Robust Superhydrophobic and Repellent Coatings Based on Micro/Nano SiO2 and Fluorinated Epoxy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fluorinated Epoxy
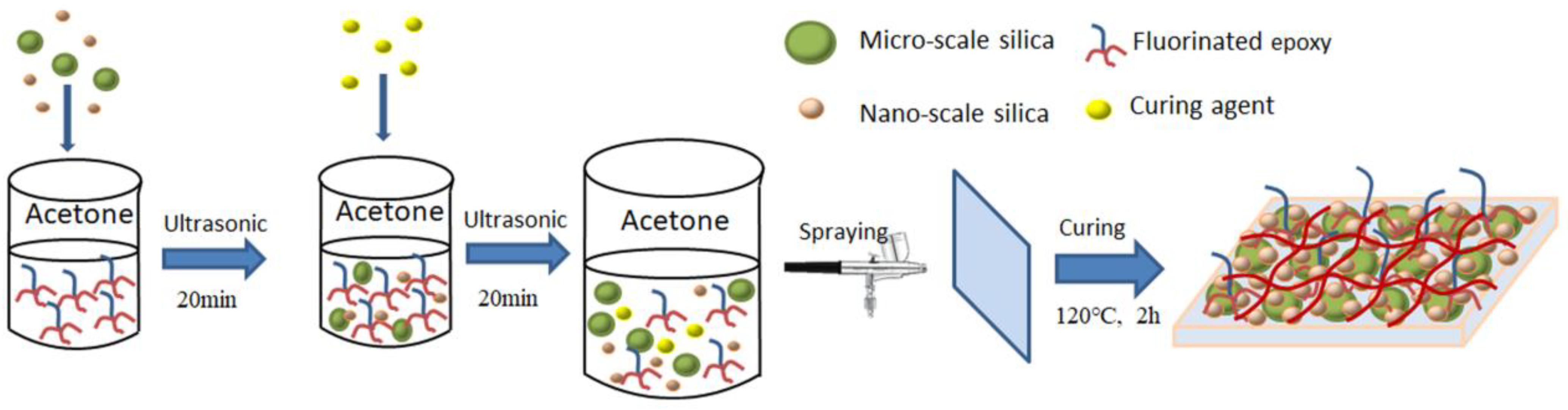
2.3. Preparation of Micro/Nano-Silica Coatings by Spray-Coating Approach
2.4. Analysis and Testing Methods
- Abrasion test
- Cross cut adhesion test
- Chemical stability test
- Self-cleaning test
- Anti-icing test
- Other characterization
3. Results and Discussion
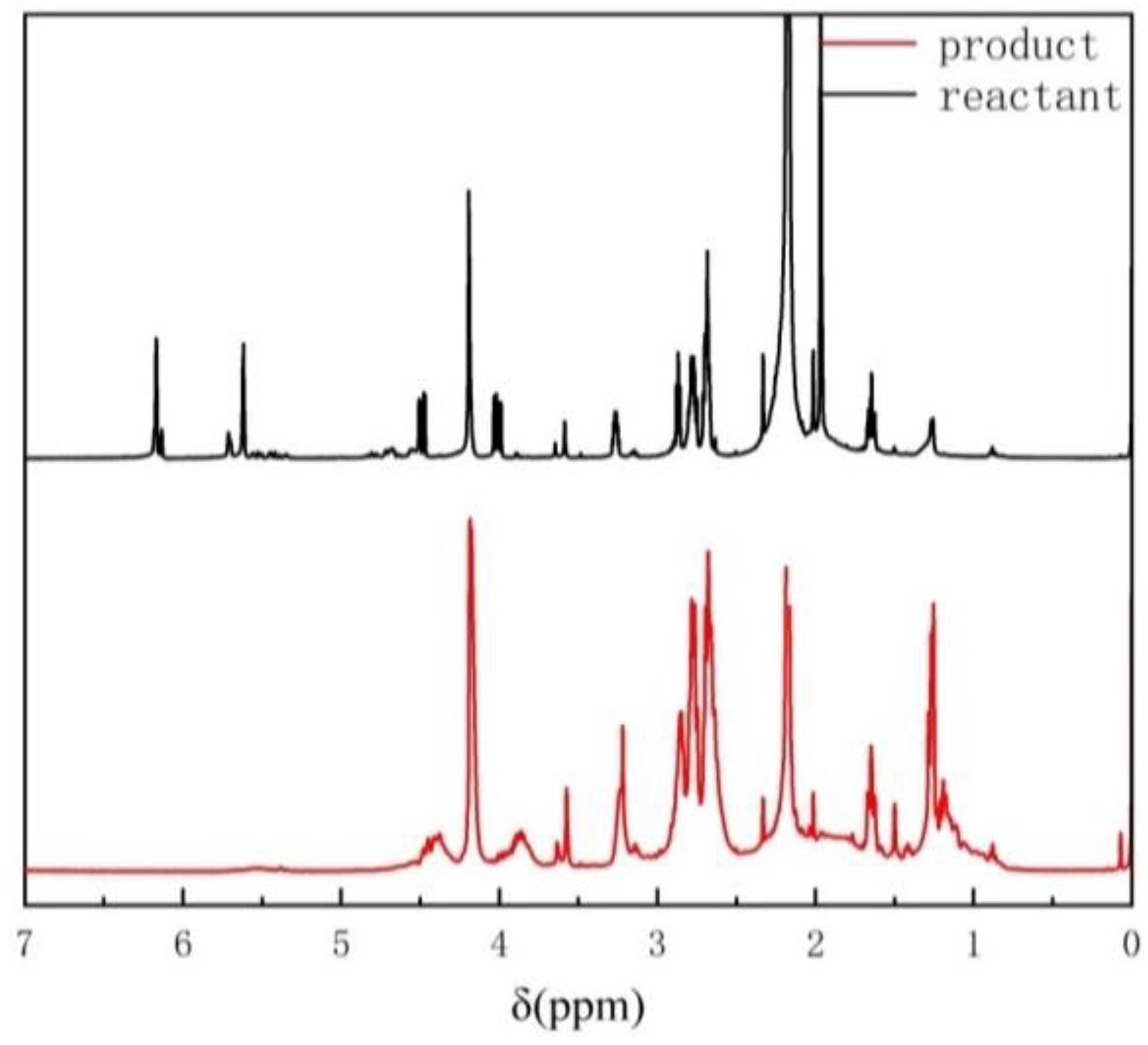
3.1. Preparation and Characterization of Fluorinated Epoxy
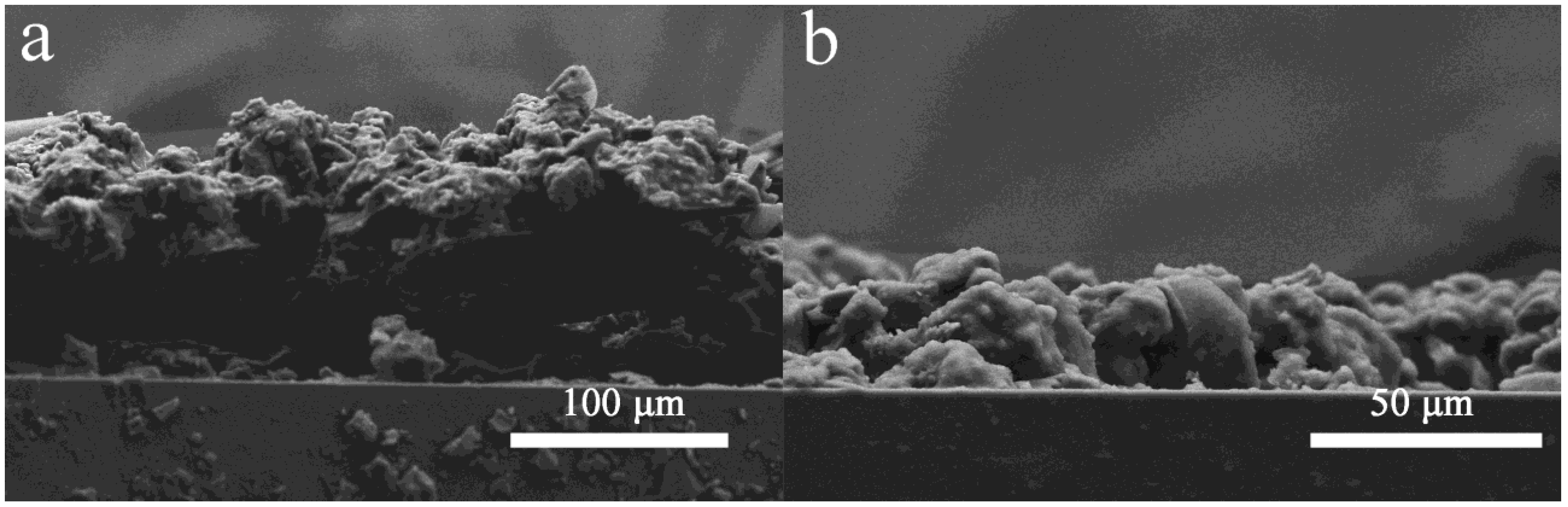
3.2. Wetting Behavior and Mechanical Durability of the Coating
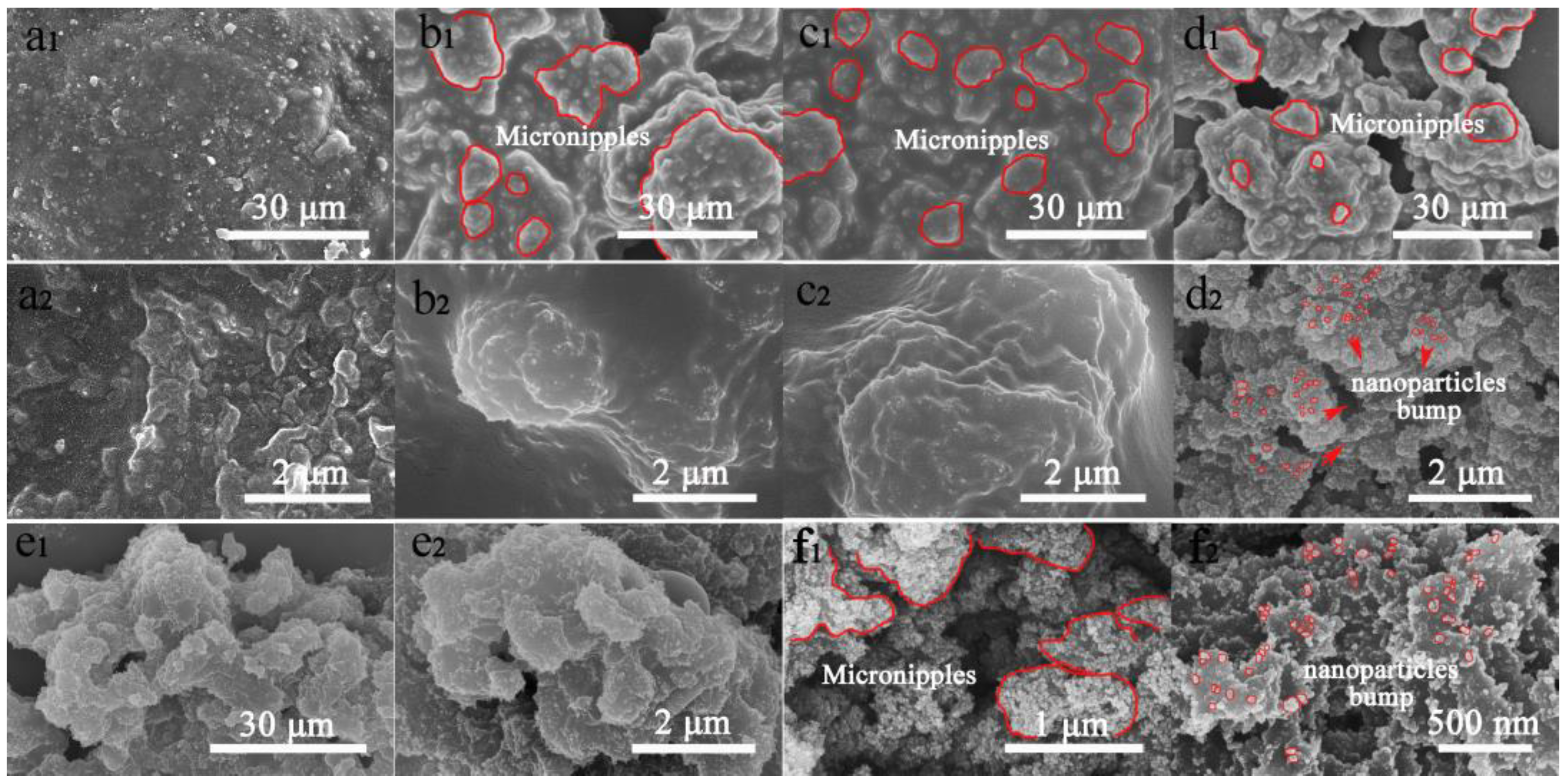
3.3. Surface Chemical Composition and Morphology
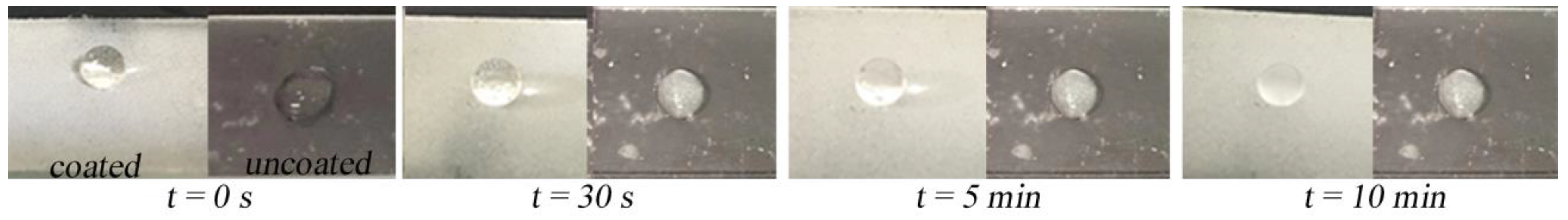
3.4. Chemical Stability
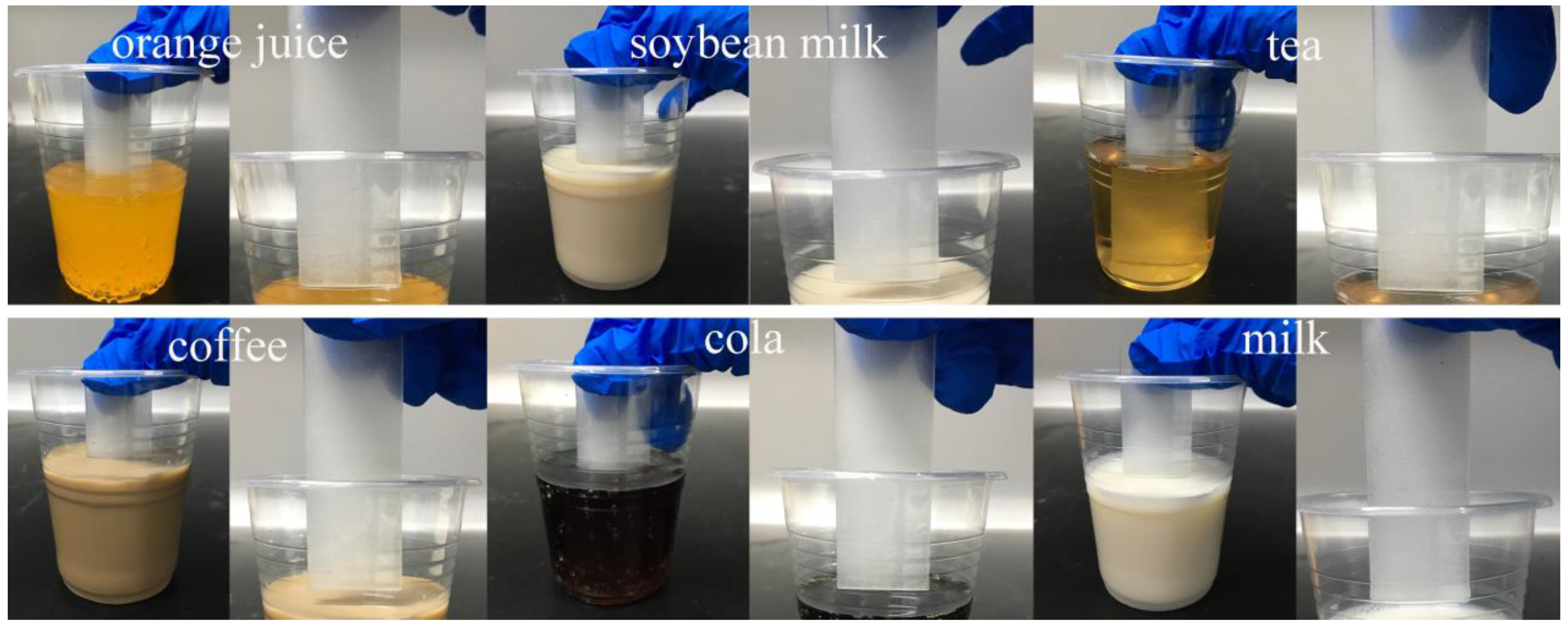
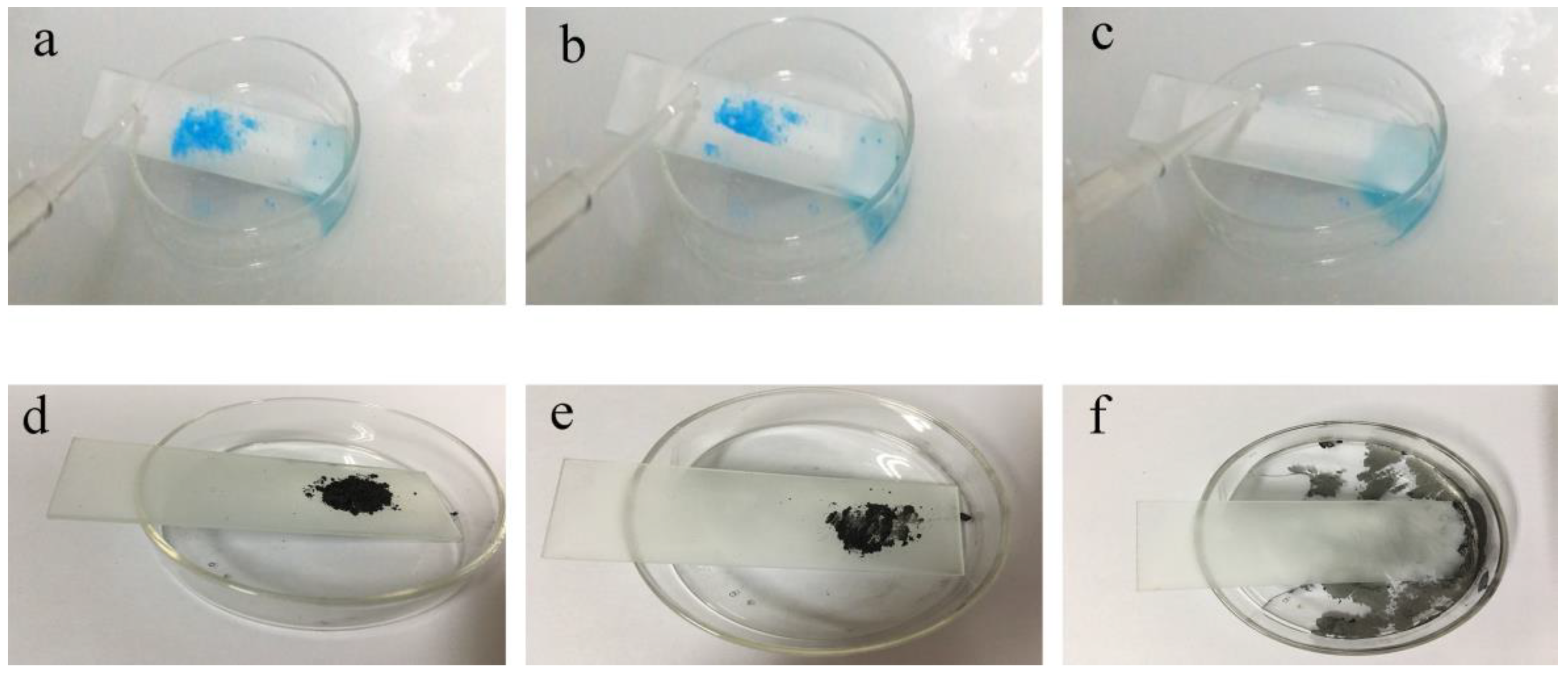
3.5. Self-Cleaning Properties
