Anti-Corrosion Behavior of Olmesartan for Soft-Cast Steel in 1 mol dm−3 HCl
Abstract
1. Introduction
2. Experimental Section
2.1. Soft Cast Steel
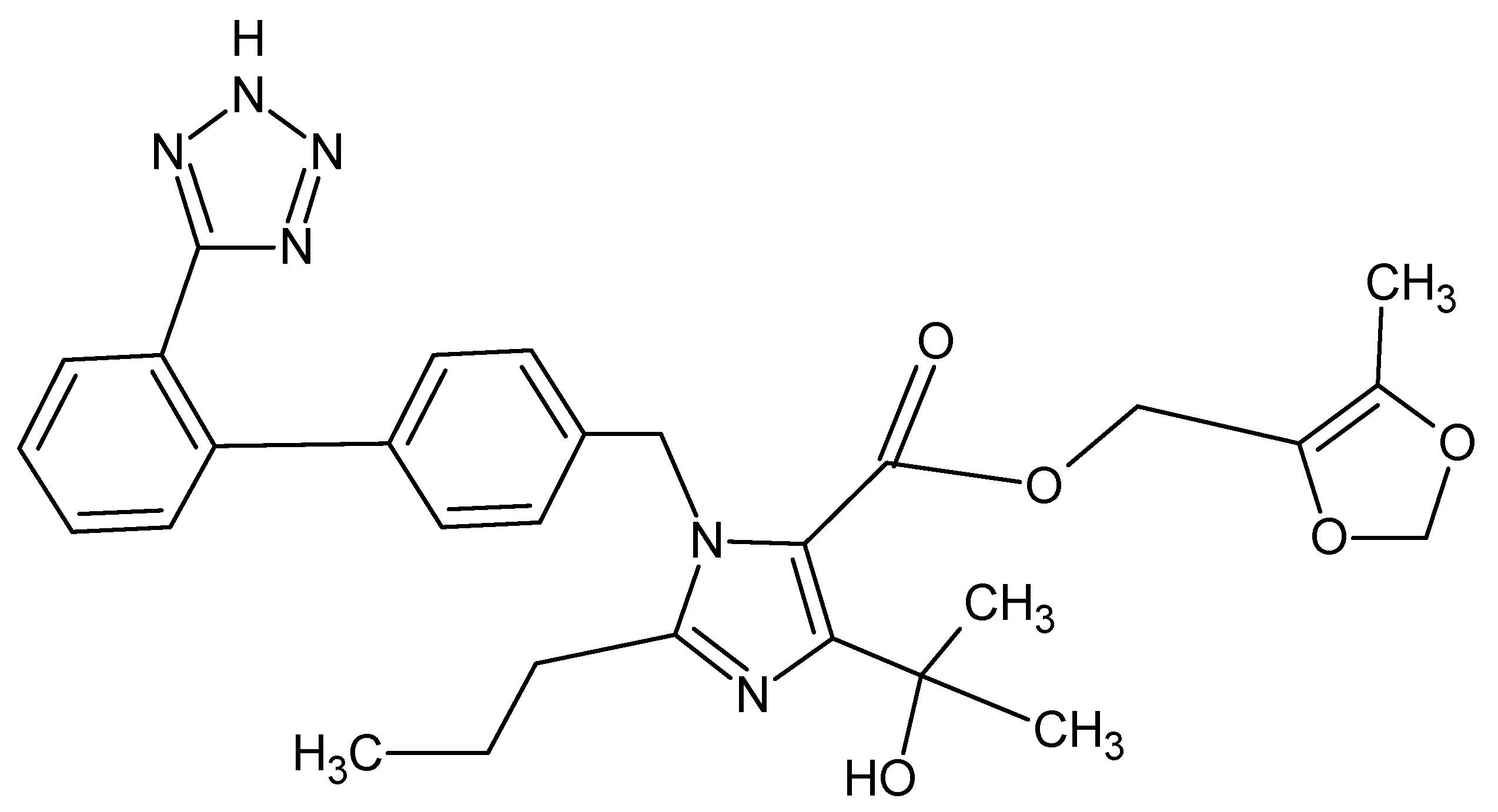
2.2. Inhibitor
2.3. Quantum Studies
2.4. Molecular Dynamics Simulations
2.5. Electrochemical Techniques
2.6. Scanning Electron Microscopy (SEM) Measurement
3. Result and Discussions
3.1. Quantum Calculations
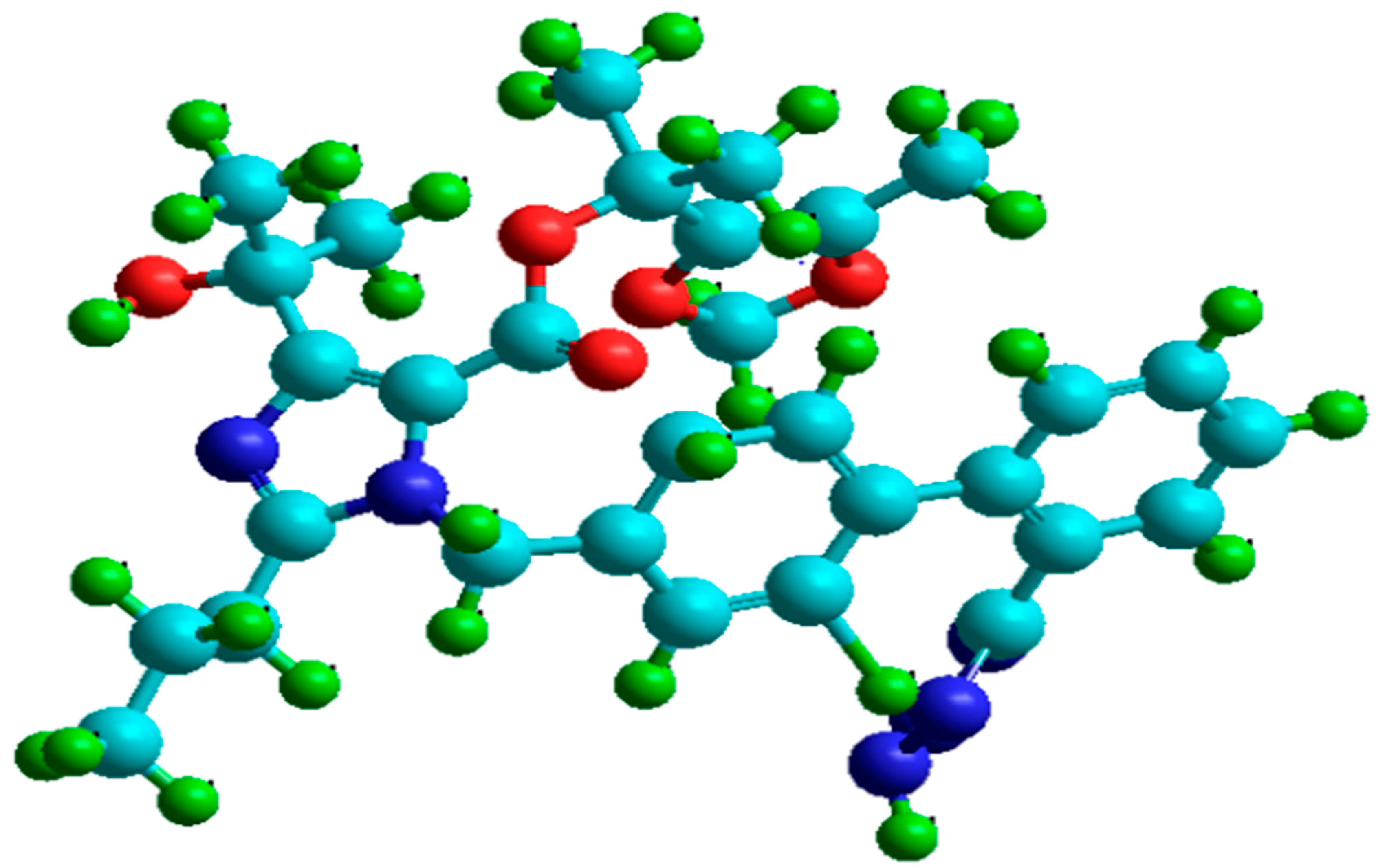
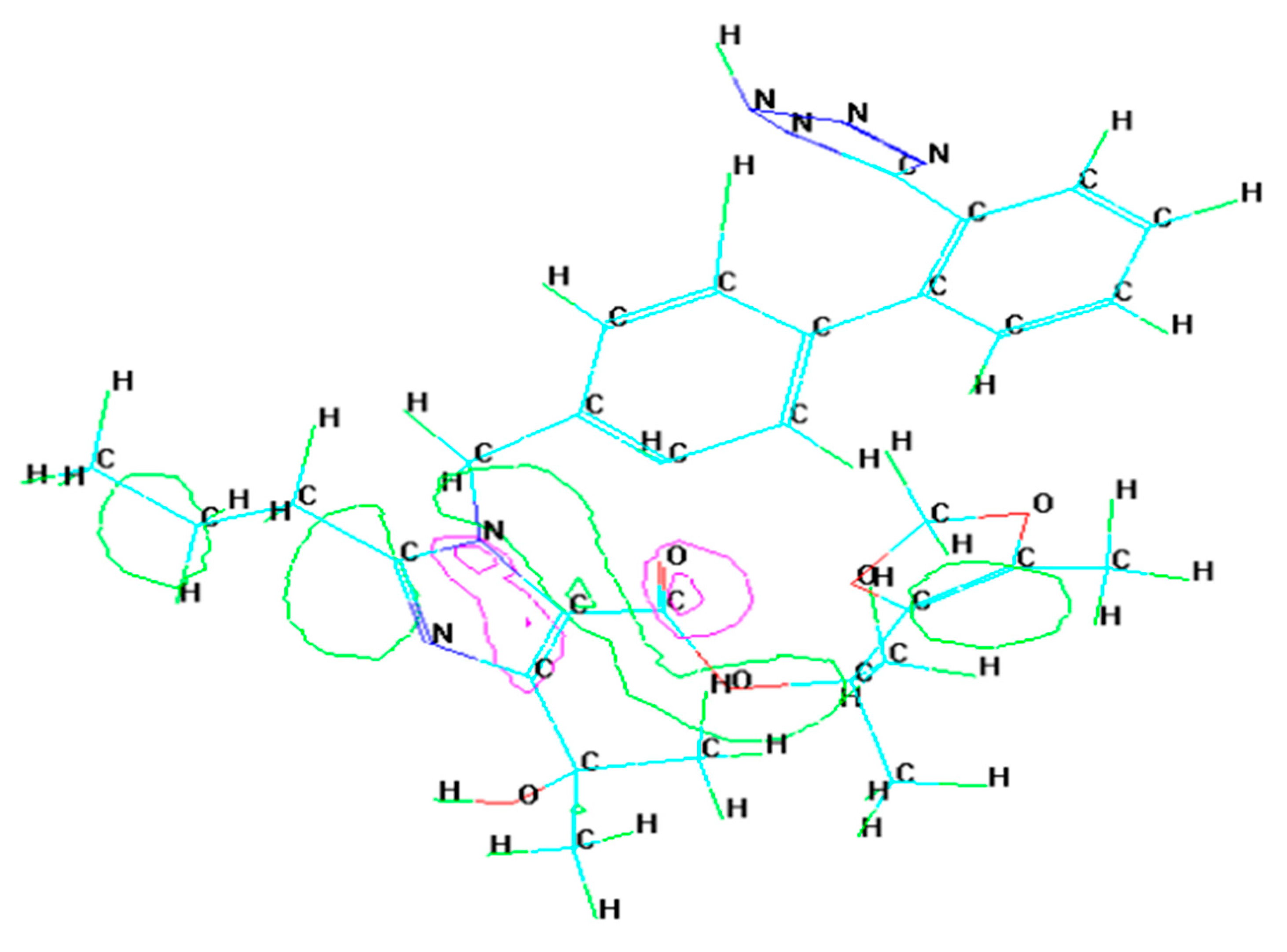
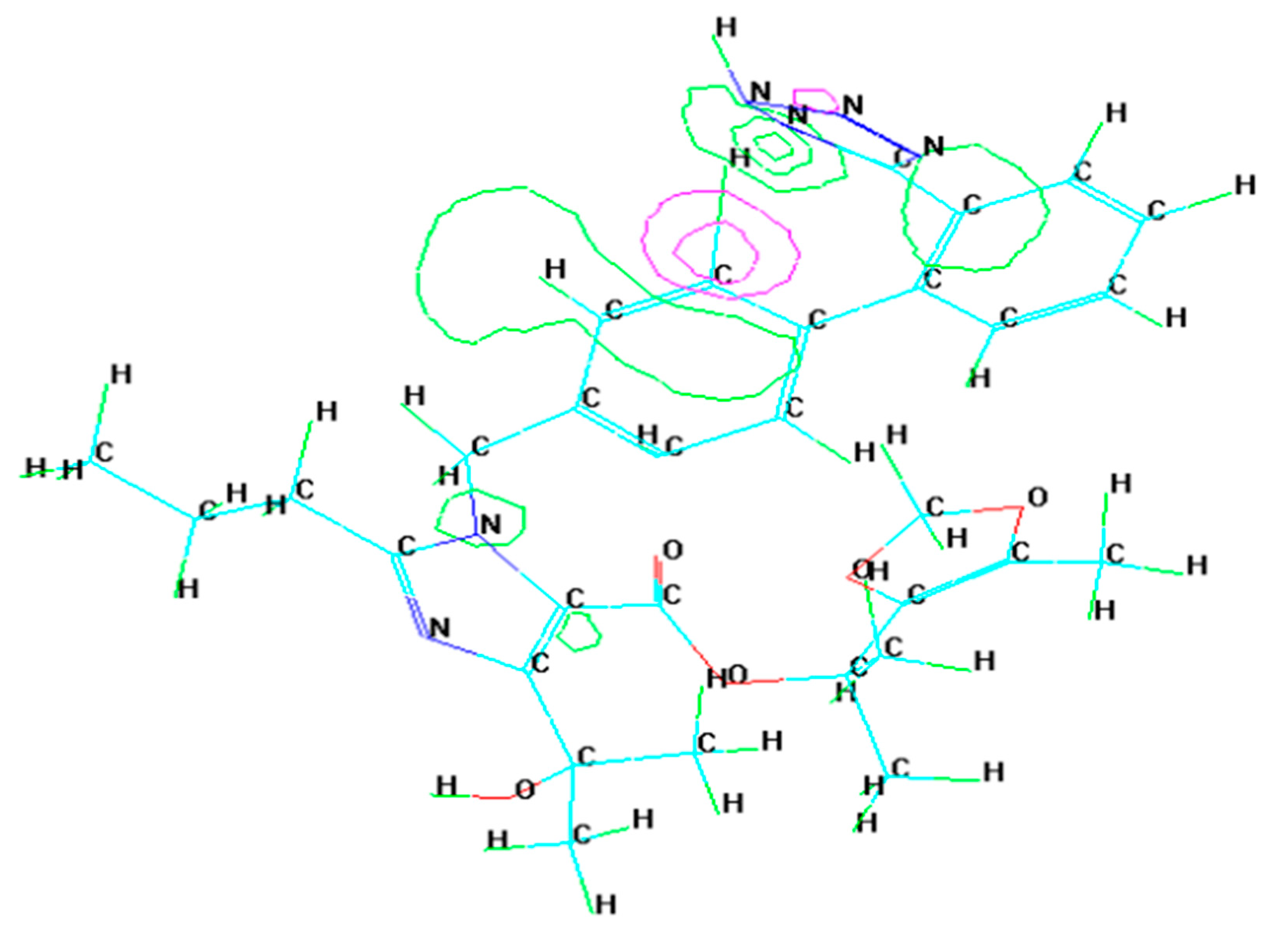
3.2. Molecular Dynamics Simulation
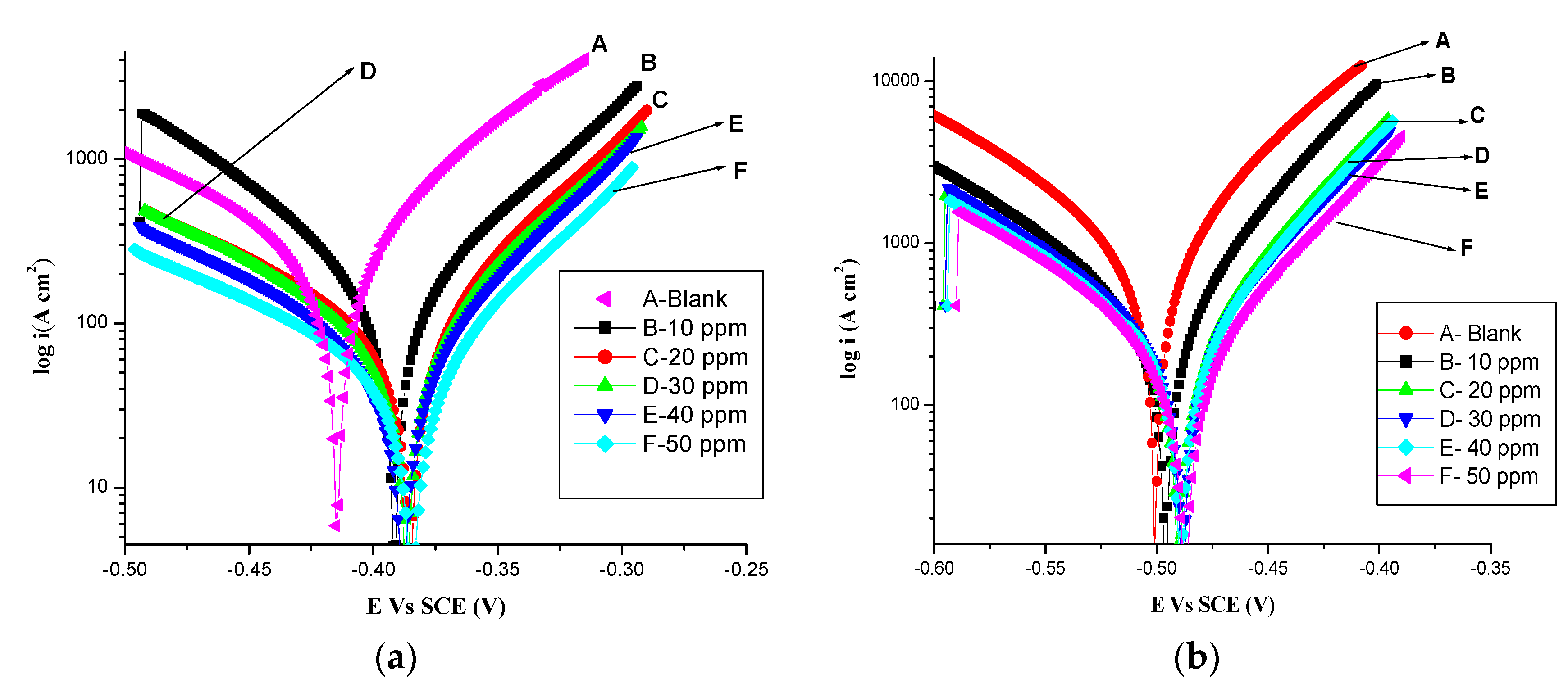
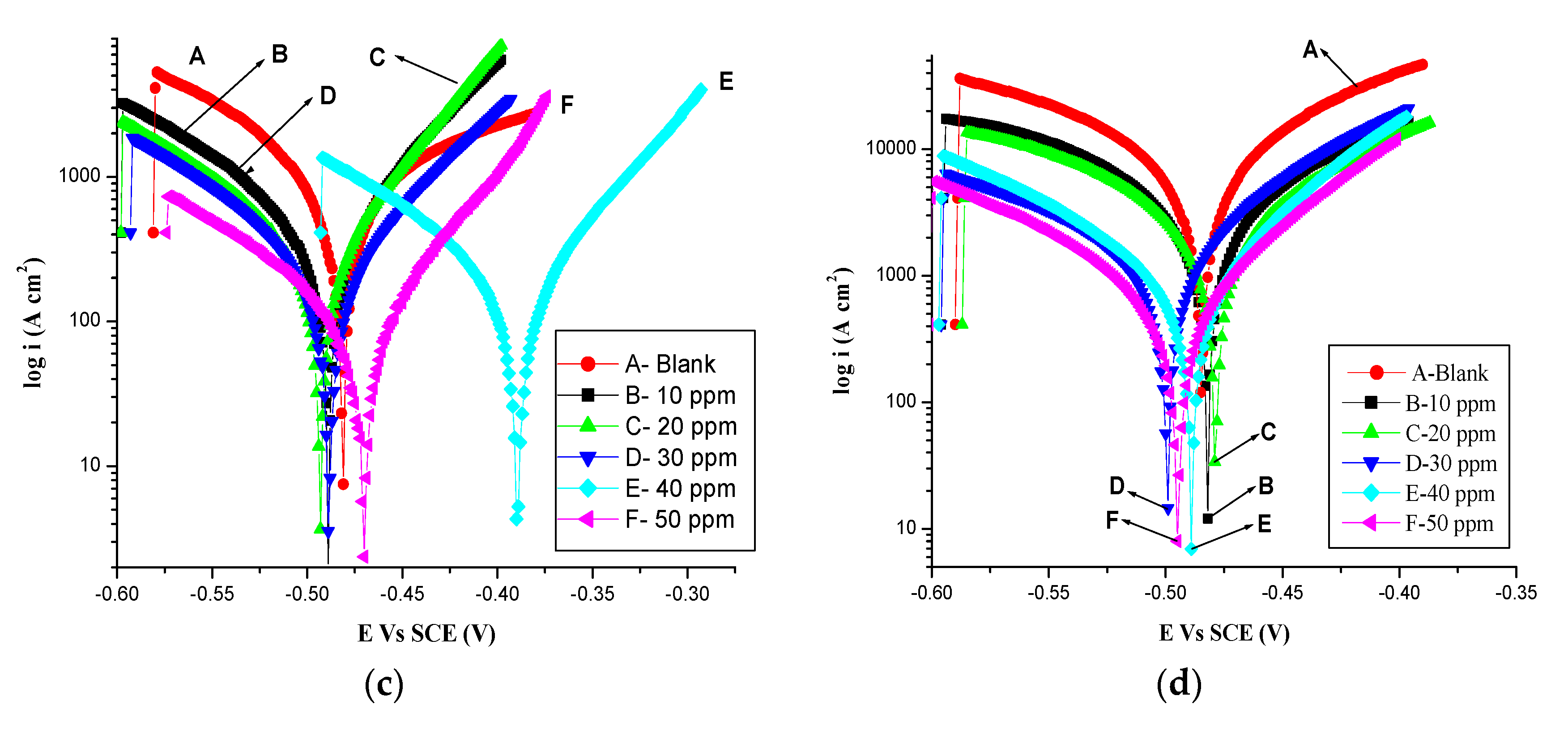
3.3. Potentiodynamic Polarization Measurements
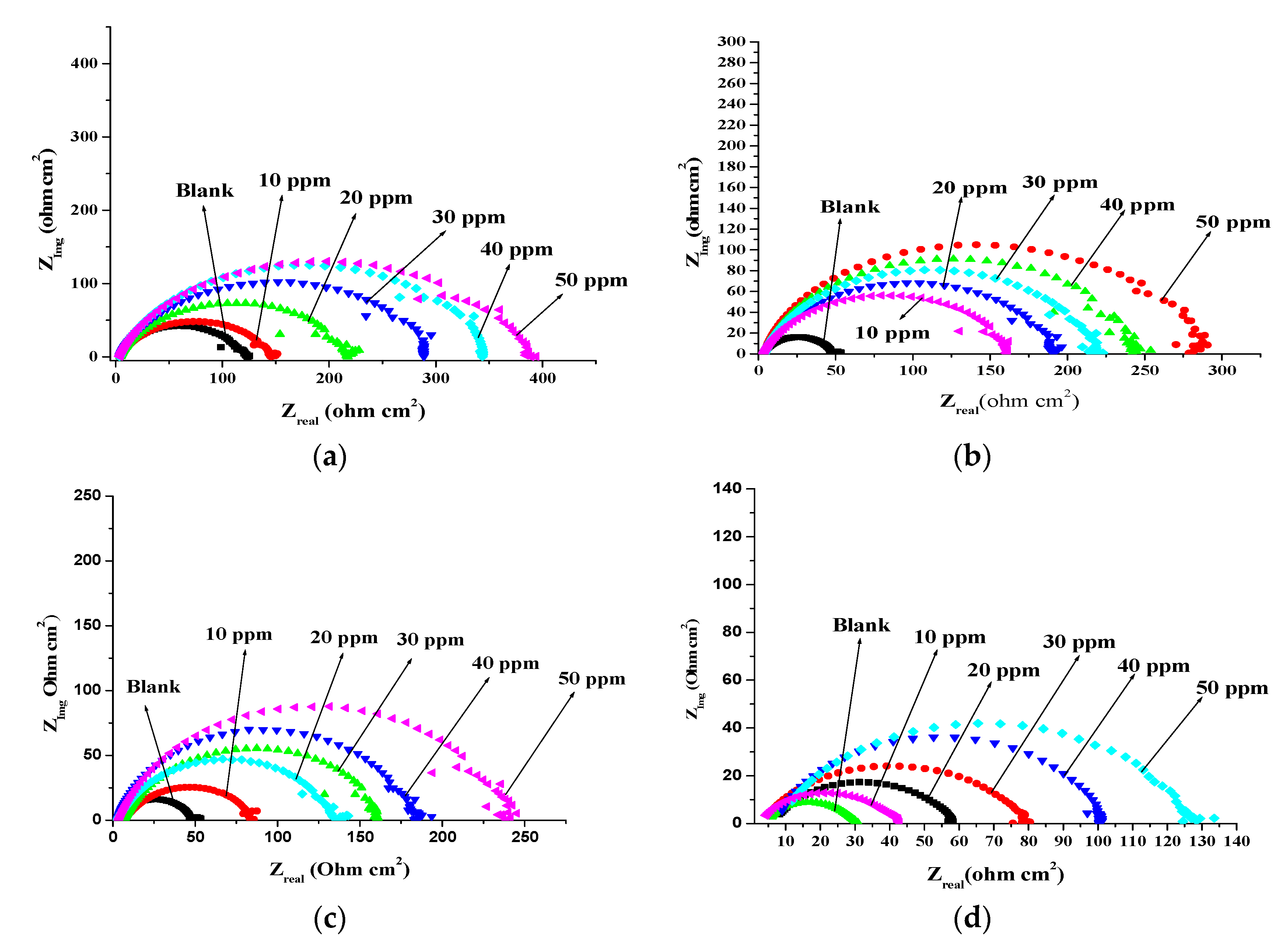
3.4. Electrochemical Impedance Spectroscopy (EIS) Measurement
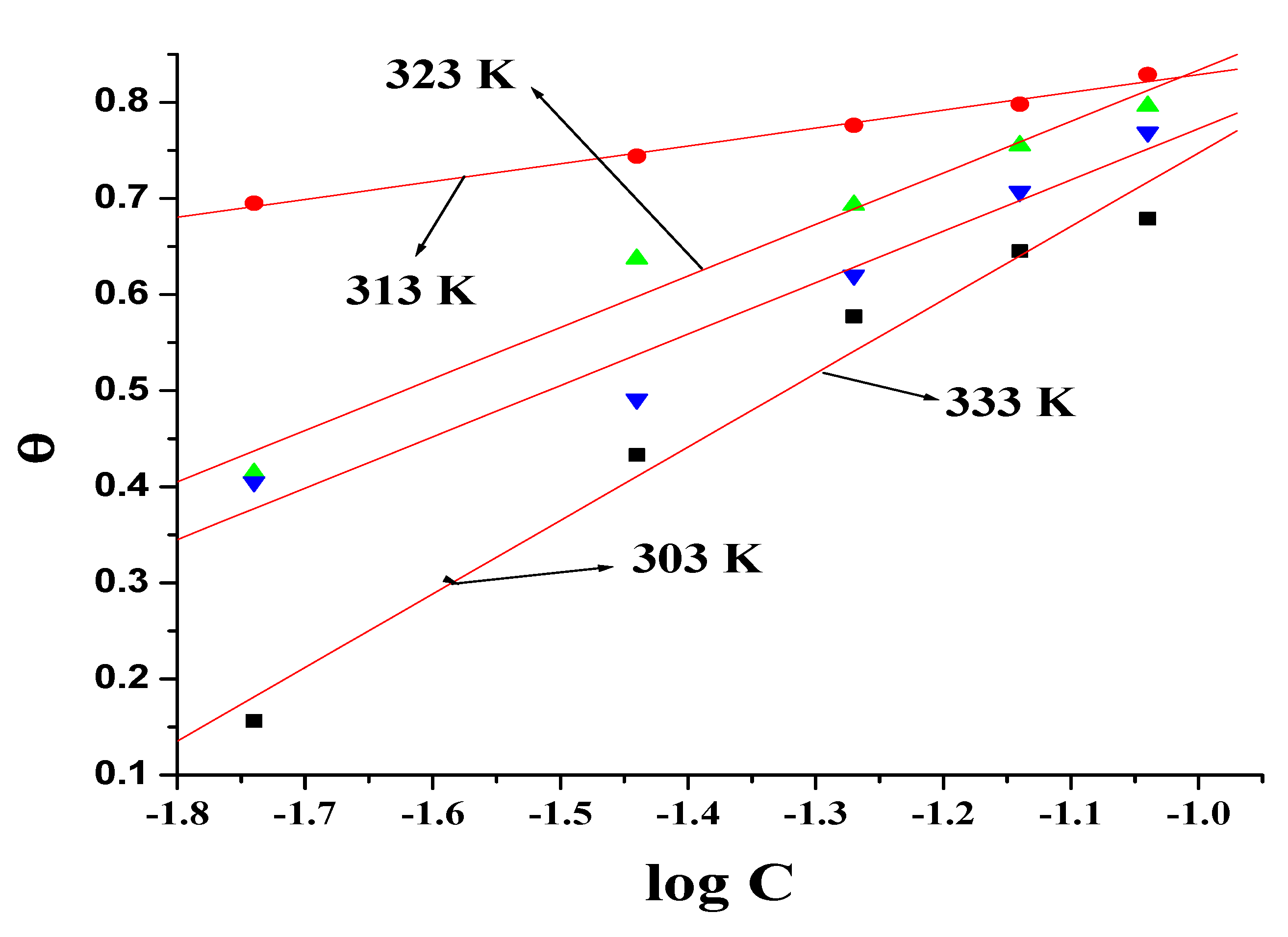
3.5. Thermodynamics
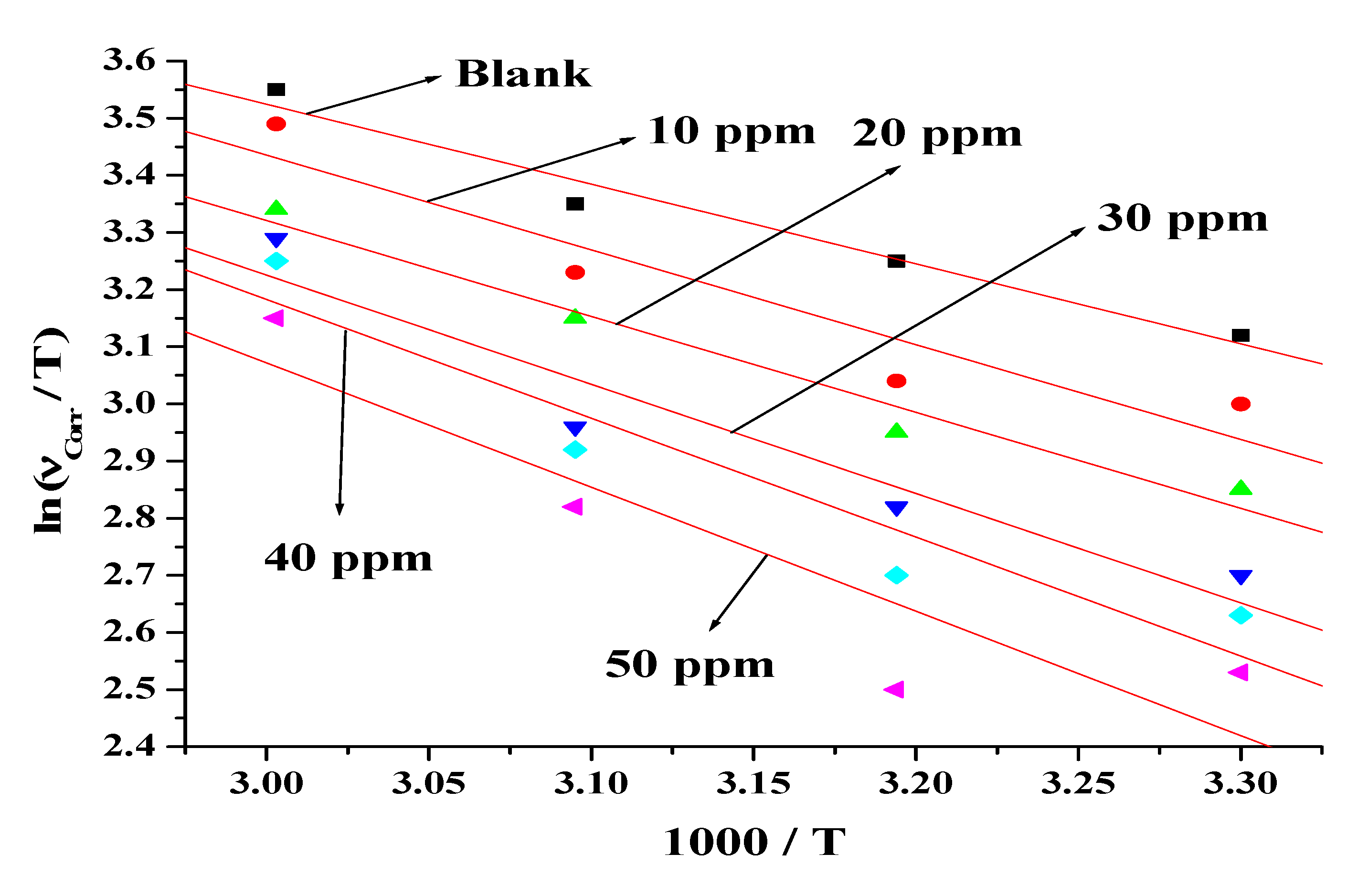
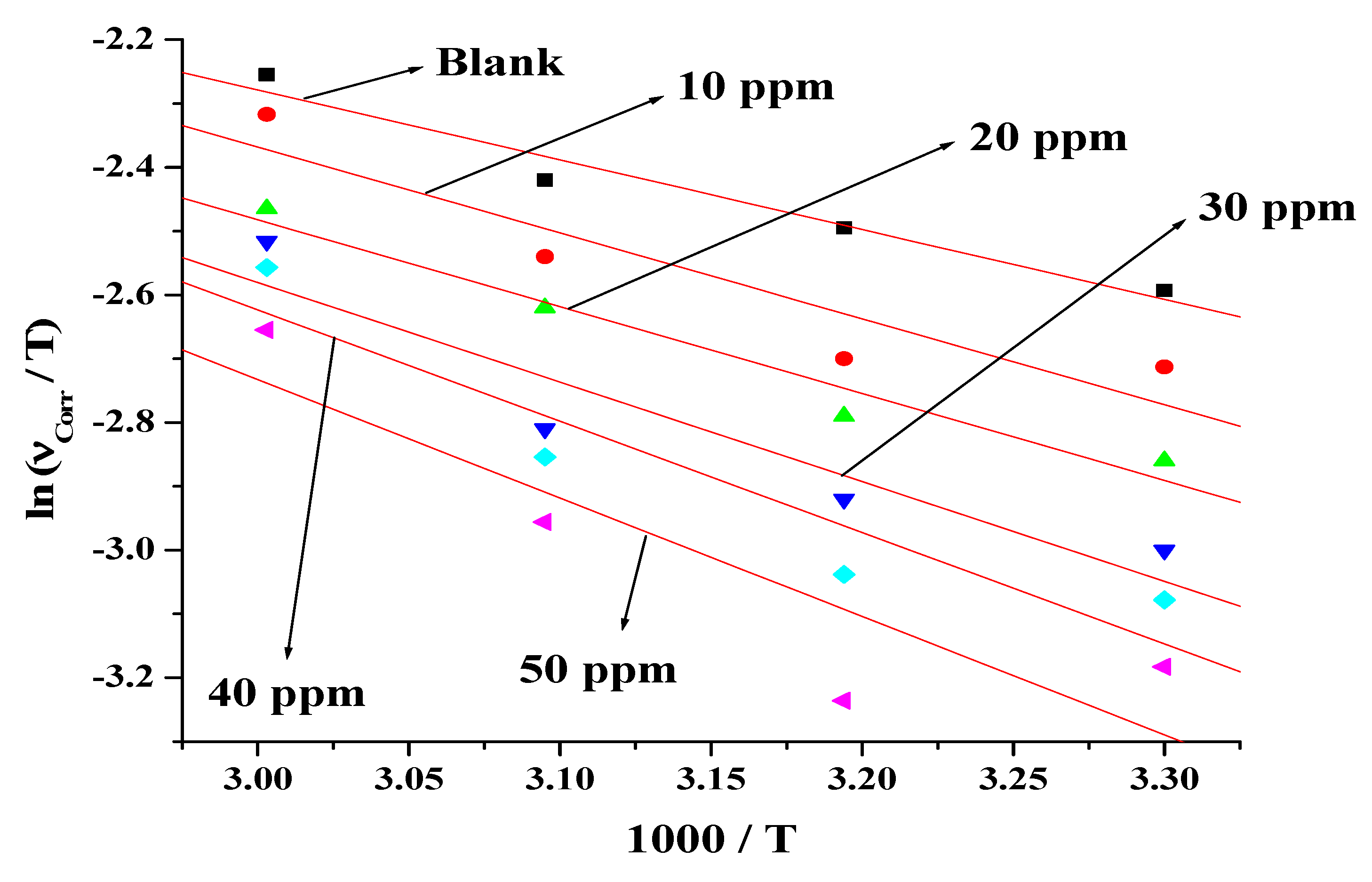
3.6. Activation Parameters
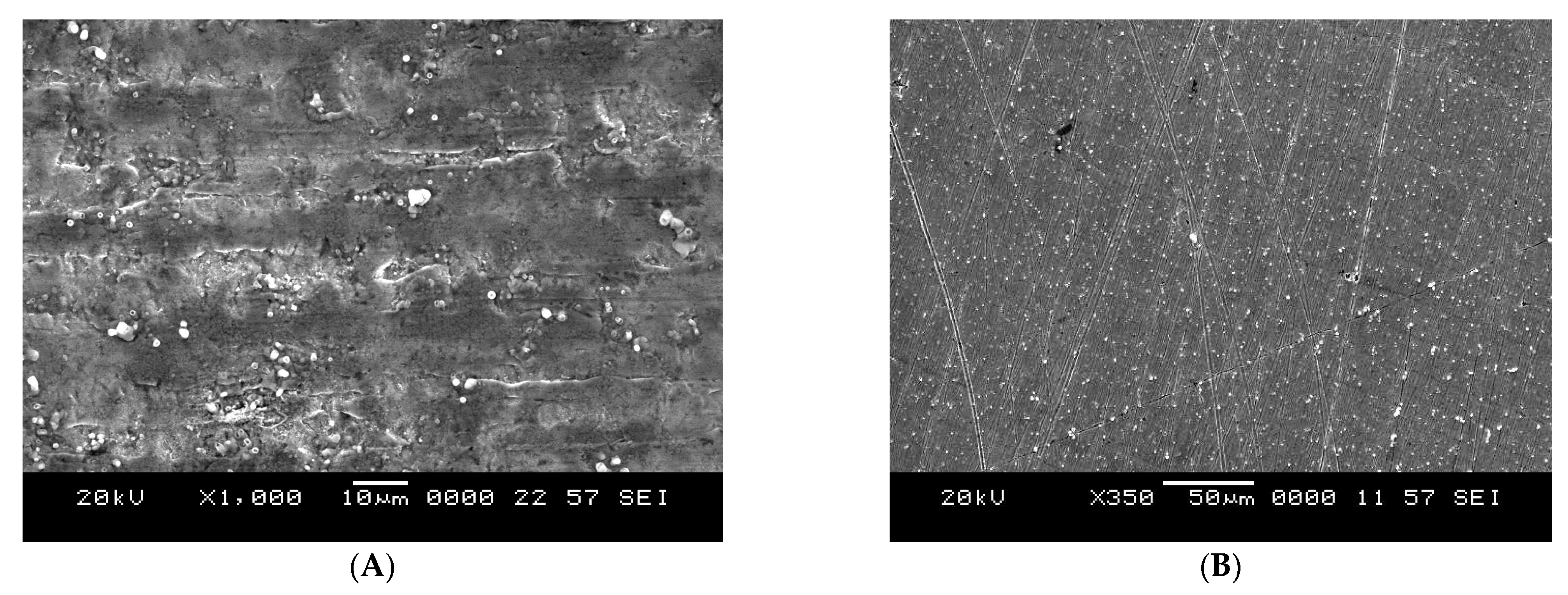
3.7. SEM Analysis
4. Conclusions
- Quantum chemical parameters of olmesartan include a low energy gap value (ΔE) = 7.026 eV and high dipole moment value (µ) = 7.83, suggesting that the olmesartan serves as an effective corrosion inhibitor for soft-cast steel in 1 mol dm−3 HCl.
- Molecular dynamic simulations anticipated spontaneous adsorption of olmesartan on the surface of soft-cast steel by involving C, N, and O elements. They also confirmed that the back-donation of electrons from the metal surface to the olmesartan molecule increases the stability of the inhibitor layer.
- Electrochemical measurements such as polarization and impedance spectroscopy suggest that an inhibitor’s inhibition efficiency increases in its increasing concentrations in 1 mol dm−3 HCl. The inhibitory activity of olmesartan is attributed to its adsorption onto the surface of soft-cast steel.
- Olmesartan adsorption obeyed Temkin’s isotherm model with the values of ΔG0ads within the −40 to −20 kJ/mol range suggesting a mixed-mode of physisorption and chemisorption.
