1. Introduction
In the past years, many studies have been conducted on the applicability of basalt fibers (BFs). As a reinforcing material for composites, BFs (mainly composed by SiO
2) can be prepared from basalt rocks using conventional equipment with the advantage on the low-cost process of melting and drawing [
1]. Compared with other fiber materials, BFs have the excellent properties such as high tensile strength, high E-modulus, high abrasion strength, high temperature resistance, high resistance to aggressive media, excellent thermal and sound insulation, good chemical stability [
2]. Moreover, the mechanical properties of BFs can be kept without significant decrease in the operating temperature range of 200–600 °C [
3]. In addition, the environmentally friendly preparation and recycling process of BFs significantly improves its demand for the modern market [
1,
4,
5,
6]. Thus, the BFs might be a promising reinforced material as the candidate of glass and carbon fibers in composite materials, and have been attractive in the application of friction materials, corrosion resistance materials, heat shields, thermal insulting barriers and hot fluid transportation pipes in many fields [
7].
Especially in the field of flywheel rotors’ structural materials, the fiber-reinforced resin matrix composite flywheel was generally to replace the traditional metal flywheel due to their high strength, high energy storage density and low specific volume [
8]. Compared with the common reinforced fibers (glass and carbon fibers), the BFs represent an alternative as the substitution due to their excellent properties.
However, the smooth and chemically inert surface of BFs [
9,
10,
11] results in the low interfacial adhesion between BFs and matrix (here below referred as interfacial adhesion) to reduce the composite materials’ performance. To enhance the interfacial adhesion, many studies have been conducted to modify the fiber surface, such as the plasma modification technology, the oxidized modification technology, the coating modification technology, and so on [
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23]. Manikandan and Jain et al. [
3,
24] increased the surface roughness of BFs by the acid and alkali chemical treatments, which improved interfacial bonding strength between fibers and matrix. Kim et al. [
11] and Ricciardi [
25] used surface treatment of BFs by low-temperature atmospheric oxygen plasma and SF
6 plasma to increase the wettability of BFs remarkably, and improve adhesive force between fiber/resin interfaces accompanied by physical etching and by the formation of chemical functional groups containing oxygen and nitrogen on the fiber surface. This increased the interlaminar fracture toughness of basalt/epoxy woven composites. Wei et al. proposed the coating modification as an effective way in improving the mechanical properties of BFs and the properties of basalt fiber/epoxy resin composites [
1,
6]. Cheng et al. [
26,
27,
28] investigated the effects of surface-treated F-12 aramid fibers and carbon fibers on the interfacial adhesion between fibers and matrix. They found that rare earth modification solution treatment can increase the concentration of reactive functional groups on fiber surface through chemical coordinating reaction. Additionally, the interfacial adhesion was promoted obviously and the tensile properties of composites improved significantly. Meanwhile, the tensile strengths of single fibers are almost not affected by rare earth modification solution treatment. It exhibits an attractive method to treat BFs with the rare earth modification solution due to its high efficiency, simple process, environmentally friendly characteristics, and preservation of fiber tensile strengths. Thus, with the few studies on BFs modified by rare earth elements, the modification study on BFs to prepare fiber-reinforced composites with excellent performance was promising.
In this study, the rare earth modification solution was synthesized by adding different concentrations of element La. The effects of the BFs surfaces with different concentration of element La were investigated. The mechanical properties of the composite materials, such as the tensile strength, the bending strength and the interlaminar shear strength (ILSS) were detected. The modification mechanism of the BFs surface was also discussed.
2. Experimental Details
2.1. Materials
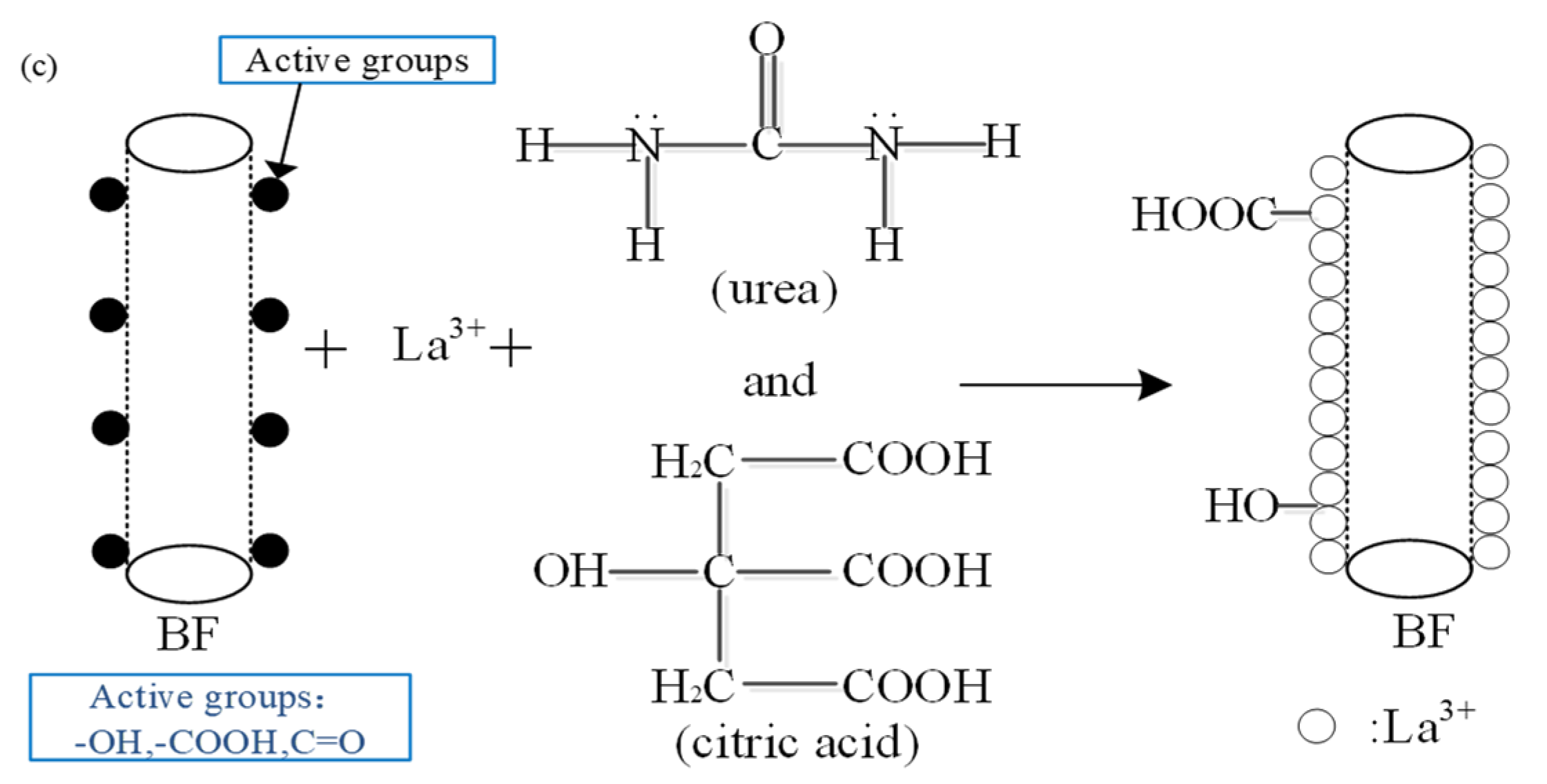
The used BFs cloth (223 g/km, unidirectional cloth) with an average fiber of diameter (5.9 µm) was manufactured by Shanxi Basalt Fiber Technology Co., Ltd., Taiyuan, China. The elastic modulus and the tensile strength of the BFs cloth were about 7.5 and 1835 MPa, respectively. The epoxy resin (LY564) and the curing agent (22964, aliphatic polyamine curing agent) were from Huntsman Co., Ltd. (The Woodlands, TX, USA). The following chemical reagents were used: acetone and nitric acid (analytical reagent, provided by Tianjin Tianda Chemical Reagent Factory, Tianjin, China), absolute ethyl alcohol (analytical reagent, produced by Tianjin Tianli Chemical Reagent Co., Ltd., Tianjin, China), citric acid and urea (analytical reagent, provided by Tianjin Zhiyuan Chemical Reagent Co., Ltd., Tianjin, China), lanthanum chloride (99.99%, manufactured by Jining Zhongkai New Type Material Co., Ltd., Jining, China).
