Tribological Behavior of Al2O3-MoO2-SiO2 Composite Ceramic Coating on Al-Zn-Mg-Cu Alloy
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Sample Preparation
2.2. Analysis of the MAO Coatings
3. Results and Discussion
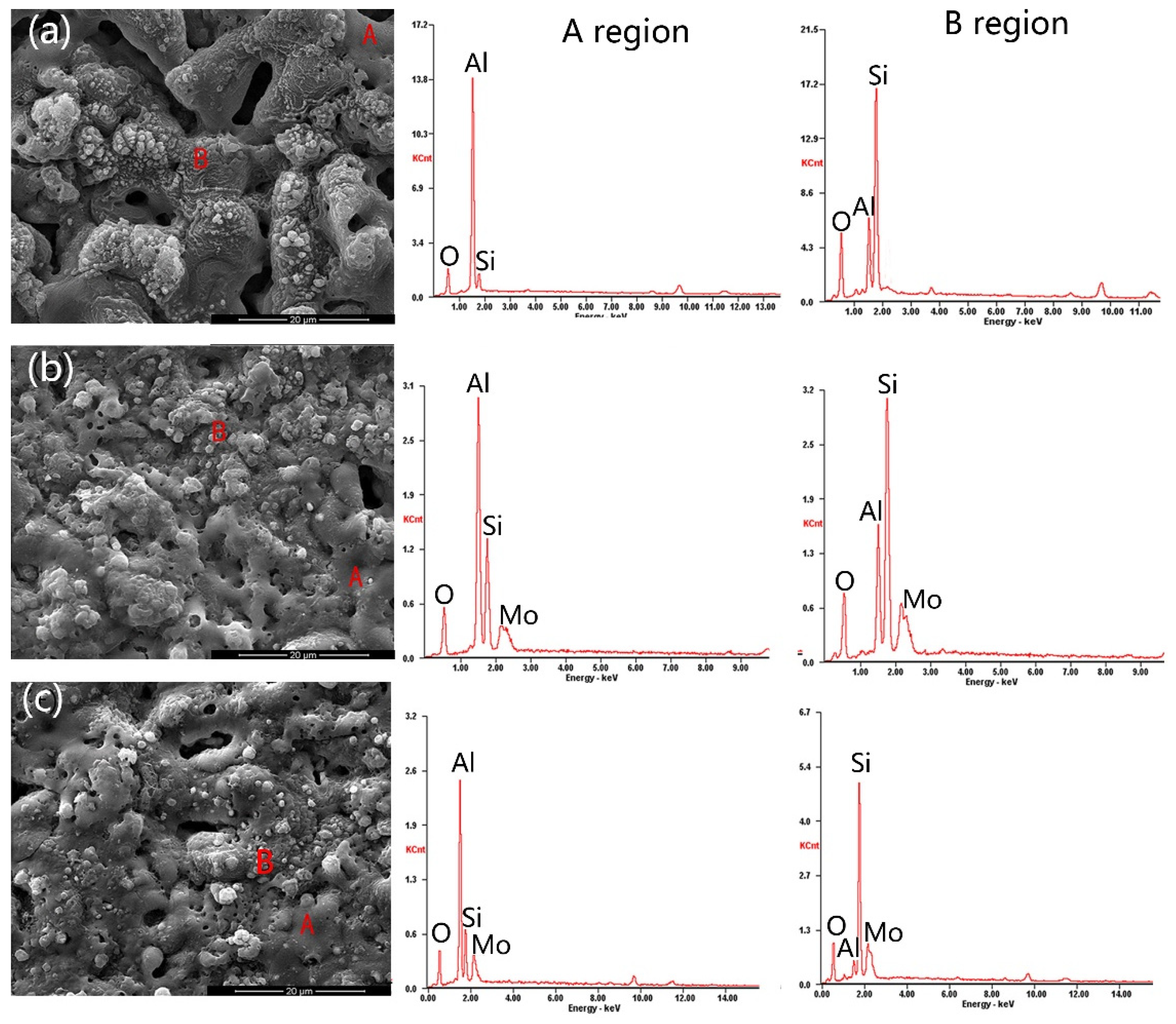
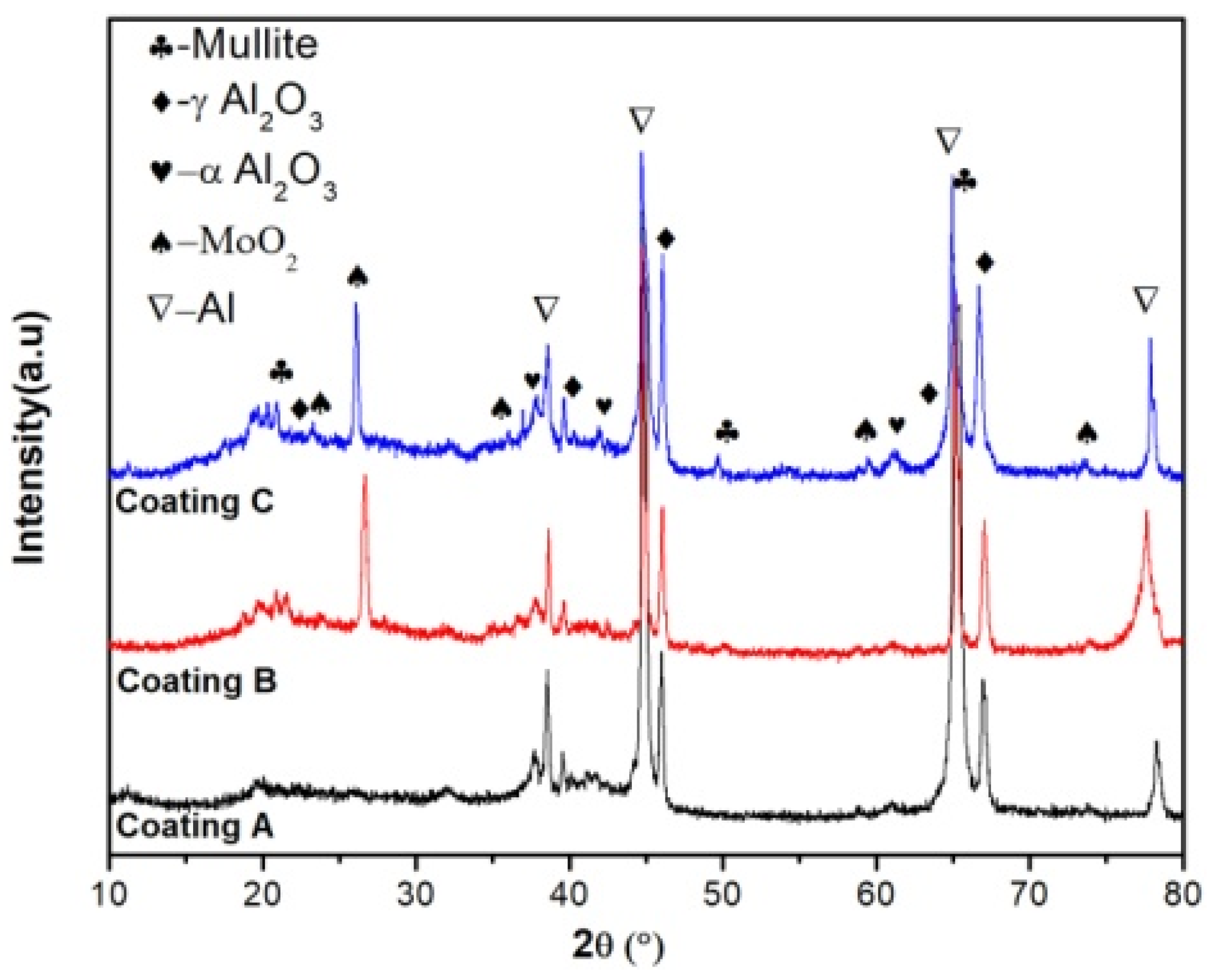
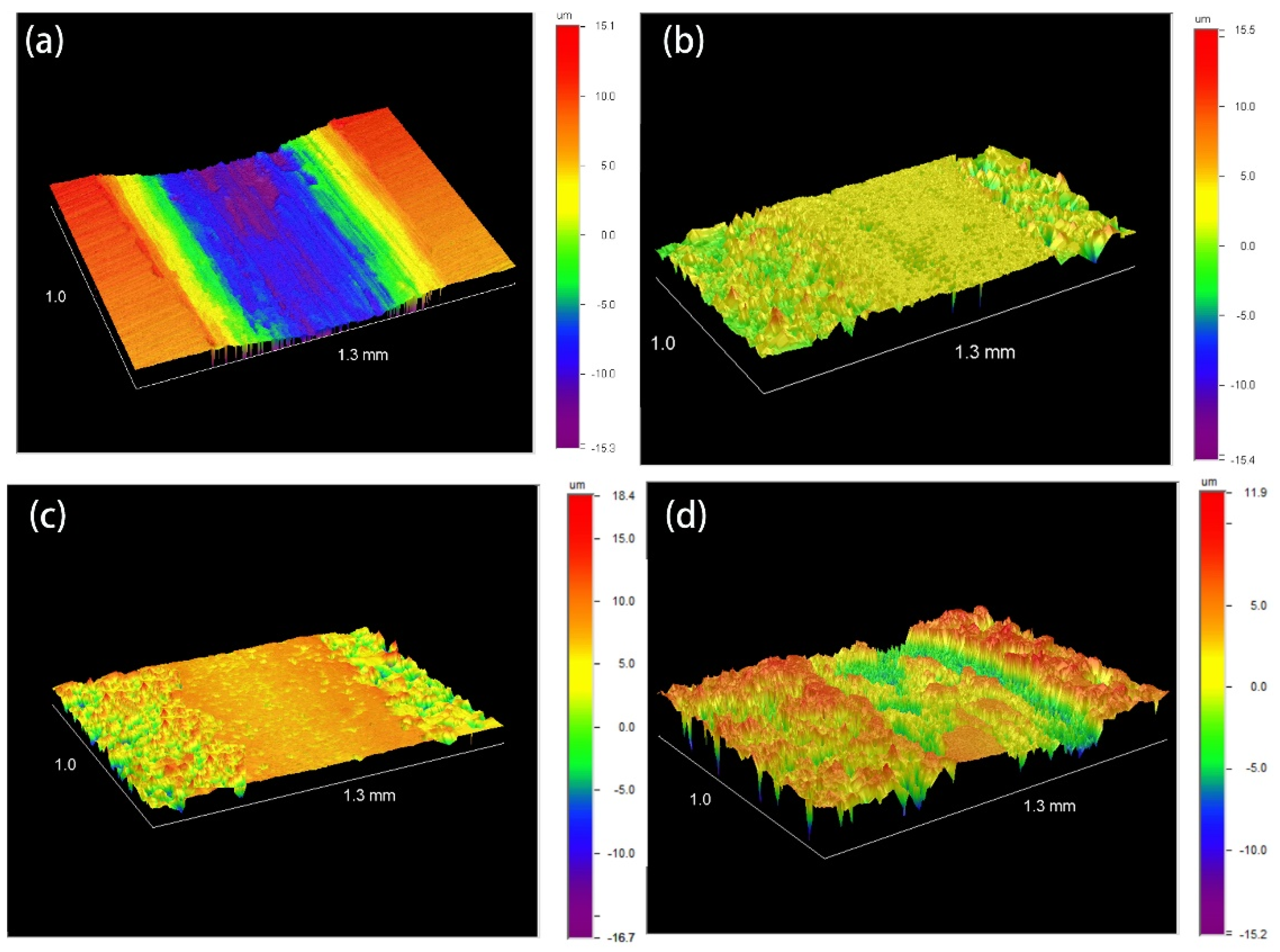
3.1. Microstructure and Phase Composition of the Coatings
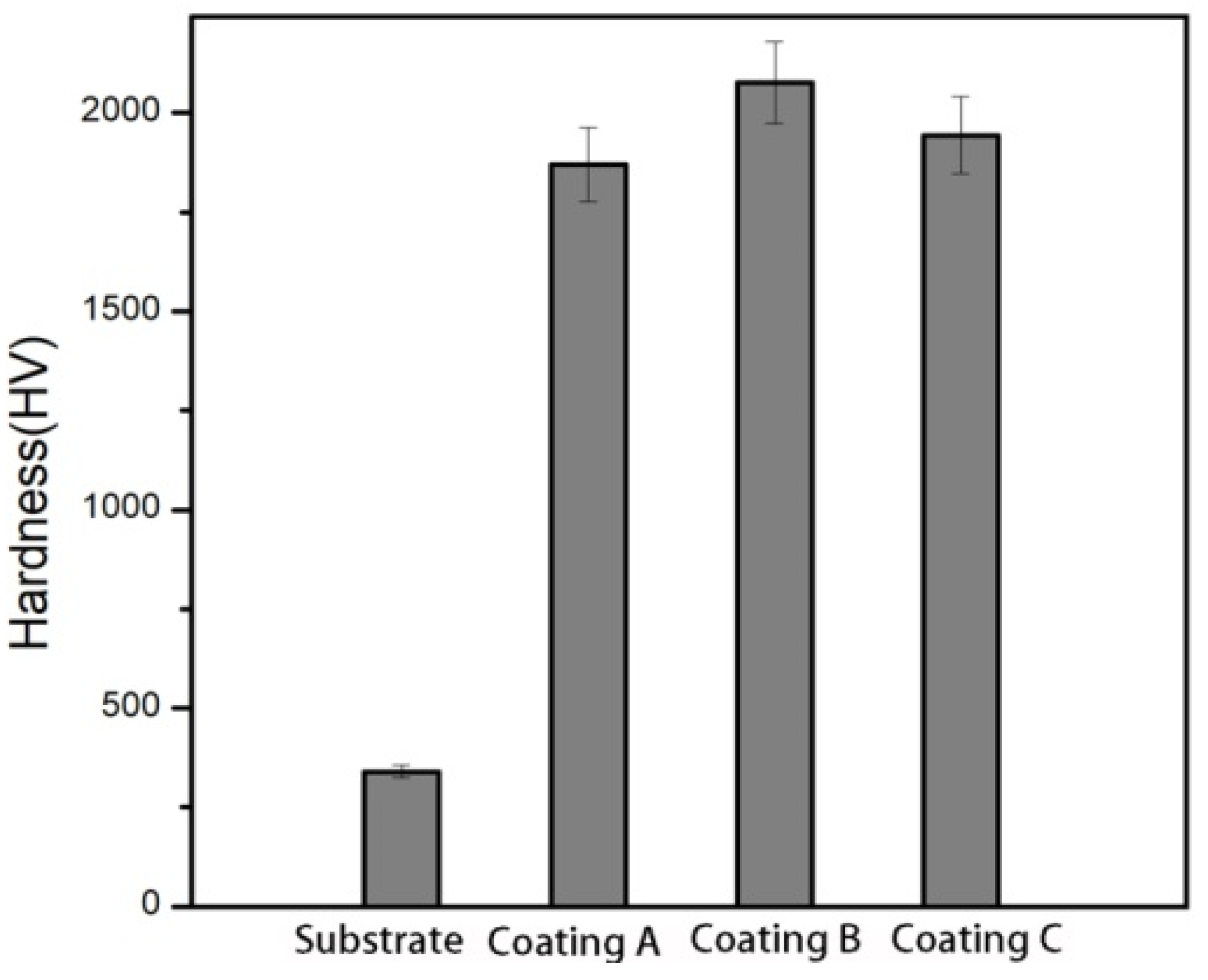
3.2. Hardness Analysis of Al-Zn-Mg-Cu Alloy and Coatings
3.3. Wear Properties
3.3.1. Friction Coefficient
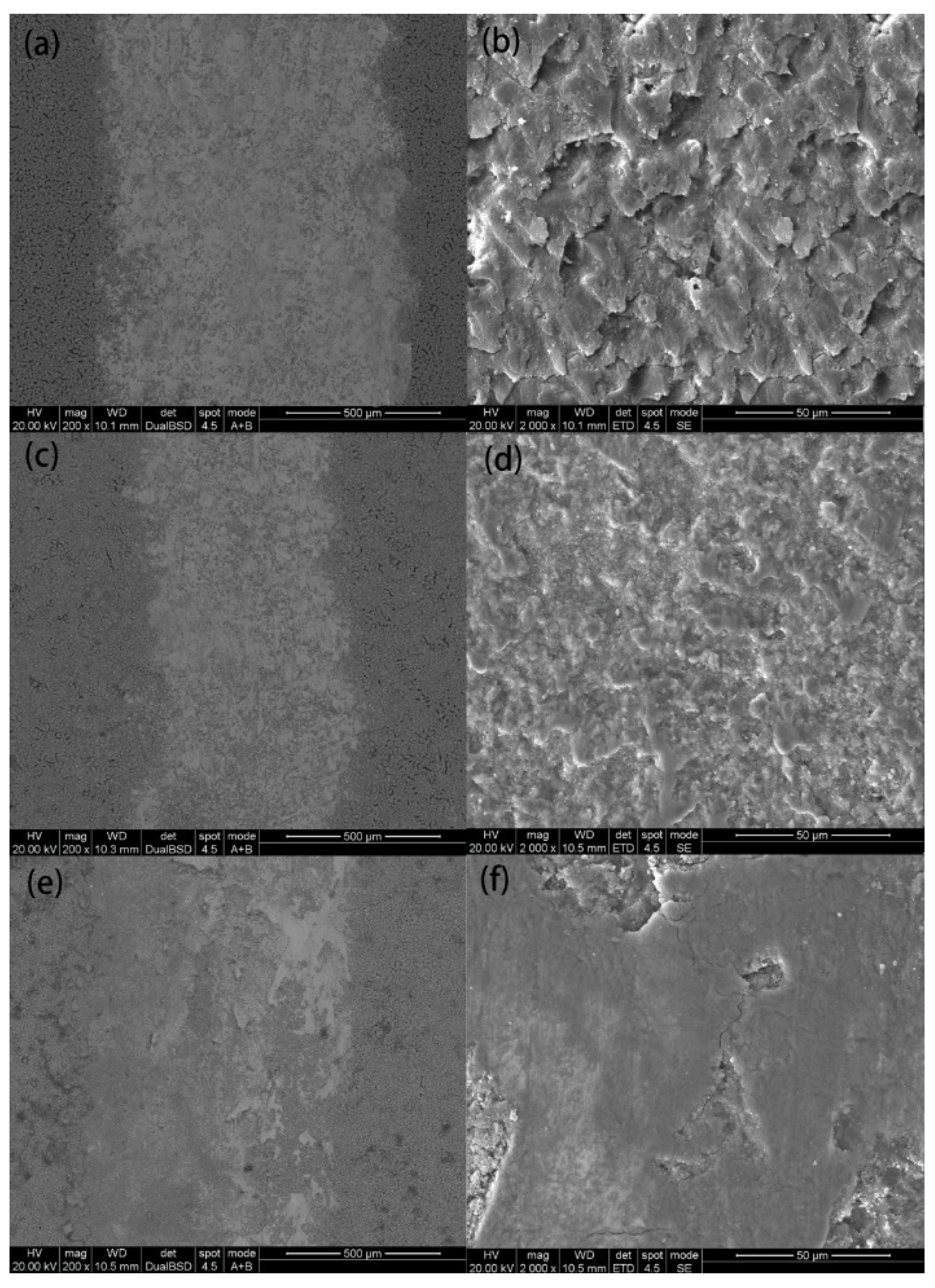
3.3.2. Wear Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gelfgat, M.Y.; Basovich, V.S.; Adelman, A. Aluminum alloy tubules for the oil and gas industry. World Oil 2006, 227, 45–51. [Google Scholar]
- Feng, C.; Shou, W.B.; Liu, H.Q.; Yi, D.Q.; Feng, Y.R. Microstructure and mechanical properties of high strength Al−Zn−Mg−Cu alloys used for oil drill pipes. Trans. Nonferrous Met. Soc. China 2015, 25, 3515–3522. [Google Scholar] [CrossRef]
- Liang, J.; Peng, L.; Sun, J.H.; Zhang, Y.Q.; Peng, L. Development of aluminum alloy drill rod material in geological drilling and the indoor experimental study. Geol. Explor. 2011, 47, 304–308. [Google Scholar]
