Thermal Spray Multilayer Ceramic Structures with Potential for Solid Oxide Cell Applications
Abstract
:1. Introduction
- (a)
- The layered structure is characteristic of the thermal spray method, based on a layer-by-layer deposition of powdered materials, until the targeted thickness is reached. In this way, multiple discrete layers of various materials can be deposited in a layer-by-layer structure, aiming for the effective adherence between them, so as to create interfaces with certain structure that will be quite effective in processing catalytic and electrochemical reactions as well as ionic and electronic charge transfer.
- (b)
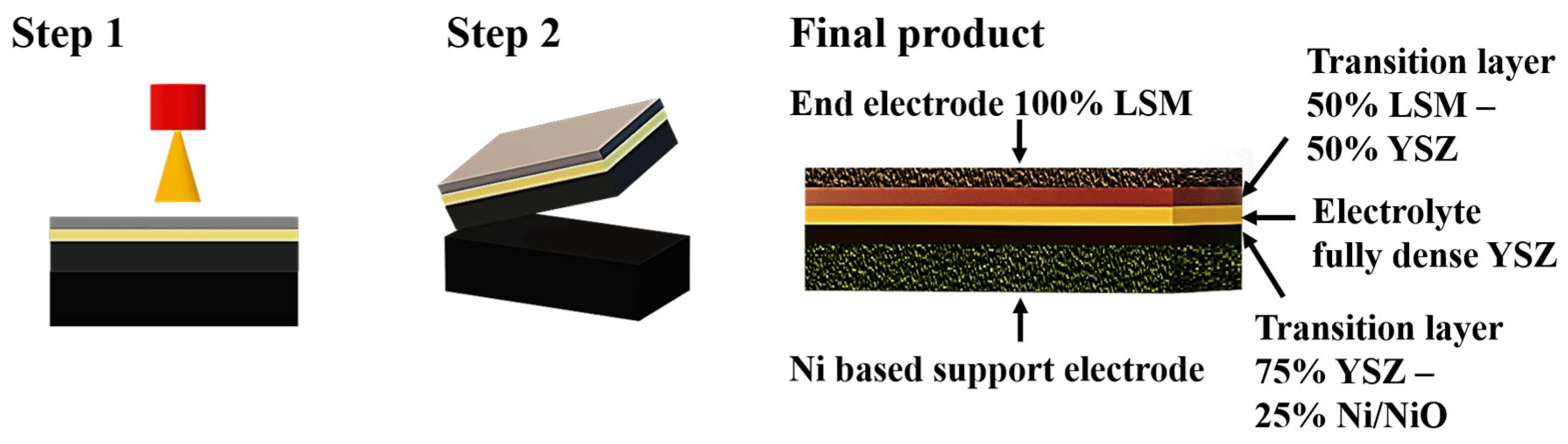