3.6. Anti-Icing Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Ma, Y.; Tan, J.J.; Fan, X.L.; Liu, Y.B.; Gu, J.W.; Zhang, B.L.; Zhang, H.P.; Zhang, Q.Y. Robust, self-healing, superhydrophobic coatings highlighted by a novel branched thiol-ene fluorinated siloxane nanocomposites. Compos. Sci. Technol. 2016, 137, 78–86. [Google Scholar] [CrossRef]
- Chen, J.H.; Liu, Z.H.; Wen, X.F.; Xu, S.P.; Wang, F.; Pi, P.H. Two-Step Approach for Fabrication of Durable Superamphiphobic Fabrics for Self-Cleaning, Antifouling, and On-Demand Oil/Water Separation. Ind. Eng. Chem. Res. 2019, 58, 5490–5500. [Google Scholar] [CrossRef]
- Wang, H.H.; Lu, H.T.; Zhang, X. Super-robust superamphiphobic surface with anti-icing property. RSC Adv. 2019, 9, 27702–27709. [Google Scholar] [CrossRef]
- Li, Y.; Hu, T.; Li, B.; Wei, J.; Zhang, J. Totally Waterborne and Highly Durable Superamphiphobic Coatings for Anti-Icing and Anticorrosion. Adv. Mater. Interfaces 2019, 6. [Google Scholar] [CrossRef]
