Influence of Alternative and Conventional Surface Treatments on the Bonding Mechanism between PEEK and Veneering Resin for Dental Application
Abstract
1. Introduction
2. Materials and Methods
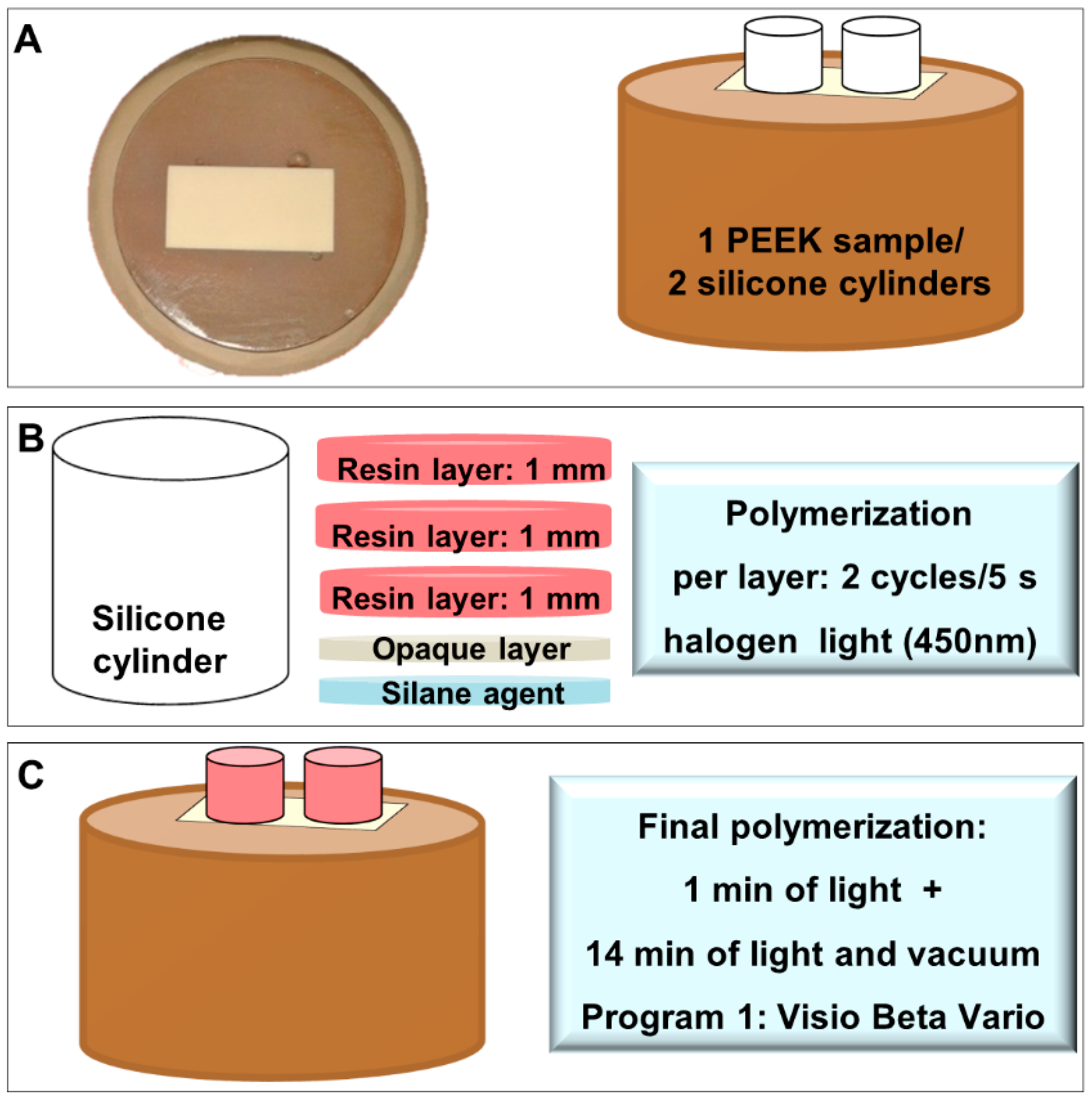
2.1. PEEK Surface Treatments
- (1)
- TBS Group (control): microblasting sand with 110 μ Al2O3 particles (Rocatec™ Pre) followed by tribochemical coating of the microblasted surface with silica-modified 110 μ aluminium oxide (Rocatec™ Plus)
- (2)
- ALO Group: sandblasting with 45 μ Al2O3 particles.
- (3)
- SA5 Group: etching with 98% sulfuric acid for 5 s and rinse in running water for 1 min.
- (4)
- SA30 Group: etching with 98% sulfuric acid for 30 s and rinse in running water for 1 min.
- (5)
- TBS-H Group: microblasting sand with 110 μ Al2O3 particles followed by silica coating and heated in an oven at 79 °C for 60 s after RelyX™ Ceramic Primer application.