- Net shape spray-forming is based on two steps: thermal spraying on specially designed molds and subsequent removal of the mold without damaging the sprayed layer. The objective of the present investigation is to thermally spray at least three different material layers (electrode-electrolyte-electrode) and then to remove this multilayer structure from the mold. The manufacturing of a five-layer structure has also been attempted, by adding two transition layers between electrodes and electrolytes (Figure 1).
2. Materials and Methods
2.1. Materials and Experimental Procedure
2.1.1. YSZ Electrolyte
2.1.2. Ni-YSZ and Ni/NiO-YSZ Supporting Electrode
2.1.3. LSM End Electrode
2.2. Characterization Techniques
3. Results and Discussion
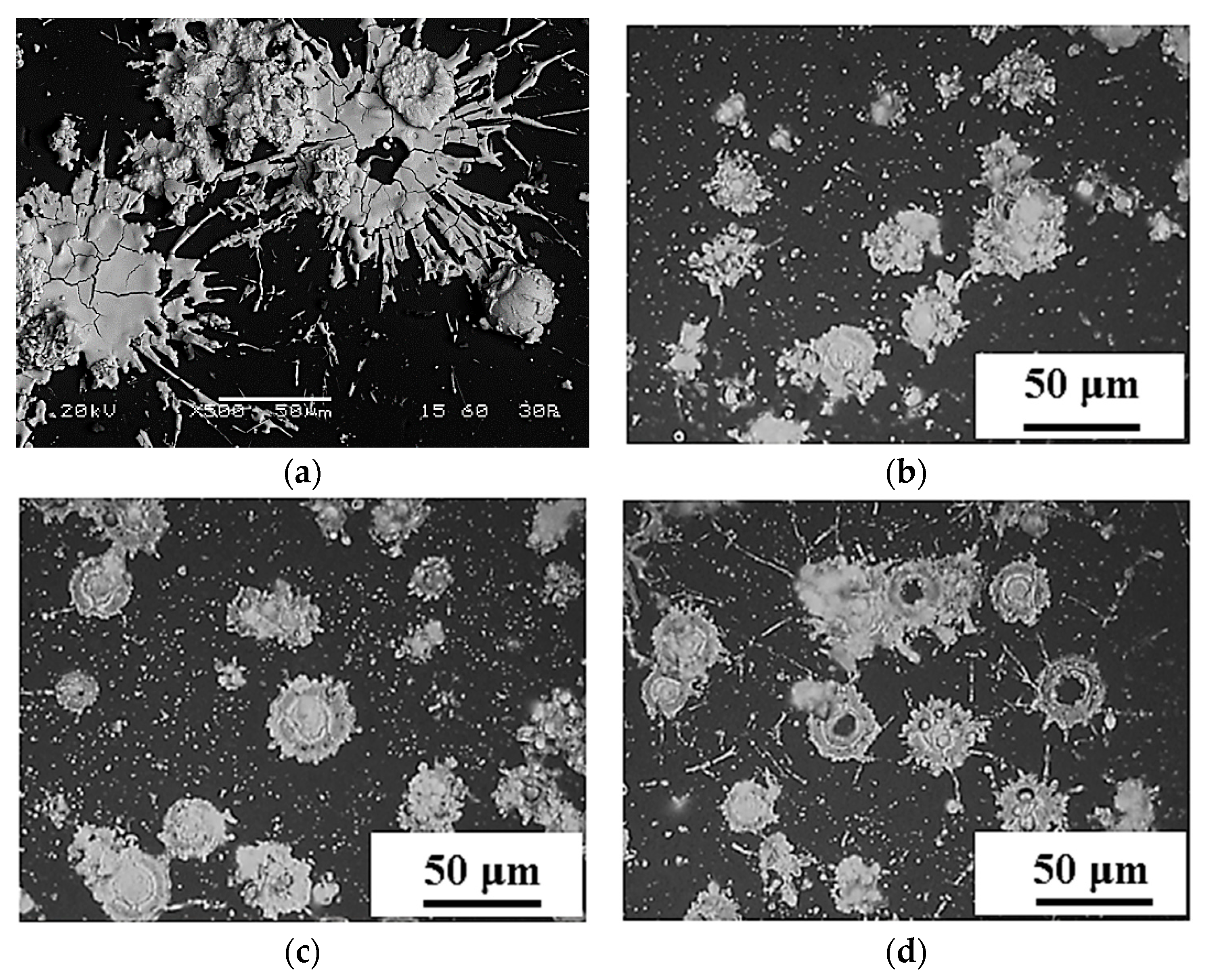