- The activation parameters determine the effect of temperature on the inhibition efficiency of olmesartan in 1 mol dm−3 HCl for soft-cast steel. The inhibition efficiency of olmesartan decreases with the increasing the temperature.
- The SEM images for soft-cast steel in the presence of olmesartan in 1 mol dm−3 HCl, indicate the smooth and uniform adsorption process of the metal surface’s protective barrier.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.B.; Hua, Y.X. Corrosion inhibition of mild steel by alkyl imidazolium ionic liquids in hydrochloric acid. Electrochem. Acta 2009, 54, 1881. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Rawat, J. Inhibition of mild steel corrosion by some macrocyclic compounds in hot and concentrated hydrochloric acid. Mater. Chem. Phys. 2002, 73, 118. [Google Scholar] [CrossRef]
- Banuprakash, G.; Prasanna, B.M.; Santhosh, B.M.; Guruprasad, A.M. Corrosion Inhibitive Capacity of Vanillin-Based Schiff Base for Steel in 1 M HCl. J. Fail. Anal. Prev. 2021, 21, 89–96. [Google Scholar] [CrossRef]
- Lagrenee, M.; Bentiss, F.; Vezin, H.; Lagrenée, M. The inhibition of mild steel corrosion in acidic solutions by 2,5-bis(4-pyridyl)-1,3,4-thiadiazole: Structure-activity correlation. Corros. Sci. 2006, 48, 1279. [Google Scholar] [CrossRef]
- Matad, P.B.; Mokshanatha, P.B.; Hebbar, N.; Venkatesha, V.T.; Tandon, H.C. Ketosulfone Drug as a Green Corrosion Inhibitor for Mild Steel in Acidic Medium. Ind. Eng. Chem. Res. 2014, 53, 8436. [Google Scholar] [CrossRef]
- Guruprasad, A.M.; Sachin, H.P.; Swetha, G.A.; Prasanna, B.M. Corrosion inhibition of zinc in 0.1 M hydrochloric acid medium with clotrimazole: Experimental, theoretical and quantum studies. Surf. Interfaces 2020, 19, 100478. [Google Scholar] [CrossRef]
- Swetha, G.A.; Sachin, H.P.; Guruprasad, A.M.; Prasanna, B.M. Rizatriptan Benzoate as Corrosion Inhibitor for Mild Steel in Acidic Corrosive Medium: Experimental and Theoretical Analysis. J. Fail. Anal. Prev. 2019, 19, 1113. [Google Scholar] [CrossRef]
- Hebbar, N.; Praveen, B.M.; Prasanna, B.M.; Deepa, A. Electrochemical and Adsorption Studies of Telmisartan for Mild Steel in Acidic Medium. J. Bio Tribo Corros. 2019, 5, 40. [Google Scholar] [CrossRef]