2.2. Resin Application
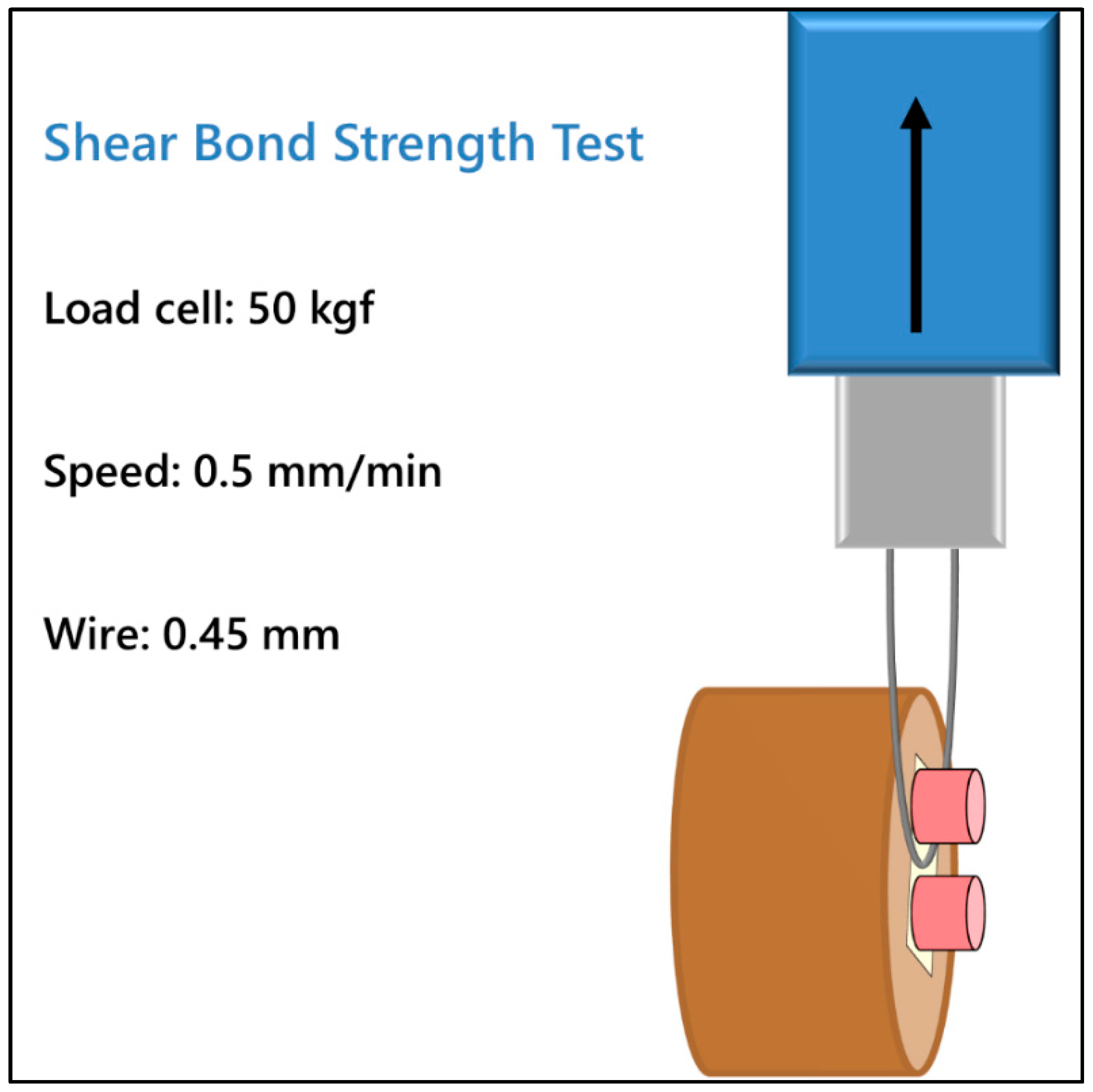
2.3. SBS Test and Failure Analysis
2.4. Contact Angle and Micromorphological Analysis
2.5. Energy-Dispersive X-ray Spectroscopy (EDS)
2.6. Data Analysis
3. Results
3.1. SBS Test and Failure Analysis
3.2. Contact Angle Measurements
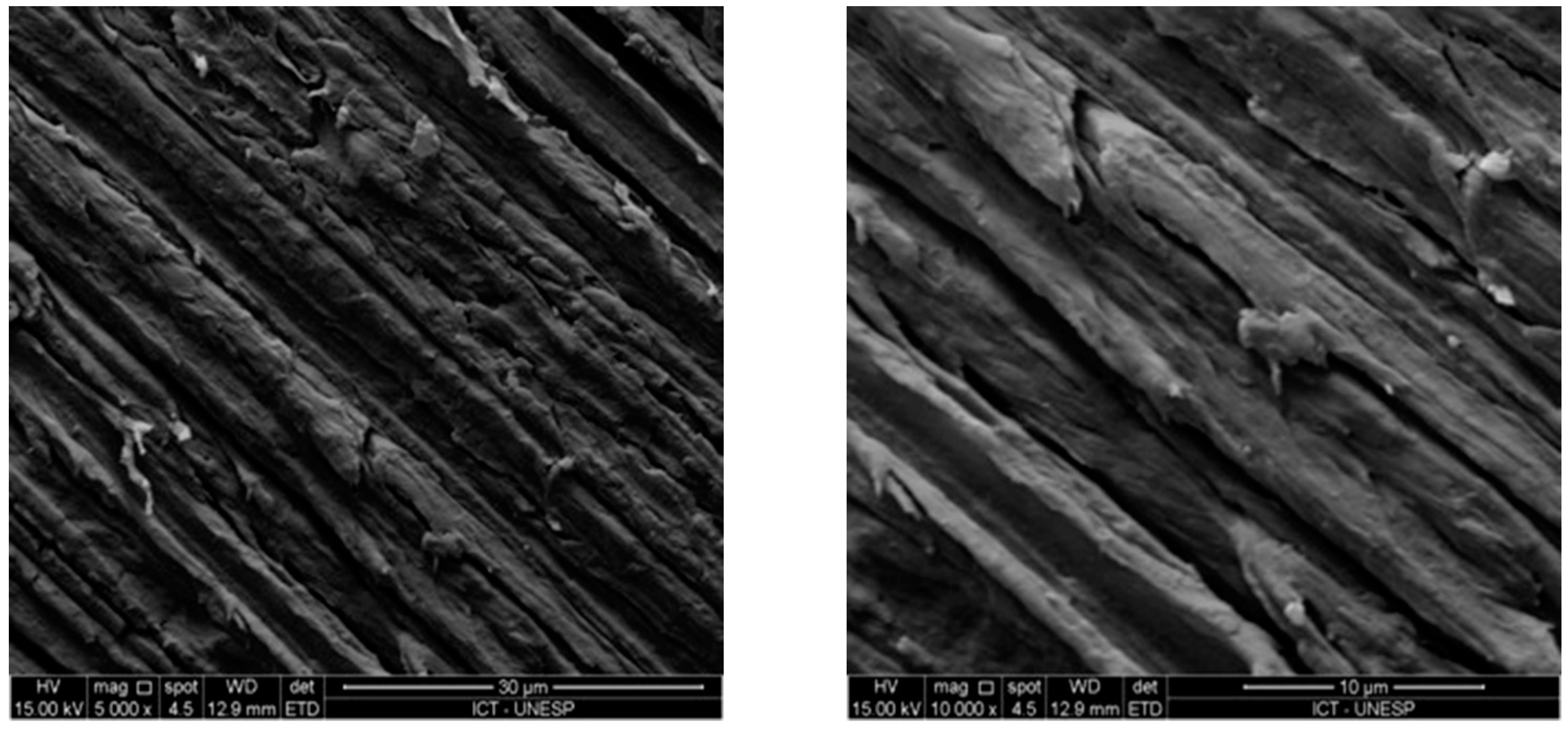
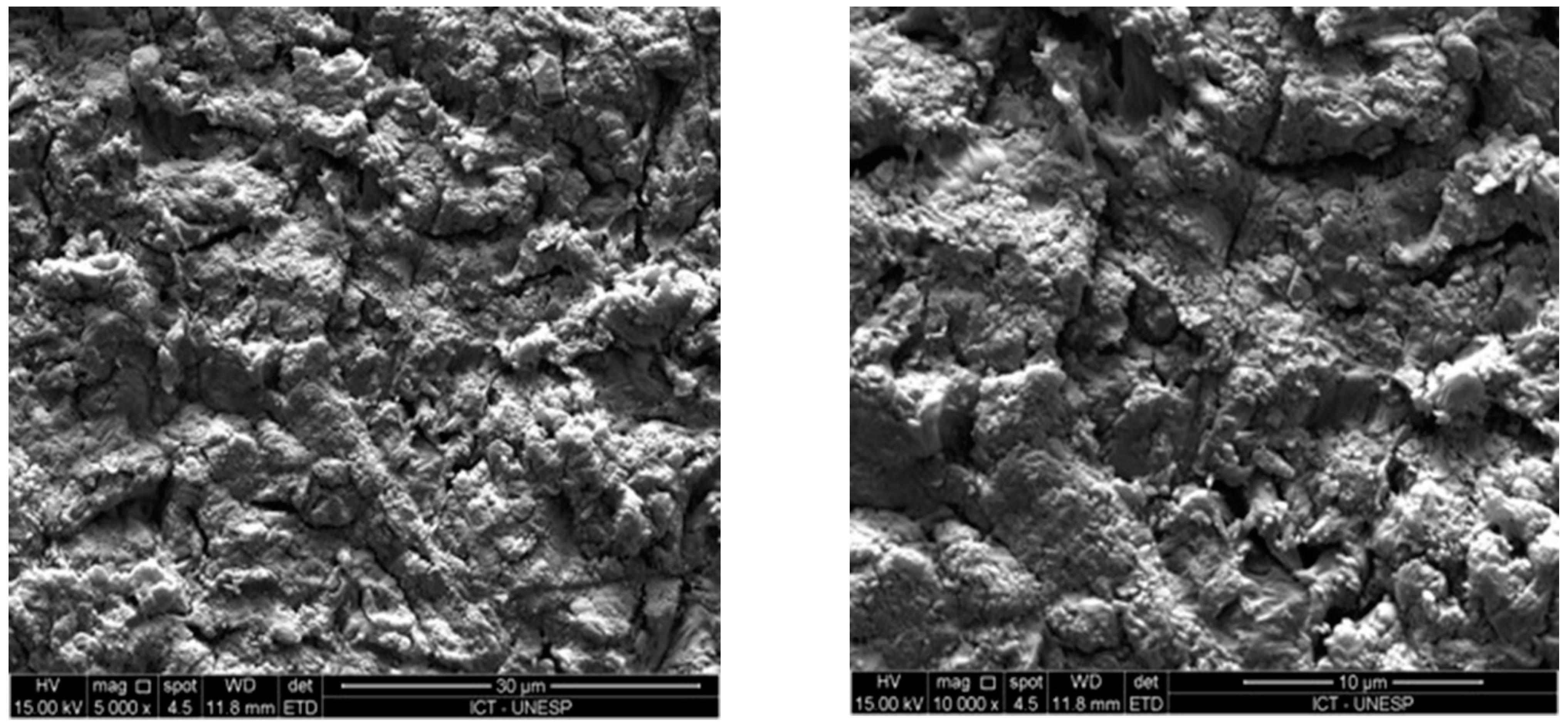
3.3. Micromorphological Analysis
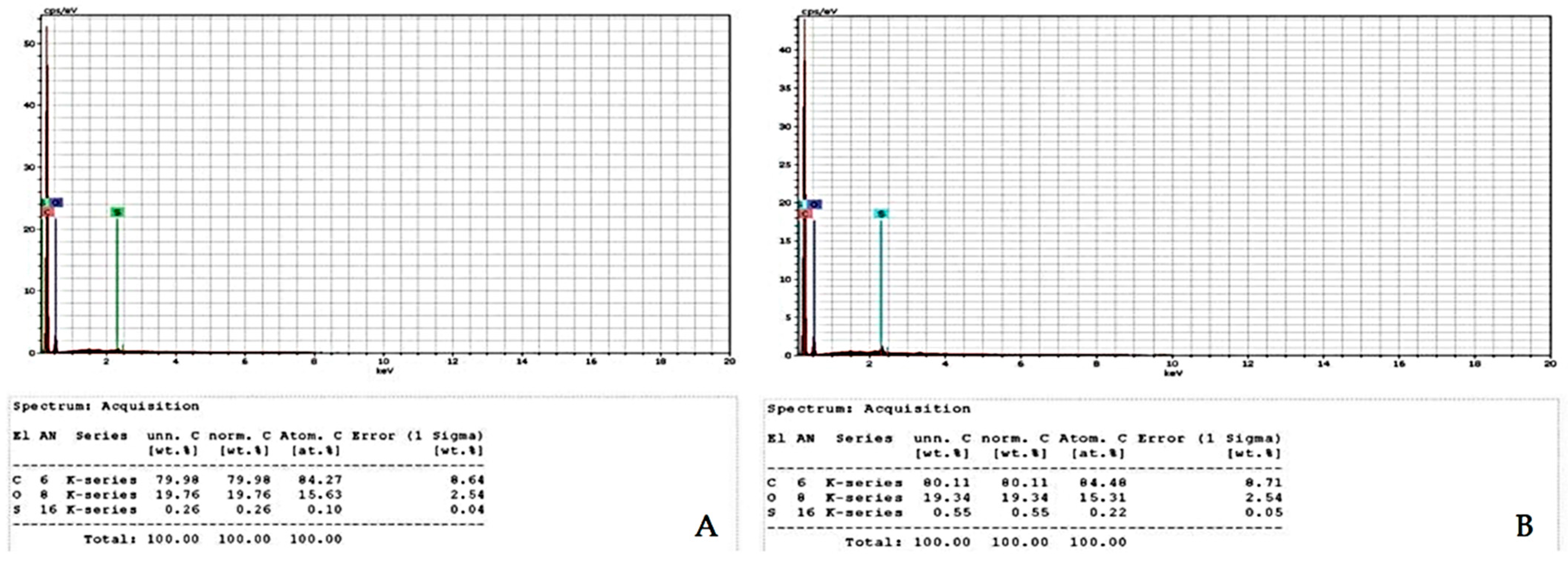
3.4. Energy-Dispersive X-ray Spectroscopy (EDS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quan, D.; Alderliesten, R.; Dransfeld, C.; Tsakoniatis, I.; Benedictus, R. Co-cure joining of epoxy composites with rapidly UV-irradiated PEEK and PPS composites to achieve high structural integrity. Compos. Struct. 2020, 251, 112595. [Google Scholar] [CrossRef]
- Heinrich, L.A. Future opportunities for bio-based adhesives-advantages beyond renewability. Green Chem. 2019, 21, 1866–1888. [Google Scholar] [CrossRef]
- Wang, X.; Lu, T.; Wen, J.; Xu, L.; Zeng, D.; Wu, Q.; Cao, L.; Lin, S.; Liu, X.; Jiang, X. Selective responses of human gingival fibroblasts and bacteria on carbon fiber reinforced polyetheretherketone with multilevel nanostructured TiO2. Biomaterials 2016, 83, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Cheng, Q.; Zhao, R.; Zhu, X.; Yang, X.; Yang, X.; Zhang, K.; Song, Y.; Zhang, X. Comparison of osteointegration property between PEKK and PEEK: Effects of surface structure and chemistry. Biomaterials 2018, 1, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Wang, L.; Zhao, R.; Yang, X.; Yang, X.; Zhu, X.; Liu, L.; Zhang, K.; Song, Y.; Zhang, X. A biomimetically hierarchical polyetherketoneketone scaffold for osteoporotic bone repair. Sci. Adv. 2020, 6, 1–16. [Google Scholar] [CrossRef]
- Arcila, L.; de Carvalho Ramos, N.; Bottino, M.; Tribst, J.P.M. Indications, materials and properties of 3D printing in dentistry: A literature overview. Res. Soc. Dev. 2020, 9, e80791110632. [Google Scholar] [CrossRef]
- Alqurashi, H.; Khurshid, Z.; Syed, A.U.Y.; Rashid Habib, S.; Rokaya, D.; Zafar, M.S. Polyetherketoneketone (PEKK): An emerging biomaterial for oral implants and dental prostheses. J. Adv. Res. 2021, 28, 87–95. [Google Scholar] [CrossRef]