2.2. Treatment of Fibers
The BFs cloth was cut into 260 × 35 mm
2. After immersed in acetone for 48 h at room temperature to remove the protective coating on the surface of BFs, the fibers were dried in a drying oven at 100 °C for 1 h. The dried fiber cloth was soaked in the concentrated nitric acid with constant temperature water bath at 60 °C for 2 h to improve the roughness and the –OH numbers of the fibers’ surfaces [
29]. Washing the fibers 3–4 times with distilled water to remove acid fluid from their surfaces, the PH value on the surface of the fibers was adjusted to 7. Finally, the fibers were dried in a drying oven at 100 °C for 1 h and conserved in the dryer.
According to a certain weight ratio, the composition of the BFs modification solution were uniformly mixed at room temperature, as shown in
Table 1. Additionally, five kinds of rare earth modification solution were prepared with different La
3+ concentrations of 0.1, 0.3, 0.5, 0.7, and 0.9 wt.% After immersed in the rare earth modification solution for 2 h, the treated BFs were dried in the drying oven at 100 °C for 1 h.
2.3. Fabrication and Measurements of Basalt Fiber/Epoxy Resin Composites
The prepared BFs was immersed in epoxy resin/curing agent mixture at volume ratio 4:1. With the hand lay-up method, the BF/ERCs were paved as three layers in the mold, and fabricated after curing in a drying oven at 120 °C for 15 min and 140 °C for 2 h, respectively.
The change of BFs’ chemical structure was determined in the transmission mode using Fourier transform infrared spectroscopy (FT-IR, Perkin Elmer 100, Waltham, MA, USA). X-ray photoelectron spectroscopy (XPS) using a PHI 5700 ESCA System equipped with an Al-Ka X-ray source (NewYork, NY, USA), analyzed the chemical composition of the BFs surfaces. The surface morphologies of BFs and the fracture surface morphologies of the composites were examined using scanning electron microscopy (SEM, JSM-6480, Tokyo, Japan).
The tensile test and the bending test were carried out to determine the tensile strength and the bending strength of BF/ERCs, respectively. The ILSS of BF/ERCs was measured by the three-point short-beam bending test. Due to the details of the mechanical tests shown in
Table 2 below, the specimens were cut according to the following dimensional requirement.
3. Results
3.1. FT-IR Spectra of BFs
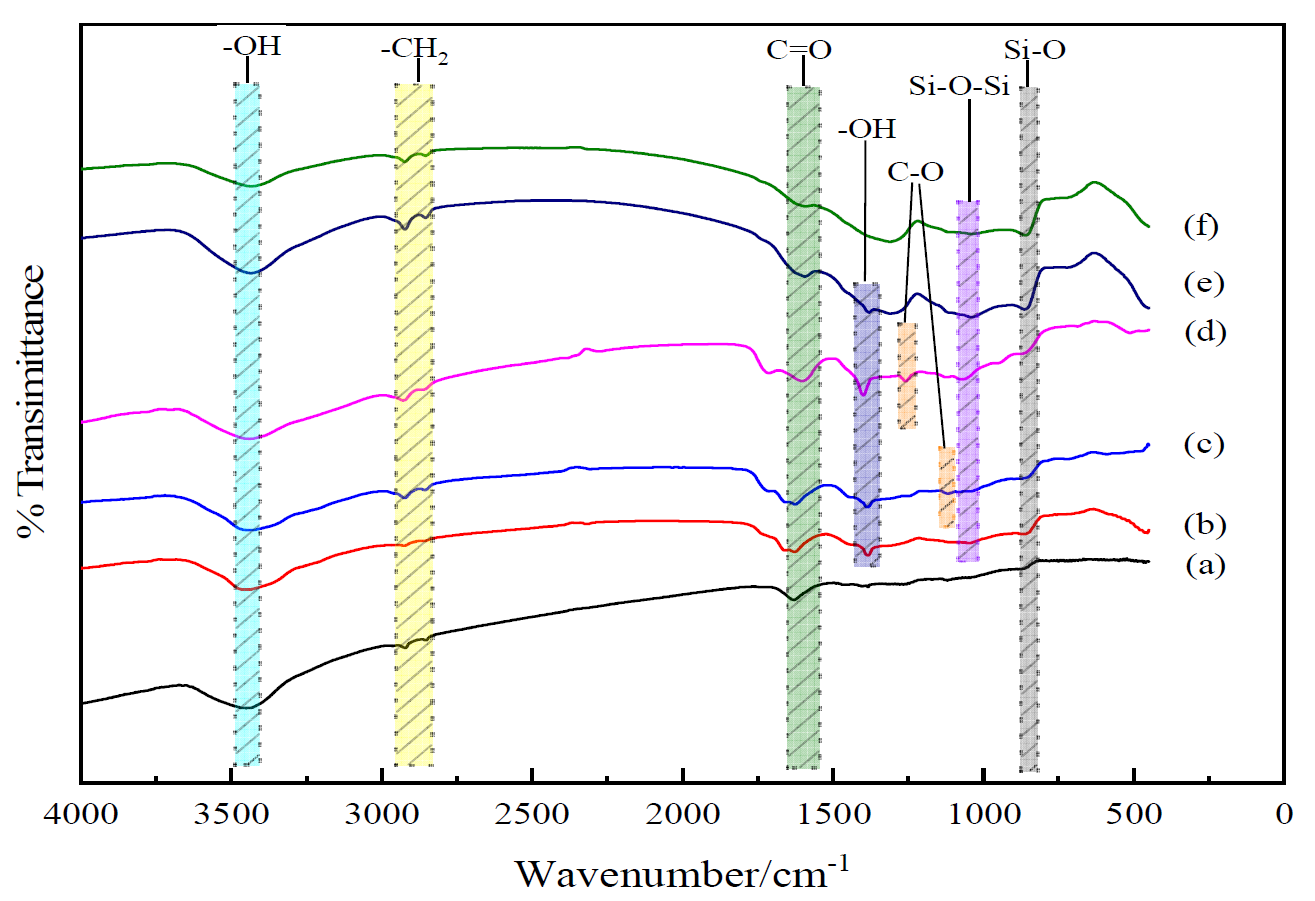
The chemical structures of untreated and treated BFs were obtained with FT-IR spectroscopy, as shown in
Figure 1. The characteristic peaks of –OH at 3450 cm
−1 and C=O at 1630 cm
−1 were observed in the untreated BFs. In addition, the peaks of –CH
2 appeared at 2921 and 2844 cm
−1, respectively. As the main composition of BFs, the peak of Si-O from SiO
2 of BFs appeared at 860 cm
−1.
For BFs treated with the 0.1 wt.% La3+ rare earth modification solution, the new absorption peaks of C=O at 1660 cm−1 and –OH at 1384 cm−1 appeared in the range of 4000–1330 cm−1, and the peaks of –CH2 at 2917 and 2845 cm−1 from the citric acid were broader than those of the untreated BFs, indicating that the La3+ was successfully absorbed on the BFs’ surface with the introduced –CH2– from the acyl group and citric acid, which also brought C=O. The absorption band at the peak of 1046 cm−1 referred to the formation of along with the peak of shifting from 860 to 863cm−1, stating that the Si–OH from the hydrolysis of SiO2 react with itself or the introduced –COOH under dehydration condensation reaction to produce Si–O–Si and more Si–O. With La3+ concentration increasing to 0.3 wt.% in the rare earth modification solution, the new peak of C–O from the citric acid along with the introduced La3+ at 1116 cm−1 appeared in the FT-IR spectrum. Moreover, the peaks of –CH2 at 2922 and 2859 cm−1 exhibited obviously. It indicated that the La3+ was absorbed to the surface with –COOH and –CH2 from citric acid. When La3+ concentration was up to 0.5 wt.%, the vibration frequency of C–O increased from 1116 to 1260 cm−1, and that of C=O stretched from 1668 to 1716 cm−1, accompanied by the urea in the modification solution. Due to the strong affinity of La3+ to nonmetal element, the La3+ could combine with urea and citric acid under the coordination reaction to introduce the C=O and C–O from the modification solution, resulting that more active oxygen-contained functional groups were introduced onto the fiber surfaces with the increasing La3+ concentration.
