Preparation and Characterization of (Al, Fe) Codoped ZnO Films Prepared by Sol–Gel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Film Preparation
2.2. Film Characterization
3. Results
3.1. Surface Morphology
3.2. XRD Analysis
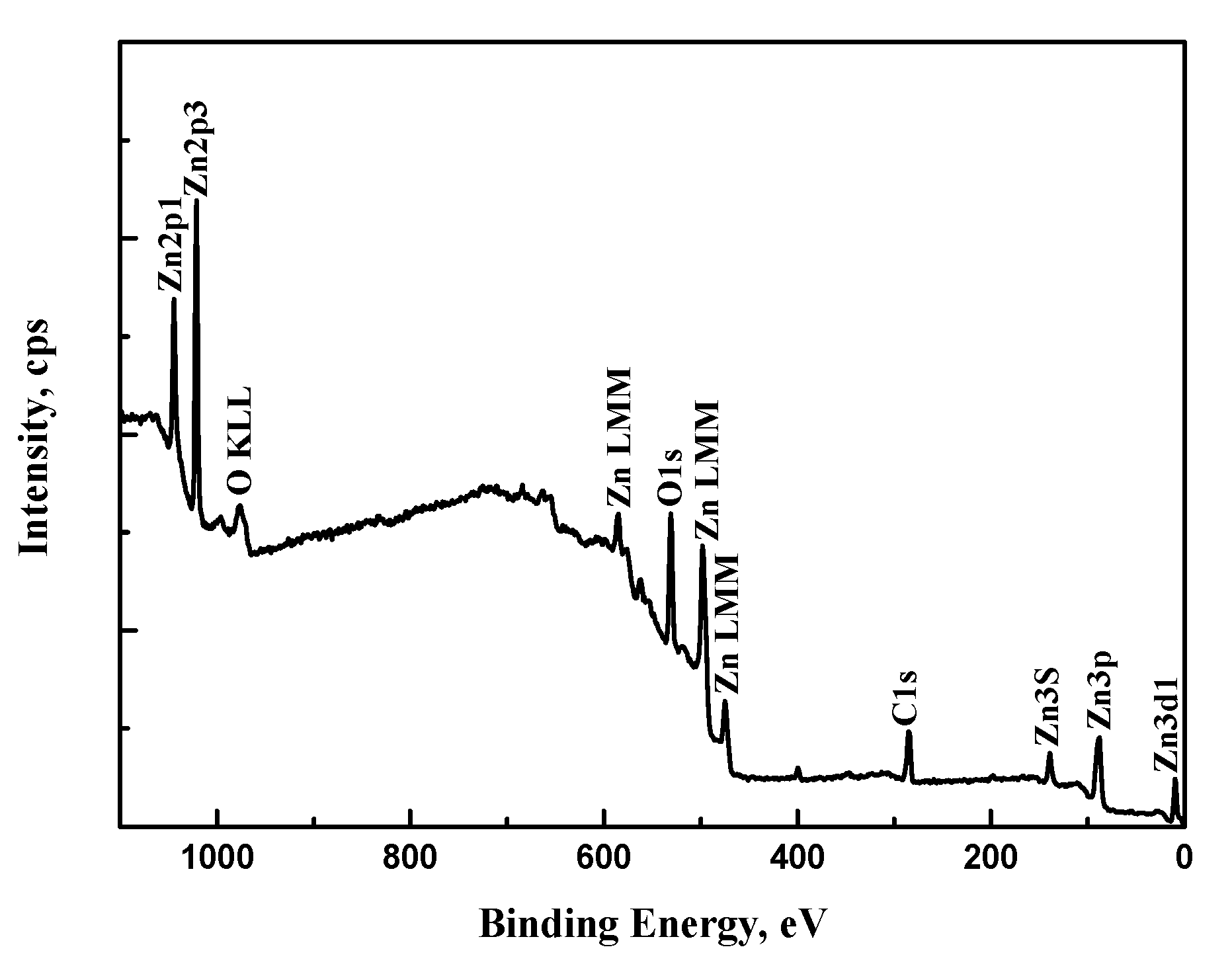
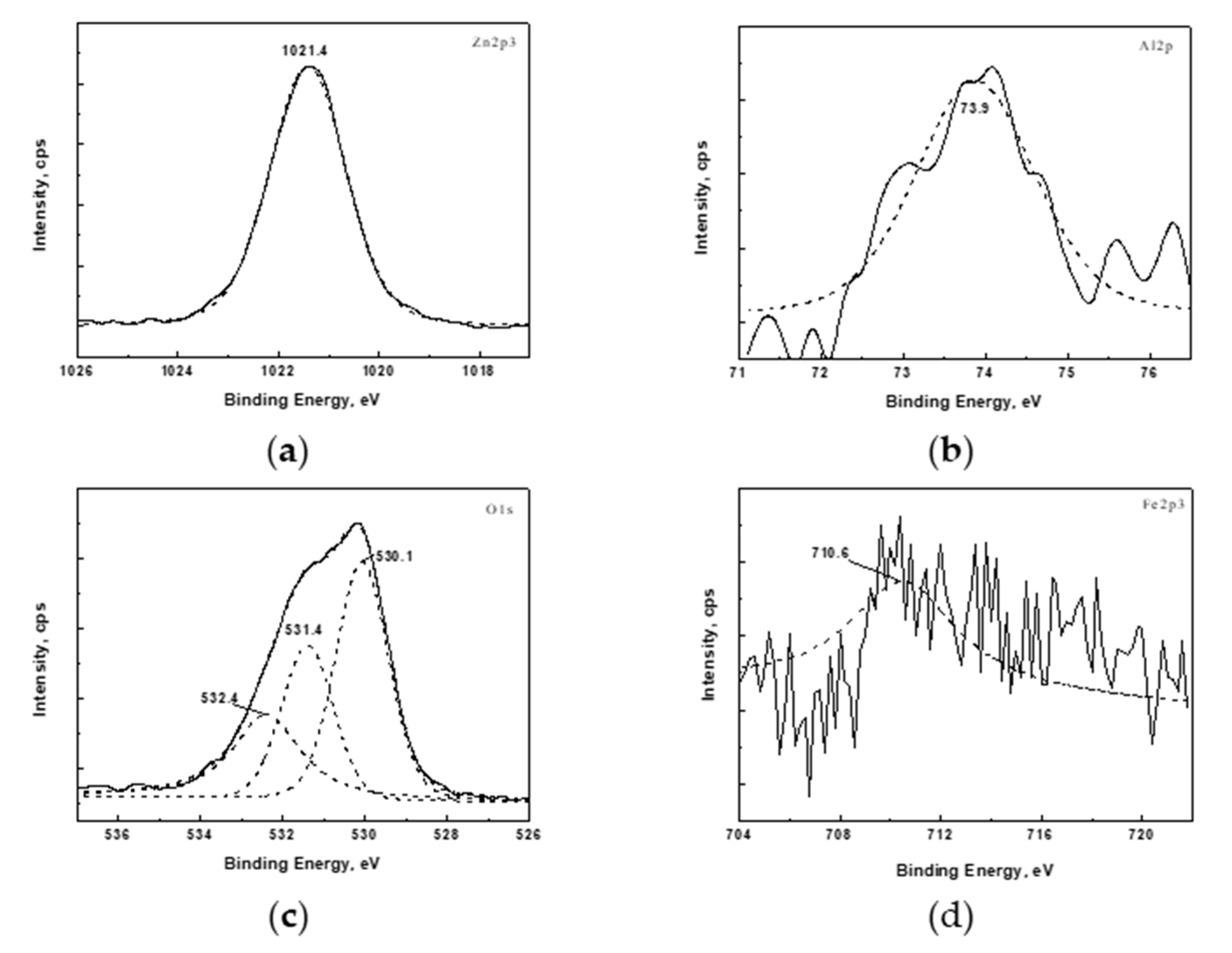
3.3. XPS Analysis
3.4. Optical Property
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nickel, N.H.; Evgenii, T. Zinc Oxide - A Material for Micro- and Optoelectronic Applications; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Znaidi, L. Sol–gel-deposited ZnO thin films: A review. Mater. Sci. Eng. B 2010, 174, 18–30. [Google Scholar] [CrossRef]
- Patil, P.S. Versatility of chemical spray pyrolysis technique. Mater. Chem. Phys. 1999, 59, 185–198. [Google Scholar] [CrossRef]
- Tsay, C.-Y.; Lee, W.-C. Effect of dopants on the structural, optical and electrical properties of sol–gel derived ZnO semiconductor thin films. Curr. Appl. Phys. 2013, 13, 60–65. [Google Scholar] [CrossRef]
- Selma, M.H.A.-J.; Sabah, H.S.; Taha, A.A.; Jassim, H.A. Studying structural, morphological and optical properties of nanocrystalline ZnO:Ag films prepared by sol–gel method for antimicrobial activity. J. Sol–Gel Sci. Technol. 2018, 87, 362–371. [Google Scholar] [CrossRef]
