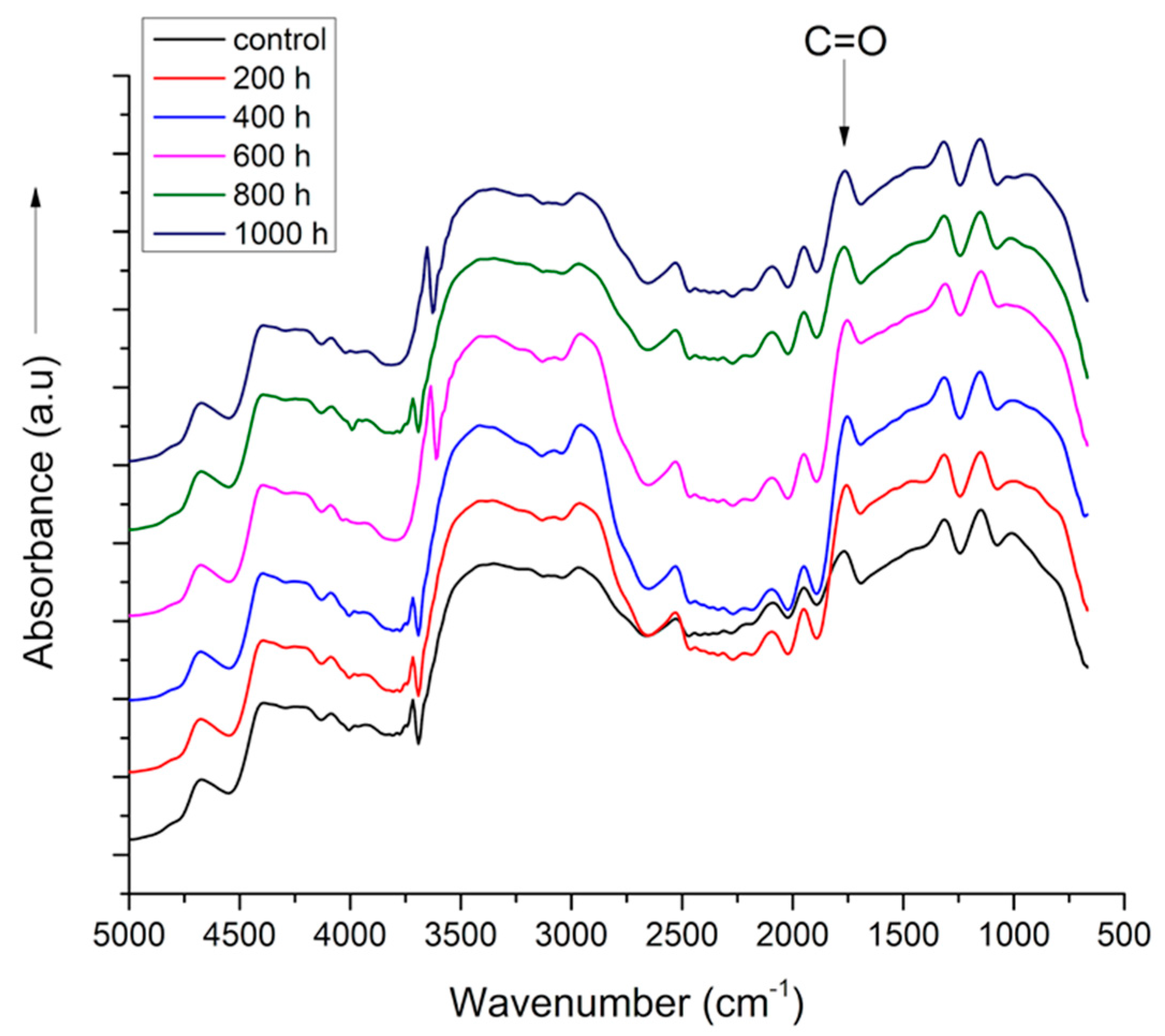
2.2. FTIR Spectra
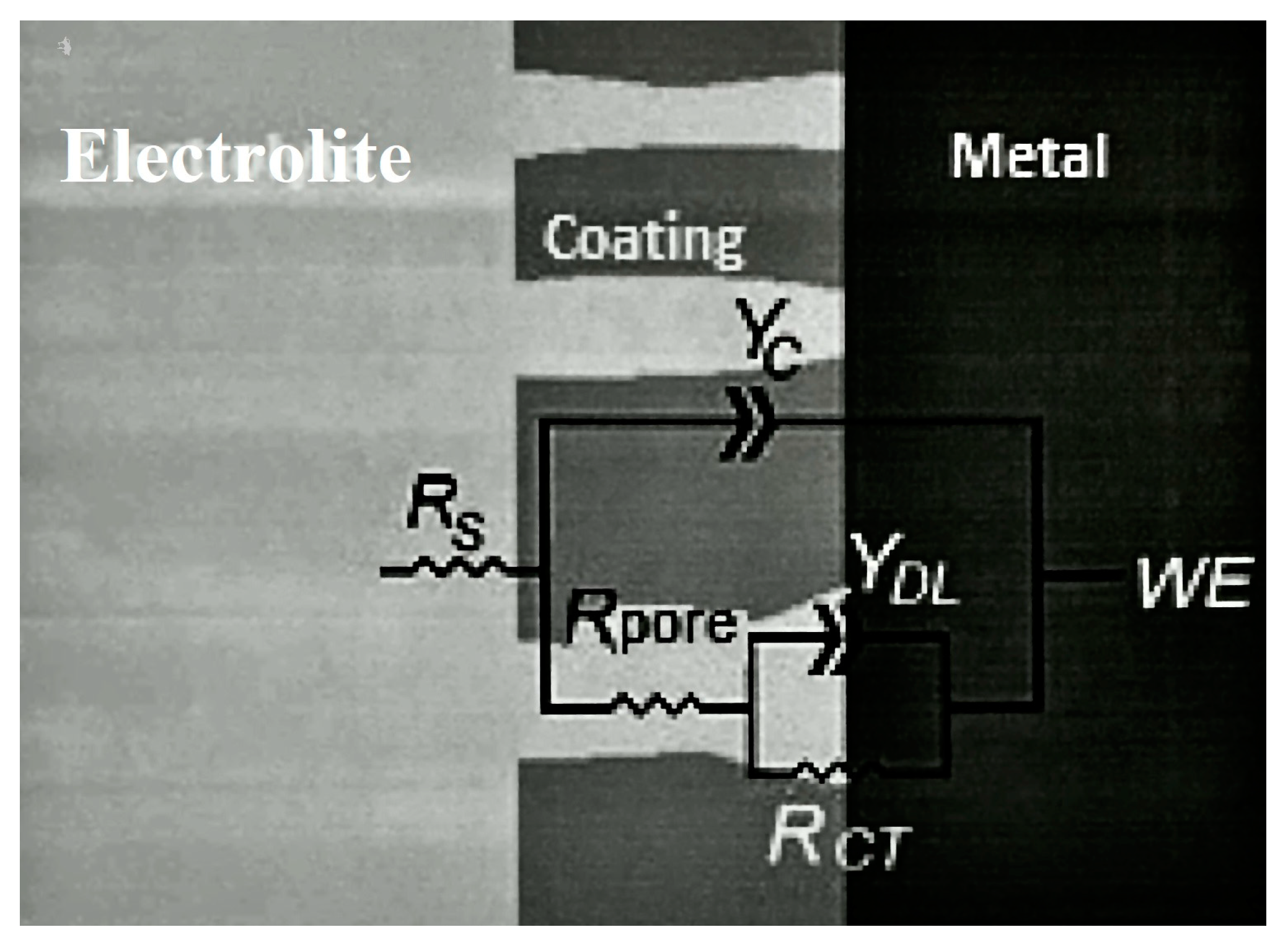
2.3. Electrochemical Tests (EIS and EN)
3. Results
3.1. Thickness and Gloss