With the La3+ concentration above 0.5 wt.%, the peak of C=O began to disappear. At the La3+ concentration of 0.9 wt.%, the peak disappearances of C–O at 1260 cm−1 and the other weakened characteristic peaks were observed, implying that superabundant active groups introduced by the excess La3+ were aggregated on the BFs to be supersaturated and further destroyed formed macromolecular membrane on the BFs’ surface to trigger the loss of active groups.
3.2. XPS Results of BFs
3.2.1. XPS Results of BFs
As shown in
Figure 2 and
Table 3, the XPS survey spectra and the detected atomic concentration of the elements C, O, N and Si on the BFs surfaces were respectively obtained to analyze the chemical composition of the BFs surface. On the surfaces of BFs treated with the rare earth modification solution, the concentration of element C decreased and then increased with the increase of La
3+ concentration, but the changes of the elements O and N were contrary. The proportions of O/C and N/C on the BFs surfaces had the same variation trend with the concentration of element C. When La
3+ concentration was 0.5 wt.%, the proportions of O/C and N/C reached maximum values, confirming that the BFs surfaces contained the largest number of active oxygen-containing groups.
3.2.2. XPS Results of La on BFs Surfaces
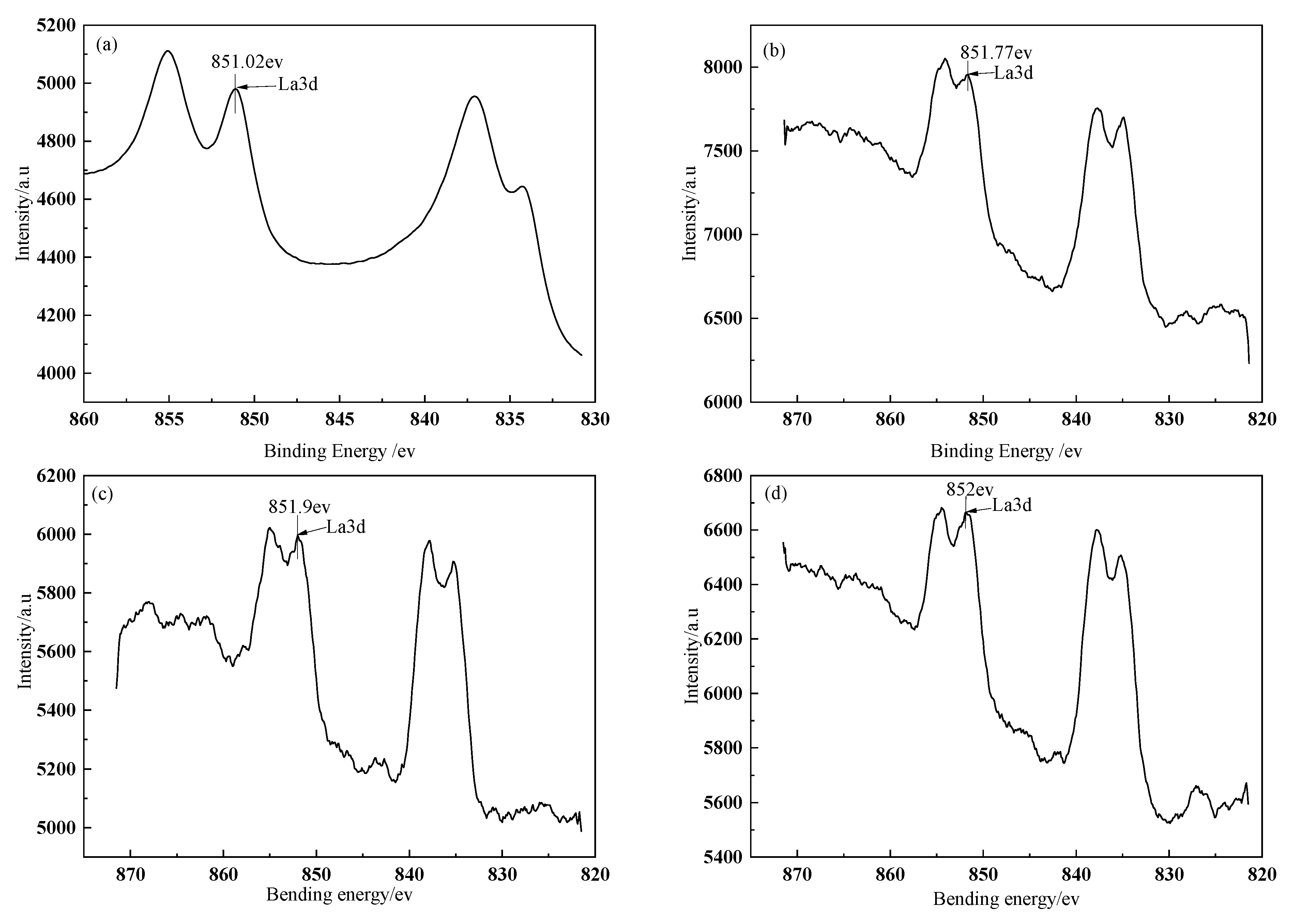
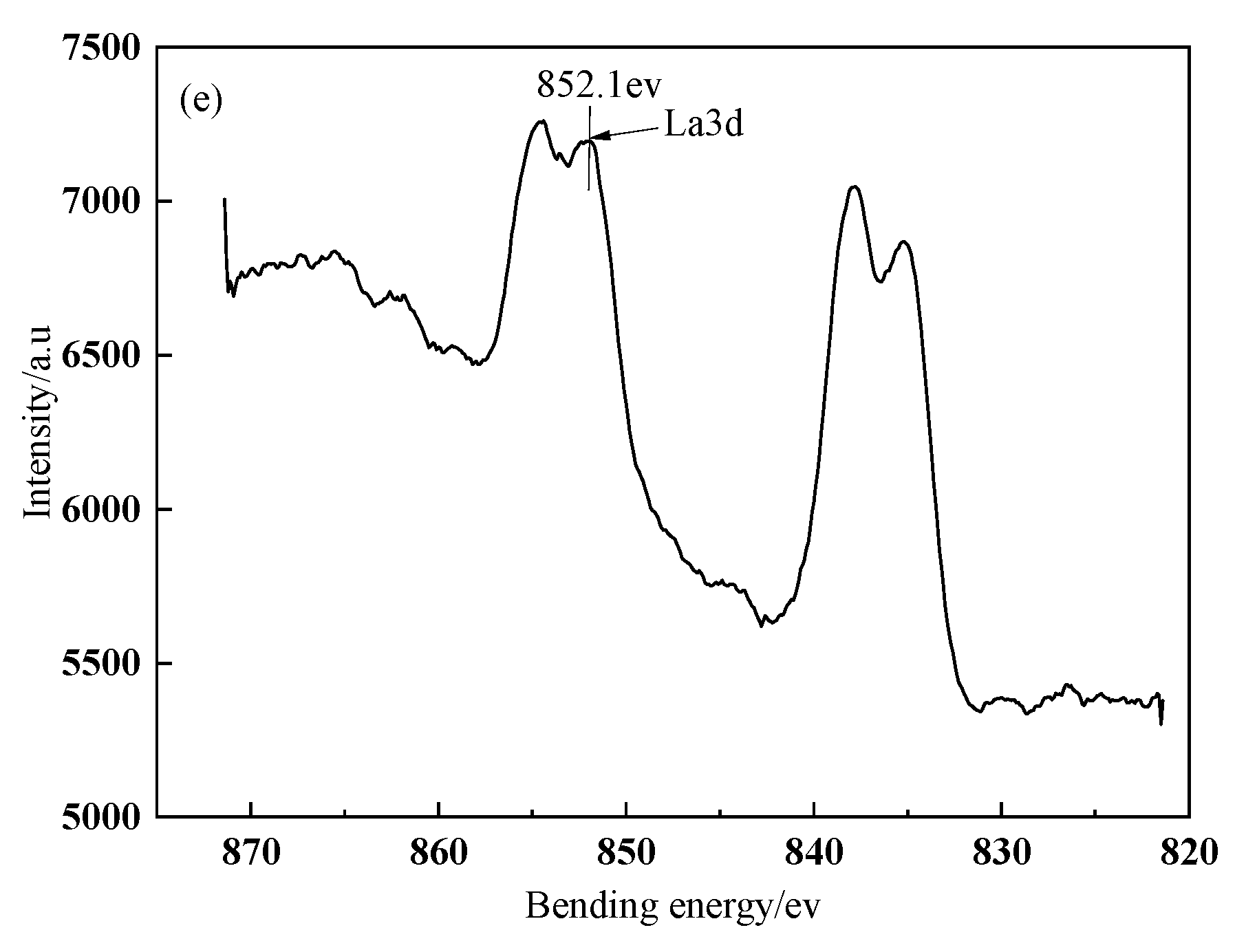
The fitted curves of La3d spectra on BFs surface treated with different modification solution containing La
3+ are presented in
Figure 3. The spectra peaks of La3d in the treated BFs with the different La
3+ modification solution concentrations (0.1, 0.3, 0.5, 0.7, and 0.9 wt.%) were corresponding to the bonding energies of 851.02, 851.77, 851.9, 852, and 852.1 ev, respectively. All the bonding energies were lower than that (853.0 ev) of the La corresponding to La3d in LaCl
3, indicating that the coordination chemical reactions occurred between the La and the O, C, N element of the treated fibers’ surfaces to the generate lanthanum coordination compounds on fibers’ surfaces.
