Abstract
In this study, different amounts of SiO2 nanoparticles (7 nm) were added to simultaneously reach high transmittance, high hardness, and high adhesion for TiO2 film prepared by the sol–gel method and coated on glass through a dip-coating technique. For the film to achieve self-cleaning, anti-fogging, superhydrophilicity, and visible photo-induced photocatalysis, TiO2-SiO2 film was modified via a rapid microwave plasma-nitridation process for efficient N-doping by various N2-containing gases (N2, N2/Ar/O2, N2/Ar). Through nitrogen plasma, the content of N atom reached 1.3% with the ratio of O/Ti atom being 2.04. The surface of the thin films was smooth, homogeneous, and did not crack, demonstrated by the root mean square (RMS) roughness of film surface being 3.29–3.94 nm. In addition, the films were composed of nanoparticles smaller than 10 nm, with a thickness of about 100 nm, as well as the crystal phase of the thin film being anatase. After the plasma-nitridation process, the visible-light transmittance of N-doped TiO2-SiO2 films was 89.7% (clean glass = 90.1%). Moreover, the anti-fogging ability was excellent (contact angle < 5°) even without light irradiation. The degradation of methylene blue showed that the photocatalytic performance of N-doped TiO2-SiO2 films was apparently superior to that of unmodified films under visible-light irradiation. Moreover, the pencil hardness and adhesion rating test of the thin films were 7H and 5B, respectively, indicating that the obtained coatings had great mechanical stability.
1. Introduction
Titanium oxide (TiO2) is a very important material due to its multifunctional application in photocatalysis, hydrophilic or hydrophobic material, gas sensors, biosensors, photovoltaic cells, photochromic/electrochromic/optical devices, corrosion protection, and bactericide [1,2,3]. Superhydrophilic and self-cleaning TiO2 film on glass can be used to decompose oily stains or air pollutants, which could have promising potential applications in industry. Moreover, through the production and modification of light-sensitive TiO2 thin films, mirrors or windows with high performance of self-cleaning and anti-fogging functions could be achieved [4,5,6].
TiO2 has a high refractive index (n = 2.52 for anatase; 2.76 for rutile), resulting in poor transmittance, no adsorption bands in the visible wavelengths, and the formation of white surface coatings [7,8]. Hence, refractive index grading at the interface of two materials is required for reaching efficient transmission of a normal-incident wave for some particular wavelengths. Since SiO2 provides a refractive index that is intermediate between that of air (index of refraction, n = 1) and that of glass (n = 1.55), SiO2 (n = 1.46), it is generally used in solar cells of silicon-based thin film [9]. A previous study proposed mixing TiO2 and SiO2 for the promotion of various unique purposes, including superhydrophilic and self-cleaning functions under UV-light illumination [10]. The results revealed that the surface oxygen vacancies are saturated by OH groups, leading to a superhydrophilic surface [11]. Hence, adding SiO2 nanoparticles into TiO2 sol–gel to elevate the transmittance of film was carried out in this study.
Modified TiO2 photocatalysts can be produced by the deposition of metal (Cu, Ag, Pt, Au, Wu, Pd) or the doping of non-metal elements (N, P, S, C, F). The photocatalysis can be achieved under ultraviolet or visible-light irradiation, which highlights the demand for them as usable materials within the energy and environmental sectors [2,12,13,14,15,16]. The production of visible-light photocatalysts can lower the threshold energy of photoexcitation, and then apparently enhance the utilization of solar energy. The doping of various elements, particularly with N, has been carried out in several studies [17,18,19]. N-doped TiO2 photocatalysts can be synthesized via gas-phase or liquid-phase reactions, including the sol–gel method, chemical treatment of bare oxide, or high-temperature oxidation [17,18,20,21]. However, the plasma N-doping technique provides a dry and rapid process.
The production of hydrophilic TiO2 photocatalyst film has been demonstrated through numerous approaches: radio-frequency magnetron sputtering, electron beam evaporation, and the sol–gel method accompanied by spin-coating or dip-coating [22,23,24,25]. Furthermore, the photocatalytic activity of TiO2 thin films doped with nitrogen can be utilized to form TiO2−xNx photocatalysts via sputtering at low pressure, heating in an NH3-contained atmosphere, oxidative annealing of TiN, and direct high-temperature nitridation [26]. As a result of plasma-nitridation, it is a relatively simple and rapid process. Ti-N bonds and oxygen vacancies in the TiO2 films were formed by using a 13.56 MHz cathodic magnetron plasma at 30–100 W, 5 Pa for 5 min [26]. For an N-TiO2/CNT electrode, nitrogen-doped TiO2 was previously synthesized by plasma-enhanced atomic layer deposition on carbon nanotubes to enhance the specific capacity [27].
Simultaneously achieving high transparency/adhesion, superhydrophilicity, and visible photo-induced photocatalysis for the TiO2-based catalysts is exceedingly difficult. Due to the refractive index of TiO2 film/glass and the use of additives for modifying the catalyst, the combined interaction apparently decreases the transmittance of films. High-transparent, superhydrophilic, visible photo-induced photocatalytic N-doped TiO2-SiO2 nano film on glass has received scarce attention within the literature, and thus is one of the focal points of the current study.