3.1. Single-Layer Structure Optimization
3.1.1. YSZ Electrolyte Layer
3.1.2. Ni-YSZ Support Electrode Layer
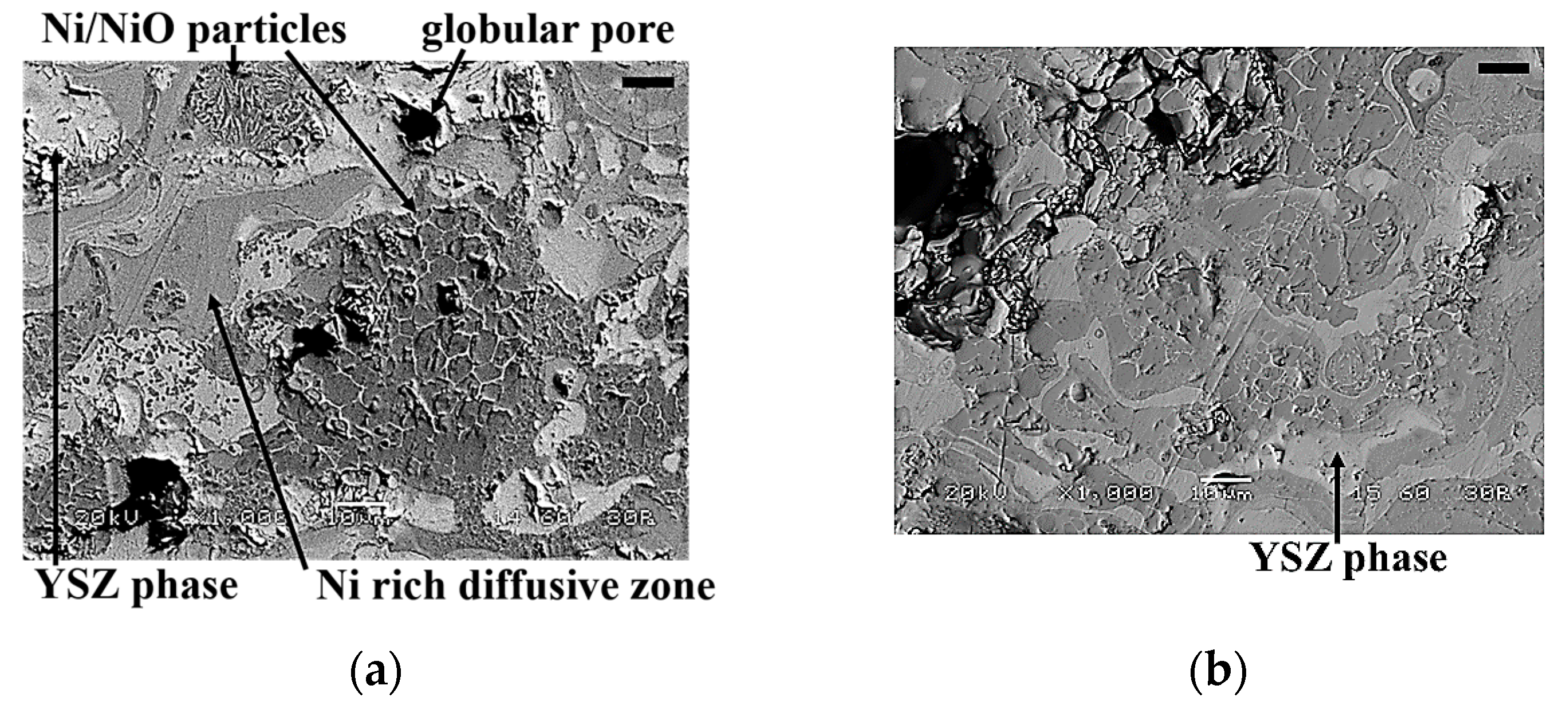
3.1.3. Ni/NiO-YSZ Support Electrode Layer
3.1.4. LSM End Electrode
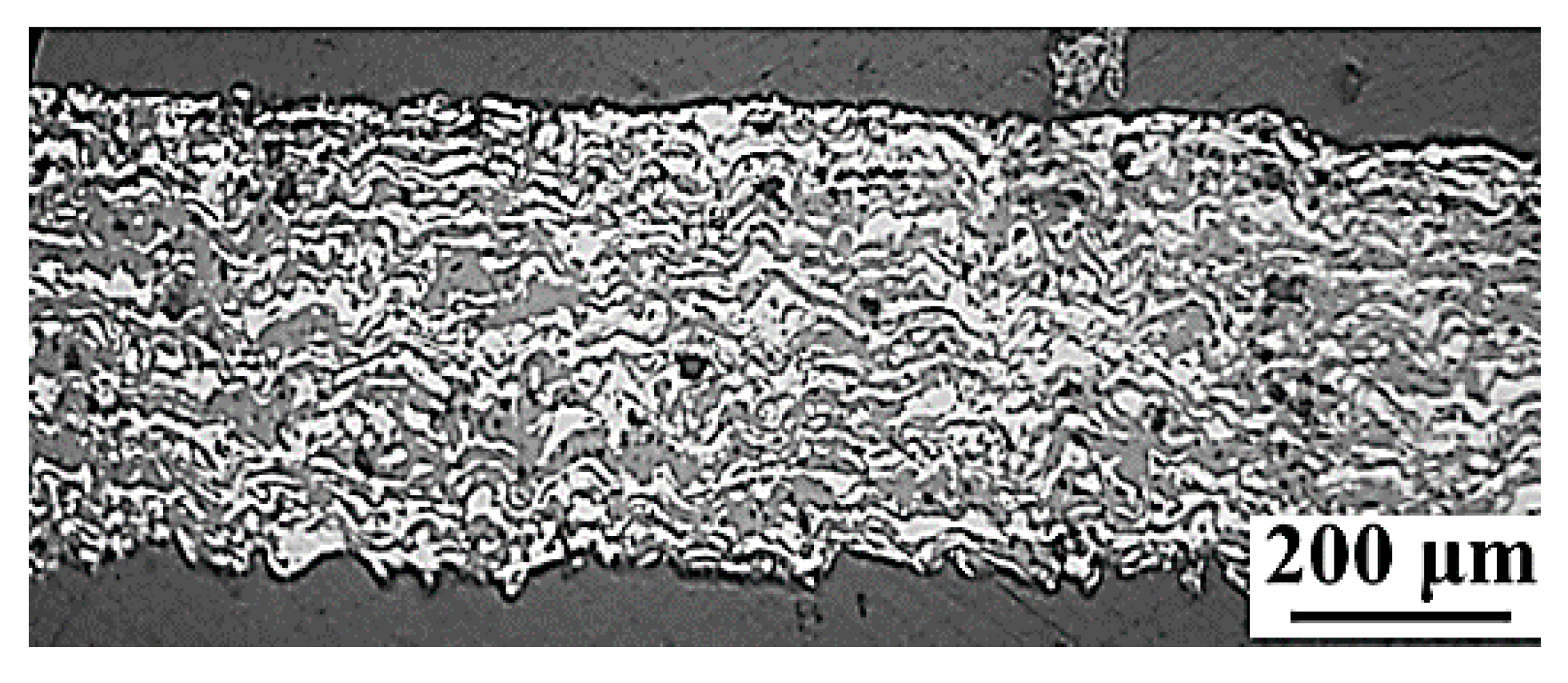
3.2. Three-Layer Structure Manufacturing (Electrode-Electrolyte-Electrode)
- Porosity had to be increased in the electrodes.
- Porosity had to be reduced in the electrolyte.
- Microcracking should not exist in the electrolyte.
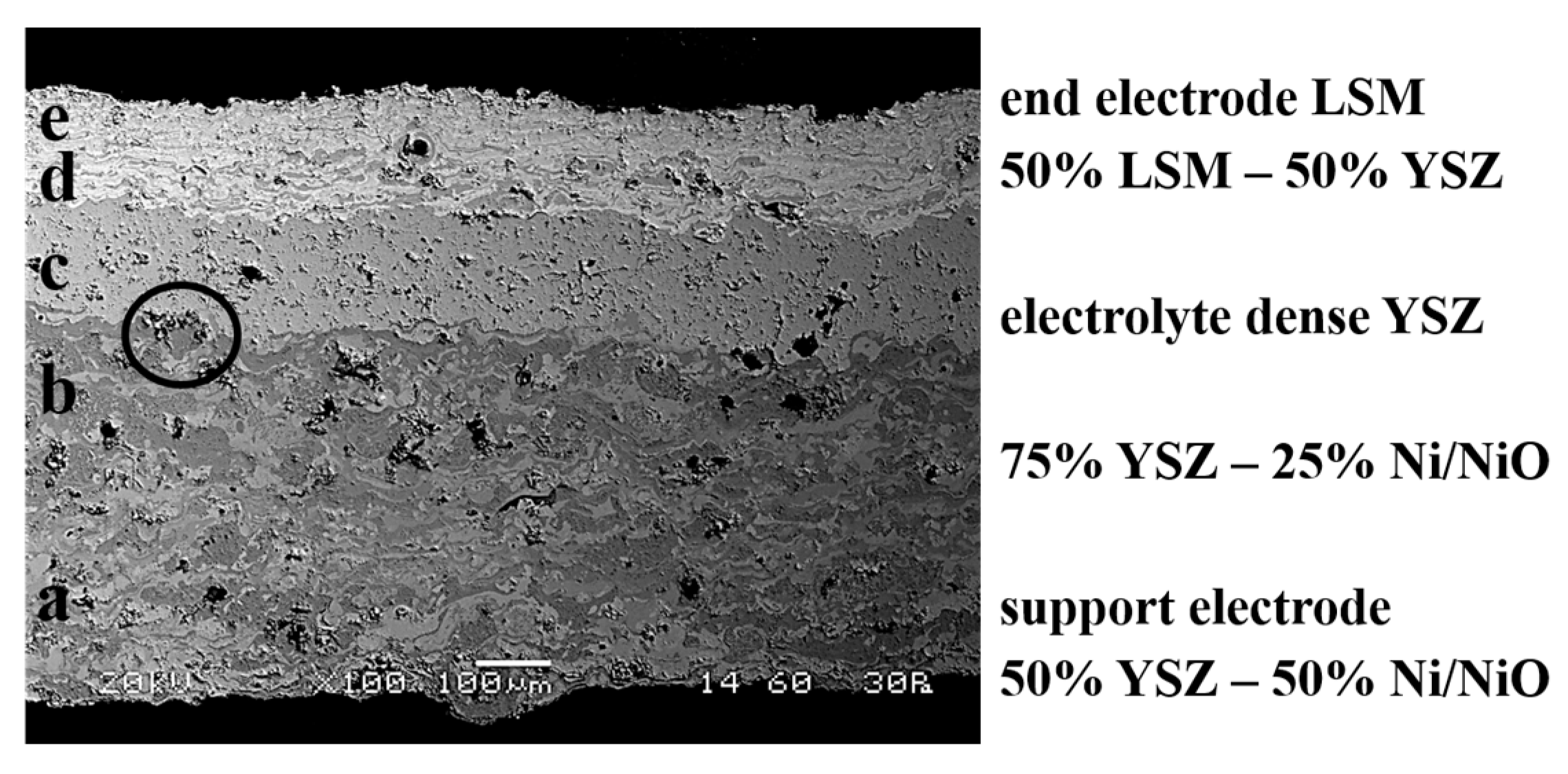
3.3. Five-Layer Structure Manufacturing (Electrode-Transition Layer-Electrolyte-Transition Layer-Electrode)
- (a)
- Support electrode: composition Ni/NiO 50%–YSZ 50%, thickness 300–350 μm, porosity 25–30%.