3.2.3. XPS Characterization of Element C on BFs Surfaces
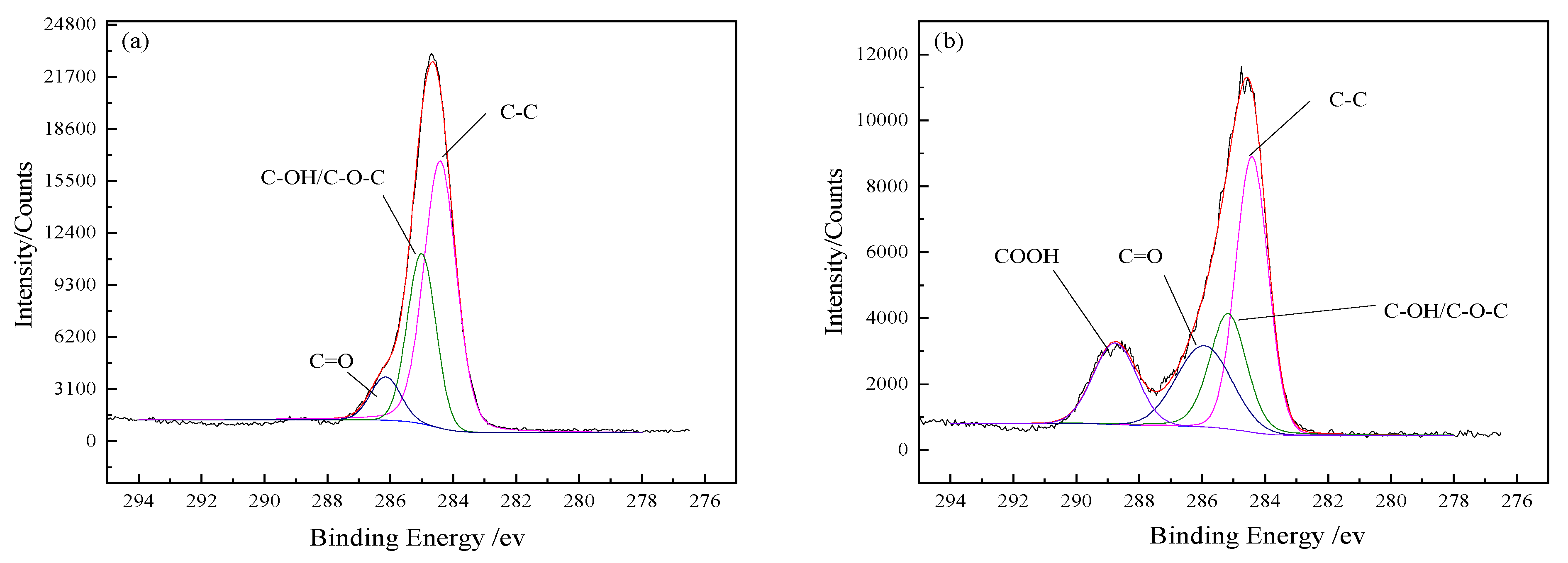
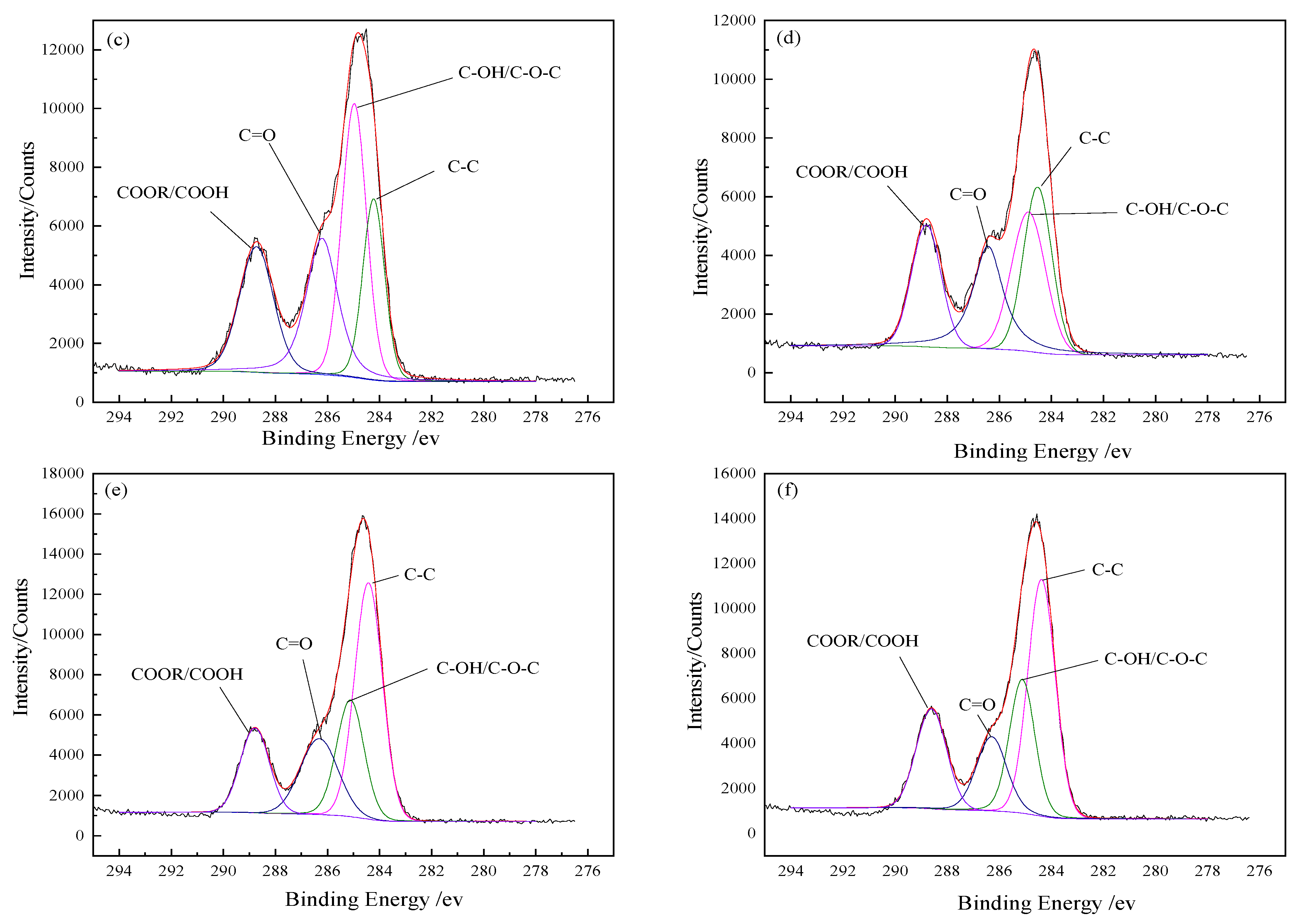
The fitted curves of C 1
s spectra and the detected functional groups contents on the BFs surfaces are presented in
Figure 4 and
Table 4, respectively. Three small peaks of C–C, C–O– and C=O appeared under the C 1
s peak of the untreated BFs surfaces in
Figure 4a, indicating that C atoms were combined with O atoms. The percentage of C atoms in the corresponding chemical state was represented with the area ratio of every small peak to the C 1
s peak. Due to the low content of element N on BFs surfaces, the C–N bond could be ignored.