2. Materials and Methods
2.1. Experimental Materials
Ti-containing gel was prepared by first mixing acetylacetone (AcAc, CH3COCH2COCH3, 98%, Fluka®, Milwaukee, WI, USA) and tetraisopropoxide (TTIP, Ti[OCH(CH3)2]4, 99.8%, Acros®, Rochester, NY, USA) and then adding ethanol (EtOH, C2H5OH, 99.5%, Shimakyu Pure Chemicals, Samut Sakhon, Thailand), hydrochloric acid (HCl, 37%, Nihon Shiyaku Reagent, Taipei, Taiwan), and H2O with a TTIP/AcAc/EtOH/HCl/H2O molar ratio of 1:5:110:0.024:5.4. The molar concentrations of AcAc, TTIP, EtOH, HCl, and H2O were 0.123, 0.615, 13.53, 0.003, and 0.664 M, respectively. The solution was stirred for 3 h at 50 °C. Then, varying amounts of silica gel (SiO2) nanoparticles (7 nm, Sigma-Aldrich, St. Louis, MO, USA) (0, 0.1, 1, 3 wt.%) were added to the sol–gel solution and stirred for 1 h to adjust the refractive index, as well as to reach the highest transmittance of film on the glass.
2.2. Method
The cleaning procedure of glass substrate (1.1 mm thick, 76.2 by 25.4 mm) was carried out first. The processes are as follows: ultrasonic shock in detergent solution for 20 min by an ultrasonic cleaner (400 W, 40 k Hz, Delta DC400H, Delta Ultrasonic Co. Ltd., New Taipei City, Taiwan); deionized (DI) water flushing; ultrasonic shock in DI water for 20 min; ultrasonic shock in methanol/HCl (v/v = 250:250 mL) solution for 20 min, followed by DI water flushing. Then, the TiO2/SiO2 films on dry glass were applied via dip-coating technology at various withdrawal speeds (WS = 5, 8, 10, 20, 40 mm/s) to examine the effects on transmittance, adhesion, and hardness of the films on glass. Consequently, the prepared thin film was calcined by a furnace (in air, 2.3 kW, Tender F-12, TenDer Co. Ltd., Kaohsiung, Taiwan) at 500 °C for 1 h with a heating rate of 2 °C/min for transforming amorphous crystallization into the anatase phase.
To induce the self-cleaning ability of the film at visible light, the sensitive photocatalyst thin films with high transmittance and superhydrophilic attributes were modified by N-doping the film via the microwave (MW) plasma-nitridation procedure using different reaction gases, including N2, N2/Ar(4.7%)/O2(2%), and N2/Ar(6.7%) at a total flow rate of 12 L/min (N2 is balanced gas) and an applied power of 900 W with a nitridation temperature of 500 °C for 1 min. The pressure used in the MW plasma system during plasma-nitridation process was at atmospheric pressure. Table 1 presents the parameters and conditions of sample preparation in this study.

Table 1.
Parameters and conditions of sample preparation.
The 2.45 GHz microwave (MW) plasma apparatus was applied to modify the thin films. The general scheme of the system is similar to the previous study [28]. The plasma system was assembled with a commercially available magnetron (YJ-1600, National Electronics, La Fox, IL, USA), with a stationary power of 5 kW (maximum) and operated in continuous-wave mode by passing microwaves through a circulator and a waveguide with a three-stub tuner, then reaching a cavity. An arc was used to ignite the plasma. A quartz tube reactor with a diameter of 2.9 cm intersected the waveguide (ASTEX WR340, MKS, Wilmington, MA, USA), and a resonator was placed perpendicular to it.
A glass dish was used for the photocatalytic experiments. The dish was cleaned by using DI water and ethanol solution, separately, with an ultrasonic oscillator for 30 min at room temperature. Then, the SiO2-TiO2 film/glass was located at the bottom of dish. The photocatalytic performance of the samples was assessed by adding 15 mL methyl blue (MB) (15 ppm) solution to the dish under visible light (Philips, T5-6WA, max. peak at 610 nm, Amsterdam, The Netherlands). The effect of N-doping on the catalysis performance of methylene blue via visible photo-induced process was examined and discussed.
2.3. Analytical Methods
The transmittance of the produced TiO2/SiO2 thin film was measured by using a UV–Vis spectrophotometer between 400 and 800 nm (Perkin Elmer/Lambda 35, Waltham, MA, USA). The thickness, morphology, and topology of thin films were characterized by scanning with an electron microscope (SEM, S3000N, Hitachi, Krefeld, Germany). The crystal structure of the synthesized TiO2-SiO2 film was determined using X-ray diffraction (XRD, RINT-2000, Rigaku, Austin, TX, USA) with CuKα radiation in the scan range of 15° to 85° (2θ). The superhydrophilic capacity of films was examined by a contact-angle analyzer (Digidrop/R&D, GBX, Dublin, Ireland). The roughness of films was analyzed by an atomic force microspore (AFM, NanoMan NS4 + D3100, Digital Instruments, Bresso, Italy). In the glow discharge zone, an optical emission spectrometer (OES, Ocean Optics, HR 4000CG, Kent, UK) was used to detect the active species involved in the plasma-nitridation process.
3. Results and Discussion
3.1. The Effects of SiO2 Addition and Withdrawal Speeds
3.1.1. Average Transmittance
After the cleaning procedure of glass substrate, the average transmittance of dry glass was 90.5% for the visible light region between 400 and 800 nm. Then, TiO2 thin films were prepared by the gel prepared from the TTIP/AcAc/EtOH/HCl/H2O solution via the dip-coating process. As a result, the average transmittance of double-sided TiO2 film/glass (without SiO2 addition) was apparently reduced. At a 5 mm/s withdrawal speed (WS), the average transmittance was only 68.3%. When WS was increased from 8 to 40 mm/s, the average transmittance was between 71.6% and 72.5% (Figure 1), significantly lower than that of original glass due to the high refractivity of TiO2 (n = 2.52 for anatase; 2.76 for rutile) compared to that of glass (n = 1.55) with a low surface roughness, which led to a reduction in transmittance for visible light.