- Arsenault, B.; Simard, S.; Marcoux, P.; Ghali, E. Stress Corrosion Cracking Mitigation of 7075-T6 Aluminium Alloy by Thermal Spray Coating. In Proceedings of the NACE International Corrosion Conference 2006, San Diego, CA, USA, 12–16 March 2006. [Google Scholar]
- Yan, T.N.; Xue, W.; Lan, K. High reliability aluminum alloy drill pipe and its application in super-deep wells and extended-reach wells. Geolog. Sci. Technol. Inform. 2010, 29, 112–115. [Google Scholar]
- Wernick, S.; Pinner, R.; Sheasby, P.G. The Surface Treatment and Finishing of Aluminum and Its Alloys; Finishing Publications Ltd.: Warrington, UK, 1987. [Google Scholar]
- Henley, V.F. Anodic Oxidation of Aluminium & Its Alloys; Pergamon Press: Oxford, UK, 1982. [Google Scholar]
- Sherif, E.M.; Park, S.M. Effects of 1, 5-naphthalenediol on aluminium corrosion as a corrosion inhibitor in 0.50 M NaCl. Electrochem. Soc. 2005, 152, B205–B211. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Li, X.G.; Dong, C.F.; Cheng, Y.F. Characterization of atmospheric corrosion of 2A12 aluminum alloy in tropical marine environment. J. Mater. Eng. Perform. 2010, 19, 591–598. [Google Scholar] [CrossRef]