As seen in
Figure 4b, with the peak appearance of –COOH in the fitted curves of C 1
s spectra of the BFs, new functional groups were generated on the surface of BFs treated with the rare earth modification solution, besides the original ones. According to the low-to-high order of binding energy, these functional groups can be arranged as alcoholic hydroxyl (C–OH) or ether bond (C–O–C), carbonyl (C=O) and carboxyl (COOH). The –C–C content on the BFs surfaces firstly decreased and then increased with La
3+ concentration increasing, but the changes of the carbon–oxygen bond were contrary. When La
3+ concentration was 0.5 wt.%, with the sum of C–OH/–C–O–C–, C=O and –COOH reaching to a maximum value (72.87%), the number of oxygen-containing groups on the BFs surfaces were the largest. As La
3+ concentration exceeded 0.5 wt.%, the content of carbon–oxygen bond decreased. That was attributed to the fact that the abundant oxygen-containing groups introduced by excess La were aggregated on the fibers’ surfaces and could not be stably absorbed to the fibers’ surfaces with the weak Van der Waals force between La
3+. Moreover, the aggregated supersaturated active oxygen-containing groups could also trigger the break of the origin formed macromolecular membrane on the fibers’ surfaces, which led to the loss of active groups.
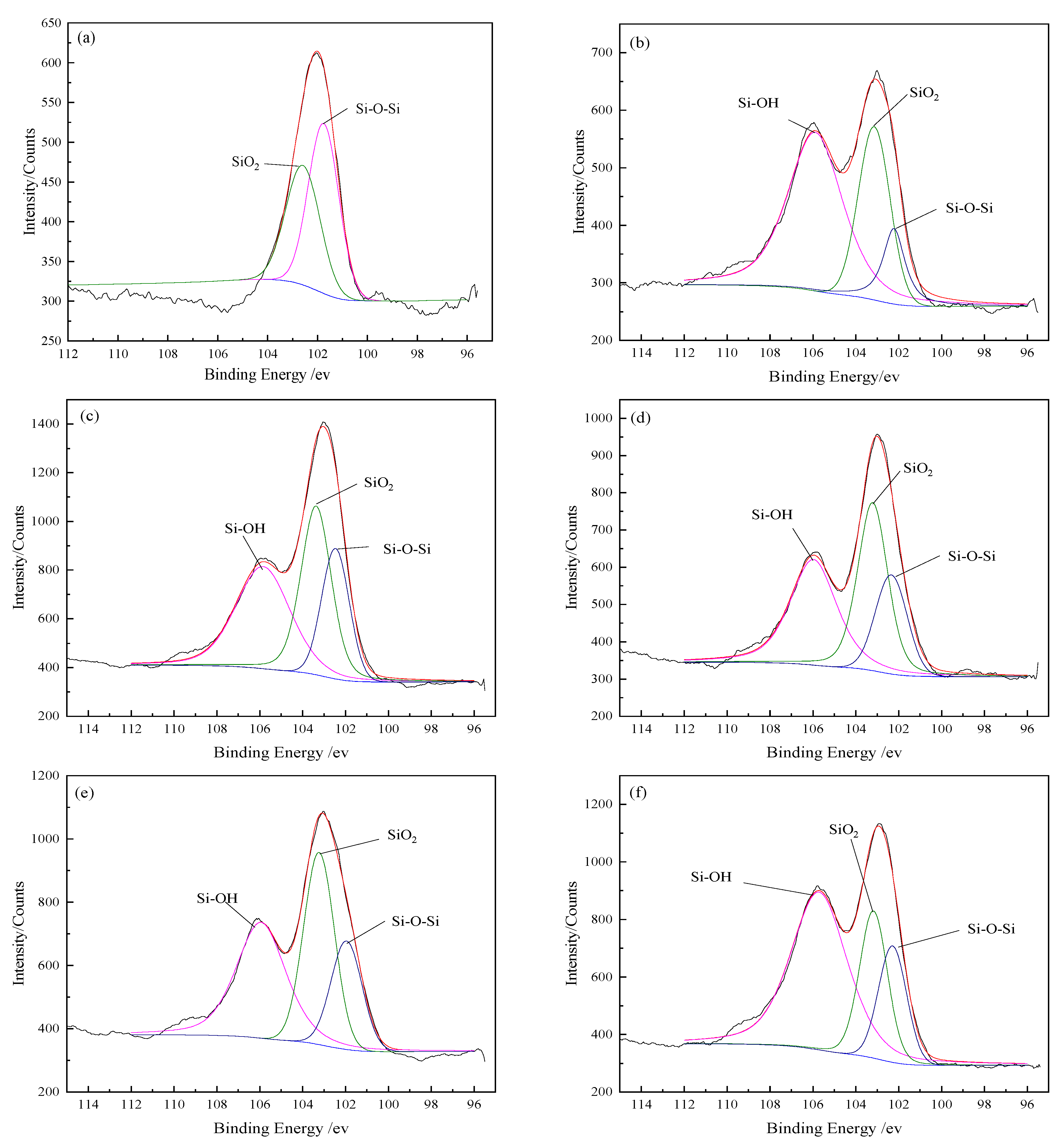
3.2.4. XPS Characterization of Si Element on BFs Surfaces
The functional group contents on the BFs surfaces are presented in
Table 5, according to the fitted curves of Si 2
p spectra in
Figure 5.
Figure 5a showed that SiO
2 and Si–O–Si bonds appeared on the untreated BFs surfaces, affirming that SiO
2 was the main chemical composition of BFs surfaces. The existence of Si–O–Si bonds was ascribed to the formation of BFs connected by [SiO
4] tetrahedron, which could further form [Si
2O
7] under interaction and even the chain structure with a higher degree of polymerization. Under the hydrolysis of SiO
2 in the modification solution, the new functional group (Si–OH) was generated on the surface of BFs treated with the rare earth modification solution along with the obvious decrease of SiO
2. However, the increase of Si–O–Si was mainly ascribed to the dehydration condensation reaction of Si–OH bonds by themselves or active organic groups in the modification solution, such as –COOH and –OH.
It can be seen that the SiO2 content on the BFs surfaces firstly decreased and then increased with La3+ concentration increasing, but the content changes of Si–O–Si and Si–OH were contrary. When La3+ concentration was 0.5 wt.%, the SiO2 content reached a minimum value (36.12%) corresponding to the highest contents of Si–O–Si and Si–OH. With the La3+ concentration beyond 0.5 wt.%, the ether bond was generated with the reaction of Si–OH and active groups in the rare earth modification solution. Accordingly, the hydrolysis of SiO2 was compressed and the content of SiO2 increased.
3.3. Basalt Fiber Surface Morphology
Figure 6 reveals the changes of BFs surface morphology after modification. The surfaces of untreated BFs were smooth without obvious grooves or salients in
Figure 6a. Some particles formed through the process of the active groups drawn onto the fiber surfaces by the rare earth element, were attached to the surfaces of BFs treated with the rare earth modification solution containing La
3+ concentration 0.1 wt.% in
Figure 6b.