- Su, F.; Yao, K. Facile fabrication of superhydrophobic surface with excellent mechanical abrasion and corrosion resistance on copper substrate by a novel method. ACS Appl. Mater. Interfaces 2014, 6, 8762–8770. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, W.; Xia, D.; Huang, Y.; Zhao, X.; Zhang, J. Spray coated superamphiphobic surface with hot water repellency and durable corrosion resistance. Colloid Surf. A 2020, 596. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, H.; Niu, H.; Zhang, J.; Du, Y.; Lin, T. Dual-Layer Superamphiphobic/Superhydrophobic-Oleophilic Nanofibrous Membranes with Unidirectional Oil-Transport Ability and Strengthened Oil-Water Separation Performance. Adv. Mater. Interfaces 2015, 2. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Wang, Z.; Liang, Y.; Cui, Z.; Zhao, J.; Li, X.; Ren, L. Multistimuli-Responsive Microstructured Superamphiphobic Surfaces with Large-Range, Reversible Switchable Wettability for Oil. ACS Appl. Mater. Inter. 2019, 11, 28478–28486. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing superoleophobic surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef]
- Telecka, A.; Li, T.; Ndoni, S.; Taboryski, R. Nanotextured Si surfaces derived from block-copolymer self-assembly with superhydrophobic, superhydrophilic, or superamphiphobic properties. RSC Adv. 2018, 8, 4204–4213. [Google Scholar] [CrossRef]
- Tuominen, M.; Teisala, H.; Haapanen, J.; Makela, J.M.; Honkanen, M.; Vippola, M.; Bardage, S.; Walinder, M.E.P.; Swerin, A. Superamphiphobic overhang structured coating on a biobased material. Appl. Surf. Sci. 2016, 389, 135–143. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Y.; Peng, Z.; Zhang, B.; Chen, S. How to Achieve a Monostable Cassie State on a Micropillar-Arrayed Superhydrophobic Surface. J. Phys. Chem. B. 2021, 125, 883–894. [Google Scholar] [CrossRef]