- Ivanova, T.; Harizanova, A.; Koutzarova, T.; Vertruyen, B. Sol–gel nanocrystalline ZnO:Ag films: Structural and optical properties. Superlattices Microstruct. 2014, 70, 1–6. [Google Scholar] [CrossRef]
- Boudjouan, F.; Chelouche, A.; Touam, T.; Djouadi, D.; Mahiou, R.; Chadeyron, G.; Fischer, A.; Boudrioua, A. Doping effect investigation of Li-doped nanostructured ZnO thin films prepared by sol–gel process. J. Mater. Sci. Mater. Electron. 2016, 27, 8040–8046. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Iram, S.; Rafi, R.; Riaz, S.; Naseem, S. Effect of Cu doping on the structural, magnetic and optical properties of ZnO thin films. Appl. Phys. A 2018, 124, 1–10. [Google Scholar] [CrossRef]
- Istrate, A.-I.; Mihalache, I.; Romanitan, C.; Tutunaru, O.; Gavrila, R.; Dediu, V. Copper doping effect on the properties in ZnO films deposited by sol–gel. J. Mater. Sci. Mater. Electron. 2021, 32, 4021–4033. [Google Scholar] [CrossRef]
- Huang, K.; Tang, Z.; Zhang, L.; Yu, J.; Lv, J.; Liu, X.; Liu, F. Preparation and Characterization of Mg-Doped ZnO Thin Films by Sol–Gel Method. Appl. Surf. Sci. 2012, 258, 3710–3713. [Google Scholar] [CrossRef]
- Mia, M.N.H.; Habiba, U.; Pervez, M.F.; Kabir, H.; Nur, S.; Hossen, M.F.; Sen, S.K.; Hossain, M.K.; Iftekhar, M.A.; Rahman, M.M. Investigation of aluminum doping on structural and optical characteristics of sol–gel assisted spin-coated nano-structured zinc oxide thin films. Appl. Phys. A 2020, 126, 1–12. [Google Scholar] [CrossRef]
- Shahzad, M.B.; Qi, Y.; Lu, H.; Wang, X. A study on the Al doping behavior with sol aging time and its effect on structural and optical properties of sol–gel prepared ZnO thin films. Thin Solid Films 2013, 534, 242–248. [Google Scholar] [CrossRef]
- Hadimani, P.L.; Ghosh, S.S.; Sil, A. Preparation of Fe doped ZnO thin films and their structural, magnetic, electrical characterization. Superlattices Microstruct. 2018, 120, 199–208. [Google Scholar] [CrossRef]
- Goktas, A.; Mutlu, I.H.; Yamada, Y. Influence of Fe-doping on the structural, optical, and magnetic properties of ZnO thin films prepared by sol–gel method. Superlattices Microstruct. 2013, 57, 139–149. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Xu, Q.; Fang, G. Preparation and characterization of Al doped ZnO thin films by sol–gel process. J. Alloys Compd. 2012, 542, 151–156. [Google Scholar] [CrossRef]