Figure 6c,d shows that a trend of increasing particles was accompanied by an increasing concentration of La
3+. When La
3+ concentration was up to 0.5 wt.%, a great number of small particles uniformly covered the fiber surfaces to form the fibers’ coatings.
As shown in
Figure 6e,f, the particles decreased on the fiber surfaces, as La
3+ concentration increased to 0.7 wt.%. When La
3+ concentration was up to 0.9 wt.%, the excess rare earth elements were attached onto the fiber surfaces with the saturation of active groups, resulting that only a few particles of inhomogeneous size were on the fibers. Moreover, these active groups were easy to bulge and even be broken under the function of tension.
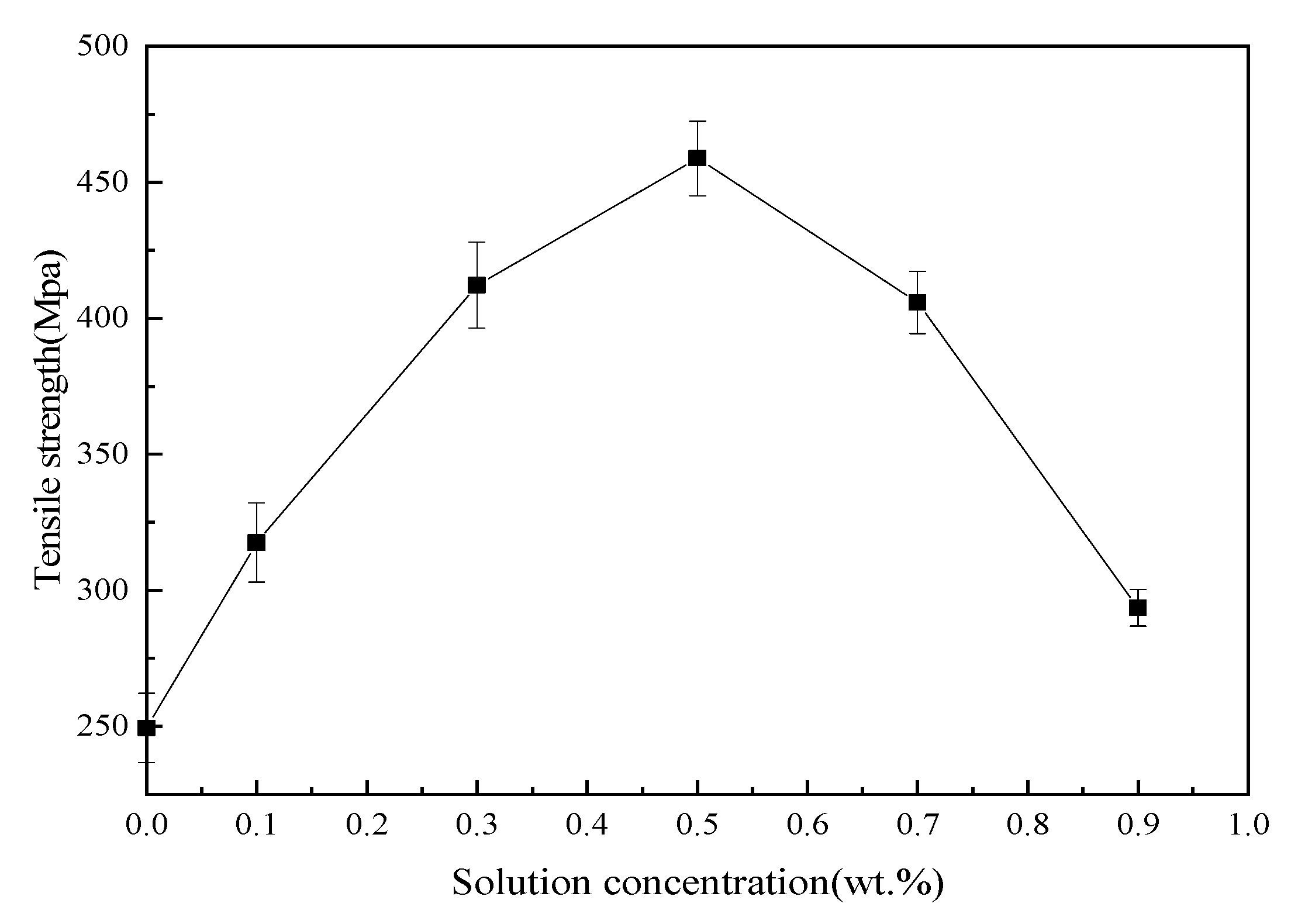
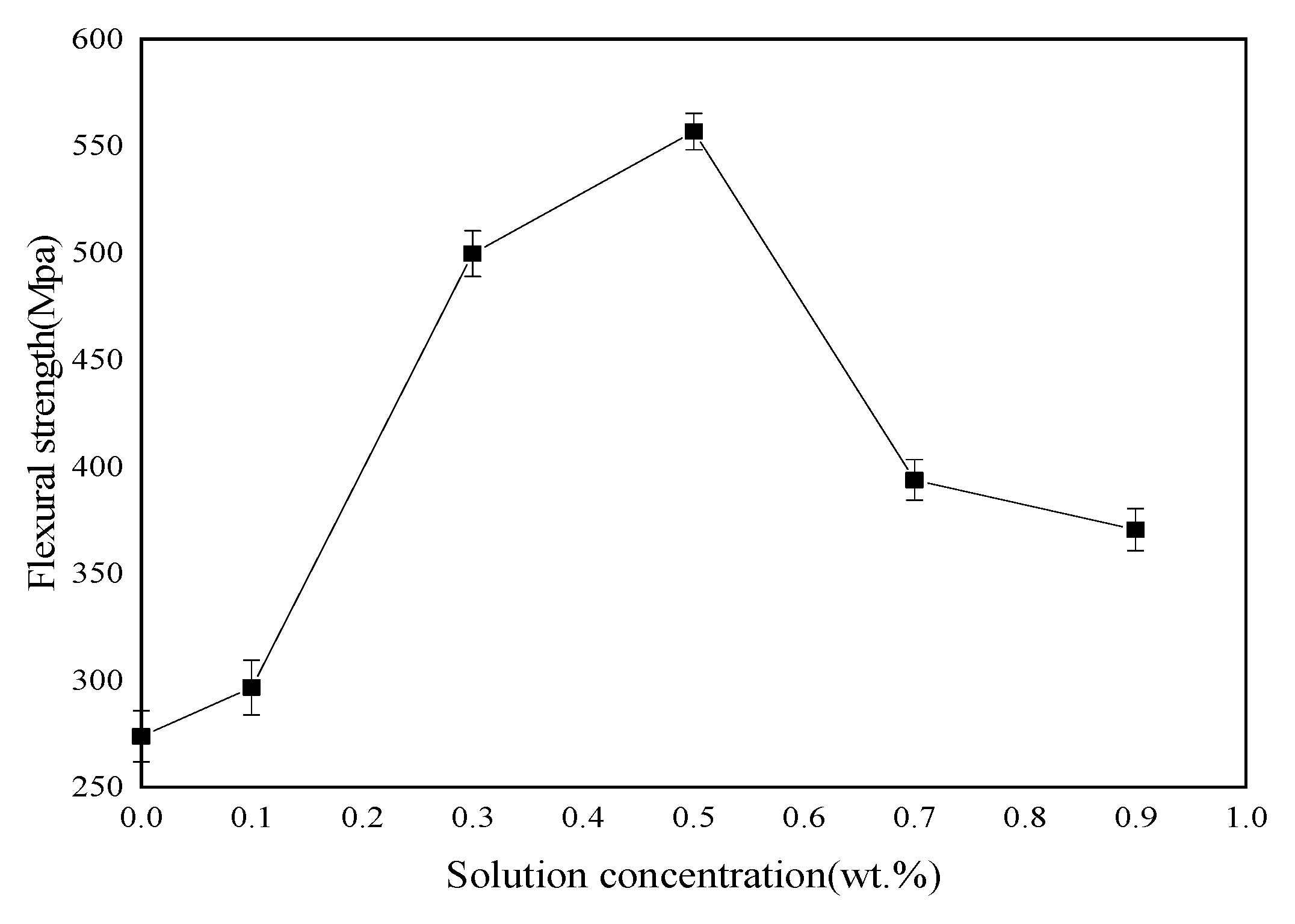
3.4. Mechanical Properties of Basalt Fibers/Epoxy Resin Composites
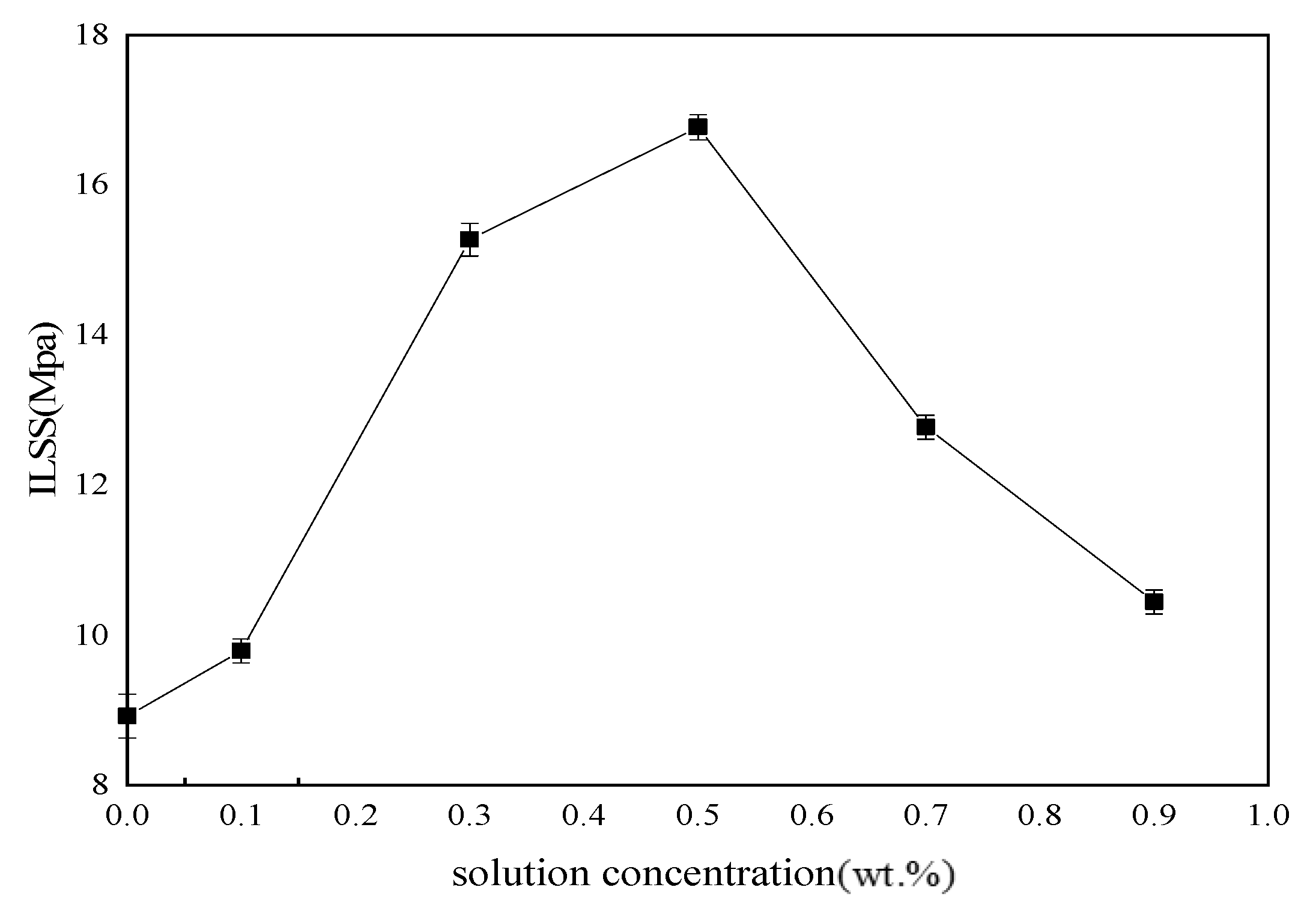
Tensile, bending and ILSS standard tests were performed to analyze mechanical behavior of BF/ERCs. According to the obtained experimental data shown in
Figure 7,
Figure 8 and
Figure 9, the mechanical properties, including the tensile strength, the bending strength and ILSS of BF/ERCs, were improved by BFs treated with La
