Abstract
The current literature lacks substantial information about the effect of denture cleansers on the color stability of denture bases formed using Computer-Aided Design/Computer-Aided Manufacturing (CAD/CAM) additive and CAD/CAM subtractive manufacturing techniques. This study aimed to assess the effect of two commercially available denture cleansers on the color stability of denture base resins fabricated using four different techniques. Forty-five disc-shaped specimens were fabricated using each technique. Initial color readings were recorded. Specimens were randomly divided into three subgroups (n = 15): a control group (distilled water) and two denture cleanser groups. They were immersed in these solutions, simulating 180 days of use. Final color readings were recorded. The color difference was calculated, and the data were statistically analyzed. For all the specimens, significant color changes were observed after immersion in denture cleanser solutions. The extent of color change varied according to the type of denture cleanser used. When placed in the denture cleanser solutions, the CAD/CAM subtractive group showed the maximum color change (−1.10 and −0.72), while the CAD/CAM additive (3D printing) groups showed the least color change (−0.48 and −0.54). Clinicians should choose appropriate denture cleansers for newly introduced denture base resins to minimize the changes in the color of the dentures.
1. Introduction
The removable dental prosthesis is a significant treatment option for replacing missing teeth in patients above 65 years old [1]. With the digitalization and advancements in material science, many new techniques and clinical protocols for fabricating dentures have evolved [2,3]. Computer-Aided Design (CAD) and Computer-Aided Machining (CAM) have revolutionized the approach to fabricating removable dental prostheses (RDP). The subtractive milling technique uses pre-polymerized polymethyl methacrylate (PMMA) blocks for fabricating RDPs [2,3,4,5,6]. More recently, additive manufacturing techniques using 3D printing have also been used to fabricate RDPs and have shown satisfactory results [7,8,9]. These new denture base resins have displayed superior mechanical properties when compared to conventional heat-polymerized PMMA [10,11,12,13,14,15]. However, the conventional compression molding technique is still the choice of technique for the fabrication of RDP worldwide [16,17]. Whichever technique or material used for fabricating RDPs should have acceptable physical and surface properties for the treatment to be a success.
The proper maintenance of denture hygiene is important to keep underlying tissues healthy. Elderly people, especially with poor manual dexterity, find it difficult to mechanically clean the denture. Therefore, chemical cleansing with denture cleansing materials alone or in addition to mechanical cleaning is generally recommended. These denture cleansers act in different ways and help in removing the stains, debris, and biofilm from the denture surfaces [18,19]. However, these denture cleansers tend to deteriorate the mechanical and physical properties of PMMA denture base materials [20,21,22].
The denture cleansers have a bleaching effect and tend to alter the color of denture base materials, thus giving them an aged and damaged look [21,23,24]. These color changes often lead to patient dissatisfaction and concerns regarding the serviceability of the prosthesis [25,26,27].
To the best of our knowledge, the current literature lacks the relevant information on the effect of denture cleansers on the color stability of CAD/CAM additive and CAD/CAM subtractive denture base resins when compared to injection-molded and conventional compression-molded denture base resins. Therefore, this study aims to compare the effect of two commonly used denture cleansers on the color stability of four denture base resins, which were fabricated using different techniques. The hypothesis to be tested was that there would be no significant change in the color stability of the CAD/CAM additive, CAD/CAM subtractive, and injection-molded denture base resin experimental groups, when compared to conventional compression, molded heat cure denture base resin (control group).
2. Materials and Methods
2.1. Materials
The research protocol was approved by the research board at the college of Dentistry, Jazan University (Reference number: CODJU-21205).
In this study, four different denture fabrication techniques (simulating the protocols for RDP fabrication) were used to prepare customized disc-shaped specimens of uniform dimension ≅ 10 mm (diameter) × 2 mm (thickness) from various commercially available denture base resins. The fabrication techniques used were: CAD/CAM additive (3D Printed), CAD/CAM subtractive, injection molding, and conventional heat polymerized compression molding. The specifications of each denture base material and denture cleanser in terms of its group, trade name, lot number, manufacturer, main composition, polymerization, and fabrication technique are listed in Table 1.

Table 1.
Commercial names and details of materials used in the study.
2.2. Specimen Preparation
The software used for sample size estimation was G*Power (version 3.1.9.4, 2019, Heinrich Heine University Düsseldorf, Düsseldorf, Germany), and it assumed a 12-group comparison. A total of 180 resin specimens were prepared using four denture fabrication techniques. A minimum sample size of 45 (15 in each subgroup) was found to be sufficient for an alpha of 0.05, power of 80%, and effect size of 0.80.