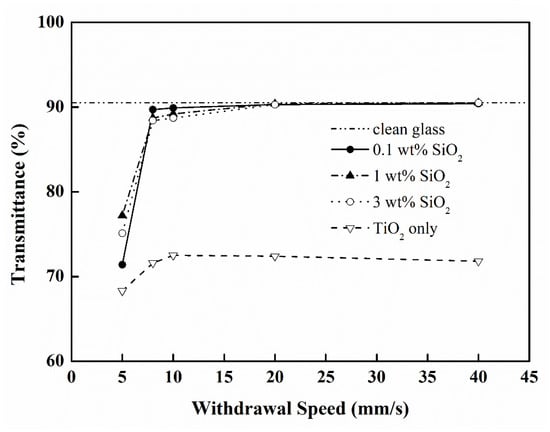
Figure 1.
The transmittance of TiO2 film/glass with the addition of various amounts of SiO2 and different withdrawal speeds.
In order to elevate the transmittance of TiO2-SiO2 thin films, the nanoparticles of SiO2 (0.1~3 wt.%) were added to the TiO2 gel. It is a similar technique with the production of anti-reflection optical film by adding the material of a lower index of refraction [29]. When the additional amount of SiO2 was in the range of 0.1~3 wt.%, the average transmittances were significantly higher than those lacking the additional SiO2 (71.6%); a secondary result yielded a slight increase from increasing the withdrawal speed from 8 mm/s to 40 mm/s (Figure 1). The high visible-light transmittance (88.0% to 90.6%) of as-prepared film/glass could be achieved (90.5% for clear glass) when SiO2 nanoparticles were added; this new value was higher than those without the addition of SiO2 due to the reduction of the index of refraction. In addition, a higher withdrawal speed led to an increase of either film thickness or roughness, resulting in higher transmittance. Due to the higher transmittance for 0.1 wt.% added SiO2 than that for other amounts of added SiO2 at a withdrawal speed of 8~40 mm/s, an additional amount of SiO2 was fixed at 0.1% for the subsequent experiments.
3.1.2. Superhydrophilic Characteristics
The angle of contact (θ) was about 10° for the glass substrate after cleaning procedures. However, it was easily dirtied when absorbing the air pollutants or organics which existed in the atmospheric environment. When the TiO2 film was coated on the glass, θ decreased from 18° to 7° when the withdrawal speed was reduced from 40 to 5 mm/s due to the elevation of the roughness of the surface, leading to an increase in hydrophilicity [30]. After 20 min of UV radiation, the angle of contact could decrease to under 5°, producing a superhydrophilic property of the film. However, θ reverted to its original status after 24 h of storage time in the dark.
When factoring in the addition of SiO2, θ decreased with the increase in the SiO2 addition, the decrease in withdrawal speed, and the UV irradiation time. At 0.1% SiO2, θ decreased from 10° to 5° by decreasing WS from 40 mm/s to 5 mm/s under the condition of no UV irradiation (Figure 2) due to the elevation of roughness. Moreover, after UV irradiation for 20 min, θ decreased to 1.5°~5° (Figure 2a), achieving superhydrophilic and anti-fogging properties, as well as reverting to its original status after 24 h of storage time in the dark (Figure 2b). Furthermore, when the addition of SiO2 was increased from 0.1 wt.% to 3 wt.%, θ only decreased 1~3° due to similar roughness [31].
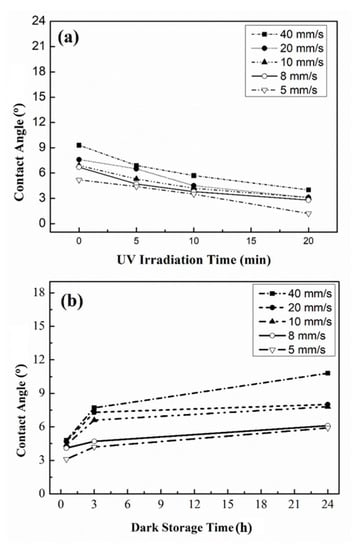
Figure 2.
Surface contact angle of TiO2-SiO2 (0.1%) film (a) at various withdrawal speeds and UV irradiation times, and (b) the effect of storage time in the dark.
3.2. Thickness, Morphology, and Topography of TiO2-SiO2 Films
Figure 3 shows the profile and thickness of the TiO2 film at SiO2 = 0.1 wt.%. The faster withdrawal speed in the dip-coating process led to a thicker film [32]. The thickness of the thin film was only 70, 90, and 110 nm at withdrawal speeds of 5, 8, and 10 mm/s, respectively.
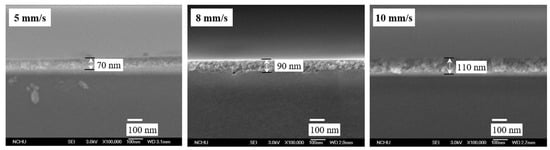
Figure 3.
Thickness of TiO2-SiO2 (0.1%) films prepared at various withdrawal speeds (SEM ×100,000).
From the morphology analysis, SEM images (Figure 4) showed that the films comprised well-distributed nanoparticles and the primary particle size was uniform and smaller than 10 nm. As the various withdrawal speeds increased, the particle size and porosity gradually became smaller, resulting in the roughness being reduced (Figure 4). The surface of the films was exceptionally smooth, and the nanoparticles were arranged compactly. Moreover, there was no aggregation or agglomeration of particles found, even after sintering at 500 °C.