- Abdul, S.R.; Rita, M.; Katiyar, P.K.; Kantesh, B. Superhydrophobic, self-cleaning carbon nanofiber CVD coating for corrosion protection of AISI 1020 steel and AZ31 magnesium alloys. Surf. Coat. Technol. 2020, 404, 126421. [Google Scholar]
- Chen, L.; Zeng, D.P.; Liu, Z.Y.; Bai, S.; Li, J. Improving the fatigue crack propagation resistance and damage tolerance of 2524-T3 alloy with amorphous electroless Ni-P coating. J. Mater. Eng. Perform. 2018, 27, 881–888. [Google Scholar] [CrossRef]
- Wojciechowski, J.J.; Szubert, K.; Peipmann, R.; Fritz, M.; Schmidt, U.; Bund, A.; Lota, G. Anti-corrosive properties of silane coatings deposited on anodised aluminium. Electrochim. Acta 2016, 220, 1–10. [Google Scholar] [CrossRef]
- Brusciotti, F.; Darya, V.; Xue, M.H.; Montemor, F.; Lamaka, S.; Ferreira, M. Hybrid epoxy-silane coating for improved corrosion protection of Mg alloy. Corros. Sci. 2013, 67, 82–90. [Google Scholar] [CrossRef]
- Sobolev, A.; Peretz, T.; Borodianskiy, K. Synthesis and growth mechanism of ceramic coatings on an Al-Cu alloy using plasma electrolytic oxidation in molten salt. J. Alloys Compd. 2021, 869, 159309. [Google Scholar] [CrossRef]
- Sieber, M.; Mehner, T.; Dietrich, D.; Alisch, G.; Nickel, D.; Meyer, D.; Scharf, I.; Lampke, T. Wear-resistant coatings on aluminium produced by plasma anodizing-A correlation of wear properties, microstructure, phasecomposition and distribution. Surf. Coat. Technol. 2014, 240, 96–102. [Google Scholar] [CrossRef]
- Lin, M.; Nemcova, A.; Voevodin, A.A.; Korenyi-Both, A.; Liskiewicz, T.W.; Laugel, N.; Matthews, A.; Yerokhin, A. Surface characteristics underpinning fretting wear performance of heavily loaded duplex chameleon/PEO coatings on Al. Tribol. Int. 2021, 154, 106723. [Google Scholar] [CrossRef]
- Sobolev, A.; Peretz, T.; Borodianskiy, K. Fabrication and characterization of ceramic coating on Al7075 alloy by plasma electrolytic oxidation in molten salt. Coatings 2020, 10, 993. [Google Scholar] [CrossRef]
- Hakimizad, A.; Raeissi, K.; Golozar, M.A. The effect of pulse waveforms on surface morphology, composition and corrosion behavior of Al2O3 and Al2O3/TiO2 nano-composite PEO coatings on 7075 aluminum alloy. Surf. Coat. Technol. 2017, 324, 208–221. [Google Scholar] [CrossRef]