2.2.1. CAD/CAM Additive (3D Printing) Group
The specimens were virtually designed using CAD software (Microsoft 3D builder, 16.0.2611.0, 2021, Microsoft Corporation, Redmond, WA, USA). The stereolithography (STL) file was uploaded in the slicing software (CHITUBOX V1.8.1, 2021, CBD-Tech, Guangdong, China). The printing direction was 45° towards the Z-axis/printing table [28]. The STL file was sent to the 3D printing software. Supporting structures were applied in the software and a 50-micrometer layer thickness was selected for printing the specimens using a direct light processing (DLP) 3D printer (NextDent™ 5100, NextDent B.V., Soesterberg, The Netherlands), with a 385 nm wavelength. The material used was NextDent Denture 3D+ (Vertex-Dental), Dimethacrylate-based resins with photo-initiator, filler, and pigments. The printed specimens were cleaned with isopropyl alcohol (99.9%) in an ultrasonic bath (FORMwash, Formlabs, Somerville, MA, USA), first for 3 min then for 2 min, to remove the excess resin. After complete drying, the specimens were polymerized in a light-curing unit (NextDent LC-PrintBox, Vertex-Dental B.V., Soesterberg, The Netherlands) according to the manufacturer’s instructions. A total of 45 specimens were fabricated (Figure 1).

Figure 1.
Specimens prepared by CAD/CAM additive manufacturing technique (3D printing).
2.2.2. CAD/CAM Subtractive (Milling) Group
The virtual design used for the additive technique was also used for the subtractive technique. The STL file was sent to the CAM software (Opera Pro-Expert-5, Euromax Monaco, Monaco). A pre-polymerized PMMA block (Wieland) was used. A total of 45 specimens were dry-milled using an Opera Pro-Expert 5 milling machine with a diamond wafering blade (Milling bur number 2.1, 1.5, 1.0) (Figure 2).
Figure 2.
Specimens prepared by CAD/CAM subtractive manufacturing technique (Milling).
2.2.3. Injection Molded Group
Disc-shaped wax patterns (45) were fabricated using metal molds, which were embedded in type IV dental stone (Octa-Rock dental stone, Kulzer, Hanau, Germany) in special metallic flasks. Each flask contained approximately 9 wax patterns. Wax sprue was positioned as recommended. After dewaxing, 2 layers of separating media were applied. For injection molding, a Thermopress 400 system version 2.62 (2019, Bredent GmbH&Co., Senden, Germany) was used. After the selection of the injecting material (Bre. flex, Bredent, Senden, Germany), the manufacturer’s instructions were followed, and the equipment was programmed as follows: temperature setting of 222 °C, the heating time of 15 min, injecting pressure 5 bar, and pressing time of 90 s. After polymerization, specimens were deflasked and were finished to the required dimension (Figure 3).

Figure 3.
Wax patterns invested for injection molding technique.
2.2.4. Conventional Heat Cure Compression Molding Group
Metal molds were used to fabricate the disc-shaped wax patterns (45) with the aforementioned dimensions. These wax patterns were embedded in type IV dental stone in metallic flasks. Each flask contained approximately 15 wax patterns. After de-waxing, two layers of separating media were applied. Heat polymerizing PMMA resin (Meliodent, Hanau, Germany; pink veined color) was mixed and packed according to the manufacturer’s instructions for the conventional compression molding technique. A long curing cycle (74 °C for 8 h followed by 100 °C for 1 h) was used to polymerize the resin. Later, the polymerized specimens were deflasked and finished manually to the required dimension. All the prepared specimens were stowed in a black container until all the specimens had been prepared (Figure 4).

Figure 4.
Wax patterns invested for conventional compression molding technique.
2.3. Finishing and Polishing of Specimens
All the finishing and polishing procedures were performed by a one trained operator. One surface of each specimen was finished using a MotoPol 8 grinder and polisher (Buehler GmbH, Dusseldorf, Germany), with wet abrasive silicon carbide discs (Buehler Ltd., Lake Bluff, IL, USA) with a grit of 1500, 2500, or 4000 [29]. For surface polishing, the following sequence was employed. Firstly, a lathe bristle brush with pumice slurry was used, followed by polishing with a muslin brush wheel and polishing compound (Hatho Polishing Compound, Keystone Industries, Gibbstown, NJ, USA). Lastly, a muslin cloth brush with polishing paste (Universal Polishing Paste, Ivoclar, Schaan, Liechtenstein) was used at 500 rpm. A digital caliper (IP67, 500-702-20, Mitutoyo, Kanagawa, Japan) was used to verify the dimensions, and loupes were used for the final quality check. Samples not meeting the standards were replaced with new specimens. The unpolished surface of each specimen was coded using a small round bur with a low-speed handpiece. All polished specimens were cleaned in an ultrasonic bath (Hygosonic, DURR Dental, Bietigheim-Bissingen, Germany) for 2 min and were dried with sterile tissue paper for initial color testing.