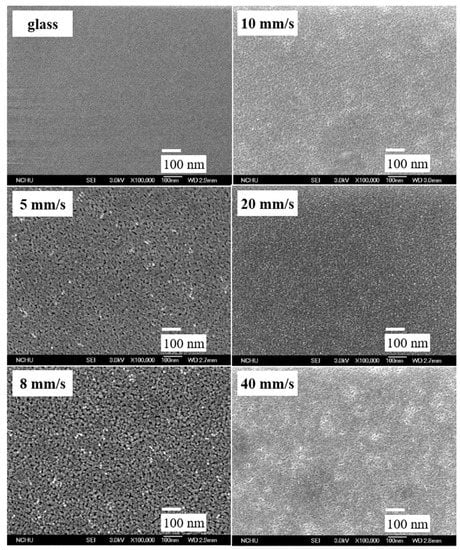
Figure 4.
Morphology of TiO2-SiO2 (0.1%) films prepared at various withdrawal speeds (SEM ×100,000).
Based on the XRD patterns of TiO2-SiO2/glass substrate (Figure 5), the amorphous TiO2 structure existed either in the as-prepared film/glass or after annealing at 200 °C. After annealing at 500 °C, characteristic peaks of main anatase TiO2 (JCPDS No. 21-1272) without rutile crystal structures were observed.
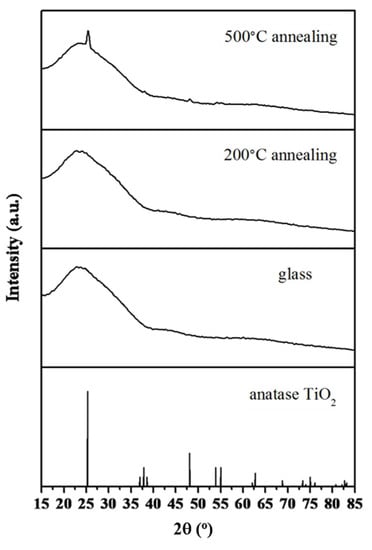
Figure 5.
Features of the XRD pattern for glass and TiO2-SiO2 film annealing at 200 °C and 500 °C.
The analysis of the atomic force microscope indicated that the root mean square roughness of the surface was only 0.25 nm for clean glass. After the coating of TiO2 with 0.1 wt.% SiO2, excluding the film thickness, the roughness was affected by withdrawal speed, reaching 5.73 nm at a WS of 5 mm/s, then decreasing to 0.61 nm at a WS of 40 mm/s (Table 2).

Table 2.
RMS roughness of surface of TiO2-SiO2 (0.1%) films prepared at various withdrawal speeds.
In order to access the adhesion between the TiO2-SiO2 (0.1%) film and clean glass, the standard test method (ASTM D3359) [33] was used by applying and removing pressure-sensitive tape over cuts made in the film. The results showed that the adhesion forces of films prepared for all withdrawal speeds are ASTM class 5B, indicating no film pull-off and representing the highest level of adhesion.
3.3. Rapid Plasma-Nitridation of Films by Different N2-Containing Gases
The superior experimental conditions when preparing the high-adhesion, transparent, and superhydrophilic TiO2-SiO2 film on glass were achieved by adding 0.1 wt.% of SiO2 at a WS of 8 mm/s. However, the photocatalytic performance should be carried out under UV irradiation. To produce a visible photo-induced photocatalytic TiO2-SiO2 film, the prepared films were modified by doping N atoms via the rapid microwave plasma-nitridation process in 1 min by inducing different N2-containing gases (N2, N2/Ar/O2, N2/Ar).
3.3.1. Surface Composition Analyses
The surface compositions and functional groups of the modified films were measured by XPS analysis. The results of chemical composition analysis showed that the content of N atoms was 1.3, 0.9, and 0.1 wt.% in the TiO2-SiO2 film by using N2, N2/Ar/O2, and N2/Ar plasma gases, respectively, indicating that more N atoms could be doped by N2 plasma.
In Figure 6, the N1S peak regions were deconvoluted into surface functional group contributions. The peaks of N1s observed with the chemical bonding energies around 398.2~399.3 eV, 400.6~401.7 eV, 402.7~403.7 eV, 407.1~407.3 eV, and 409.1 eV when using N2 or N2/Ar/O2 gases indicate the possible presence of N-TiO2 or N–O bonds (TiNxOy), adsorbed N2, TiO-N, N2O adsorption, and NO3− (surface nitrate), respectively (Figure 6a,b) [34,35,36,37]. However, by using N2/Ar as plasma gases (Figure 6c), it is similar to unmodified films (Figure 6d), lacking an apparent N peak region that can be deconvoluted into surface functional group contributions, which may be caused by the reduction in density of high energetic N-containing species due to easier energy transfer to Ar than to N2 molecules in the discharge zone. The main functional groups for the plasma N-doped films showed that O can be substituted rapidly for the N in TiO2 via the formation of oxygen vacancy [38] by using an atmospheric-pressure MW plasma coupled with plasma-nitridation gases.
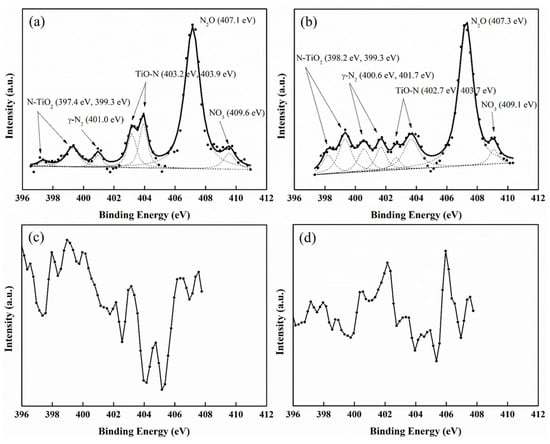
Figure 6.