- Xu, L.; Li, X. Influence of Fe-doping on the structural and optical properties of ZnO thin films prepared by sol–gel method. J. Cryst. Growth 2010, 312, 851–855. [Google Scholar] [CrossRef]
- Shakti, N.; Gupta, P.S. Structural and optical properties of sol–gel prepared ZnO thin film. App. Phys. Res. 2010, 2, 19. [Google Scholar] [CrossRef] [Green Version]
- Zi-qiang, X.; Hong, D.; Yan, L.; Hang, C. Al-doping effects on structure, electrical and optical properties of c-axis-orientated ZnO:Al thin films. Mater. Sci. Semicond. Process. 2006, 9, 132–135. [Google Scholar] [CrossRef]
- Namoune, A.; Touam, T.; Chelouche, A. Thickness, annealing and substrate effects on structural, morphological, optical and waveguiding properties of RF sputtered ZnO thin films. J. Mater. Sci. Mater. Electron. 2017, 28, 12207–12219. [Google Scholar] [CrossRef]
- Cui, L.; Wang, G.-G.; Zhang, H.-Y.; Sun, R.; Kuang, X.-P.; Han, J.-C. Effect of film thickness and annealing temperature on the structural and optical properties of ZnO thin films deposited on sapphire (0001) substrates by sol–gel. Ceram. Int. 2013, 39, 3261–3268. [Google Scholar] [CrossRef]