- Lümkemann, N.; Eichberger, M.; Stawarczyk, B.; Azimian, F.; Klosa, K.; Kern, M.; Stawarczyk, B.; Taufall, S.; Roos, M.; Schmidlin, P.R.; et al. Polyetheretherketone—A suitable material for fixed dental prostheses? Dent. Mater. J. 2012, 28, 2–7. [Google Scholar] [CrossRef]
- Panayotov, I.V.; Orti, V.; Cuisinier, F.; Yachouh, J. Polyetheretherketone (PEEK) for medical applications. J. Mater. Sci. Mater. Med. 2016, 27. [Google Scholar] [CrossRef] [PubMed]
- Giudice, R.L.; Tribst, J.P.M. Dental materials coatings: Effect on the clinical behavior. Coatings 2020, 10, 1229. [Google Scholar] [CrossRef]
- Hahnel, S.; Wieser, A.; Lang, R.; Rosentritt, M. Biofilm formation on the surface of modern implant abutment materials. Clin Oral Implant. Res 2015, 26, 1297–1301. [Google Scholar] [CrossRef]
- Dal Piva, A.M.; Tribst, J.P.; Borges, A.L.; e Souza, R.O.; Bottino, M.A. CAD-FEA modeling and analysis of different full crown monolithic restorations. Dent. Mater. 2018, 34, 1342–1350. [Google Scholar] [CrossRef]
- Campaner, L.M.; Silveira, M.P.M.; de Andrade, G.S.; Borges, A.L.S.; Bottino, M.A.; de Dal Piva, A.M.O.; Lo Giudice, R.; Ausiello, P.; Tribst, J.P.M. Influence of polymeric restorative materials on the stress distribution in posterior fixed partial dentures: 3D finite element analysis. Polymers 2021, 13, 758. [Google Scholar] [CrossRef]
- Villefort, R.F.; Tribst, J.P.M.; Dal Piva, A.M.O.; Borges, A.L.; Binda, N.C.; Ferreira, C.E.A.; Bottino, M.A.; von Zeidler, S.L.V. Stress distribution on different bar materials in implant-retained palatal obturator. PLoS ONE 2020, 15. [Google Scholar] [CrossRef]
- Tribst, J.; Dal Piva, A.; Borges, A.; Araújo, R.; da Silva, J.; Bottino, M.; Kleverlaan, C.; de Jager, N. Effect of different materials and undercut on the removal force and stress distribution in circumferential clasps during direct retainer action in removable partial dentures. Dent. Mater. 2020, 36, 179–186. [Google Scholar] [CrossRef] [PubMed]
- de Araújo Nobre, M.; Moura Guedes, C.; Almeida, R.; Silva, A.; Sereno, N. Hybrid Polyetheretherketone (PEEK)–Acrylic Resin Prostheses and the All-on-4 Concept: A Full-Arch Implant-Supported Fixed Solution with 3 Years of Follow-Up. J. Clin. Med. 2020, 9, 2187. [Google Scholar] [CrossRef]