2.4. Initial Color Testing
A portable colorimeter (CS-10, Hangzhou Quality Lab Scientific Co. Ltd., Hangzhou, China) was used to record the initial color measurements immediately following the preparation of specimens. The instrument was calibrated according to the manufacturer’s instructions. Each specimen was placed against a standard white background while its color was measured to avoid potential absorption effects on any of the measured color parameters [30]. Three readings from three different sites were recorded for each specimen (one at the center and another two randomly from off-centric areas), and the obtained values were averaged. The color coordinates were detailed via the CIELAB color system.
2.5. Immersion Procedures
The specimens from each group were randomly divided into three subgroups (n = 15) and submerged in either one of the two denture cleanser solutions or distilled water.
- Subgroup A: Control group (distilled water).
- Subgroup B: Polident 3 min daily denture cleanser.
- Subgroup C: Fixodent Plus Scope denture cleanser.
For the control group, accelerated aging (equivalent to 180 days) of the specimens was carried out in a thermocycling machine (Model 1100, SD Mechatronik, Bayern, Germany).
For subgroups B and C, as per the manufacturer’s instructions, each type of denture cleansing tablet was placed individually in 250 mL of warm distilled water (40 °C) and stirred for 30 s. The specimens from each group were submerged in the solution with the polished surface facing upwards [31]. For both denture cleansers, the submersion time was 3 min, as recommended by the manufacturer. After the stipulated time, the specimens were removed from the cleansing solution, washed in running water, and submerged again in a new solution for the next cycle. Thirty submersion cycles (3 min each) were performed each day for 6 days to imitate 180 days of prosthesis submersion by the patient [22]. The specimens were stored in distilled water at room temperature between the submersion procedures.
2.6. Final Color Testing
Final color measurements were taken after the submersion procedures had been completed using the same protocol as was used for the initial color measurement. The CIE2000 formula Equation (1) was used to calculate the color difference [32,33].
where ∆L′, ∆C′, and ∆H′ are the difference in lightness, saturation, and hue, respectively, with correction performed with weighing coefficients (SL, SC, and SH) and parametric coefficients (kL, kC, and kH) as the constants.
The CIE2000 calculator (http://colormine.org, accessed on 25 May 2021) was used for the sake of ease in the calculation of ∆E00. It uses color coordinates derived from the CIELAB color system (ΔL*, Δa*, and Δb* are the differences in the L*, a*, and b* values, measured before and after submersion, where L* represents perceptual lightness, and a* and b* represent four unique colors of human vision: red, green, blue, and yellow). All the initial and final measurements were recorded by a single trained operator.
Surface roughness was measured using a 3D optical non-contact surface profiler (UMT 1, Campbell, CA, USA) both before and after immersion in solutions, to find any correlation between change in color and change in surface roughness.
2.7. Data Analysis
The collected data were tabulated in a Microsoft Excel spreadsheet (version 1910, 2019, Microsoft Inc., Redmond, WA, USA), and statistical analysis was performed using SPSS version 24.0 (IBM SPSS Statistics for Windows, Version 24.0., 2016, IBM Corp., Armonk, NY, USA). The Shapiro–Wilk test (with p < 0.05) was performed to check the normality of distribution in the data. Later, a two-way ANOVA test was carried out, followed by a post hoc Tukey HSD test (α level = 0.05).
3. Results
The mean color change (∆E00) among the groups is presented in Table 2. The color change (∆E00) was greater in solution B, followed by solution C, and the least color change was observed in solution A (control group). When placed in solution A, the greatest color change was seen in group 4 (0.23 ± 0.77), followed by group 1 (−0.22 ± 0.53). However, when placed in denture cleanser solutions B and C, group 2 showed the greatest color change (−1.10 ± 0.57 and −0.72 ± 0.46), and group 1 showed the least color change (−0.48 ± 0.88 and −0.54 ± 0.93) (Figure 5). The post hoc analysis showed a statistically significant difference among all the groups concerning the color change (Table 2).

Table 2.
Mean color change (ΔE00) among the groups.
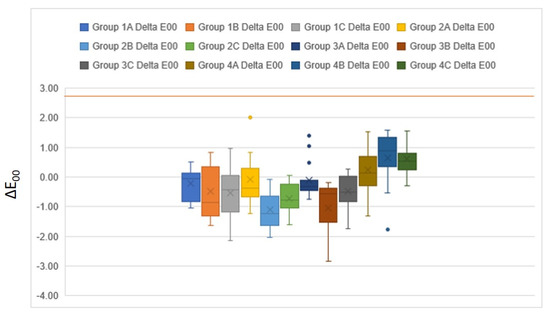
Figure 5.
Effect of denture cleansers on the color of different resin groups. The brown line represents the clinically acceptable threshold (ΔE00 = 2.8).
Table 3 depicts the two-way ANOVA comparison among the three groups concerning color change. A statistically significant color change occurred within each group after immersion in different solutions (p = 0.0001). Additionally, when the four groups were compared, a statistically significant color change among the groups was observed (p = 0.0001).

Table 3.