- (b)
- Support electrode/electrolyte transition layer: composition Ni/NiO 25–YSZ 75%, thickness 100–120 μm, porosity 20–25%.
- (c)
- Electrolyte: composition YSZ 100%, thickness 100–120 μm, porosity < 5%, not connected.
- (d)
- Electrolyte/end electrode transition layer: composition 50% YSZ–50% LSM, thickness 80–100 μm, porosity 20–25%.
- (e)
- End electrode: composition LSM 100%, thickness 70–90 μm, porosity 25–30%.
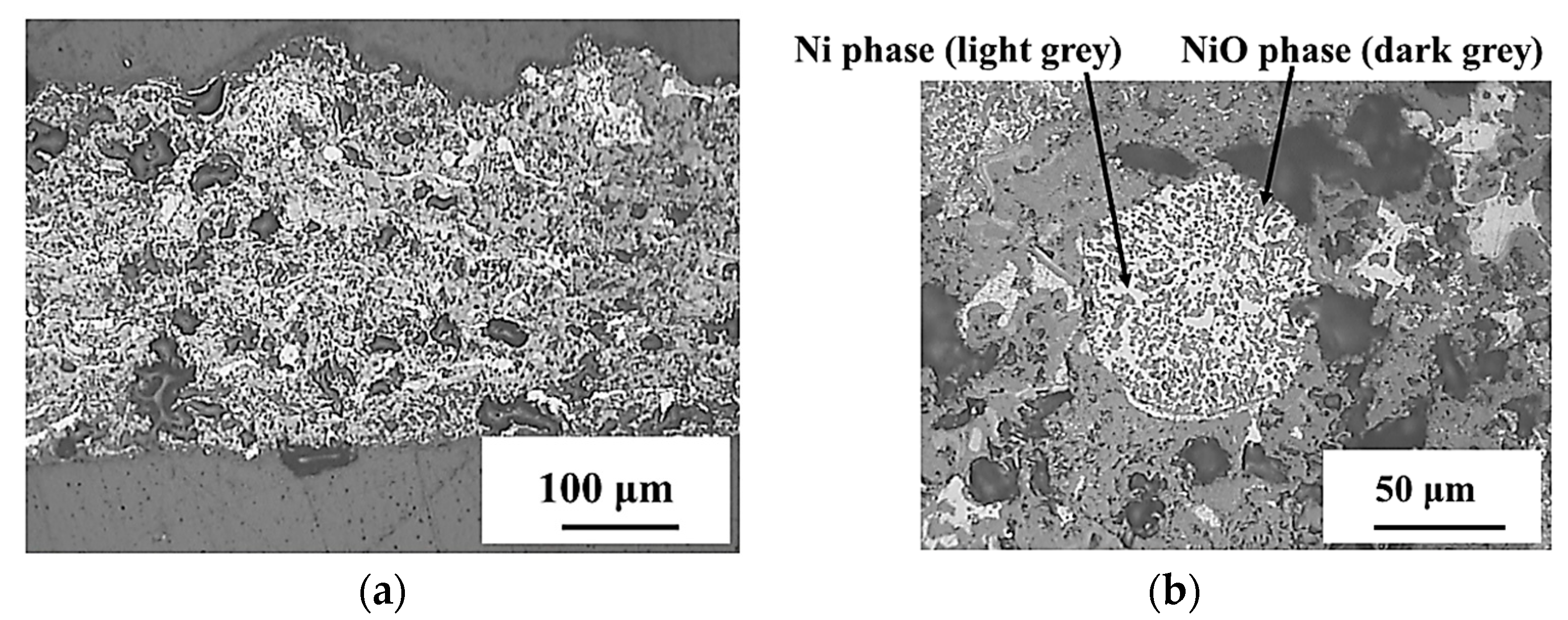
3.3.1. Support Electrode Layer
3.3.2. Support Electrode-Electrolyte Transition Layer
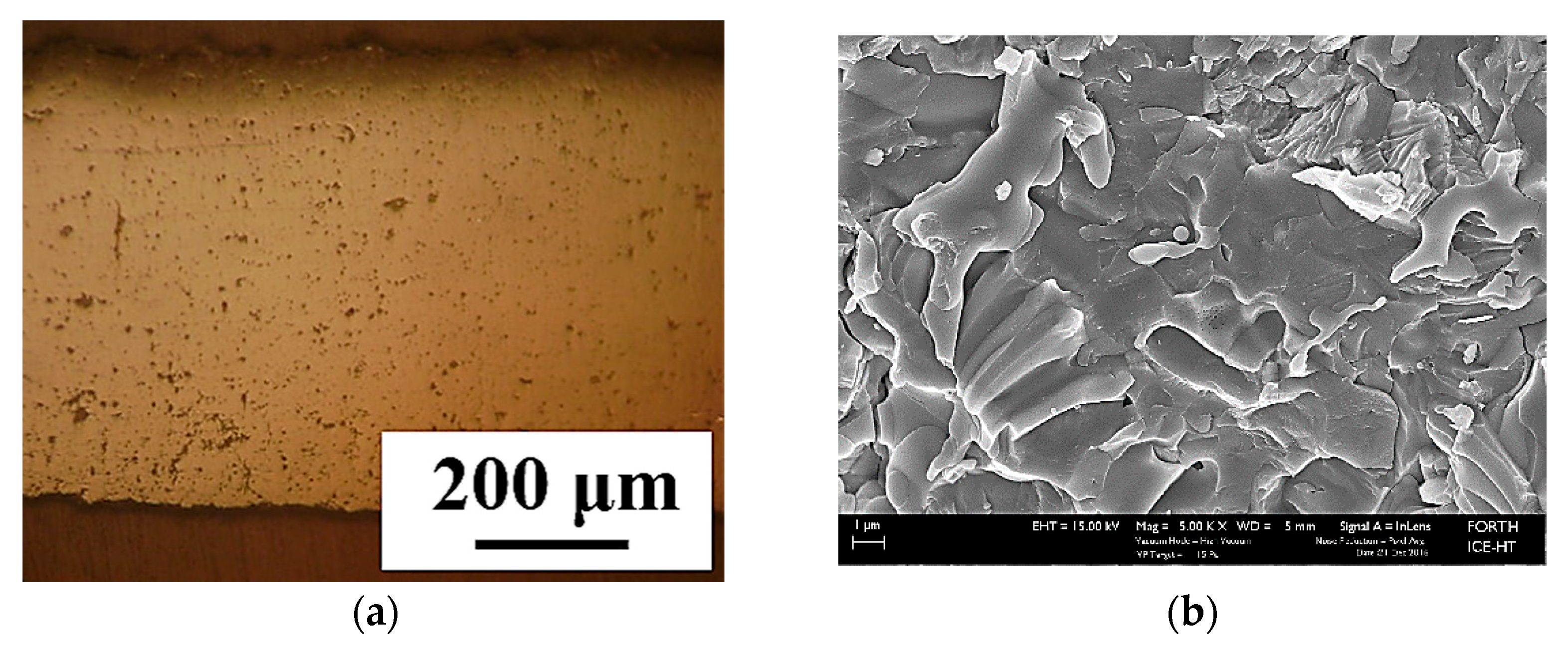
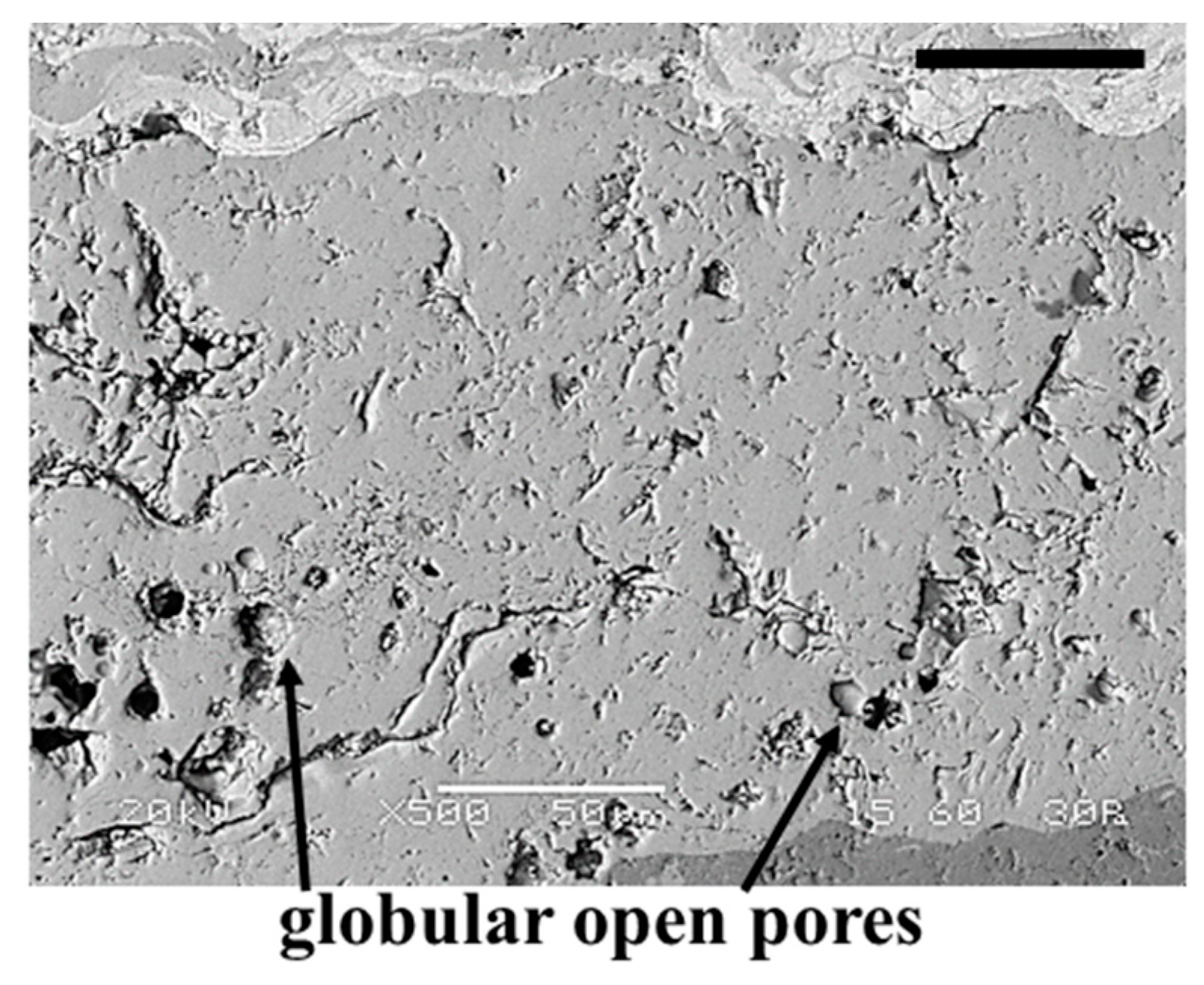
3.3.3. Electrolyte Layer
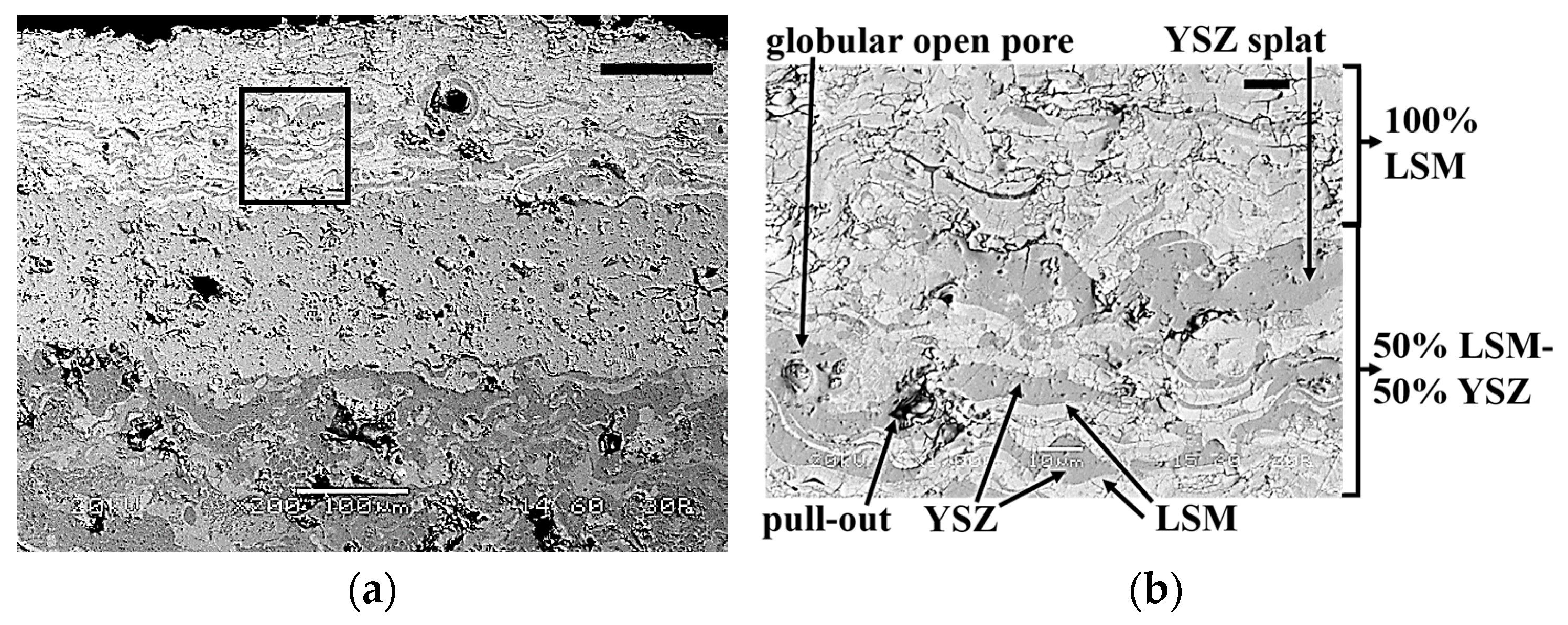
3.3.4. Electrolyte-End Electrode Transition Layer
3.3.5. End Electrode Layer
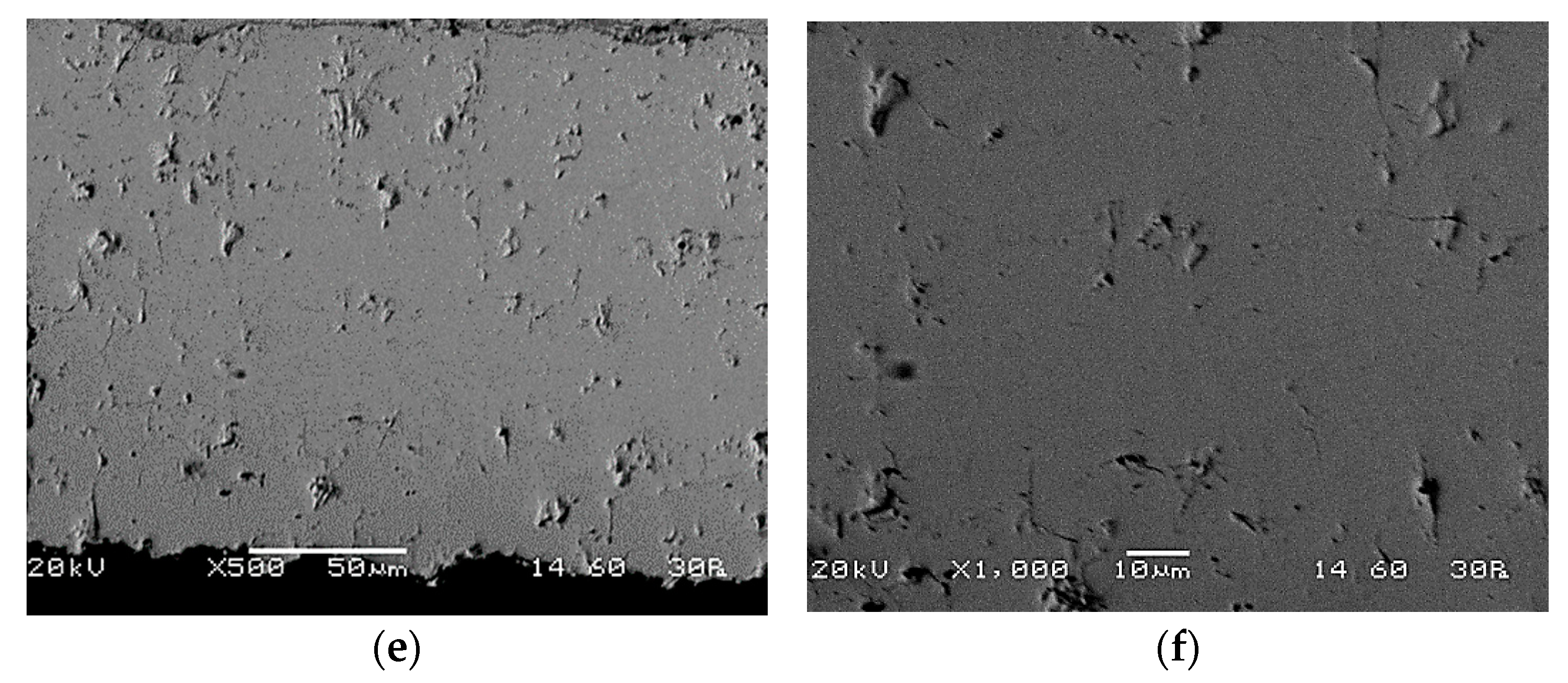
3.4. Electrolyte Permeability Test (Acetone Permeability Comparative Test, APCT) and Results
3.5. Improvement of Electrolyte’s Gas Tightness
4. Conclusions
- Three-layer and five-layer free-standing electrode-supported SOCs were manufactured exclusively by the use of the thermal spray technique.