- Costa-Palau, S.; Torrents-Nicolas, J.; Brufau-De Barberà, M.; Cabratosa-Termes, J. Use of polyetheretherketone in the fabrication of a maxillary obturator prosthesis: A clinical report. J. Prosthet. Dent. 2014, 112, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Tribst, J.P.M.; de Oliveira Dal Piva, A.M.; Borges, A.L.S.; Rodrigues, V.A.; Bottino, M.A.; Kleverlaan, C.J. Does the prosthesis weight matter? 3D finite element analysis of a fixed implant-supported prosthesis at different weights and implant numbers. J. Adv. Prosthodont. 2020, 12, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Mertens, C.; de San Jose Gonzalez, J.; Freudlsperger, C.; Bodem, J.; Krisam, J.; Hoffmann, J.; Engel, M. Implant-prosthetic rehabilitation of hemimaxillectomy defects with CAD/CAM suprastructures. J. Cranio-Maxillofac. Surg. 2016, 44, 1812–1818. [Google Scholar] [CrossRef] [PubMed]
- Rauch, A.; Hahnel, S.; Günther, E.; Bidmon, W.; Schierz, O. Materials Tooth-Colored CAD/CAM Materials for Application An Illustrated Clinical Comparison. Materials 2020, 13, 5588. [Google Scholar] [CrossRef] [PubMed]
- Alghazzawi, T.F.; Lemons, J.; Liu, P.R.; Essig, M.E.; Bartolucci, A.A.; Janowski, G.M. Influence of Low-Temperature Environmental Exposure on the Mechanical Properties and Structural Stability of Dental Zirconia. J. Prosthodont. 2012, 21, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.F.V.; Anami, L.C.; Campos, T.M.B.; de Melo, R.M.; de e Souza, R.O.A.; Bottino, M.A. Bonding of the polymer polyetheretherketone (PEEK) to human dentin: Effect of surface treatments. Braz. Dent. J. 2016, 27, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Qian, Y.; Zhu, Y.; Liu, H.; Gan, K.; Guo, J. The effect of different surface treatments on the bond strength of PEEK composite materials (DEMA-D-13-00481). Dent. Mater. 2014, 30, e209–e215. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Di, L.; Yang, D. Study on the pretreatment of poly(ether ether ketone)/multiwalled carbon nanotubes composites through environmentally friendly chemical etching and electrical properties of the chemically metallized composites. ACS Appl. Mater. Interfaces 2013, 5, 12499–12509. [Google Scholar] [CrossRef]
- Chaijareenont, P.; Prakhamsai, S.; Silthampitag, P.; Takahashi, H.; Arksornnukit, M. Effects of different sulfuric acid etching concentrations on PEEK surface bonding to resin composite. Dent. Mater. J. 2018, 37, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wong, H.; Wang, W.; Li, P.; Xu, Z.; Chong, E.; Yan, C.; Yeung, K.; Chu, P. Cytocompatibility, osseointegration, and bioactivity of three-dimensional porous and nanostructured network on polyetheretherketone. Biomaterials 2013, 34, 9264–9277. [Google Scholar] [CrossRef]