Two-way ANOVA for mean color change among the four groups. Dependent variable: ∆E00. df—degrees of freedom, F—ratio of two variances, Sig.—statistical significance.
The mean surface roughness change (ΔSa) among the groups is presented in Table 4. Increase in surface roughness was greater in solution C, followed by solution B, whereas immersion in solution A reduced the surface roughness. When placed in solution A, the greatest change in surface roughness was seen in group 4 (−0.012 ± 0.004). However, when placed in denture cleanser solution B and C, group 1 showed the greatest change in surface roughness (0.071 ± 0.012 and 0.17 ± 0.019).

Table 4.
Mean surface roughness change (ΔSa) among the four groups.
4. Discussion
This study analyzed the effects of denture cleansers on the color stability of four denture base resins fabricated using different techniques. A significant color change was observed when the four groups were compared. Therefore, the hypothesis can be rejected; however, the extent of these color changes varied according to fabrication technique and type of denture base resin.
Denture cleansers help eliminate stains, debris, and biofilm from denture surfaces [18,19]. The effects of denture cleansers on heat cure acrylic are well documented [22,23,24,34,35,36,37,38,39,40,41,42,43], but none of these studies have focused on the effect of denture cleansers on 3D-printed and milled denture base resins.
In this study, specimens were submerged for 3 min in warm water (40 °C) containing a denture cleanser. Thirty submersion cycles were repeated each day for 6 days, simulating 180 days of prosthesis submersion. This was done following an earlier study [22].
The change in color of the denture base resins due to the bleaching effect of the denture cleansers is key to evaluating the serviceability of the material, especially from the patient’s perspective [25,26,27]. A colorimeter, which is one of the most effective instruments for evaluating color, was used in this study [44,45,46]. The color difference was calculated using the CIE2000 formula. This formula uses CIELAB values to calculate color changes. This is the most complicated but most accurate CIE color difference algorithm available. When used for assessing the color change of dental tissues, the CIELAB2000 formula is considered better than the classical CIELAB formula in assessing the color difference that can be perceived by the human eye [32,47,48,49].
There are differences in opinion between various investigators regarding which ∆E00 value should be considered clinically acceptable (50:50% acceptability threshold) [50,51,52]. Perez et al. reported ∆E00 values below 2.8 to be clinically acceptable for gingiva-colored materials [52]. In the current study, the ∆E00 values were far below the clinically acceptable range for each of the four denture base resins after immersion in denture cleansers.
When immersed in solution B (Polident denture cleanser), the greatest change in E was observed in group 2 (−1.10), followed by group 3 (−1.04), then group 4 (0.65), while group 1 showed the least color change (−0.48); whereas when immersed in solution C (Fixodent denture cleanser), the greatest change in E was observed in group 2 (−0.72), followed by group 4 (−0.61), then group 1 (0.54), and group 3 showed the least color change (−0.47). The hypothesis for color change was rejected, as there was a statistically significant difference in ∆E00 between all the groups. Denture cleanser B caused much higher changes in E compared to denture cleanser C. This may be due to differences in the chemical composition of the denture cleansers.
Changes in E were also observed when specimens were thermocycled (control group). The highest change was observed in group 4 (0.23), followed by group 1 (−0.22), then group 3 (−0.12), while group 2 showed the least change (−0.09). Although these changes were statistically significant, they were far smaller than the changes in E observed in specimens immersed in denture cleansers.
A direct comparison of the present study with any other study cannot be made as this is the first study to evaluate the change in color of 3D-printed and milled denture base materials. There are studies in the literature that evaluate the effects of different denture cleansers on other denture base materials. Percaini et al. measured the color change in heat-polymerized acrylic resin after its immersion in denture cleansers, simulating 180-day use, and they found insignificant changes in color [22]. The results of the present study are following the study by Ozyilmaz et al., who evaluated the effects of various denture cleansers on the color stability of polyetherketoneketone, polyamide, and polymethylmethacrylate resins, finding that all denture cleansers increased the ΔE values relative to the baseline values [23]. Hong et al. performed a study to determine the effects of denture cleansers on the color stability of three different types of acrylic resin (heat-polymerized, autopolymerized, and visible light-polymerized). They concluded that the ΔE values of all denture base acrylic resins increased with time. They stated that the color change was due to the monomer leaching out and water being absorbed [34]. Polyzois et al. [35] studied the effect of peroxide and hypochlorite cleansers on the color of an acetal denture base material and found changes in ΔE values ranging from 2.64 to 7.64 for acrylic and 2.77 to 26.54 for acetal resin. The immersion of acetal resin in the hypochlorite cleansers gave rise to a clinically unacceptable ΔE. Zidan et al. [24] assessed the color stability of heat-polymerized denture base acrylic resin impregnated with zirconia nanoparticles after immersion in denture cleaners for 180 days. The greatest color changes were observed in the groups with 7 wt.% and 10 wt.% zirconia nanoparticles; furthermore, the types of denture cleanser used influenced the color stability. The results of all these studies are following the present study.