3+ concentration increasing from 0.1 to 0.5 wt.%. The active functional groups attached to the fibers’ surfaces, such as C=O and O–H, improved the fiber surface roughness and activity, and thus enhanced the adhesion between fibers and the composite matrix. The maximum values of the tensile strength, the bending strength and ILSS were up to 458.7, 556.7 and 16.77 Mpa, and increased by 56.22%, 103.32% and 88% compared with those of the unmodified ones, respectively.
When La3+ concentration exceeded 0.5 wt.%, the mechanical strength of composites decreased as La3+ concentration increased. This may be ascribed to the fact that the excess active groups introduced by La3+ were aggregated to be supersaturated, and the triggered break of saturated active groups happened, leading to the loss of active groups. Moreover, the bits of grains on the interface of composite formed by the introduction of the excess La3+ to the fibers’ surface also reduced the interface bonding strength.
3.5. Fracture Surfaces Morphology of Basalt Fibers/Epoxy Resin Composites
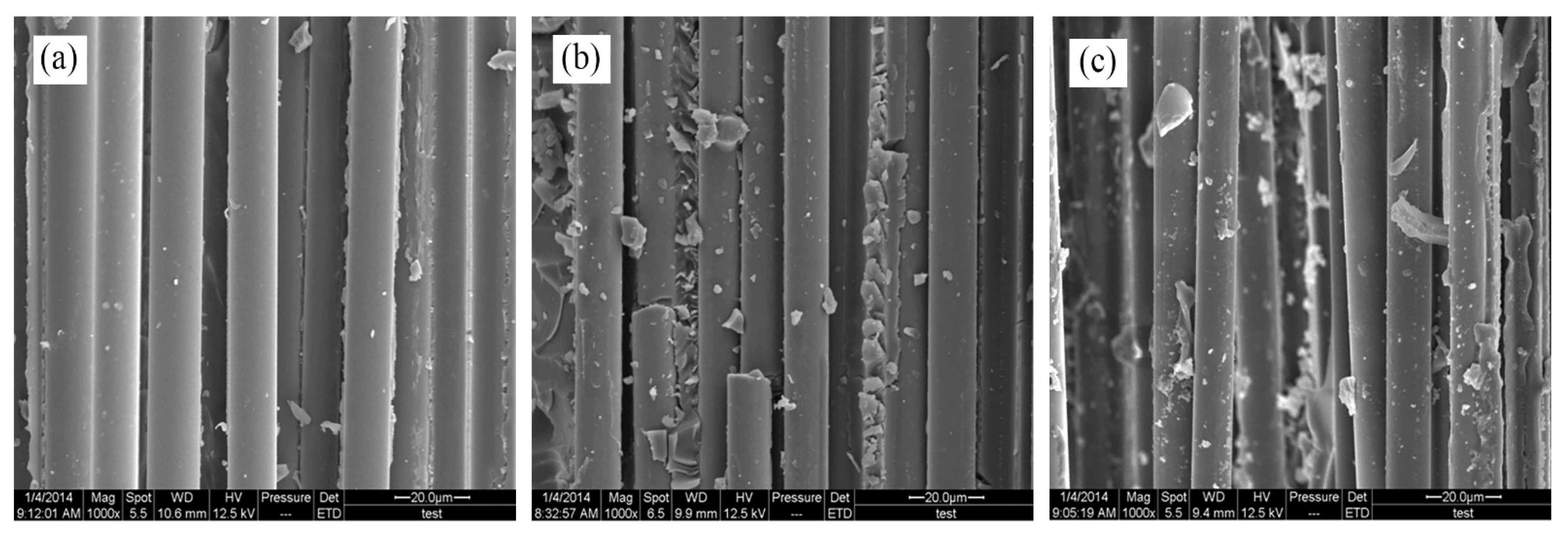
The fracture surfaces of untreated and treated BF/ERCs in the tensile test was observed by SEM. In
Figure 10a, smooth fibers and grooves can be seen on the fracture surfaces of untreated composites. Caused by the worse interfacial adhesion between fibers and the resin matrix, the fibers can be easily separated or pulled out from the resin matrix, exhibiting the low tensile strength of composites.