XPS spectra of N1s for as-prepared SiO2-TiO2 films modified by different plasma gases: (a) N2, (b) N2/Ar/O2, (c) N2/Ar, and (d) without nitridation (dots: measured XPS data; dashed lines: fittings of binding energy spectra to XPS data; solid lines: sum of the fitted data).
3.3.2. Photocatalytic Performance of As-Modified Films under Visible-Light Irradiation
Under the condition of UV-light irradiation, the TiO2-SiO2 films will be superhydrophilic (θ < 5°). However, for utilization under a visible photo-induced environment, for example, indoors or a cloudy day, the superhydrophilic visible-induced TiO2-SiO2 (0.1 wt.%) films on glass with high adhesion, transparent, and anti-fogging ability are prepared via the rapid (1 min) plasma N-doping process by different plasma gases, including N2, N2/Ar/O2, and N2/Ar.
The analysis of UV-visible spectra of the non-doped and N-doped TiO2-SiO2 films (Figure 7) indicated that nitrogen plasma modification promoted visible-light absorption and red shift. The non-doped TiO2-SiO2 film shows the absorbance edge around 380 nm. A red shift in the absorption edge toward the visible-light region (λ is about 410 nm) is evident in the N-doped TiO2-SiO2 film modified by N2 plasma at 0.9 kW for 1 min due to the incorporation of nitrogen atoms into the lattice of TiO2.
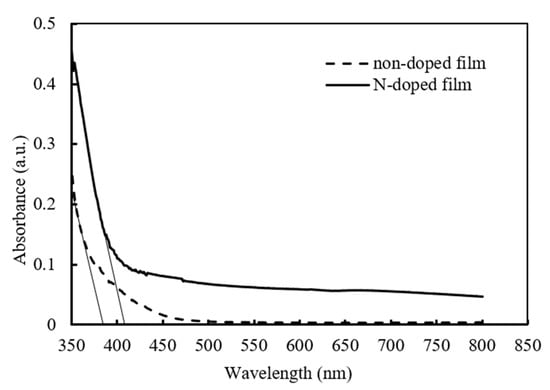
Figure 7.
UV-vis absorption spectra of the non-doped and N-doped films.
The removal efficiency of methylene blue (MB) in a glass reactor was carried out using visible-light irradiation, to evaluate the performance of the individual N-doped films [39]. The results of degradation for the photocatalytic activity of the TiO2-SiO2 (0.1 wt.%) films modified by different plasma gases are compared in Figure 8. Under visible-light degradation, the orders of conversion of MB are the films modified by N2 (84.3%) > N2/Ar/O2 > N2/Ar >> without nitridation (14.8%) after a visible-light irradiation of 500 min, indicating that the photocatalytic performance of TiO2-SiO2 films modified by N2 plasma was slightly higher than that of those by N2/Ar/O2 or N2/Ar plasma; however, this was much higher than that of those without N-doped film.
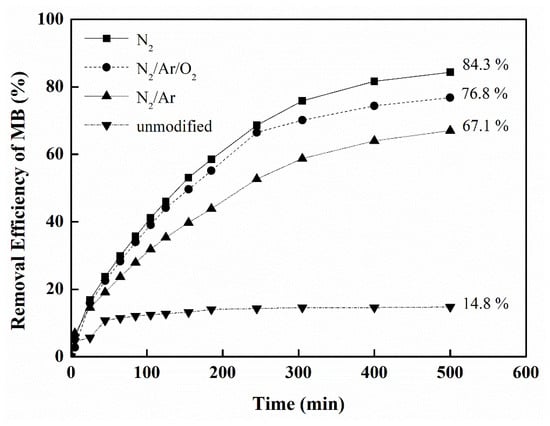
Figure 8.
Photocatalytic conversion of methylene blue under visible-light irradiation for the TiO2-SiO2 (0.1 wt.%) films modified by different plasma gases (N2, N2/Ar/O2, N2/Ar, unmodified) (MB = 15 ppm, MB solution = 15 mL).
The results revealed that a higher removal efficiency of methylene blue for visible-light induced degradation was found at a higher content of N atoms in TiO2-SiO2 film, because the red shift of N-doped films into the visible light absorption range was achieved. The results also indicate that the plasma-nitridation process can be used for rapid N-doped modification of films. Moreover, the photocatalytic reaction of MB is usually fitted with the pseudo-first-order reaction rate under visible light irradiation [28].
3.3.3. Characterization of Optical Emission Spectra
An MW plasma can be successfully used to rapidly dope N atoms into TiO2-SiO2 films in 1 min of residence time. Resulting from active or energetic N-containing species, including atomic N, excited N2, N ions, and N2 ions, are generated from nitrogen and ammonia gas in the glow discharge zone [40,41]. These energetic N-containing species can thermodynamically react with TiO2 more easily than N2 molecules, forming TiO2−xNx.