- Ling-Yun, A.N.; Ying, M.A.; Yan, X.X.; Wang, S.; Wang, Z.-Y. Effects of electrical parameters and their interactions on plasma electrolytic oxidation coatings on aluminum substrates. Trans. Nonferrous Met. Soc. China 2020, 30, 883–895. [Google Scholar]
- Baratia, N.; Meletisb, E.I.; Farda, F.G.; Yerokhin, A.; Rastegari, S.; Faghihi-Sani, M.A. Al2O3–ZrO2 nanostructured coatings using DC plasma electrolyticoxidation to improve tribological properties of Al substrates. Appl. Surf. Sci. 2015, 356, 927–934. [Google Scholar] [CrossRef]
- Vatan, H.N.; Kahrizsangi, R.E.; Asgarani, M.K. Structural, tribological and electrochemical behavior of SiC nanocomposite oxide coatings fabricated by plasma electrolytic oxidation (PEO) on AZ31 magnesium alloy. J Alloys Compd. 2016, 683, 241–255. [Google Scholar] [CrossRef]
- Krishna, L.R.; Gupta, P.S.V.N.B.; Sundararajan, G. The influence of phase gradient within the micro arc oxidation (MAO) coatings on mechanical and tribological behaviors. Surf Coat. Technol. 2015, 269, 54–63. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, Z.; Wei, D.; Ma, D.; Lu, X.; Zhu, X. Fabrication of WO3/Al2O3 composite ceramic coatings on 6063 aluminum alloy by ultrasound-enhanced micro-arc oxidation. Int. J. Electrochem. Sci. 2021, 16, 21063. [Google Scholar] [CrossRef]
- Hadraba, H.; Drdlik, D.; Chlup, Z.; Maca, K.; Dlouhy, I.; Cihlar, J. Laminated alumina/zirconia ceramic composites prepared by electrophoretic deposition. J. Eur. Ceram. Soc. 2012, 32, 2053–2056. [Google Scholar] [CrossRef]
- Polat, A.; Makaraci, M.; Usta, M. Influence of sodium silicate concentration on structural and tribological properties of microarc oxidation coatings on 2017A aluminum alloy substrate. J. Alloys Compd. 2010, 504, 519–526. [Google Scholar] [CrossRef]
- Li, Z.J.; Yuan, Y.; Jing, X.Y. Comparison of plasma electrolytic oxidation coatings on Mg-Li alloy formed in molybdate/silicate and aluminate/silicate composite electrolytes. Mater. Corros. 2014, 65, 493–501. [Google Scholar] [CrossRef]
- Kaseem, M.; Kamil, M.P.; Ko, Y.G. Electrochemical response of MoO2-Al2O3 oxide films via plasma electrolytic oxidation. Surf. Coat. Technol. 2017, 322, 163–173. [Google Scholar] [CrossRef]