The result of the present study partially agrees with those from the study by Polychronakis et al., who reported a greater color change in nylon denture base materials as compared to heat-cured acrylic resin [36]. In the present study, when the specimens were immersed in solution C (Fixodent denture cleanser), the amide-based denture base resins showed less color change when compared to heat-cure acrylic resins, whereas when immersed in solution B, the greater color change was observed in the amide-based denture bases.
Further, the studies by Iazetti et al. [37] and Hersek et al. [38] reported more significant color changes in hydrophilic materials when compared to hydrophobic materials. The monomers used in the light-cured acrylic resins are HDMA (hexamethylene glycol dimethacrylate) [39,40], whereas heat-cured resins use MMA (methyl methacrylate). The difference in color change between groups could be due to the differences in the hydrophilic nature of these monomers. The solubility and water sorption of, and presence of less residual monomer in, light-polymerized resins compared to those of heat-polymerized resins may also cause differences in color change between the groups [34,41].
The composition of the denture cleanser also affects the degree of color change. Denture base materials can be damaged by high peroxide contents, the level of oxygenation, and the pH of the solution [42,43]. Polymerized acrylic resin can undergo hydrolysis and decomposition by denture cleansers [34]. The pH of Polident was reported to be between 6.4 and 6.63 [43], while that of Fixodent was around 9.5 [53]. This difference in pH, along with the differences in peroxide content, of the two denture cleansers used, may explain the different extent of color change they caused.
Denture cleansers can deteriorate the surface of the denture base materials which also can be a cause of change in the color. In the current study no correlation was observed between alterations in surface roughness and color of denture base materials after immersion in different denture cleansing solutions. These results agreed with Ayaz et al. [54], who concluded that not only the surface roughness but also the inherent nature of some materials influence the color change.
Denture cleansers have oxide-releasing agents and enzymes, which can affect the pigments present in the denture base materials. Commonly used pigments in denture base materials are organic dyes, salts of cadmium, mercury, and iron [55]. Robinson et al. [56] reported that denture cleansers cause expansion of intermolecular spaces, which can aid in leaching out of intrinsic pigments and penetration of extrinsic colorants present in denture cleansing solutions. The difference in composition, crosslinking, and the presence or absence of water-soluble pigments can also be probable causes of the difference in extent of color change in these resin materials. Additionally, Polident denture cleanser (solution B), used in the current study, has various pigments, which could explain why denture base materials immersed in this solution displayed a maximum change in color.
The results of the present study show that denture cleansers affect the color stability of the tested denture base materials fabricated using different techniques. The limitations of the present study, and recommendations for future research, are listed below:
- The buffering action of saliva, staining from food ingredients, and the effects of temperature change could not be replicated in this in vitro study. Further studies could be conducted using denture bases used by patients;
- The present study only evaluated the effect of the chemical method of cleansing on the denture base materials, for six months. Further studies could be conducted wherein chemical methods are combined with mechanical cleaning methods, and for longer duration;
- Lastly, the present study only tested the color change. The effect of the denture cleanser on other important aspects, such as surface roughness, gloss, surface hardness, and flexural strength could also be investigated. Additional clinical studies using denture cleansers of different compositions should also be conducted on these new denture base materials.
This in vitro study can guide dentists in selecting the appropriate denture cleanser for recently introduced denture base materials, thus minimizing the change in color of the material and averting the dentures’ early replacement for esthetic reasons.
5. Conclusions
Within the limitations of this in vitro study, the following conclusions can be drawn:
- There was a significant change in color in all the denture base resins fabricated using different techniques after immersion in denture cleanser solutions. However, the ∆E00 values were far below the clinically acceptable range for all four denture base resins;
- Both the Polident and Fixodent denture cleansers gave rise to the highest ∆E00 values for the CAD/CAM milled denture base resins;
- The CAD/CAM additive (3D printed) denture base resins showed the lowest ∆E00 values when immersed in the Polident denture cleanser, and the second-lowest values when immersed in the Fixodent denture cleanser;
- The ∆E00 values of injection-molded denture base resins were lower compared to the conventional heat-polymerized resins for both the denture cleanser solutions;
- The extent of color change varied according to the type of denture cleanser used. The ∆E00 values were higher with Polident compared to Fixodent for all the denture base resins.