3.2. Fourier Transformed Infra-Red Spectra
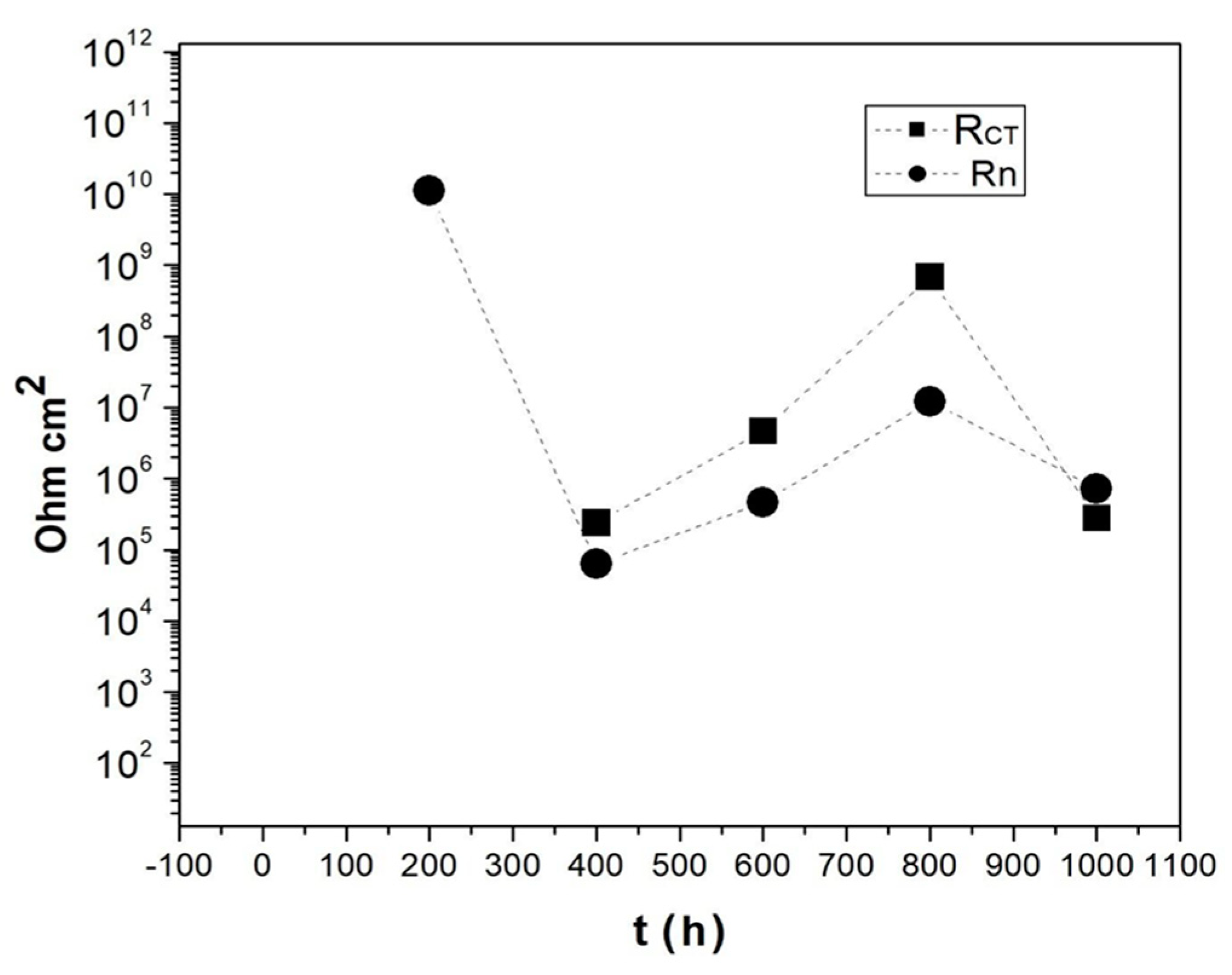
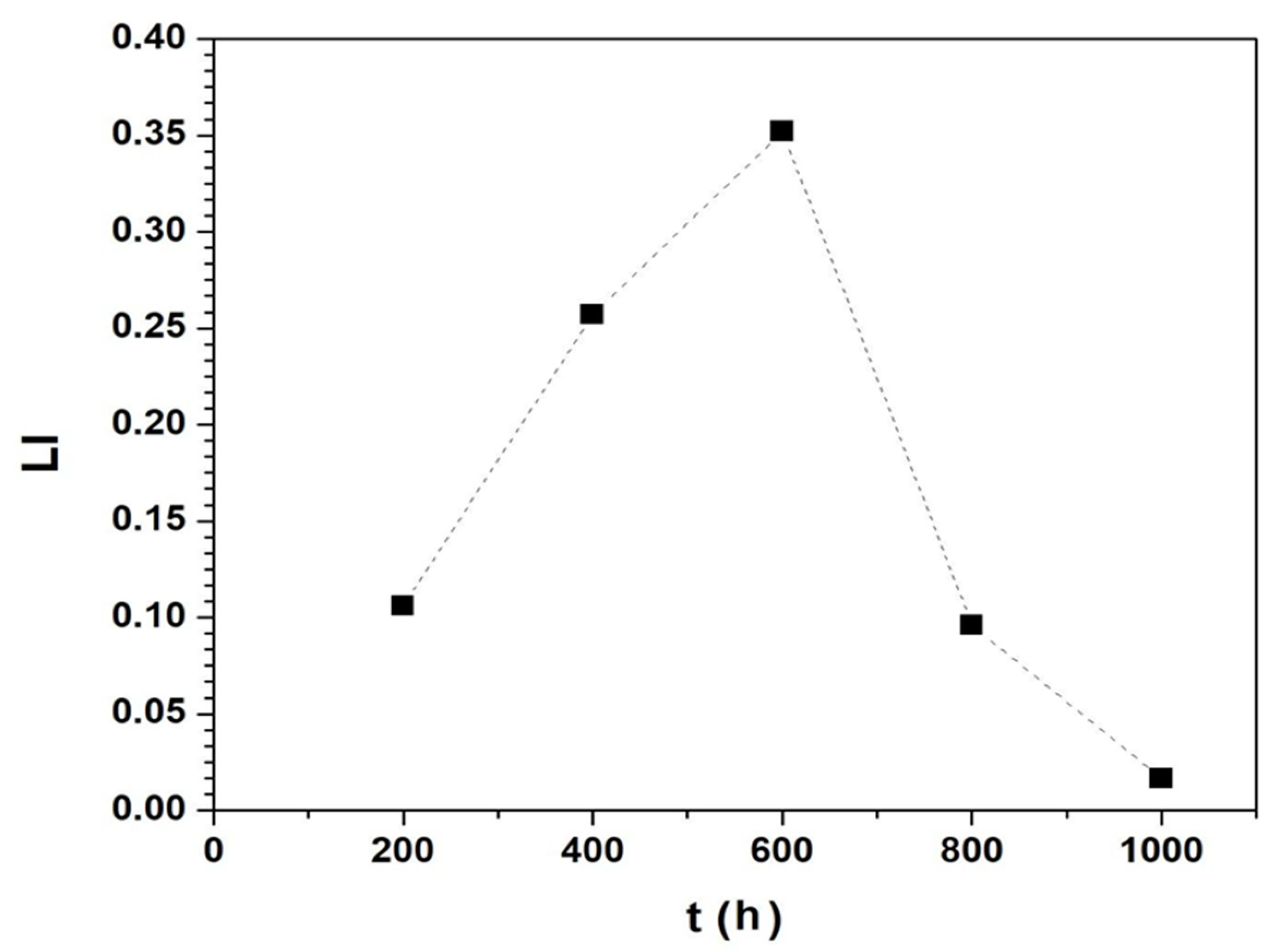
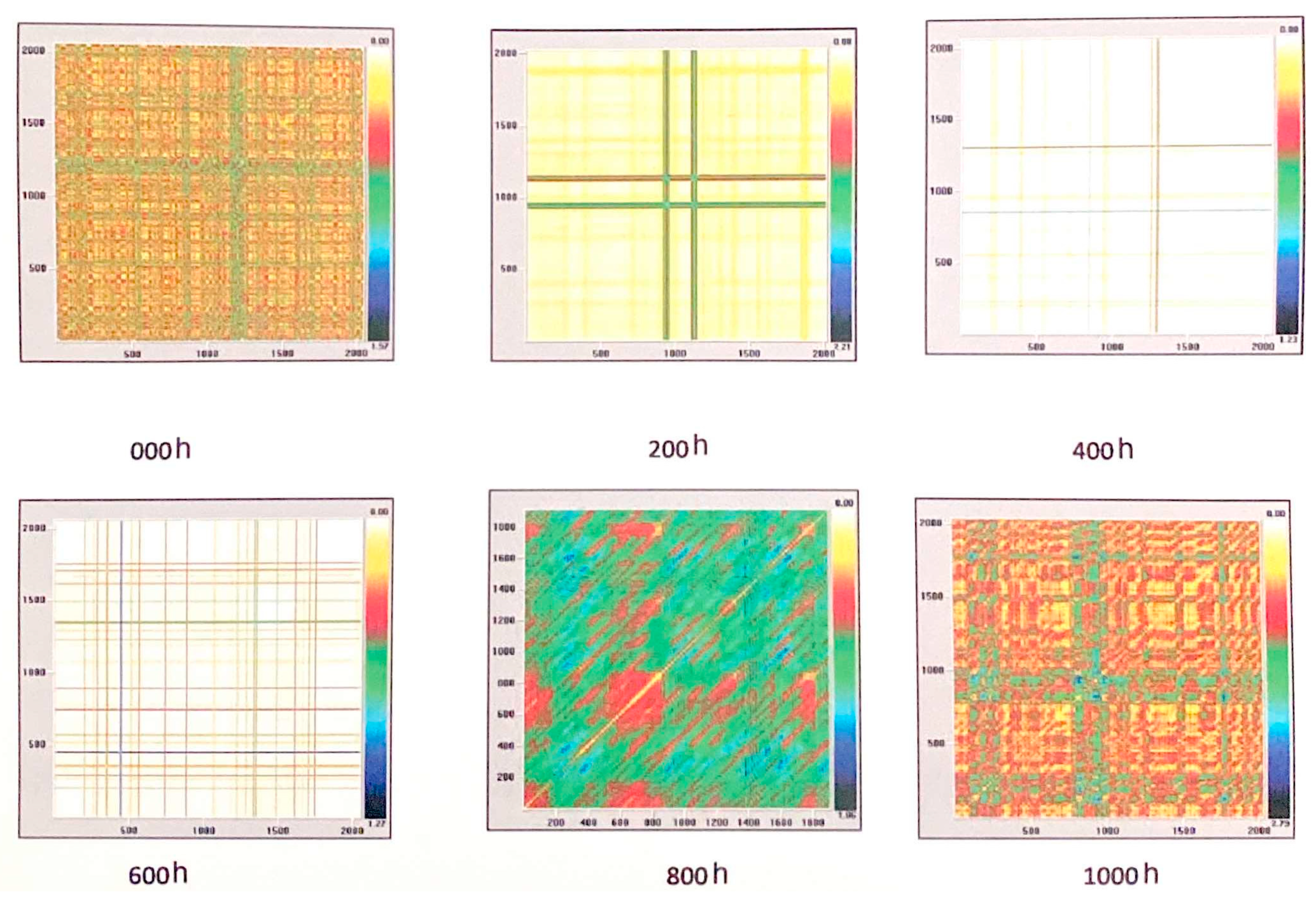
3.3. Electrochemical Tests (EIS and EN)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopes, T.D.S.; Martins, D.; Carneiro, C.; Machado, J.; Mendes, A. Accelerated aging of anticorrosive coatings: Two-stage approach to the AC/DC/AC electrochemical method. Prog. Org. Coat. 2020, 138, 105365. [Google Scholar] [CrossRef]
- ISO 9227:2014; Corrosion Tests in Artificial Atmospheres—Salt Spray Tests. ISO: Geneva, Switzerland, 2014.
- ISO 4628; Paints and Varnishes—Evaluation of Degradation of Coatings—Part 1 to 10. ISO: Geneva, Switzerland, 2016.