- Zhu, C.; Gao, Y.; Huang, Y.; Li, H.; Meng, S.; Francisco, J.S.; Zeng, X.C. Controlling states of water droplets on nanostructured surfaces by design. Nanoscale 2017, 9, 18240–18245. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, Z.; Liu, W. Biomimetic Multi-Functional Superamphiphobic FOTS-TiO2 Particles beyond Lotus Leaf. ACS Appl. Mater. Inter. 2016, 8, 27188–27198. [Google Scholar] [CrossRef]
- Yao, W.; Li, L.; Li, O.L.; Cho, Y.-W.; Jeong, M.-Y.; Cho, Y.-R. Robust, self-cleaning, amphiphobic coating with flower-like nanostructure on micro-patterned polymer substrate. Chem. Eng. J. 2018, 352, 173–181. [Google Scholar] [CrossRef]
- Li, H.; Yu, S.; Han, X. Fabrication of CuO hierarchical flower-like structures with biomimetic superamphiphobic, self-cleaning and corrosion resistance properties. Chem. Eng. J. 2016, 283, 1443–1454. [Google Scholar] [CrossRef]
- Velayi, E.; Norouzbeigi, R. Single-step prepared hybrid ZnO/CuO nanopowders for water repellent and corrosion resistant coatings. Ceram. Int. 2019, 45, 16864–16872. [Google Scholar] [CrossRef]
- Ge, D.; Yang, L.; Zhang, Y.; Rahmawan, Y.; Yang, S. Transparent and Superamphiphobic Surfaces from One-step Spray Coating of Stringed Silica Nanoparticle/Sol Solutions. Part. Part. Syst. Char. 2014, 31, 763–770. [Google Scholar] [CrossRef]
- Zhi, S.; Wang, G.; Zeng, Z.; Zhu, L.; Liu, Z.; Zhang, D.; Xu, K.; Xue, Q. 3D mossy structures of zinc filaments: A facile strategy for superamphiphobic surface design. J. Colloid Interf. Sci. 2018, 526, 106–113. [Google Scholar] [CrossRef]
- Hyuneui, L. Beyond a nature-inspired lotus surface: Simple fabrication approach part I. Superhydrophobic and transparent biomimetic glass part II. Superamphiphobic web of nanofibers. In Advances in Biomimetics; InTech Open: London, UK, 2011; pp. 145–158. [Google Scholar]