- Schmidlin, P.R.; Stawarczyk, B.; Wieland, M.; Attin, T.; Hämmerle, C.H.F.; Fischer, J. Effect of different surface pre-treatments and luting materials on shear bond strength to PEEK. Dent. Mater. 2010, 26, 553–559. [Google Scholar] [CrossRef]
- Ourahmoune, R.; Salvia, M.; Mathia, T.G.; Berthel, B.; Fouvry, S.; Mesrati, N. Effect of sandblasting substrate treatment on single lap shear strength of adhesively bonded PEEK and its composites. In Proceedings of the 18th International Conference on Composite Materials, Jeju Island, Korea, 21–26 August 2011; pp. 2–7. [Google Scholar]
- Ourahmoune, R.; Salvia, M.; Mathia, T.G.; Mesrati, N. Surface morphology and wettability of sandblasted PEEK and its composites. Scanning 2014, 36, 64–75. [Google Scholar] [CrossRef]
- Bahbou, M.F.; Nylén, P.; Wigren, J. Effect of grit blasting and spraying angle on the adhesion strength of a plasma-sprayed coating. J. Therm. Spray Technol. 2004, 13, 508–514. [Google Scholar] [CrossRef]
- Ivosevic, M.; Gupta, V.; Baldoni, J.A.; Cairncross, R.A.; Twardowski, T.E.; Knight, R. Effect of substrate roughness on splatting behavior of HVOF sprayed polymer particles: Modeling and experiments. Proc. Int. Therm. Spray Conf. 2006, 15, 725–730. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Jordan, P.; Schmidlin, P.R.; Roos, M.; Eichberger, M.; Gernet, W.; Keul, C. PEEK surface treatment effects on tensile bond strength to veneering resins. J. Prosthet. Dent. 2014, 112, 1278–1288. [Google Scholar] [CrossRef]
- Sproesser, O.; Schmidlin, P.R.; Uhrenbacher, J.; Roos, M.; Gernet, W.; Stawarczyk, B. Effect of sulfuric acid etching of polyetheretherketone on the shear bond strength to resin cements. J. Adhes Dent. 2014, 16, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Keul, C.; Liebermann, A.; Schmidlin, P.R.; Roos, M.; Sener, B.; Stawarczyk, B. Influence of PEEK surface modification on surface properties and bond strength to veneering resin composites. J. Adhes Dent. 2014, 16, 383–392. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Bähr, N.; Beuer, F.; Wimmer, T.; Eichberger, M.; Gernet, W.; Jahn, D.; Schmidlin, P.R. Influence of plasma pretreatment on shear bond strength of self-adhesive resin cements to polyetheretherketone. Clin. Oral Investig. 2014, 18, 163–170. [Google Scholar] [CrossRef]