Author Contributions
Conceptualization, S.J., A.A. and M.S.; methodology, S.J., W.M.A., A.H.A.H. and N.M.A.N.; software, A.H.A.H., N.M.A.N. and S.J.; validation, A.A., M.S. and S.J.; formal analysis, S.J., M.S., W.M.A., S.B. and S.P.; investigation, S.J., M.S., A.H.A.H., N.M.A.N. and W.M.A.; resources, W.M.A., N.M.A.N., A.H.A.H. and A.A.; data curation, S.J. and A.A.; writing—original draft preparation, S.J. and A.A.; writing—review and editing, S.J., M.S., W.M.A., A.H.A.H., N.M.A.N., A.A., S.B. and S.P.; visualization, S.J., A.H.A.H., N.M.A.N. and A.A.; supervision, S.J., S.B. and M.S.; project administration, S.J. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The research protocol was approved by the research board at the College of Dentistry, Jazan University (Reference number: CODJU-21205).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Commission of Oral Health; Research and Epidemiology Report of a Working Group Oral health needs of the elderly--an international review. Commission of Oral Health, Research and Epidemiology Report of a Working Group. Int. Dent. J. 1993, 43, 348–354. [Google Scholar]
- Bidra, A.S.; Taylor, T.D.; Agar, J.R. Computer-aided technology for fabricating complete dentures: Systematic review of historical background, current status, and future perspectives. J. Prosthet. Dent. 2013, 109, 361–366. [Google Scholar] [CrossRef]
- Kattadiyil, M.T.; Jekki, R.; Goodacre, C.J.; Baba, N.Z. Comparison of treatment outcomes in digital and conventional complete removable dental prosthesis fabrications in a predoctoral setting. J. Prosthet. Dent. 2015, 114, 818–825. [Google Scholar] [CrossRef]
- Srinivasan, M.; Kalberer, N.; Naharro, M.; Marchand, L.; Lee, H.; Müller, F. CAD-CAM milled dentures: The Geneva protocols for digital dentures. J. Prosthet. Dent. 2020, 123, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Schweiger, J.; Stumbaum, J.; Edelhoff, D.; Güth, J.-F. Systematics and concepts for the digital production of complete dentures: Risks and opportunities. Int. J. Comput. Dent. 2018, 21, 41–56. [Google Scholar]
- Millet, C.; Virard, F.; Dougnac-Galant, T.; Ducret, M. CAD-CAM immediate to definitive complete denture transition: A digital dental technique. J. Prosthet. Dent. 2020, 124, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-S.; Harris, B.T.; Pellerito, J.; Morton, D. Fabrication of an interim complete removable dental prosthesis with an in-office digital light processing three-dimensional printer: A proof-of-concept technique. J. Prosthet. Dent. 2018, 120, 331–334. [Google Scholar] [CrossRef]
- Unkovskiy, A.; Wahl, E.; Zander, A.T.; Huettig, F.; Spintzyk, S. Intraoral scanning to fabricate complete dentures with functional borders: A proof-of-concept case report. BMC Oral Health 2019, 19, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristache, C.M.; Totu, E.E.; Iorgulescu, G.; Pantazi, A.; Dorobantu, D.; Nechifor, A.C.; Isildak, I.; Burlibasa, M.; Nechifor, G.; Enachescu, M. Eighteen Months Follow-Up with Patient-Centered Outcomes Assessment of Complete Dentures Manufactured Using a Hybrid Nanocomposite and Additive CAD/CAM Protocol. J. Clin. Med. 2020, 9, 324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, M.; Gjengedal, H.; Cattani-Lorente, M.; Moussa, M.; Durual, S.; Schimmel, M.; Müller, F. CAD/CAM milled complete removable dental prostheses: An in vitro evaluation of biocompatibility, mechanical properties, and surface roughness. Dent. Mater. J. 2018, 37, 526–533. [Google Scholar] [CrossRef] [Green Version]
- Alp, G.; Murat, S.; Yilmaz, B. Comparison of Flexural Strength of Different CAD/CAM PMMA-Based Polymers. J. Prosthodont. 2019, 28, E491–E495. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.; Murat, S.; Alp, G.; Zaimoglu, A. Evaluation of flexural strength and surface properties of prepolymerized CAD/CAM PMMA-based polymers used for digital 3D complete dentures. Int. J. Comput. Dent. 2018, 21, 31–40. [Google Scholar] [PubMed]
- Alammari, M.R. The influence of polishing techniques on pre-polymerized CAD\CAM acrylic resin denture bases. Electron. Physician 2017, 9, 5452–5458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Dwairi, Z.N.; Tahboub, K.Y.; Baba, N.Z.; Goodacre, C.J.; Özcan, M. A Comparison of the Surface Properties of CAD/CAM and Conventional Polymethylmethacrylate (PMMA). J. Prosthodont. 2019, 28, 452–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinmassl, O.; Dumfahrt, H.; Grunert, I.; Steinmassl, P.-A. Influence of CAD/CAM fabrication on denture surface properties. J. Oral Rehab. 2018, 45, 406–413. [Google Scholar] [CrossRef] [Green Version]