- Guruprasad, A.M.; Sachin, H.P.; Swetha, G.A.; Prasanna, B.M. Adsorption and inhibitive properties of Seroquel drug for zinc corrosion in 0.1 M hydrochloric acid solution. Int. J. Ind. Chem. 2019, 10, 17. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Praveen, B.M.; Hebbar, N.; Pavitra, M.K.; Manjunatha, T.S.; Malladi, R.S. Theoretical and experimental approach of inhibition effect by sulfamethoxazole on mild steel corrosion in 1-M HCl. Surf. Interface Anal. 2018, 50, 779. [Google Scholar] [CrossRef]
- Praveen, B.M.; Prasanna, B.M.; Hebbar, N.; Kumar, P.S.; Jagadeesh, M.R. Experimental and Theoretical Studies on Inhibition Effect of the Praziquantel on Mild Steel Corrosion in 1 M HCl. J. Bio Tribo Corros. 2018, 4, 21. [Google Scholar] [CrossRef]
- Hebbar, N.; Praveen, B.M.; Prasanna, B.M.; Venkatarangaiah, V.T.; Abd Hamid, S.B. Adsorption, thermodynamic, and electrochemical studies of Ketosulfide for mild steel in acidic medium. J. Adhes. Sci. Technol. 2015, 29, 2692. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Praveen, B.M.; Hebbar, N.; Venkatesha, T.V.; Tandon, H.C.; Abd Hamid, S.B. Electrochemical study on the inhibitory effect of Aspirin on mild steel in 1M hydrochloric acid. J. Assoc. Arab. Univ. Basic Appl. Sci. 2017, 22, 62. [Google Scholar] [CrossRef]
- Hebbar, N.; Praveen, B.M.; Prasanna, B.M.; Venkatesha, T.V.; Abd Hamid, S.B. Anthranilic Acid as Corrosion Inhibitor for Mild Steel in Hydrochloric Acid Media. Procedia Mater. Sci. 2014, 5, 712. [Google Scholar] [CrossRef][Green Version]
- Praveen, B.M.; Prasanna, B.M.; Mallikarjuna, N.M.; Jagadeesh, M.R.; Hebbar, N.; Rashmi, D. Investigation of anticorrosive behaviour of novel tert-butyl 4-[(4-methyl phenyl) carbonyl] piperazine-1-carboxylate for carbon steel in 1M HCl. Heliyon 2021, 7, e06090. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjuna, N.M.; Keshavayya, J.; Prasanna, B.M.; Praveen, B.M.; Tandon, H.C. Synthesis, Characterization, and Anti-Corrosion Behavior of Novel Mono Azo Dyes Derived from 4,5,6,7-Tetrahydro-1,3-benzothiazole for Mild Steel in Acid Solution. J. Bio Tribo Corros. 2020, 6, 9. [Google Scholar] [CrossRef]