- Zakir, M.; Ashraf, U.; Tian, T.; Han, A.; Qiao, W.; Jin, X.; Zhang, M.; Tsoi, J.K.H.; Matinlinna, J.P. The Role of Silane Coupling Agents and Universal Primers in Durable Adhesion to Dental Restorative Materials—A Review. Curr. Oral Health Rep. 2016, 3, 244–253. [Google Scholar] [CrossRef]
- Queiroz, J.; Benetti, P.; Ozcan, M.; de Oliveira, L.; Della Bona, A.; Takahashi, F.; Bottino, M. Surface characterization of feldspathic ceramic using ATR FT-IR and ellipsometry after various silanization protocols. Dent. Mater. 2012, 28, 189–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corazza, P.; Cavalcanti, S.; Queiroz, J.; Bottino, M.; Valandro, L. Effect of post-silanization heat treatments of silanized feldspathic ceramic on adhesion to resin cement. J. Adhes Dent. 2013, 15, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Campos, T.M.B.; Cividanes, L.S.; Machado, J.P.B.; Simonetti, E.A.N.; Rodrigues, L.A.; Thim, G.P. Mullite crystallization using fully hydrolyzed silica sol: The gelation temperature influence. J. Sol-Gel Sci. Technol. 2014, 72, 219–226. [Google Scholar] [CrossRef]
- Caglar, I.; Ates, S.; Yesil Duymus, Z. An in vitro evaluation of the effect of various adhesives and surface treatments on bond strength of resin cement to polyetheretherketone. J. Prosthodont. 2019, 28, e342–e349. [Google Scholar] [CrossRef]
- Kern, M.; Lehmann, F. Influence of surface conditioning on bonding to polyetheretherketon (PEEK). Dent. Mater. 2012, 28, 1280–1283. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Beuer, F.; Wimmer, T.; Jahn, D.; Sener, B.; Roos, M.; Schmidlin, P.R. Polyetheretherketone—A suitable material for fixed dental prostheses? J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1209–1216. [Google Scholar] [CrossRef]
- Lümkemann, N.; Eichberger, M.; Stawarczyk, B. Bonding to different PEEK compositions: The impact of dental light curing units. Materials 2017, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Shukla, D.; Negi, Y.S.; Kumar, V. Modification of Poly(ether ether ketone) Polymer for Fuel Cell Application. J. Appl. Chem. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Kubiak, K.; Wilson, M.; Mathia, T.; Carval, P. Wettability versus roughness of engineering surfaces. Wear 2010, 271, 523–528. [Google Scholar] [CrossRef]