- Jin, C.G.; Gao, Y.; Wu, X.M.; Cui, M.L.; Zhuge, L.J.; Chen, Z.C.; Hong, B. Structural and magnetic properties of transition metal doped ZnO films. Thin Solid Films 2010, 518, 2152–2156. [Google Scholar] [CrossRef]
- Wang, J.; Cai, J.; Lin, Y.-H.; Nan, C.-W. Room-temperature ferromagnetism observed in Fe-doped NiO. Appl. Phys. Lett. 2005, 87, 202501. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Chen, J.T.; Wang, J.; Zhang, F.; Zhang, G.A.; Wu, Z.G.; Yan, P.X. The effect of La doping concentration on the properties of zinc oxide films prepared by the sol–gel method. J. Cryst. Growth 2008, 310, 2627–2632. [Google Scholar] [CrossRef]
- Li, J.H.; Shen, D.Z.; Zhang, J.Y.; Zhao, D.X.; Li, B.S.; Lu, Y.M.; Liu, Y.C.; Fan, X.W. Magnestism origin of Mn-doped ZnO nanoclusters. J. Magn. Magn. Mater. 2006, 302, 118–121. [Google Scholar] [CrossRef]
- Wang, X.B.; Song, C.; Li, D.M.; Geng, K.W.; Zeng, F.; Pan, F. The influence of different doping elements on microstructure, piezoelectric coefficient and resistivity of sputtered ZnO film. Appl. Surf. Sci. 2006, 253, 1639–1643. [Google Scholar] [CrossRef]