- Javidi, M.; Fadaee, H. Plasma electrolytic oxidation of 2024-T3 aluminum alloy andinvestigation on microstructure and wear behavior. Appl. Surf. Sci. 2013, 286, 212–219. [Google Scholar] [CrossRef]
- Hussein, R.; Nie, X.; Northwood, D.; Yerokhin, A.; Matthews, A. Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation (PEO) process. Appl. Phys. 2010, 43, 105203. [Google Scholar] [CrossRef]
- Kang, M.L.; Byung, U.L.; Sang, Y.; Eung, S.L.; Bongyoung, Y.; Dong, H.S. Evaluation of plasma temperature during plasma oxidation processing of AZ91 Mg alloy through analysis of the melting behavior of incorporated particles. Electrochim. Acta 2012, 67, 6–11. [Google Scholar]
- Dehnavi, V.; Liu, X.Y.; Luan, B.L.; Shoesmith, D.W.; Rohani, S. Phase transformation in plasma electrolytic oxidation coatings on 6061 aluminum alloy. Surf. Coat. Technol. 2014, 251, 106–114. [Google Scholar] [CrossRef]
- Wang, K.; Koo, B.H.; Lee, C.G.; Kim, Y.-J.; Lee, S.-H.; Byon, E. Effects of electrolytes variation on formation of oxide layers of 6061 al alloys by plasma electrolytic oxidation. Trans. Nonferrous Met. Soc. China 2009, 19, 866–870. [Google Scholar] [CrossRef]