- Murakami, N.; Wakabayashi, N.; Matsushima, R.; Kishida, A.; Igarashi, Y. Effect of high-pressure polymerization on mechanical properties of PMMA denture base resin. J. Mech. Behav. Biomed. Mater. 2013, 20, 98–104. [Google Scholar] [CrossRef]
- Nogueira, S.S.; Ogle, R.E.; Davis, E.L. Comparison of accuracy between compression- and injection-molded complete dentures. J. Prosthet. Dent. 1999, 82, 291–300. [Google Scholar] [CrossRef]
- Paranhos, H.F.O.; Silva-Lovato, C.H.; Souza, R.F.; Cruz, P.C.; Freitas, K.M.; Peracini, A. Effects of mechanical and chemical methods on denture biofilm accumulation. J. Oral Rehabil. 2007, 34, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Saraç, D.; Saraç, Y.S.; Kurt, M.; Yüzbaşioğlu, E. The effectiveness of denture cleansers on soft denture liners colored by food colorant solutions. J. Prosthodont. 2007, 16, 185–191. [Google Scholar] [CrossRef]
- Robinson, J.G.; McCabe, J.F.; Storer, R. Denture bases: The effects of various treatments on clarity, strength and structure. J. Dent. 1987, 15, 159–165. [Google Scholar] [CrossRef]
- Berger, J.C.; Driscoll, C.F.; Romberg, E.; Luo, Q.; Thompson, G. Surface roughness of denture base acrylic resins after processing and after polishing. J. Prosthodont. 2006, 15, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Peracini, A.; Davi, L.R.; de Queiroz Ribeiro, N.; de Souza, R.F.; da Silva, C.H.L.; de Freitas Oliveira Paranhos, H. Effect of denture cleansers on physical properties of heat-polymerized acrylic resin. J. Prosthodont. Res. 2010, 54, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Ozyilmaz, O.Y.; Kara, O.; Akin, C. Evaluation of various denture cleansers on color stability and surface topography of polyetherketoneketone, polyamide, and polymethylmethacrylate. Microsc. Res. Tech. 2021, 84, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zidan, S.; Silikas, N.; Haider, J.; Yates, J. Effect of Cleansers on the Colour Stability of Zirconia Impregnated PMMA Bio-Nanocomposite. Nanomaterials 2020, 10, 1757. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Lim, B.-S.; Kim, C.-W. Influence of illuminating and viewing aperture size on the color of dental resin composites. Dent. Mater. 2004, 20, 116–123. [Google Scholar] [CrossRef]
- Shotwell, J.L.; Razzoog, M.E.; Koran, A. Color stability of long-term soft denture liners. J. Prosthet. Dent. 1992, 68, 836–838. [Google Scholar] [CrossRef]
- Canay, S.; Hersek, N.; Tulunoğlu, I.; Uzun, G. Evaluation of colour and hardness changes of soft lining materials in food colorant solutions. J. Oral Rehab. 1999, 26, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Hada, T.; Kanazawa, M.; Iwaki, M.; Arakida, T.; Soeda, Y.; Katheng, A.; Otake, R.; Minakuchi, S. Effect of Printing Direction on the Accuracy of 3D-Printed Dentures Using Stereolithography Technology. Materials 2020, 13, 3405. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S.; Kamnoedboon, P.; Özcan, M.; Srinivasan, M. CAD/CAM Complete Denture Resins: An In Vitro Evaluation of Color Stability. J. Prosthodont. 2021, 30, 430–439. [Google Scholar] [CrossRef]
- Uchida, H.; Vaidyanathan, J.; Viswanadhan, T.; Vaidyanathan, T.K. Color stability of dental composites as a function of shade. J. Prosthet. Dent. 1998, 79, 372–377. [Google Scholar] [CrossRef]
- Ma, T.; Johnson, G.H.; Gordon, G.E. Effects of chemical disinfectants on the surface characteristics and color of denture resins. J. Prosthet. Dent. 1997, 77, 197–204. [Google Scholar] [CrossRef]
- Luo, M.; Cui, G.; Rigg, B. The development of the CIE 2000 colour-difference formula: CIEDE2000. Color Res. Appl. 2001, 26, 340–350. [Google Scholar] [CrossRef]
- Gómez-Polo, C.; Montero, J.; Gómez-Polo, M.; Martin Casado, A. Comparison of the CIELab and CIEDE 2000 Color Difference Formulas on Gingival Color Space. J. Prosthodont. 2020, 29, 401–408. [Google Scholar] [CrossRef]
- Hong, G.; Murata, H.; Li, Y.; Sadamori, S.; Hamada, T. Influence of denture cleansers on the color stability of three types of denture base acrylic resin. J. Prosthet. Dent. 2009, 101, 205–213. [Google Scholar] [CrossRef]