- Rashid, A.R.A.; Othman, N.S.; Dasuki, K.A. Effect of solution molarity on optical properties of Al doped ZnO thin films. J. Phys. Conf. Ser. 2019, 1204, 012068. [Google Scholar] [CrossRef]
- Liu, W.L.; Zhang, Y.F. Blueshift of absorption edge and photoluminescence in Al doped ZnO thin films. Integr. Ferroelectr. 2018, 188, 112–120. [Google Scholar] [CrossRef]
- Tsay, C.-Y.; Fan, K.-S.; Lei, C.-M. Synthesis and characterization of sol–gel derived gallium-doped zinc oxide thin films. J. Alloys Compd. 2012, 512, 216–222. [Google Scholar] [CrossRef]
- Wang, L.-M.; Liao, J.-W.; Peng, Z.-A.; Lai, J.-H. Doping effects on the characteristics of Fe:ZnO films: Valence transition and hopping transport. J. Electrochem. Soc. 2009, 156, H138–H142. [Google Scholar] [CrossRef]
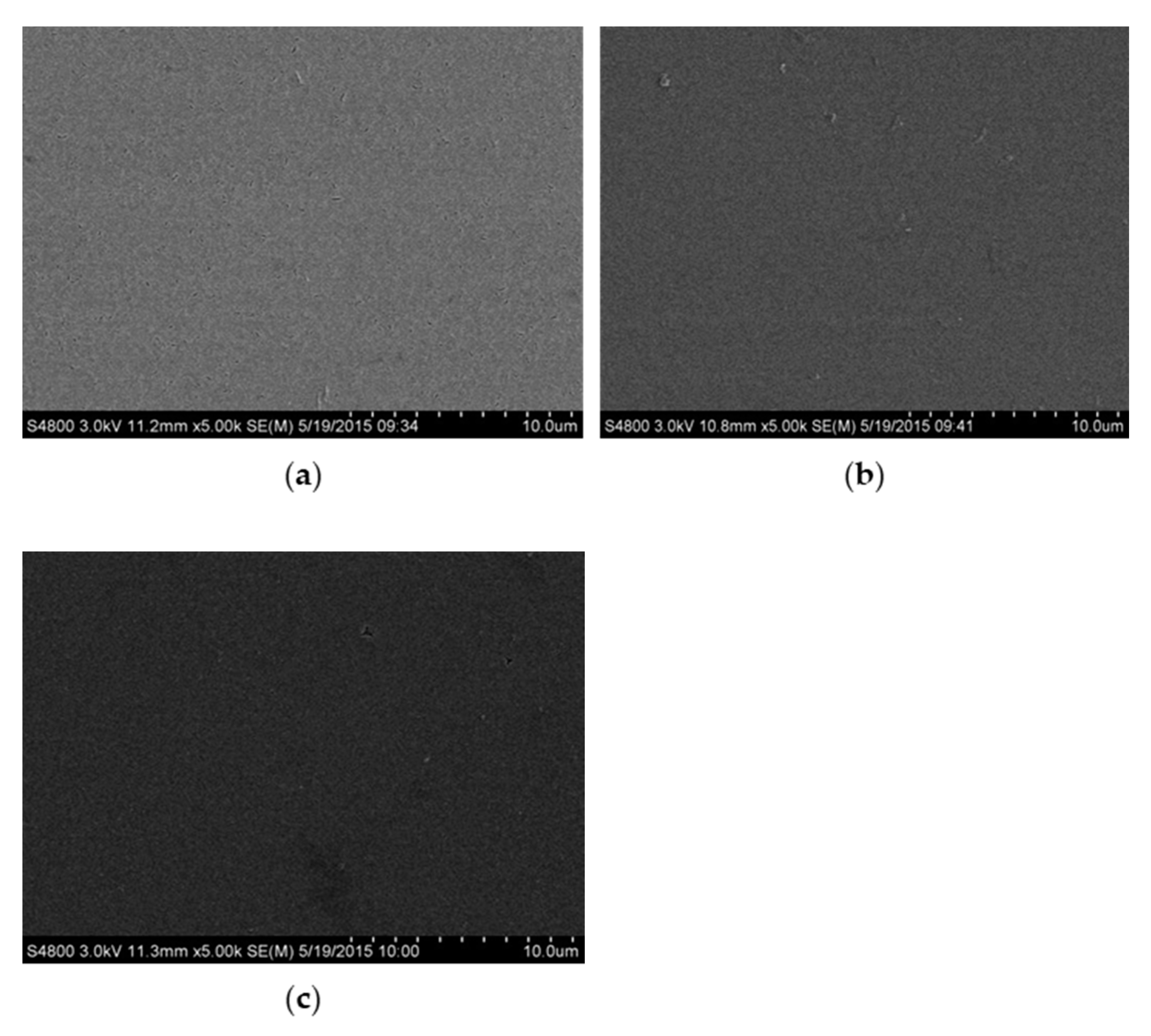
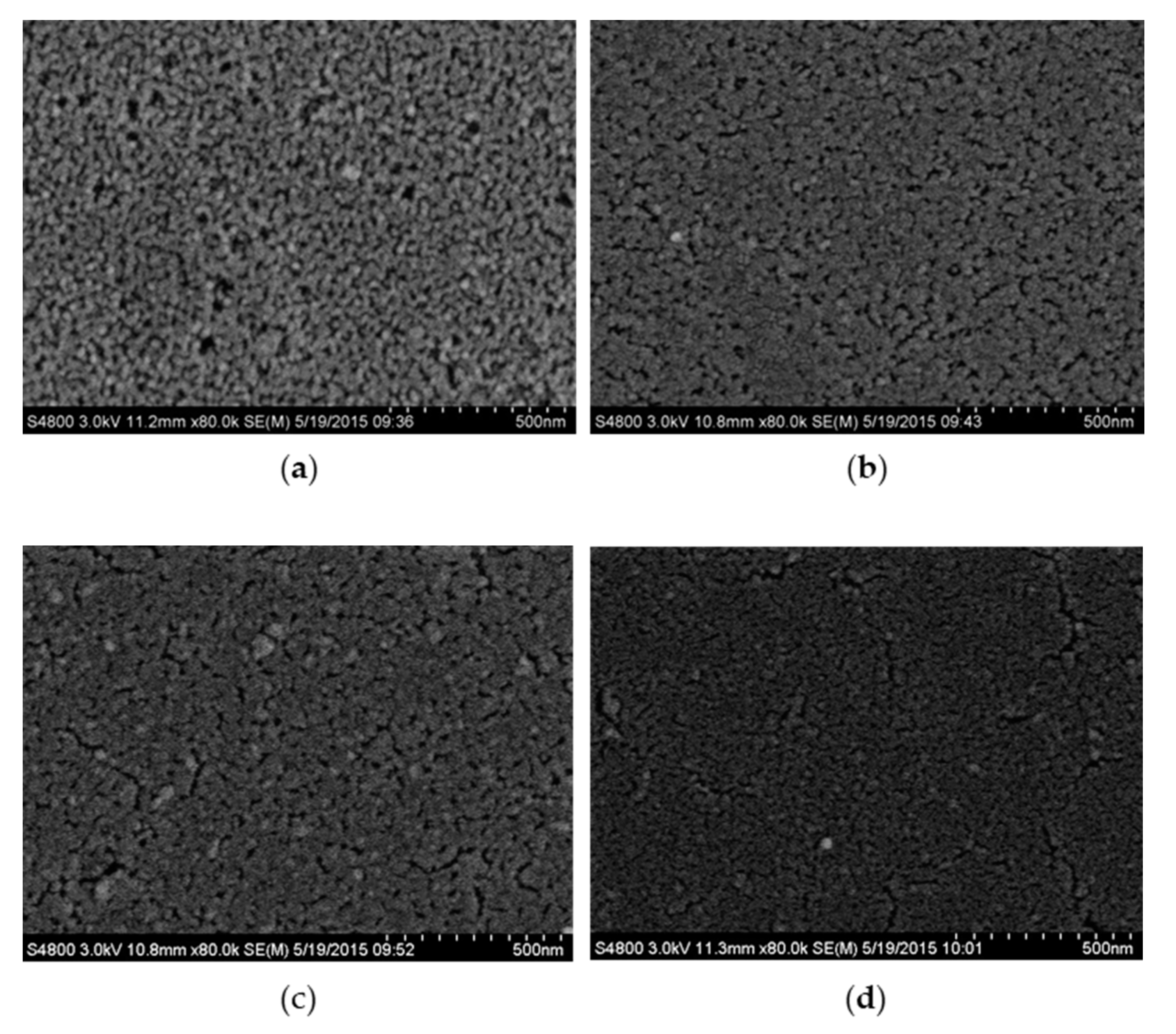

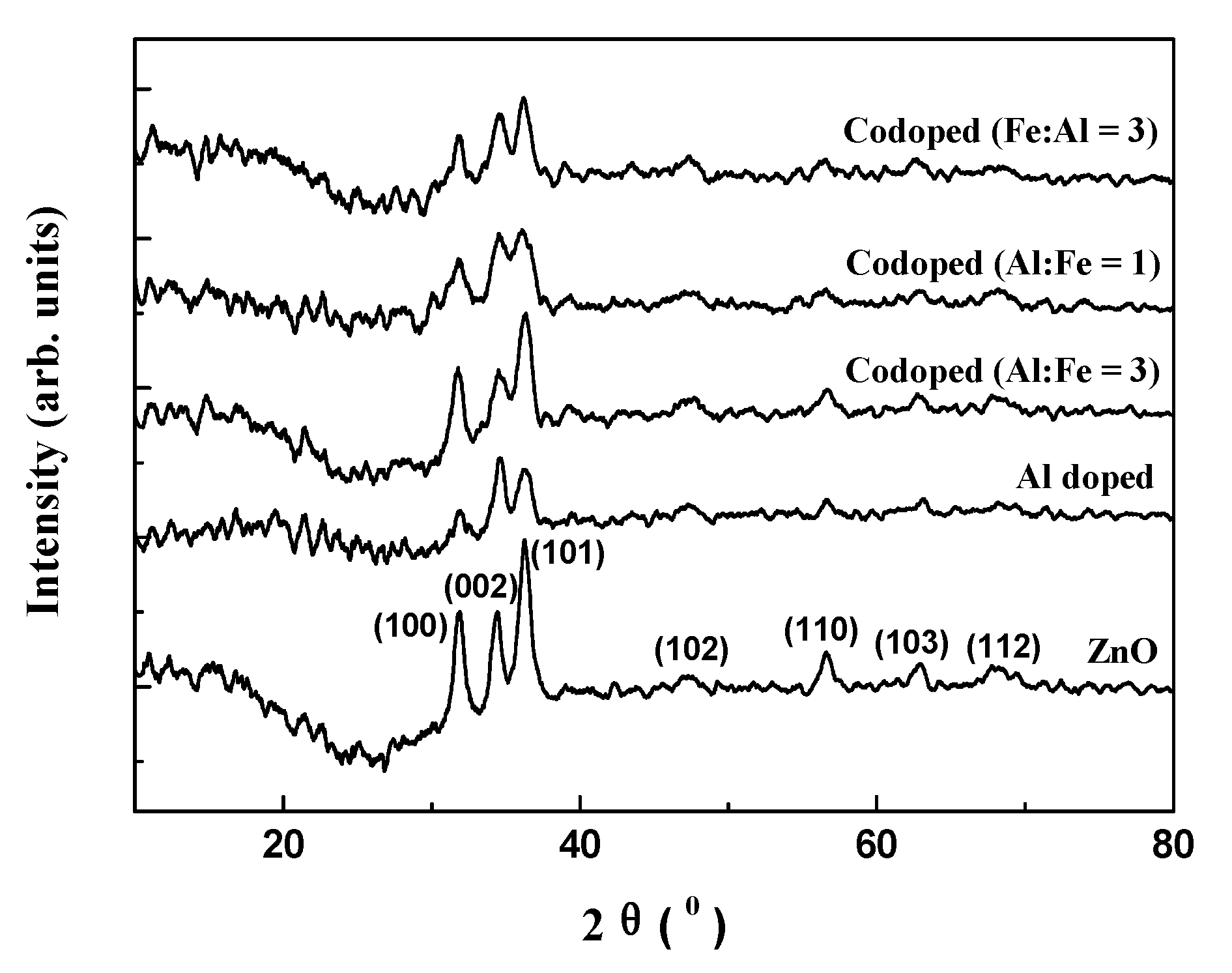

| Films | ZnO | Al Doped | Codoped (Al:Fe = 3) | Codoped (Al:Fe = 1) | Codoped (Fe:Al = 3) |
|---|---|---|---|---|---|
| 2θ value | 34.376° | 34.552° | 34.551° | 34.552° | 34.677° |