- Archard, J.F. Contact and rubbing of flat surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]



| Zn | Mg | Cu | Cr | Fe | Si | Al |
|---|---|---|---|---|---|---|
| 6.28 | 2.19 | 1.6 | 0.15 | 0.4–0.6 | 0.4–0.6 | Bal. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, D.; Liu, Z.; Zou, L.; Wu, H. Tribological Behavior of Al2O3-MoO2-SiO2 Composite Ceramic Coating on Al-Zn-Mg-Cu Alloy. Coatings 2021, 11, 915. https://doi.org/10.3390/coatings11080915
Zeng D, Liu Z, Zou L, Wu H. Tribological Behavior of Al2O3-MoO2-SiO2 Composite Ceramic Coating on Al-Zn-Mg-Cu Alloy. Coatings. 2021; 11(8):915. https://doi.org/10.3390/coatings11080915
Chicago/Turabian StyleZeng, Diping, Zhiyi Liu, Lihua Zou, and Haijiang Wu. 2021. "Tribological Behavior of Al2O3-MoO2-SiO2 Composite Ceramic Coating on Al-Zn-Mg-Cu Alloy" Coatings 11, no. 8: 915. https://doi.org/10.3390/coatings11080915
APA StyleZeng, D., Liu, Z., Zou, L., & Wu, H. (2021). Tribological Behavior of Al2O3-MoO2-SiO2 Composite Ceramic Coating on Al-Zn-Mg-Cu Alloy. Coatings, 11(8), 915. https://doi.org/10.3390/coatings11080915