- Padmashree, B.; Manjunatha, K.; Prasanna, B.M. Electrochemical Behavior of 1,3-bis(1-Phenylethyl) Urea as a Corrosion Inhibitor for Carbon Steel in 1 M HCl. J. Fail. Anal. Prev. 2020, 20, 226–234. [Google Scholar] [CrossRef]
- Rajendraprasad, S.; Ali, S.; Prasanna, B.M. Electrochemical Behavior of N1-(3-Methylphenyl) Piperidine-1,4-Dicarboxamide as a Corrosion Inhibitor for Soft-Cast Steel Carbon Steel in 1 M HCl. J. Fail. Anal. Prev. 2020, 20, 235. [Google Scholar] [CrossRef]
- Narayana, H.; Praveen, B.M.; Prasanna, B.M.; Vishwanath, P. Electrochemical and adsorption studies of 4-Chloro, 8-(Trifluoromethyl) Quinoline (CTQ) for mild steel in acidic media. J. Fail. Anal. Prev. 2020, 20, 1516–1523. [Google Scholar] [CrossRef]
- Haris, N.I.N.; Sobri, S.; Yusof, Y.A.; Kassim, N.K. An Overview of Molecular Dynamic Simulation for Corrosion Inhibition of Ferrous Metals. Metals 2021, 11, 46. [Google Scholar] [CrossRef]
- Maricica, S.; Lina, M.; Almira, R.; Petru, A.; Geta, C.; Rodica, D.; Jaroslav, V.; Arūnas, R. Corrosion Study of Stainless Steel Incubated in Solutions Consisting of Biocide (Oxonia-Active) and Aspergillus niger Suspension. Chemija 2012, 23, 180. [Google Scholar]
- Hu, Y.; Xin, L.; Liu, T.; Lu, Y. Corrosion Behavior of L245N Standard Steel in CO2 Saturated Brine under Flow Condition. Metals 2021, 11, 880. [Google Scholar] [CrossRef]
- Mu, G.N.; Li, X.; Li, F. Synergistic inhibition between o-phenanthroline and chloride ion on cold-rolled steel corrosion in phosphoric acid. Mater. Chem. Phys. 2004, 86, 59. [Google Scholar] [CrossRef]
- Ji, G.; Shukla, S.K.; Dwivedi, P.; Sundaram, S.; Prakash, R. Inhibitive Effect of Argemone mexicana Plant Extract on Acid Corrosion of Mild Steel. Ind. Eng. Chem. Res. 2011, 50, 11954–11959. [Google Scholar] [CrossRef]
- Yadav, M.; Behera, D.; Kumar, S.; Sinha, R.R. Studied corrosion inhibition performance of three Benzimidazole derivatives for mild steel in HCl. Ind. Eng. Chem. Res. 2013, 52, 6318. [Google Scholar] [CrossRef]
- Zhang, J.; Gong, X.L.; Yu, H.H.; Du, M. The inhibition mechanism of imidazoline phosphate inhibitor for Q235 steel in hydrochloric acid medium. Corros. Sci. 2011, 53, 3324. [Google Scholar] [CrossRef]