| interplanar spacing | 0.2607 nm | 0.2594 nm | 0.2594 nm | 0.2594 nm | 0.2585 nm |
| grain size | 8.984 nm | 8.544 nm | 6.335 nm | 6.239 nm | 5.856 nm |
| Element | C 1s | O 1s | Al 2p | Fe 2p3 | Zn 2p3 |
|---|---|---|---|---|---|
| Content (%) | 41.42 | 40.30 | 1.04 | 0.44 | 16.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Shen, W.; Zhang, X.; Li, J.; Ma, J. Preparation and Characterization of (Al, Fe) Codoped ZnO Films Prepared by Sol–Gel. Coatings 2021, 11, 946. https://doi.org/10.3390/coatings11080946
Wang J, Shen W, Zhang X, Li J, Ma J. Preparation and Characterization of (Al, Fe) Codoped ZnO Films Prepared by Sol–Gel. Coatings. 2021; 11(8):946. https://doi.org/10.3390/coatings11080946
Chicago/Turabian StyleWang, Jiangang, Wenjing Shen, Xin Zhang, Jianhui Li, and Jing Ma. 2021. "Preparation and Characterization of (Al, Fe) Codoped ZnO Films Prepared by Sol–Gel" Coatings 11, no. 8: 946. https://doi.org/10.3390/coatings11080946
APA StyleWang, J., Shen, W., Zhang, X., Li, J., & Ma, J. (2021). Preparation and Characterization of (Al, Fe) Codoped ZnO Films Prepared by Sol–Gel. Coatings, 11(8), 946. https://doi.org/10.3390/coatings11080946