Figure 9 shows the optical emission intensity of intermediate species that were detected in the glow discharge zone at 0.9 kW. For nitrogen plasma, the active or energetic species can be produced via the complex plasma-chemical reactions, such as electron-impact processes, dissociative ionization, ionization, excitation reactions, and Penning ionization reaction. The characteristic emission lines of N2 from the first positive band (B3Πg → A3Σu+ transition, at 550–900 nm) and the second positive band (C3Πu → B3Πg transition, at 300–400 nm), and the emission lines of N2+ from the first negative band (B2Σu+ → X2Σg+ transition, at 381–470 nm) [40,41,42], and nearly all the peaks correspond to molecular nitrogen and ionic molecular nitrogen for N2 or N2/Ar plasma gases (Figure 9) can be identified. However, the atomic nitrogen metastable molecule and ionic atomic nitrogen can not to be observed in the discharge zone. Moreover, weak representative optical emission spectra of Ar in the range of 700~850 nm can also be identified. Except for N2 or N band, Figure 9 also shows that N–O bands (γ system, 226.9, 237.0, 247.9, 259.6, 272.2, 286.0 nm) and very weak O peaks (777.2, 777.4 nm) can be observed in the O2− containing (N2/Ar/O2) MW plasma [43,44].
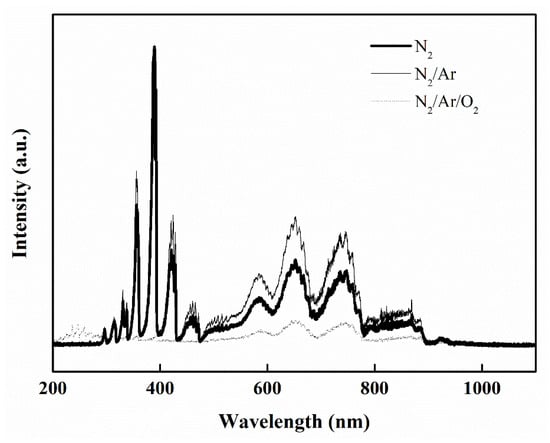
Figure 9.
Optical emission spectra for plasma-nitridation by different gases (N2, N2/Ar, N2/Ar/O2) at 760 Torr, 0.9 kW.
3.4. Characteristics of TiO2-SiO2 Films after Plasma-Nitridation
θ was about 10° for the clean glass substrate, 7~18° for TiO2 film/glass, 5~10° for TiO2-SiO2 (0.1 wt.%) film/glass, and lower than 5° after UV radiation of 20 min for TiO2-SiO2 (0.1 wt.%) film. The TiO2-SiO2 (0.1 wt.%) film was prepared via modification by a rapid (1 min) microwave plasma-nitridation process using different N2-containing gases, resulting in θ being lower than 5° without visible-light irradiation, as shown in Table 2. After the plasma-nitridation process, the regular, porous, and smooth surface was formed, leading to an increase in hydrophilicity [45]. Moreover, θ was still decreased to about 3° after visible-light irradiation for 20 min, regardless of what kind of plasma gases were utilized (Table 3), revealing that the modified superhydrophilic and anti-fogging films could be used in a visible photo-induced environment.

Table 3.
Contact angle (θ) for different N-containing gases after various visible-light irradiation times (min) by MW plasma for TiO2-SiO2 (0.1%) films.
After the plasma-nitridation process, the RMS roughness of the film surface slightly decreased when compared to that without plasma-nitridation, from 3.94 nm (unmodified) to 3.54 nm (N2 plasma), 3.50 nm (N2/Ar/O2 plasma), and 3.29 nm (N2/Ar) (Table 3). Hence, the RMS roughness of films was not affected significantly by plasma-nitridation. Visible-light transmittance was also unchanged by the different plasma gases, more so than that of the unmodified TiO2-SiO2 film or clean glass, reaching a range of 89.8~90.1% (Table 3). Both the film being modified at low temperature (500 °C) and a short modification time (1 min) resulted in the roughness not being significantly changed, and the film surface was porous and smooth with a high visible-light transmittance.
In order to assess the hardness of the TiO2-SiO2 (0.1%) film on the glass, the standard test method (ASTM D3363-05) [46] was carried out by drawing with pencil leads of known hardness. This ranges from 9H (hardest), 8H, 7H, … 7B, 8B to 9B (softest). The results showed that the pencil hardness of all prepared films for various plasma nitridation gases was 7H (Table 4), indicating good sol–gel coatings on glass (9H) due to the in situ prepared nanoparticles with only 0.1% SiO2 nanoparticles due to the nature of networking [47].

Table 4.
Characteristics of TiO2-SiO2 (0.1%) films after plasma-nitridation modified by various plasma gases (withdrawal speed = 8 mm/s).
4. Conclusions
This study successfully developed procedures to optimize the operational conditions for the sol–gel dip-coating method combined with a rapid plasma-nitridation technique to produce N-doped TiO2-SiO2 nano film on glass, while simultaneously achieving a high transmittance (~90%), high hardness (7H), superhydrophilicity (θ < 5°), and good adhesion (5B) for the applications of self-cleaning and anti-fogging. Moreover, the high photocatalytic performance of the film/glass can be easily accomplished under visible photo-induced irradiation after the rapid plasma-nitridation process.