- Polyzois, G.; Niarchou, A.; Ntala, P.; Pantopoulos, A.; Frangou, M. The effect of immersion cleansers on gloss, colour and sorption of acetal denture base material. Gerodontology 2013, 30, 150–156. [Google Scholar] [CrossRef]
- Polychronakis, N.C.; Polyzois, G.L.; Lagouvardos, P.E.; Papadopoulos, T.D. Effects of cleansing methods on 3-D surface roughness, gloss and color of a polyamide denture base material. Acta Odontol. Scand. 2015, 73, 353–363. [Google Scholar] [CrossRef]
- Iazzetti, G.; Burgess, J.O.; Gardiner, D.; Ripps, A. Color stability of fluoride-containing restorative materials. Oper. Dent. 2000, 25, 520–525. [Google Scholar]
- Hersek, N.; Canay, S.; Uzun, G.; Yildiz, F. Color stability of denture base acrylic resins in three food colorants. J. Prosthet. Dent. 1999, 81, 375–379. [Google Scholar] [CrossRef]
- Arima, T.; Murata, H.; Hamada, T. Properties of highly cross-linked autopolymerizing reline acrylic resins. J. Prosthet. Dent. 1995, 73, 55–59. [Google Scholar] [CrossRef]
- Arima, T.; Murata, H.; Hamada, T. Analysis of composition and structure of hard autopolymerizing reline resins. J. Oral Rehab. 1996, 23, 346–352. [Google Scholar] [CrossRef]
- Truong, V.T.; Thomasz, F.G. Comparison of denture acrylic resins cured by boiling water and microwave energy. Aust. Dent. J. 1988, 33, 201–204. [Google Scholar] [CrossRef]
- Nikawa, H.; Hamada, T.; Yamashiro, H.; Kumagai, H. A review of in vitro and in vivo methods to evaluate the efficacy of denture cleansers. Int. J. Prosthodont. 1999, 12, 153–159. [Google Scholar] [PubMed]
- Nikawa, H.; Iwanaga, H.; Hamada, T.; Yuhta, S. Effects of denture cleansers on direct soft denture lining materials. J. Prosthet. Dent. 1994, 72, 657–662. [Google Scholar] [CrossRef]
- Okubo, S.R.; Kanawati, A.; Richards, M.W.; Childress, S. Evaluation of visual and instrument shade matching. J. Prosthet. Dent. 1998, 80, 642–648. [Google Scholar] [CrossRef]
- Chang, J.; Da Silva, J.D.; Sakai, M.; Kristiansen, J.; Ishikawa-Nagai, S. The optical effect of composite luting cement on all ceramic crowns. J. Dent. 2009, 37, 937–943. [Google Scholar] [CrossRef]
- Brewer, J.D.; Wee, A.; Seghi, R. Advances in color matching. Dent. Clin. N. Am. 2004, 48, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Wee, A.G.; Lindsey, D.T.; Shroyer, K.M.; Johnston, W.M. Use of a porcelain color discrimination test to evaluate color difference formulas. J. Prosthet. Dent. 2007, 98, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Polo, C.; Portillo Muñoz, M.; Lorenzo Luengo, M.C.; Vicente, P.; Galindo, P.; Martín Casado, A.M. Comparison of two color-difference formulas using the Bland-Altman approach based on natural tooth color space. J. Prosthet. Dent. 2016, 115, 482–488. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Tatol, M. Color difference ΔE—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Ren, J.; Lin, H.; Huang, Q.; Zheng, G. Determining color difference thresholds in denture base acrylic resin. J. Prosthet. Dent. 2015, 114, 702–708. [Google Scholar] [CrossRef]
- Paravina, R.D.; Ghinea, R.; Herrera, L.J.; Bona, A.D.; Igiel, C.; Linninger, M.; Sakai, M.; Takahashi, H.; Tashkandi, E.; Perez, M. Color difference thresholds in dentistry. J. Esthet. Restor. Dent. 2015, 27, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.M.; Ghinea, R.; Herrera, L.J.; Carrillo, F.; Ionescu, A.M.; Paravina, R.D. Color difference thresholds for computer-simulated human Gingiva. J. Esthet. Restor. Dent. 2018, 30, E24–E30. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, F.C.T.; Rocha, M.M.; Oliveira, V.C.; Macedo, A.P.; Pagnano, V.O.; Silva-Lovato, C.H.; Paranhos, H.d.F.O. Antimicrobial activity of effervescent denture tablets on multispecies biofilms. Gerodontology 2021, 38, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, E.A.; Altintas, S.H.; Turgut, S. Effects of cigarette smoke and denture cleaners on the surface roughness and color stability of different denture teeth. J. Prosthet. Dent. 2014, 112, 241–248. [Google Scholar] [CrossRef]
- Elshereksi, N.W.; Ghazali, M.J.; Muchtar, A.; Azhari, C.H. Perspectives for titanium-derived fillers usage on denture base composite construction: A review article. Adv. Mater. Sci. Eng. 2014, 2014, 13. [Google Scholar] [CrossRef] [Green Version]
- Robinson, J.G.; McCabe, J.F.; Storer, R. The whitening of acrylic dentures: The role of denture cleansers. Br. Dent. J. 1985, 159, 247–250. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).