- Nataraja, S.E.; Venkatesha, T.V.; Manjunatha, K.; Poojary, B.; Pavithra, M.K.; Tandon, H.C. Inhibition of the corrosion of steel in hydrochloric acid solution by some organic molecules containing the methylthiophenyl moiety. Corros. Sci. 2011, 58, 2651–2659. [Google Scholar] [CrossRef]
- Badiea, A.M.; Mohana, K.N. Effect of temperature and fluid velocity on corrosion mechanism of low carbon steel in the presence of 2-hydrazino-4,7-dimethylbenzothiazole in industrial water medium. Corros. Sci. 2009, 51, 2231. [Google Scholar] [CrossRef]
- Hebbar, N.; Praveen, B.M.; Prasanna, B.M.; Sachin, H.P. Anticorrosion Potential of Flectofenine on Mild Steel in Hydrochloric Acid Media: Experimental and Theoretical Study. J. Fail. Anal. Prev. 2018, 18, 371. [Google Scholar] [CrossRef]
- Firdhouse, M.J.; Nalini, D. Corrosion Inhibition of Mild Steel in Acidic Media by 5′-Phenyl-2′,4′-dihydrospiro[indole-3,3′-pyrazol]-2(1H)-one. J. Chem. 2013, 2013, 1. [Google Scholar] [CrossRef]
- Laarej, K.; Bouachrine, M.; Radi, S.; Kertit, S.; Hammouti, B.E. Quantum Chemical Studies on the Inhibiting Effect of Bipyrazoles on Steel Corrosion in HCl. J. Chem. 2010, 7, 419. [Google Scholar] [CrossRef]
- Ali, S.A.; El-Shareef, A.M.; Al-Ghandi, R.F.; Saeed, M.T. Synthesis of aza-pseudo peptides and the evaluation of their inhibiting efficacy of mild steel corrosion in 1.0 M HCl. Corros. Sci. 2005, 47, 2659. [Google Scholar] [CrossRef]
- Jayaperumal, D. Effects of alcohol-based inhibitors on corrosion of mild steel in hydrochloric acid. Mater. Chem. Phys. 2010, 119, 478. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Praveen, B.M.; Hebbar, N.; Venkatesha, T.V.; Tandon, H.C. Inhibition study of mild steel corrosion in 1 M hydrochloric acid solution by 2-chloro 3-formyl quinoline. Int. J. Ind. Chem. 2016, 7, 9. [Google Scholar] [CrossRef]
- Ahmad, I.; Prasad, R.; Quraishi, M.A. Adsorption and inhibitive properties of some new Mannich bases of Isatin derivatives on corrosion of mild steel in acidic media. Corros. Sci. 2010, 52, 1472. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, G. The synergistic inhibition effect of oleic-based imidazoline and sodium benzoate on mild steel corrosion in a CO2-saturated brine solution. Electrochim. Acta 2012, 69, 247. [Google Scholar] [CrossRef]