- Ni and NiO were used in combination with YSZ as support electrode material. Ni/NiO-YSZ feedstock powder delivered higher porosity layers than the ones provided by metallic Ni with YSZ.
- The complete density and gas tightness of the YSZ electrolyte was not achieved for thicknesses below 100 μm.
- The permeability of the YSZ electrolyte was significantly reduced when small fused particles and high deposition rates were used, coupled with heat management to avoid microcrack formation. However, the layers were still permeable, although hardly. The permeability is rather attributed to the existence of microcracks, than to connected porosity.
- A fully non-permeable YSZ electrolyte was achieved for thicknesses in the 150–200 μm range.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Liu, T.; Lei, L.; Chen, F. High temperature solid oxide H2O/CO2 co-electrolysis for syngas production. Fuel Process. Technol. 2017, 161, 248–258. [Google Scholar] [CrossRef]
- Onbilgin, S.; Timurkutluk, B.; Timurkutluk, C.; Celik, S. Comparison of electrolyte fabrication techniques on the performance of anode supported solid oxide fuel cells. Int. J. Hydrogen Energy 2020, 45, 35162–35170. [Google Scholar] [CrossRef]
- Orsini, A.; Medaglia, P.G.; Sanna, S.; Traversa, E.; Licoccia, S.; Tebano, A.; Balestrino, G. Epitaxial superlattices of ionic conductor oxides. Superlattices Microstruct. 2009, 46, 223–226. [Google Scholar] [CrossRef] [Green Version]
- Nesaraj, A.S.; Raj, I.A.; Pattabiraman, R. Tape casting of alternate electrolyte components for solid oxide fuel cells. Indian J. Eng. Mater. Sci. 2006, 13, 347–356. Available online: http://nopr.niscair.res.in/handle/123456789/7567 (accessed on 3 May 2021).
- Chen, R.; Zhang, S.-L.; Li, C.-J.; Li, C.-X. Plasma-sprayed high-performance (Bi2O3)0.75(Y2O3)0.25 electrolyte for intermediate-temperature solid oxide fuel cells (IT-SOFCs). J. Therm. Spray Technol. 2021, 30, 196–204. [Google Scholar] [CrossRef]
- Torrell, M.; Cano, I.G.; Miguel, J.M.; Guilemany, J.M.; Laguna-Bercero, M.A.; Orera, V.M. Solid Oxide Fuel Cells produced by Atmospheric Plasma Spray technology: Structural and electrochemical characterization. In Proceedings of the International Thermal Spray Conference and Exposition, Houston, TX, USA, 21–24 May 2012; Available online: http://hdl.handle.net/10261/103043 (accessed on 3 May 2021).
- Li, C.-J.; Ning, C.-X.; Ning, X.-J. Performance of YSZ electrolyte layer deposited by atmospheric plasma spraying for cermet-supported tubular SOFC. Vacuum 2004, 73, 699–703. [Google Scholar] [CrossRef]
- Tucker, M.C. Progress in metal-supported solid oxide electrolysis cells: A review. Int. J. Hydrogen Energy 2020, 45, 24203–24218. [Google Scholar] [CrossRef]
- Costa, R.; Ansar, A. “Evolved materials and innovative design for high performance, durable and reliable SOFC cell and stack” presentation and status of the european project EVOLVE. ECS Trans. 2013, 57, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Ilhan, Z.; Ansar, A.; Soysal, D. Recent Progress in Intermediate Temperature SOFCs (ITSOFCs) Development at DLR. In Proceedings of the Advances and Innovations in SOFCs 2: From Materials to Systems Conference, Katarino, Bulgaria, 11–16 September 2011; Available online: https://elib.dlr.de/71020/ (accessed on 3 May 2021).