- Ramdé, T.; Ecco, L.G.; Rossi, S. Visual appearance durability as function of natural and accelerated ageing of electrophoretic styrene-acrylic coatings: Influence of yellow pigment concentration. Prog. Org. Coat. 2017, 103, 23–32. [Google Scholar] [CrossRef]
- Batista, M.A.; Moraes, R.P.; Barbosa, J.C.; Oliveira, P.C.; Santos, A.M. Effect of the polyester chemical structure on the stability of polyester–melamine coatings when exposed to accelerated weathering. Prog. Org. Coat. 2011, 71, 265–273. [Google Scholar] [CrossRef]
- Chen, F.; Breedon, M.; Sapper, E.; Ganther, W.; Lau, D.; Cole, I. A microclimate model to simulate neutral salt spray testing for corrosion inhibitor evaluation and functional coating development. Prog. Org. Coat. 2017, 111, 327–335. [Google Scholar] [CrossRef]
- Lalic, M.M.; Martinez, S. A Novel Application of EIS for Quantitative Coating Quality Assessment During Neutral Salt Spray Testing of High-Durability Coatings. Acta Chim. Slov. 2019, 66, 513–522. [Google Scholar] [CrossRef]
- Wan, S.; Chen, H.; Cai, G.; Liao, B.; Guo, X. Functionalization of h-BN by the exfoliation and modification of carbon dots for enhancing corrosion resistance of waterborne epoxy coating. Prog. Org. Coat. 2022, 165, 106757. [Google Scholar] [CrossRef]
- Zeng, Q.; Min, X.; Luo, Z.; Dai, H.; Liao, B. In-situ preparation of superhydrophobic Zn-Al layered double hydroxide coatings for corrosion protection of aluminum alloy. Mater. Lett. 2022, 328, 133077. [Google Scholar] [CrossRef]
- Arellano-Pérez, J.; Escobar-Jiménez, R.; Granados-Lieberman, D.; Gómez-Aguilar, J.; Uruchurtu-Chavarín, J.; Alvarado-Martínez, V. Electrochemical noise signals evaluation to classify the type of corrosion using Synchrosqueezing transform. J. Electroanal. Chem. 2019, 848, 113249. [Google Scholar] [CrossRef]
- Haruna, T.; Morikawa, Y.; Fujimoto, S.; Shibata, T. Electrochemical noise analysis for estimation of corrosion rate of carbon steel in bicarbonate solution. Corros. Sci. 2003, 45, 2093–2104. [Google Scholar] [CrossRef]
- Al-Mazeedi, H.; Cottis, R. A practical evaluation of electrochemical noise parameters as indicators of corrosion type. Electrochim. Acta 2004, 49, 2787–2793. [Google Scholar] [CrossRef]
- Hou, Y.; Aldrich, C.; Lepkova, K.; Kinsella, B. Detection of under deposit corrosion in CO2 environment by electrochemical noise and recurrence quantification analysis. Electrochim. Acta 2018, 274, 160–169. [Google Scholar] [CrossRef]
- Xia, D.-H.; Song, S.-Z.; Behnamian, Y. Detection of corrosion degradation using electrochemical noise (EN): Review of signal processing methods for identifying corrosion forms. Corros. Eng. Sci. Technol. 2016, 51, 527–544. [Google Scholar] [CrossRef]
- Smulko, J.; Darowicki, K.; Zieliński, A. Pitting corrosion in steel and electrochemical noise intensity. Electrochem. Commun. 2002, 4, 388–391. [Google Scholar] [CrossRef]
- Zaveri, N.; Sun, R.; Zufelt, N.; Zhou, A.; Chen, Y. Evaluation of microbially influenced corrosion with electrochemical noise analysis and signal processing. Electrochim. Acta 2007, 52, 5795–5807. [Google Scholar] [CrossRef]
- Turcio-Ortega, D.; Pandiyan, T.; Cruz, J.; Garcia-Ochoa, E. Interaction of Imidazoline Compounds with Fen. (n = 1−4 Atoms) as a Model for Corrosion Inhibition: DFT and Electrochemical Studies. J. Phys. Chem. C 2007, 111, 9853–9866. [Google Scholar] [CrossRef]
- Cottis, R.; Turgoose, S. Electrochemical Impedance and Noise; NACE: Houston, TX, USA, 1999. [Google Scholar]