- Swetha, G.A.; Sachin, H.P.; Guruprasad, A.M.; Prasanna, B.M.; Sudheer Kumar, K.H. Use of Seroquel as an Effective Corrosion Inhibitor for Low Carbon Steel in 1 M HCl. J. Bio Tribo Corros. 2018, 4, 57. [Google Scholar] [CrossRef]
- Banuprakash, G.; Prasanna, B.M.; Hebbar, N.; Manjunatha, T.S. Inhibitive Capability of a Novel Schiff Base for Steel in 1 M HCl Media. J Fail. Anal. Prev. 2020, 20, 572–579. [Google Scholar] [CrossRef]
- Umoren, S.A.; Ogbobe, O.; Igwe, I.O.; Ebenso, E.E. Inhibition of mild steel corrosion in acidic medium using synthetic and naturally occurring polymers and synergistic halide additives. Corros. Sci. 2008, 50, 1998. [Google Scholar] [CrossRef]
- Badiea, A.M.; Mohana, M.N. Novel cationic surfactants from fatty acids and their corrosion inhibition efficiency for carbon steel pipelines in 1 M HCl. Corros. Sci. 2009, 51, 2231. [Google Scholar] [CrossRef]
- Ghasemi1, O.; Danaee1, I.; Rashed, G.R.; RashvandAvei, M.; Maddahy, M.H. Inhibition effect of a synthesized N, N′-bis(2-hydroxybenzaldehyde)-1,3-propandiimine on corrosion of mild steel in HCl. J. Cent. South Univ. 2013, 20, 301–311. [Google Scholar] [CrossRef]
- Fouda, A.S.; Heakal, F.E.; Radwan, M.S. Role of some thiadiazole derivatives as inhibitors for the corrosion of C-steel in 1 M H2SO4. J. Appl. Electrochem. 2009, 39, 391. [Google Scholar] [CrossRef]
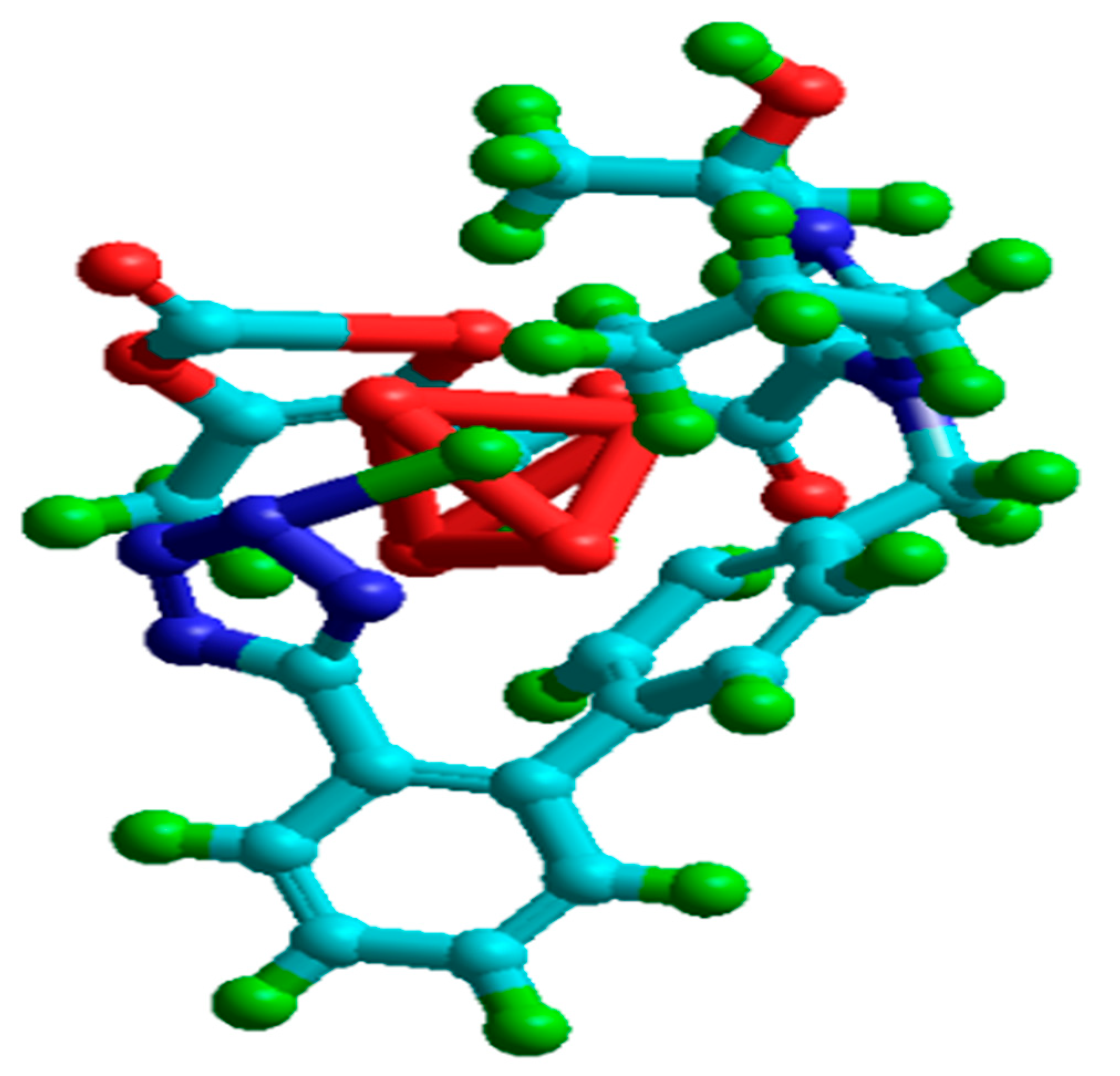
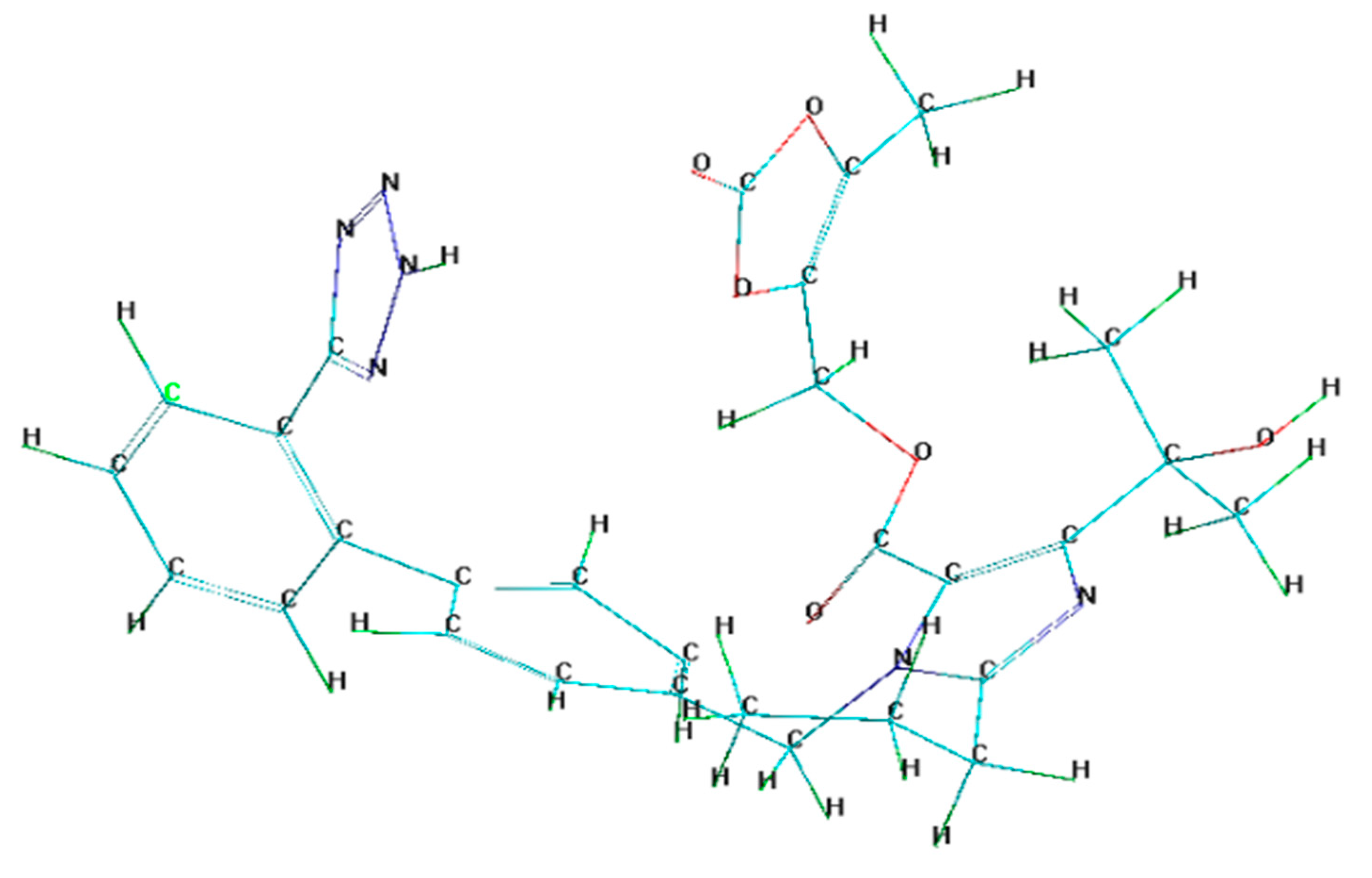
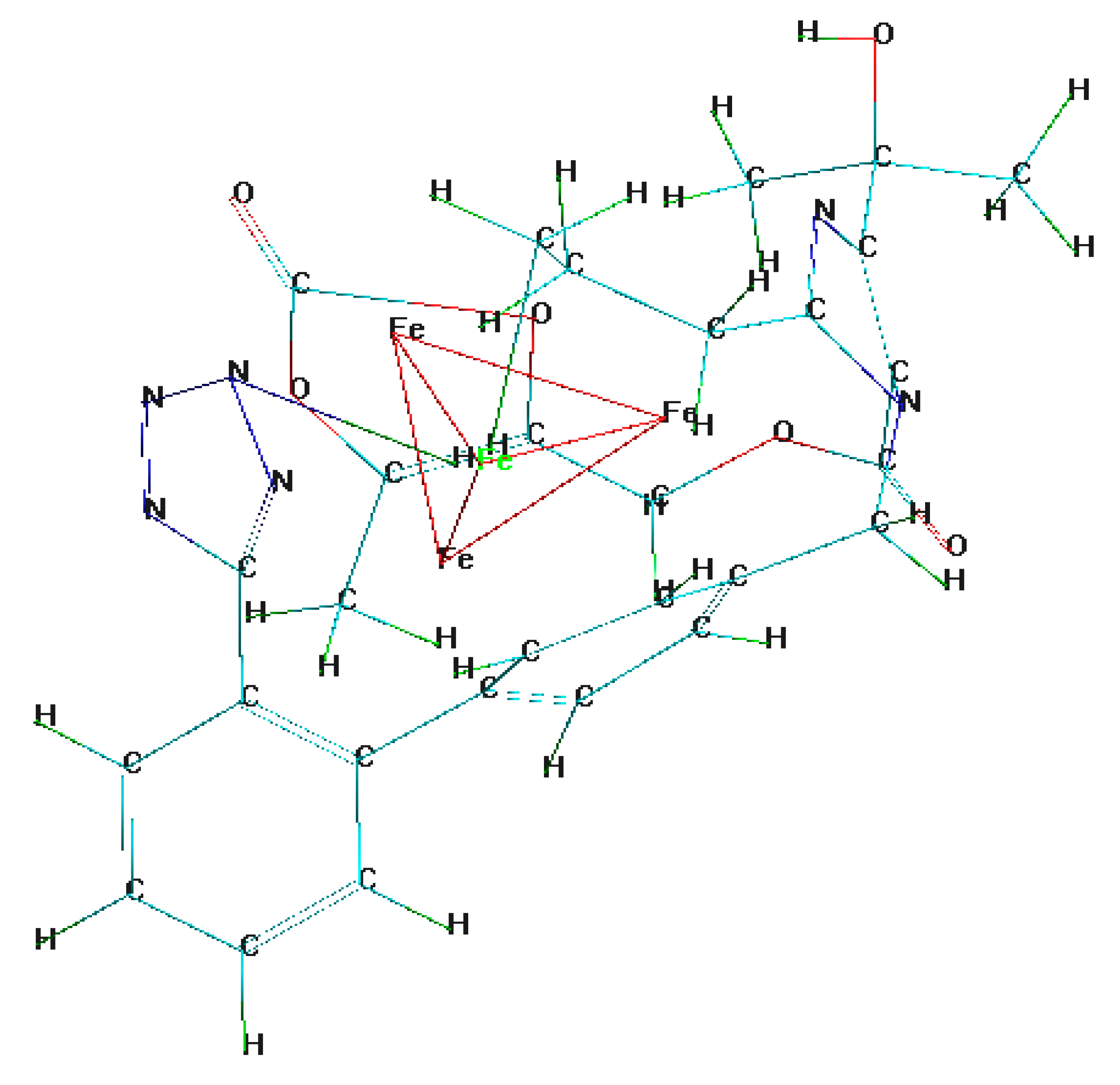

| Elements | C | Mn | P | S | (Fe) |
|---|---|---|---|---|---|
| (%) | 0.41 | 0.029 | 0.031 | 0.04 | Rest |
| Sl. No. | Quantum Chemical Parameters | Olmesartan |
|---|---|---|
| 1 | Molecular Formula | C29H32N6O5 |
| 2 | Molecular Weight | 544.60 amu |
| 3 | Total Energy | −244.84 a.u |
| 4 | EHOMO | −8.182 eV |
| 5 | ELUMO | −1.156 eV |
| 6 | ΔE = ELUMO − EHOMO (eV) | 7.026 eV |
| 7 | Dipole Moment (µ) | 7.83 Debye |
| 8 | Ionization Potential, (I) | 8.182 |
| 9 | Electron Affinity (A) | 1.156 |