- Szabo, P.; Ansar, A.; Franco, T.; Gindrat, M.; Kiefer, T. Stack Tests of Metal-Supported Plasma-Sprayed SOFC. In Proceedings of the 10th European SOFC Forum Conference, Lucerne, Switzerland, 26–29 June 2012; Available online: https://elib.dlr.de/78828/ (accessed on 3 May 2021).
- Fang, X.; Zhu, J.; Lin, Z. Effects of electrode composition and thickness on the mechanical performance of a solid oxide fuel cell. Energies 2018, 11, 1735. [Google Scholar] [CrossRef] [Green Version]
- National Energy Technology Laboratory. Seventh Edition Fuel Cell Handbook. Available online: https://netl.doe.gov/sites/default/files/netl-file/FCHandbook7.pdf (accessed on 3 May 2021).
- Muhoza, S.P.; Lee, S.; Song, X.; Guan, B.; Yang, T.; Gross, M.D. Enhancing activity, charge transport, power production, and stability of commercial solid oxide fuel cells with yttria-stabilized zirconia nanoparticles. J. Electrochem. Soc. 2020, 167, 024517. [Google Scholar] [CrossRef]
- Gupta, M.; Weber, A.; Markocsan, N.; Gindrat, M. Electrochemical performance of plasma sprayed metal supported planar solid oxide fuel cells. J. Electrochem. Soc. 2016, 163, F1059–F1065. [Google Scholar] [CrossRef] [Green Version]
- Jarligo, M.O.; Mauer, G.; Bram, M.; Baumann, S.; Vaßen, R. Plasma spray physical vapor deposition of La1−xSrxCoyFe1−yO3−δ thin-film oxygen transport membrane on porous metallic supports. J. Therm. Spray Technol. 2014, 23, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Waldbillig, D.; Kesler, O. Characterization of metal-supported axial injection plasma sprayed solid oxide fuel cells with aqueous suspension plasma sprayed electrolyte layers. J. Power Source 2009, 191, 320–329. [Google Scholar] [CrossRef]
- Károly, Z.; Szépvölgyi, J. Hollow alumina microspheres prepared by RF thermal plasma. Powder Technol. 2003, 132, 211–215. [Google Scholar] [CrossRef]
- Fauchais, P.; Montavon, G.; Bertrand, G. From powders to thermally sprayed coatings. J. Therm. Spray Technol. 2010, 19, 56–80. [Google Scholar] [CrossRef] [Green Version]
- Solonenko, O.P.; Gulyaev, I.P.; Smirnov, A.V. Thermal plasma processes for production of hollow spherical powders: Theory and experiment. J. Therm. Sci. Technol. 2011, 6, 219–234. [Google Scholar] [CrossRef] [Green Version]
- Odhiambo, J.G.; Li, W.; Zhao, Y.; Li, C. Porosity and its significance in plasma-sprayed coatings. Coatings 2019, 9, 460. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, S.; Kulkarni, A.; Sampath, S.; Herman, H. Application of image analysis for characterization of porosity in thermal spray coatings and correlation with small angle neutron scattering. Surf. Coat. Technol. 2004, 187, 6–16. [Google Scholar] [CrossRef]
- Kuroda, S.; Watanabe, M.; Kim, K.; Katanoda, H. Current status and future prospects of warm spray technology. J. Therm. Spray Technol. 2011, 20, 653–676. [Google Scholar] [CrossRef] [Green Version]
- Ctibor, P.; Roussel, O.; Tricoire, A. Unmelted particles in plasma sprayed coatings. J. Eur. Ceram. Soc. 2003, 23, 2993–2999. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, S.; Keshri, A.K.; Simpson, A.; Agarwal, A.; Balani, K. Nanomechanical properties and thermal conductivity estimation of plasma-sprayed, solid-oxide fuel cell components: Ceria-doped, yttria-stabilized zirconia electrolyte. J. Miner. Met. Mater. Soc. 2013, 65, 749–762. [Google Scholar] [CrossRef]
- Zhang, C.; Li, W.-Y.; Liao, H.; Li, C.-J.; Li, C.-X.; Coddet, C. Microstructure and electrical conductivity of atmospheric plasma-sprayed LSM/YSZ composite cathode materials. J. Therm. Spray Technol. 2007, 16, 1005–1010. [Google Scholar] [CrossRef]
- Fukumoto, M.; Huang, Y. Flattening mechanism in thermal sprayed nickel particle impinging on flat substrate surface. J. Therm. Spray Technol. 1999, 8, 427–432. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vardavoulias, M.; Gkomoza, P.; Arkas, M.; Niakolas, D.K.; Neophytides, S.G. Thermal Spray Multilayer Ceramic Structures with Potential for Solid Oxide Cell Applications. Coatings 2021, 11, 682. https://doi.org/10.3390/coatings11060682
Vardavoulias M, Gkomoza P, Arkas M, Niakolas DK, Neophytides SG. Thermal Spray Multilayer Ceramic Structures with Potential for Solid Oxide Cell Applications. Coatings. 2021; 11(6):682. https://doi.org/10.3390/coatings11060682
Chicago/Turabian StyleVardavoulias, Michail, Paraskevi Gkomoza, Michael Arkas, Dimitrios K. Niakolas, and Stylianos G. Neophytides. 2021. "Thermal Spray Multilayer Ceramic Structures with Potential for Solid Oxide Cell Applications" Coatings 11, no. 6: 682. https://doi.org/10.3390/coatings11060682
APA StyleVardavoulias, M., Gkomoza, P., Arkas, M., Niakolas, D. K., & Neophytides, S. G. (2021). Thermal Spray Multilayer Ceramic Structures with Potential for Solid Oxide Cell Applications. Coatings, 11(6), 682. https://doi.org/10.3390/coatings11060682