- Torres-Mendoza, V.; Rodríguez-Gómez, F.; García-Ochoa, E.; Genesca, J. The assessment of natural atmosphere corrosivity by the use of electrochemical noise analysis. Anti-Corros. Methods Mater. 2006, 53, 348–356. [Google Scholar] [CrossRef]
- Lee, C.; Mansfeld, F. Analysis of electrochemical noise data for a passive system in the frequency domain. Corros. Sci. 1987, 40, 959–962. [Google Scholar] [CrossRef]
- Sazou, D.; Pagitsas, M. Non-linear dynamics of the passivity breakdown of iron in acidic solutions. Chaos Solitons Fractals 2003, 17, 505–522. [Google Scholar] [CrossRef]
- Stringer, J.; Markworth, A. Applications of deterministic chaos theory to corrosion. Corros. Sci. 1993, 35, 751–760. [Google Scholar] [CrossRef]
- Chen, A.; Cao, F.; Liao, X.; Liu, W.; Zheng, L.; Zhang, J.; Cao, C. Study of pitting corrosion on mild steel during wet–dry cycles by electrochemical noise analysis based on chaos theory. Corros. Sci. 2013, 66, 183–195. [Google Scholar] [CrossRef]
- Aldrich, C.; Qi, B.; Botha, P. Analysis of electrochemical noise data with phase space methods. Miner. Eng. 2006, 19, 1402–1409. [Google Scholar] [CrossRef]
- Bahena, D.; Rosales, I.; Sarmiento, O.; Guardián, R.; Menchaca, C.; Uruchurtu, J. Electrochemical Noise Chaotic Analysis of NiCoAg Alloy in Hank Solution. Int. J. Corros. 2011, 2011, 491564. [Google Scholar] [CrossRef]
- Parkhutik, V.P. Oscillations of Open-Circuit Potential during Immersion Plating of Silicon in CuSO4/HF Solutions. Rus. J. Electrochem. 2006, 42, 512–522. [Google Scholar] [CrossRef]
- Eckmann, J.-P.; Kamphorst, S.O.; Ruelle, D. Recurrence Plots of Dynamical Systems. EPL Europhys. Lett. 1987, 4, 973–977. [Google Scholar] [CrossRef]
- Hou, Y.; Aldrich, C.; Lepkova, K.; Kinsella, B. Identifying corrosion of carbon steel buried in iron ore and coal cargoes based on recurrence quantification analysis of electrochemical noise. Electrochim. Acta 2018, 283, 212–220. [Google Scholar] [CrossRef]
- Zbilut, J.P.; Webber, C.L., Jr. Embeddings and delays as derived from quantification of recurrence plots. Phys. Lett. A 1992, 171, 199–203. [Google Scholar] [CrossRef]
- Zbilut, J.P.; Giuliani, A.; Webber, C.L. Recurrence quantification analysis and principal components in the detection of short complex signals. Phys. Lett. A 1998, 237, 131–135. [Google Scholar] [CrossRef]
- Cazares-Ibáñez, E.; Vázquez-Coutiño, G.; García-Ochoa, E. Application of recurrence plots as a new tool in the analysis of electrochemical oscillations of copper. J. Electroanal. Chem. 2005, 583, 17–33. [Google Scholar] [CrossRef]
- Zeidabadinejad, M.; Shahidi-Zandi, M.; Foroughi, M.M.; Asadollahzadeh, H. Advantages of asymmetrical over symmetrical cells for detection of under deposit CO2 corrosion using electrochemical noise. Mater. Corros. 2019, 70, 1999–2008. [Google Scholar] [CrossRef]
- Zhang, T.; Cong, Y.; Shao, Y.; Meng, G.; Wang, F. Electrochemical noise analysis on the crevice corrosion behavior of Ni–Cr–Mo–V high strength steel using recurrence plots. J. Appl. Electrochem. 2011, 41, 289–298. [Google Scholar] [CrossRef]
- Hou, Y.; Aldrich, C.; Lepkova, K.; Machuca, L.; Kinsella, B. Monitoring of carbon steel corrosion by use of electrochemical noise and recurrence quantification analysis. Corros. Sci. 2016, 112, 63–72. [Google Scholar] [CrossRef]
- Liu, W.; Wang, D.; Chen, X.; Wang, C.; Liu, H. Electrochemical noise monitoring of sulphur corrosion for nickel alloy 718. Corros. Eng. Sci. Technol. 2016, 51, 545–549. [Google Scholar] [CrossRef]
- Rossi, S.; Calovi, M.; Dalpiaz, D.; Fedel, M. The Influence of NIR Pigments on Coil Coatings’ Thermal Behaviors. Coatings 2020, 10, 514. [Google Scholar] [CrossRef]