| 10 | Electronegativity (χ) | 4.669 |
| 11 | Global hardness (ŋ) | 3.513 |
| 12 | Global Softness (σ) | −3.513 |
| 13 | Chemical Potential (α) | −4.669 |
| Temp (K) | Concentration of Olmesartan (ppm) | Ecorr (V) | icorr (μA cm−2) | νcorr (mpy) | βc (mV/dec) | βa mV/dec | ηp % |
|---|---|---|---|---|---|---|---|
| 303 | Blank | −0.415 | 0.228 | 0.369 | −121 | 75 | - |
| 10 | −0.389 | 0.180 | 0.211 | −84 | 74 | 21.05 | |
| 20 | −0.388 | 0.100 | 0.121 | −121 | 68 | 56.14 | |
| 30 | −0.387 | 0.090 | 0.105 | −111 | 70 | 60.52 | |
| 40 | −0.386 | 0.084 | 0.082 | −117 | 64 | 63.15 | |
| 50 | −0.383 | 0.059 | 0.070 | −135 | 67 | 69.29 | |
| 313 | Blank | −0.500 | 0.730 | 1.81 | −102 | 70 | - |
| 10 | −0.495 | 0.297 | 0.481 | −099 | 59 | 59.25 | |
| 20 | −0.486 | 0.220 | 0.356 | −109 | 62 | 69.81 | |
| 30 | −0.486 | 0.211 | 0.341 | −102 | 65 | 71.09 | |
| 40 | −0.486 | 0.200 | 0.324 | −106 | 61 | 72.50 | |
| 50 | −0.484 | 0.172 | 0.278 | −103 | 65 | 76.38 | |
| 323 | Blank | −0.481 | 0.733 | 8.514 | −104 | 156 | - |
| 10 | −0.486 | 0.327 | 0.547 | −66 | 105 | 55.33 | |
| 20 | −0.489 | 0.250 | 0.415 | −60 | 106 | 65.89 | |
| 30 | −0.485 | 0.241 | 0.312 | −71 | 102 | 67.12 | |
| 40 | −0.386 | 0.230 | 0.248 | −67 | 105 | 68.62 | |
| 50 | −0.468 | 0.202 | 0.146 | −65 | 109 | 72.44 | |
| 333 | Blank | −0.481 | 0.903 | 10.68 | −129 | 96 | - |
| 10 | −0.472 | 0.495 | 4.776 | −90 | 136 | 45.18 | |
| 20 | −0.472 | 0.445 | 3.672 | −128 | 92 | 50.71 | |
| 30 | −0.502 | 0.402 | 2.074 | −120 | 81 | 55.48 | |
| 40 | −0.487 | 0.302 | 1.270 | −96 | 61 | 66.55 | |
| 50 | −0.491 | 0.281 | 0.908 | −101 | 64 | 68.88 |
| Temp | Concentration of Olmesartan (ppm) | Rp Ω cm2 | Cdl (F/cm2) | ηz (%) | Surface Coverage θ |
|---|---|---|---|---|---|
| 303 K | Blank | 120.7 | 0.032 | - | - |
| 10 | 143.1 | 0.028 | 15.65 | 0.156 | |
| 20 | 212.9 | 0.026 | 43.30 | 0.433 | |
| 30 | 285.7 | 0.013 | 57.75 | 0.577 | |
| 40 | 340.0 | 0.010 | 64.50 | 0.645 | |
| 50 | 376.9 | 0.010 | 67.97 | 0.679 | |
| 313 K | Blank | 48.12 | 0.021 | - | - |
| 10 | 158.00 | 0.014 | 69.54 | 0.695 | |
| 20 | 188.00 | 0.013 | 74.40 | 0.744 | |