There were a few resins on the fiber surfaces of composites treated with the rare earth modification solution containing La
3+ concentration 0.1 wt.%, as shown in
Figure 10b. Resins increased and grooves decreased on the fracture surfaces with the increase of La
3+ concentration, as seen in
Figure 10c,d. These residual resins on the fibers’ surfaces were attributed in the improved adhesion between fibers and the resin matrix, when fibers were stretched from the resin matrix under the stress. When La
3+ concentration was up to 0.5 wt.%, the gaps between fibers were filled with the resins in
Figure 10d. BFs and the resin matrix adhered so tightly that external load was delivered to the fibers from the matrix, and the fibers mainly tolerated the breaking stress. It exhibited the obviously improved mechanical properties of BF/ERCs in the better reinforcing effect.
When La
3+ concentration exceeded 0.5 wt.%, the decrease in resins on the fiber surfaces with increasing La
3+ concentration led to the reappeared grooves on the resin matrix, when the smooth fibers pulled out, as seen in
Figure 10e,f. It demonstrated that the interfacial strength between the fibers and the resin matrix reduced, and the mechanical properties of composites correspondingly decreased.
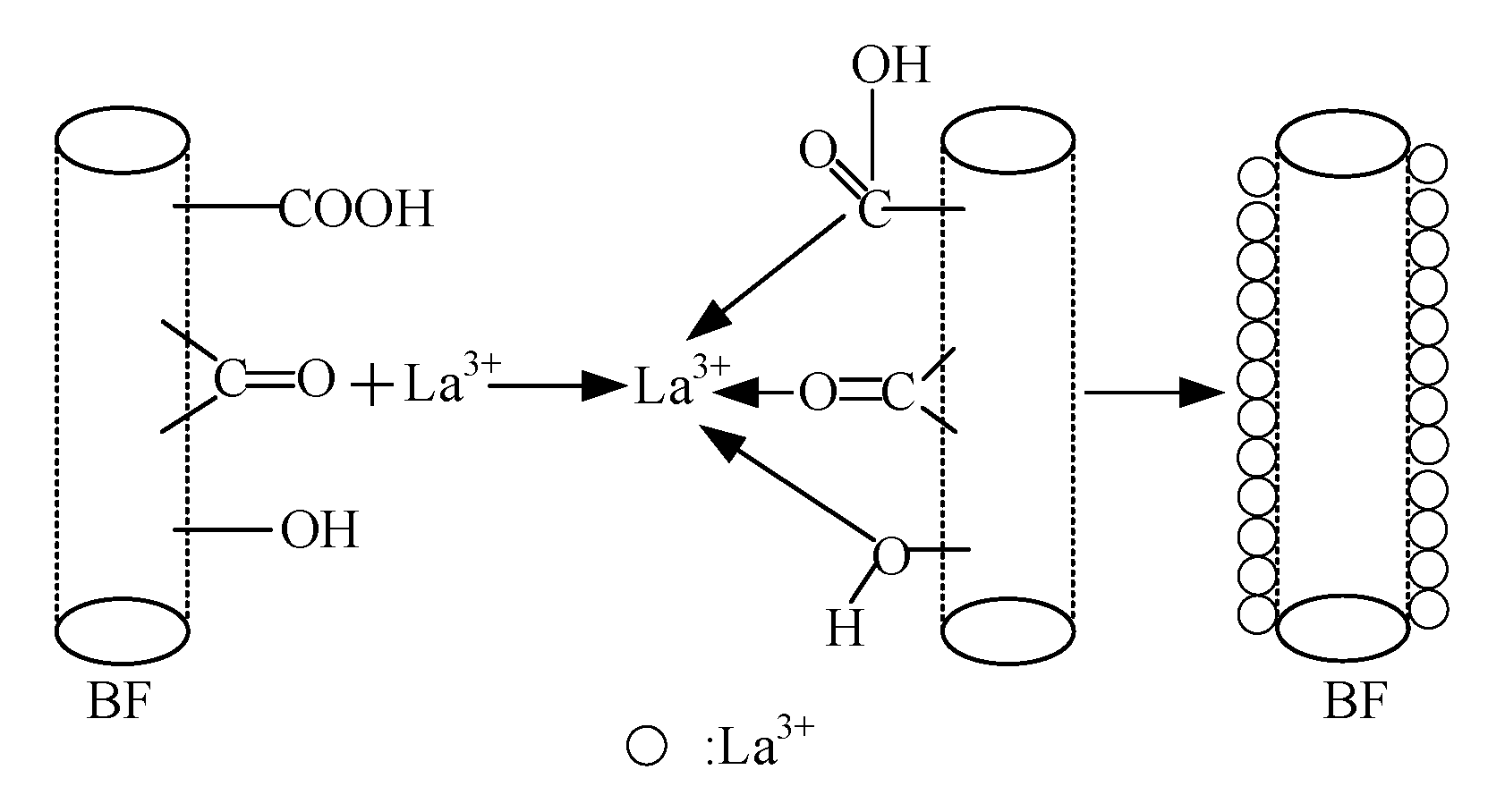
The monomolecular layer theory can be supplemented to analyze the effect of element La concentration on the tensile performances of BF/ERCs with the model shown in
Figure 11. At low concentrations, few La
3+ were absorbed onto the BFs surfaces. As shown in
Figure 11a, the arrangement of La atoms on BFs surfaces was discontinuous with a lot of voids, resulting in the failure of BF/ERCs occurring firstly on the location without La atoms under external load. Thus, the discontinuous interface between BFs and the epoxy resin is insufficient to improve the mechanical performance of BF/ERCs obviously. When La element increased to the best concentration, 0.5 wt.%, the uniform and compact monomolecular layer on the BFs surfaces exhibits high adhesive strength between BFs and the matrix, as shown in
Figure 11b. The mechanical performances of BF/ERCs reached to the highest value, including the tensile strength, the bending strength, and ILSS.
When La
3+ concentration exceeded 0.5 wt.%, excessive La atoms were assembled on the BFs surfaces to form multimolecular layer, as shown in
Figure 12. Under external load, failure of BF/ERCs firstly occurred on the interlayer of La atoms interlinked by weak Van der Waals force, resulting in the decreased performance of BF/ERCs.
5. Conclusions
In this research, the modification of basalt fibers with the modification solution of different La3+ concentrations was carried out. Moreover, the effect of modification with different La3+ concentrations and the mechanical properties for basalt fiber/epoxy resin composites modified with La3+ were evaluated.
The study about the chemical composition, the functional groups and the morphology of untreated and treated BFs’ surface with the rare earth modification solution using several analytical techniques (FTIR, XPS, and SEM), confirmed that La element in the rare earth modification solution could link active oxygen-containing functional groups to the BFs surfaces to improve the roughness and the activity of the fiber surfaces. When the La3+ concentration in the rare earth modification solution was 0.5 wt.%, the activity of BFs’ surface reached the strongest with the most introduced active groups. It is also determined that the mechanical performances of BF/ERCs, including the tensile strength, the bending strength and ILSS, could be significantly enhanced by the modified BFs due to the formed chemical reaction between resin matrix and the more active groups introduced by La3+. With the La3+ concentration in the rare earth modification solution reaching 0.5 wt.%, the bonding between the resin matrix and BFs is demonstrated to be the best and the tensile strength, the bending strength and ILSS were up to 458.7, 556.7 and 16.77 Mpa, and improved by 56.22%, 103.32% and 88% compared with those of the unmodified ones, respectively.
In addition, the modification mechanism of La3+ and enhancement mechanism of BFs/ECR’s mechanical property modified with La3+ were stated with the strong affinity of La to the nonmetal elements, indicating that the modification of BFs with La3+ was effective and the mechanical property of the prepared BFs/ECR composites was excellent.