Author Contributions
B.-J.L.: investigation, formal analysis, validation, writing—review and editing; K.-T.L.: conceptualization, formal analysis, writing—review and editing; Y.-M.K.: resources, supervision, project administration, writing—review and editing; C.-H.T.: investigation, visualization, data curation, methodology, writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to thank the Ministry of Science and Technology of Taiwan for its financial support under grants MOST 107-2221-E-992-003 and MOST 107-2622-E-992-017-CC3.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors gratefully acknowledge the use of EM000600 and EM000600 of MOST 108-2731-M-006-001 belonging to the Core Facility Center of National Cheng Kung University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bolis, V.; Busco, C.; Ciarletta, M.; Distasi, C.; Erriquez, J.; Fenoglio, I.; Livraghi, S.; Morel, S. Hydrophilic/hydrophobic features of TiO2 nanoparticles as a function of crystal phase, surface area and coating, in relation to their potential toxicity in peripheral nervous system. J. Colloid Interface Sci. 2012, 369, 28–39. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Nazeeruddin, M.K.; Baranoff, E.; Gratzel, M. Dye-sensitized solar cells: A brief overview. Sol. Energy 2011, 85, 1172–1178. [Google Scholar] [CrossRef]
- de Jesus, M.A.M.L.; Neto, J.T.d.S.; Timò, G.; Paiva, P.R.P.; Dantas, M.S.S.; Ferreira, A.d.M. Superhydrophilic self-cleaning surfaces based on TiO2 and TiO2/SiO2 composite films for photovoltaic module cover glass. Appl. Adhes. Sci. 2015, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Kameya, Y.; Yabe, H. Optical and superhydrophilic characteristics of TiO2 coating with subwavelength surface structure consisting of spherical nanoparticle aggregates. Coatings 2019, 9, 547. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.; Tang, Y.; Gong, J.; Gong, D.; Chi, L.; Lin, C.; Chen, Z. Transparent superhydrophobic/superhydrophilic TiO2-based coatings for self-cleaning and anti-fogging. J. Mater. Chem. 2012, 22, 74209–77426. [Google Scholar] [CrossRef]
- Chen, T.L.; Hirose, Y.; Hitosugi, T.; Hasegawa, T. One unit-cell seed layer induced epitaxial growth of heavily nitrogen doped anatase TiO2 films. J. Phys. D Appl. Phys. 2008, 41, 062005. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, P.; Li, B.; Zhu, N.; Dang, Z. In-depth study on intercalating threonine into layered double hydroxides. Appl. Clay Sci. 2011, 53, 615–620. [Google Scholar] [CrossRef]
- Lin, Y.S.; Lien, S.Y.; Wuu, D.S.; Huang, Y.X.; Kung, C.Y. Improvement in performance of Si-based thin film solar cells with a nanocrystalline SiO2-TiO2 layer. Thin Solid Films 2014, 570, 200–203. [Google Scholar] [CrossRef]
- Arin, M.; Watté, J.; Pollefeyt, G.; Buysser, K.D.; Driessche, I.V.; Lommens, P. Low temperature deposition of TiO2 layers from nanoparticle containing suspensions synthesized by microwave hydrothermal treatment. J. Sol. Gel Sci. Technol. 2013, 66, 100–111. [Google Scholar] [CrossRef]
- Parkin, P.; Palgrave, R.G. Self-cleaning coatings. J. Mater. Chem. 2005, 15, 1689–1695. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for environmental photocatalytic applications: A review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/Visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- Rampaul, A.; Parkin, I.P.; O’Neill, S.A.; DeSouza, J.; Mills, A.; Elliott, N. Titania and tungsten doped titania thin films on glass; active photocatalysts. Polyhedron 2003, 22, 35–44. [Google Scholar] [CrossRef]
- Seery, M.K.; George, R.; Floris, P.; Pillai, S.C. Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J. Photochem. Photobiol. A 2007, 189, 258–263. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Ma, J.; Li, K.; Li, J. Hydrothermal synthesis of S-doped TiO2 nanoparticles and their photocatalytic ability for degradation of methyl orange. Ceram. Int. 2009, 35, 1289–1292. [Google Scholar] [CrossRef]
- Bakara, A.B.; Ribeiro, C. Nitrogen-doped titanium dioxide: An overview of material design and dimensionality effect over modern applications. J. Photochem. Photobiol. C 2016, 27, 1–29. [Google Scholar] [CrossRef]
- Sato, S.; Nakamura, R.; Abe, S. Visible-light sensitization of TiO2 photocatalysts by wet-method N doping. Appl. Catal. A 2005, 284, 131–137. [Google Scholar] [CrossRef]
- Wawrzyniak, B.; Morawski, A.W. Solar-light-induced photocatalytic decomposition of two azo dyes on new TiO2 photocatalyst containing nitrogen. Appl. Catal. B 2006, 62, 150–158. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Nosaka, Y.; Matsushita, M.; Nasino, J.; Nosaka, A.Y. Nitrogen-doped titanium dioxide photocatalysts for visible response prepared by using organic compounds. Sci. Technol. Adv. Mater. 2005, 6, 143–148. [Google Scholar] [CrossRef]
- Chekini, M.; Mohammadizadeh, M.R.; Allaei, S.M.V. Photocatalytic and superhydrophilicity properties of N-doped TiO2 nano thin films. Appl. Surf. Sci. 2011, 257, 7179–7183. [Google Scholar] [CrossRef]