- de Souza, M. Organic coil coating of metal. In High-Performance Organic Coatings; Khanna, K.S., Ed.; Woodhead Publishing on Materials: Boca Raton, FL, USA, 2008; Chapter 17. [Google Scholar]
- Murray, I. Forage Analysis by Near Infra-Red Reflectance Spectroscopy. In Sward Measurement Handbook, 2nd ed.; Davies, A., Baker, R.D., Grant, S.A., Laidlaw, A.S., Eds.; British Grassland Soc.: Gateshead, UK, 1993; pp. 285–312. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies—Tables and Charts; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Fockaert, L.; Pletincx, S.; Ganzinga-Jurg, D.; Boelen, B.; Hauffman, T.; Terryn, H.; Mol, J. Chemisorption of polyester coatings on zirconium-based conversion coated multi-metal substrates and their stability in aqueous environment. Appl. Surf. Sci. 2019, 508, 144771. [Google Scholar] [CrossRef]
- Stuart, B.H. Organic molecules. In Infrared Spectroscopy: Fundamentals and Applications; Ando, D.J., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2004; Volume 1, pp. 71–80, 83. [Google Scholar]
- Mahdavian, M.; Attar, M. Another approach in analysis of paint coatings with EIS measurement: Phase angle at high frequencies. Corros. Sci. 2006, 48, 4152–4157. [Google Scholar] [CrossRef]
- Hernández, M.; Genescá, J.; Uruchurtu, J.; Barba, A. Correlation between electrochemical impedance and noise measurements of waterborne coatings. Corros. Sci. 2009, 51, 499–510. [Google Scholar] [CrossRef]
- Fei, Z.; Hudson, J.L. Chaotic Oscillations on Arrays of Iron Electrodes. Ind. Eng. Chem. Res. 1998, 37, 2172–2179. [Google Scholar] [CrossRef]


| Exposure Time in NSS | 400 h | 600 h | 800 h | 1000 h |
|---|---|---|---|---|
| Rs Ωcm2 | 88 | 1076 | 1450 | 320 |
| Yc Ω−1cm−2sNc | 3.439 × 10−6 | 2.486 × 10−6 | 3.057 × 10−7 | 2.973 × 10−6 |
| Nc | 0.882 | 0.882 | 0.872 | 0.812 |
| Rpore Ωcm2 | 209 | 4.03 × 104 | 1.563 × 107 | 1673 |
| YDL Ω−1cm−2sNDL | 3.419 × 10−6 | 5.068 × 10−5 | 45.51 | 8.862 × 10−7 |
| NDL | 0.861 | 0.999 | 0.475 | 0.960 |
| RCT Ωcm2 | 2.398 × 105 | 47.34 | 6.860 × 108 | 2.767 × 105 |
| Chi-square | 0.0018 | 0.0013 | 0.0012 | 0.0031 |
| Time of Exposure in NSS | 0 h | 200 h | 400 h | 600 h | 800 h | 1000 h |
|---|---|---|---|---|---|---|
| %R | 13.60 | 86.96 | 94.58 | 90.82 | 0.17 | 3.48 |
| %D | 0.004 | 95.36 | 97.58 | 92.69 | 18.49 | 0.00 |
| Ent(bit) | 0.0 | 7.42 | 5.93 | 5.57 | 2.93 | 0.00 |
| ML | 11 | 910 | 370 | 189 | 22 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Ochoa, E.M.; Suárez-Corrales, X.I.; Maldonado-Rivas, P.J.; Talavera-Pech, W.A.; Corvo, F. Non-Linear Evaluation of Coatings Performance: Evaluation of Polyester/Melamine Coil Coating Hydrolysis in NSS Test. Coatings 2023, 13, 1327. https://doi.org/10.3390/coatings13081327
García-Ochoa EM, Suárez-Corrales XI, Maldonado-Rivas PJ, Talavera-Pech WA, Corvo F. Non-Linear Evaluation of Coatings Performance: Evaluation of Polyester/Melamine Coil Coating Hydrolysis in NSS Test. Coatings. 2023; 13(8):1327. https://doi.org/10.3390/coatings13081327
Chicago/Turabian StyleGarcía-Ochoa, Esteban M., Xenia I. Suárez-Corrales, Pablo J. Maldonado-Rivas, William A. Talavera-Pech, and Francisco Corvo. 2023. "Non-Linear Evaluation of Coatings Performance: Evaluation of Polyester/Melamine Coil Coating Hydrolysis in NSS Test" Coatings 13, no. 8: 1327. https://doi.org/10.3390/coatings13081327
APA StyleGarcía-Ochoa, E. M., Suárez-Corrales, X. I., Maldonado-Rivas, P. J., Talavera-Pech, W. A., & Corvo, F. (2023). Non-Linear Evaluation of Coatings Performance: Evaluation of Polyester/Melamine Coil Coating Hydrolysis in NSS Test. Coatings, 13(8), 1327. https://doi.org/10.3390/coatings13081327