- Mata, F.; Gaitonde, V.N.; Karnik, S.R.; Davim, J.P. Influence of cutting conditions on machinability aspects of PEEK, PEEK CF 30 and PEEK GF 30 composites using PCD tools. J. Mater. Process. Technol. 2009, 209, 1980–1987. [Google Scholar] [CrossRef]
- Gama, L.T.; Duque, T.M.; Özcan, M.; Philippi, A.G.; Mezzomo, L.A.M.; Gonçalves, T.M.S.V. Adhesion to high-performance polymers applied in dentistry: A systematic review. Dent. Mater. 2020, 36, e93–e108. [Google Scholar] [CrossRef] [PubMed]




| Trade Names | Manufacturers | Batch Number | Chemical Composition |
|---|---|---|---|
| Aluminum oxide | Polidental Ind. Com. Ltda | 43126 | 45 μm Aluminum oxide sand (extra-fine) |
| Rocatec Pre + Rocatec Plus | 3M ESPE | 1036301855 | High-purity 110 μm aluminum oxide High-purity aluminium oxide 110 μm, modified with silicon dioxide (SiO2) |
| Sulfuric acid PA | F.Maia Ind. Com. Ltda | 33860 | 98% H2SO4 |
| RelyX™Ceramic Primer | 3M ESPE | N 406345 | Ethyl alcohol, water, methacryloxypropyltrimethoxysilane |
| Sinfony™ Opaquer Powder | 3M ESPE | 524838 | Silane-treated quartz, calcium fluoride, titanium dioxide, lauroyl peroxide, isobutylmalonyl-N,N′-dicyclohexyl-sulfamide, silane treated silica, c.i. pigment yellow 42, iron hydroxide oxide |
| Sinfony™ Activator Liquid | 3M ESPE | 518926 | Dicyclopentyldimethylene diacrylate, methyl methacrylate, monoacrylate acetate, vinyl acetate polymer, phosphine oxide, N,N-dibutylphenylethylamine hydrochloride |
| Sinfony™ | 3M ESPE | 523076 | Silane-treated glass powder, diurethane dimethacrylate, dicyclopentyldimethylene diacrylate, silane-treated silica, glass ionomer filler, 2-hydroxyethyl methacrylate |
| Procedures | Materials | Techniques | Tools | Specifications | Time |
|---|---|---|---|---|---|
| Surface treatments | Rocatec™ Pre + Plus | (Pre) Microblasting sand | Rocatec™ Jr | 1 cm distance, 2.8 bar | 15 s |
| (Plus) Silica coating | Rocatec™ Jr | 1 cm distance, 2.8 bar | 15 s | ||
| Aluminum oxide | Sandblasting | Rocatec™ Jr | 1 cm distance, 2.8 bar | 15 s | |
| 98% sulfuric acid | Swelling | Petri dish | Contact the sample surface with H2SO4 PA | 5 or 30 s | |
| Silanization | RelyX ceramic primer | Brushing | #1 brush | Homogeneous layer Wait for drying | 5 min |
| Opacification | Sinfony™ Opaquer Powder + Sinfony™ Activator Liquid | Mixing and brushing | Plastic spatula Ceramic mixing plate | 1:1 liquid—powder proportion Spatulation to cream consistency | 45 s |