- Mechiakh, R.; Sedrine, N.B.; Chtourou, R.; Bensaha, R. Correlation between microstructure and optical properties of nano-crystalline TiO2 thin films prepared by sol-gel dip coating. Appl. Surf. Sci. 2010, 257, 670–676. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, D.J.; Hahn, S.H.; Kim, E.J. Comparison of optical and photocatalytic properties of TiO2 thin films prepared by electron-beam evaporation and sol-gel dip-coating. Mater. Lett. 2003, 57, 4151–4155. [Google Scholar] [CrossRef]
- Tajima, K.; Yamada, Y.; Bao, S.; Okada, M.; Yoshimura, K. Solid electrolyte of tantalum oxide thin film deposited by reactive DC and RF magnetron sputtering for all-solid-state switchable mirror gass. Sol. Energy Mater. Sol. Cells 2008, 92, 120–125. [Google Scholar] [CrossRef]
- Yamada, K.; Yamane, H.; Matsushima, S.; Nakamura, H.; Sonoda, T.; Miura, S.; Kumada, K. Photocatalytic activity of TiO2 thin films doped with nitrogen using a cathodic magnetron plasma treatment. Thin Solid Films 2008, 516, 7560–7564. [Google Scholar] [CrossRef]
- Lin, J.; Ma, D.; Li, Y.; Zhang, P.; Mi, H.; Deng, L.; Sun, L.; Ren, X. In situ nitrogen doping of TiO2 by plasma enhanced atomic layer deposition for enhanced sodium storage performance. Dalton Trans. 2017, 46, 13101–13107. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Wang, Y.F.; Tsai, Y.I.; Tsai, C.H. A simple process for synthesizing nano Pt- and/or N-doped titanium dioxide powders by microwave plasma torch. J. Alloys Compd. 2014, 617, 834–840. [Google Scholar] [CrossRef]
- Zhang, X.T.; Sato, O.; Taguchi, M.; Einaga, Y.; Murakami, T.; Fujishima, A. Self-cleaning particle coating with antireflection properties. Chem. Mater. 2005, 17, 696–700. [Google Scholar] [CrossRef]
- Pandiyaraj, K.N.; Selvarajan, V.; Pavese, M.; Falaras, P.; Tsoukleris, D. Investigation on surface properties of TiO2 films modified by DC glow discharge plasma. Curr. Appl. Phys. 2009, 9, 1032–1037. [Google Scholar] [CrossRef]
- Machida, M.; Norimoto, K.; Watanabe, T.; Hashimoto, K.; Fujishima, A. The effect of SiO2 addition in super-hydrophilic property of TiO2 photocatalyst. J. Mater. Sci. 1999, 34, 2569–2574. [Google Scholar] [CrossRef]
- Chen, D. Anti-refection (AR) coatings made by sol-gel processes: A review. Sol. Energy Mater. Sol. Cells 2001, 68, 313–336. [Google Scholar] [CrossRef]
- ASTM D3359-02 Standard Test Methods for Measuring Adhesion by Tape Test; ASTM International: West Conshohocken, PA, USA, 2004.
- Baltrusaitis, J.; Jayaweera, P.M.; Grassian, V.H. XPS study of nitrogen dioxide adsorption on metal oxide particle surfaces under different environmental conditions. Phys. Chem. Chem. Phys. 2009, 11, 8295–8305. [Google Scholar] [CrossRef]
- Chen, C.; Bai, H.; Chang, C. Effect of plasma processing gas composition on the nitrogen-doping status and visible light photocatalysis of TiO2. J. Phys. Chem. C 2007, 111, 15228–15235. [Google Scholar] [CrossRef]
- Jain, N.; Paul, A.K.; Srivastava, T.S. Synthesis, characterization, cytotoxicity and DNA binding studies of diamminediethyldithiocarbamato-platinum(II) nitrate. J. Inorg. Biochem. 1992, 45, 123–127. [Google Scholar] [CrossRef]
- Pashutski, A.; Folman, M. Low temperature XPS studies of NO and N2O adsorption on Al(100). Surf. Sci. 1989, 216, 395–408. [Google Scholar] [CrossRef]
- Huang, C.M.; Chen, L.C.; Cheng, K.W.; Pan, G.T. Effect of nitrogen-plasma surface treatment to the enhancement of TiO2 photocatalytic activity under visible light irradiation. J. Mol. Catal. A Chem. 2007, 261, 218–224. [Google Scholar] [CrossRef]
- Kim, C.; Choi, M.; Jang, J. Nitrogen-doped SiO2/TiO2 core/shell nanoparticles as highly efficient visible light photocatalyst. Catal. Commun. 2010, 11, 378–382. [Google Scholar] [CrossRef]
- Hsueh, H.P.; McGrath, R.T.; Ji, B.; Felker, B.S.; Langan, J.G.; Karwacki, E.J. Ion energy distribution and optical emission spectra in NF3-based process chamber plasma. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2001, 19, 1346–1357. [Google Scholar] [CrossRef]
- Sun, Q.; Zhu, A.M.; Yang, X.F.; Niu, J.H.; Xu, Y.; Song, Z.M.; Liu, J. Plasma-catalytic selective reduction of NO with C2H4 in the presence of excess oxygen. Chin. Chem. Lett. 2005, 16, 839–842. [Google Scholar]
- Jeong, B.Y.; Kim, M.H. Effects of the process parameters on the layer formation behavior of plasma nitrided steels. Surf. Coat. Technol. 2001, 141, 182–186. [Google Scholar] [CrossRef]
- Goujon, M.; Belmonte, T.; Henrion, G. OES and FTIR diagnostics of HMDSO/O2 gas mixture for SiOx deposition assisted by RF plasma. Surf. Coat. Technol. 2004, 188–189. [Google Scholar] [CrossRef]
- Timmermans, E.A.H.; Jonkers, J.; Rodero, A.; Quintero, M.C.; Sola, A.; Gamero, A.; Schram, D.C.; van der Mullen, J.A.M. The behavior of molecules in microwave-induced plasma studies by optical emission spectroscopy. 2: Plasma at reduced pressure. Spectrochim. Acta B 1999, 54, 1085–1098. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- ASTM D3363-05 Standard Test Method for Film Hardness by Pencil Test; ASTM International: West Conshohocken, PA, USA, 2011.
- Kalidindi, R.S.R.; Subasri, R. Sol-gel nanocomposite hard coatings, anti-abrasive nanocoatings. Curr. Future Appl. 2015, 105–136. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).