| 30 | 215.00 | 0.010 | 77.61 | 0.776 | |
| 40 | 238.60 | 0.012 | 79.83 | 0.798 | |
| 50 | 281.80 | 0.009 | 82.92 | 0.829 | |
| 323 K | Blank | 48.12 | 0.024 | - | - |
| 10 | 82.22 | 0.023 | 41.47 | 0.414 | |
| 20 | 132.80 | 0.020 | 63.76 | 0.637 | |
| 30 | 157.20 | 0.017 | 69.38 | 0.693 | |
| 40 | 196.50 | 0.015 | 75.51 | 0.755 | |
| 50 | 236.00 | 0.013 | 79.61 | 0.796 | |
| 333 K | Blank | 30.35 | 0.0321 | - | - |
| 10 | 41.25 | 0.0315 | 40.51 | 0.405 | |
| 20 | 59.68 | 0.0305 | 49.14 | 0.491 | |
| 30 | 79.98 | 0.0236 | 62.05 | 0.620 | |
| 40 | 101.10 | 0.0226 | 70.75 | 0.707 | |
| 50 | 131.60 | 0.0212 | 76.93 | 0.769 |
| Temperature (K) | Kads (kJ/mol) | ΔG0ads (kJ/mol) |
|---|---|---|
| 303 | 661.37 | −26.47 |
| 313 | 986.19 | −28.39 |
| 323 | 730.46 | −28.49 |
| 333 | 765.11 | −29.50 |
| Concentration of Olmesartan (ppm) | Ea* (kJ/mol) | A (g/cm2/h) | ΔH* (kJ/mol) | ΔS* (J/mol/K−1) |
|---|---|---|---|---|
| Blank | 11.61 | 22.46 × 102 | 9.09 | −22.80 |
| 10 | 13.79 | 45.05 × 102 | 11.27 | −22.10 |
| 20 | 13.95 | 42.59 × 102 | 11.44 | −22.16 |
| 30 | 15.89 | 78.00 × 102 | 13.11 | −21.65 |
| 40 | 17.29 | 123.82 × 102 | 14.44 | −21.21 |
| 50 | 18.07 | 146.76 × 102 | 15.48 | −20.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praveen, B.M.; Alhadhrami, A.; Prasanna, B.M.; Hebbar, N.; Prabhu, R. Anti-Corrosion Behavior of Olmesartan for Soft-Cast Steel in 1 mol dm−3 HCl. Coatings 2021, 11, 965. https://doi.org/10.3390/coatings11080965
Praveen BM, Alhadhrami A, Prasanna BM, Hebbar N, Prabhu R. Anti-Corrosion Behavior of Olmesartan for Soft-Cast Steel in 1 mol dm−3 HCl. Coatings. 2021; 11(8):965. https://doi.org/10.3390/coatings11080965
Chicago/Turabian StylePraveen, B. M., A. Alhadhrami, B. M. Prasanna, Narayana Hebbar, and Radhakrishna Prabhu. 2021. "Anti-Corrosion Behavior of Olmesartan for Soft-Cast Steel in 1 mol dm−3 HCl" Coatings 11, no. 8: 965. https://doi.org/10.3390/coatings11080965
APA StylePraveen, B. M., Alhadhrami, A., Prasanna, B. M., Hebbar, N., & Prabhu, R. (2021). Anti-Corrosion Behavior of Olmesartan for Soft-Cast Steel in 1 mol dm−3 HCl. Coatings, 11(8), 965. https://doi.org/10.3390/coatings11080965