| Photopolymerization | Halogen light | Two cycles | 5 s | ||
| Veneering resin application | Sinfony™ | Incremental layers | #1 brush | Homogeneous layer | |
| Individual layer photopolymerization | Halogen light | Two cycles | 5 s | ||
| Final photopolymerization | Visio.Beta | Program #1 | 1 min of light followed by 14 min of light and vacuum |
| Surface Treatment | Mean and SD (MPa) * |
|---|---|
| Aluminum oxide (ALO) | 4.2 ± 2 d |
| ** Tribochemical silica coating (TBS) | 4.4 ± 2 d |
| Tribochemical silica coating + heated silane (TBS-H) | 6.5 ± 2.3 c |
| Sulfuric acid 5 s (SA5) | 12.6 ± 2 a |
| Sulfuric acid 30 s (SA30) | 10.3 ± 2.9 b |
| Surface Treatment | Mean and SD (°) * |
|---|---|
| ** Control (CTL) | 88.9 ± 6.0 ab |
| Aluminum oxide (ALO) | 127.4 ± 5.7 d |
| Tribochemical silica coating (TBS) | 93.3 ± 3.8 bc |
| Sulfuric acid 5 s (SA5) | 98.2 ± 4.3 c |
| Sulfuric acid 30 s (SA30) | 83.9 ± 2.9 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villefort, R.F.; Anami, L.C.; Campos, T.M.B.; Melo, R.M.; Valandro, L.F.; von Zeidler, S.L.V.; Bottino, M.A. Influence of Alternative and Conventional Surface Treatments on the Bonding Mechanism between PEEK and Veneering Resin for Dental Application. Coatings 2021, 11, 719. https://doi.org/10.3390/coatings11060719
Villefort RF, Anami LC, Campos TMB, Melo RM, Valandro LF, von Zeidler SLV, Bottino MA. Influence of Alternative and Conventional Surface Treatments on the Bonding Mechanism between PEEK and Veneering Resin for Dental Application. Coatings. 2021; 11(6):719. https://doi.org/10.3390/coatings11060719
Chicago/Turabian StyleVillefort, Regina F., Lilian C. Anami, Tiago M. B. Campos, Renata M. Melo, Luiz F. Valandro, Sandra L. V. von Zeidler, and Marco A. Bottino. 2021. "Influence of Alternative and Conventional Surface Treatments on the Bonding Mechanism between PEEK and Veneering Resin for Dental Application" Coatings 11, no. 6: 719. https://doi.org/10.3390/coatings11060719
APA StyleVillefort, R. F., Anami, L. C., Campos, T. M. B., Melo, R. M., Valandro, L. F., von Zeidler, S. L. V., & Bottino, M. A. (2021). Influence of Alternative and Conventional Surface Treatments on the Bonding Mechanism between PEEK and Veneering Resin for Dental Application. Coatings, 11(6), 719. https://doi.org/10.3390/coatings11060719