- Deng, X.; Mammen, L.; Butt, H.J.; Vollmer, D. Candle soot as a template for a transparent robust superamphiphobic coating. Science 2012, 335, 67–70. [Google Scholar] [CrossRef]
- Liu, Z.J.; Wang, H.Y.; Wang, W.Q.; Zhang, X.G.; Yuan, R.X.; Zhu, Y.J. Superhydrophobic poly(vinylidene fluoride) membranes with controllable structure and tunable wettability prepared by one-step electrospinning. Polymer 2016, 82, 105–113. [Google Scholar] [CrossRef]
- Ganesh, V.A.; Dinachali, S.S.; Nair, A.S.; Seeram, R. Robust Superamphiphobic Film from Electrospun TiO2 Nanostructures. ACS Appl. Mater. Inter. 2013, 5, 1527–1532. [Google Scholar] [CrossRef]
- Li, T.; Paliy, M.; Wang, X.; Kobe, B.; Lau, W.-M.; Yang, J. Facile One-Step Photolithographic Method for Engineering Hierarchically Nano/Microstructured Transparent Superamphiphobic Surfaces. ACS Appl. Mater. Inter. 2015, 7, 10988–10992. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, X.; Li, Q.; Liu, C.; Ye, T.; Liu, J.; Xu, H.; Zhang, X.; Liu, J.; Jiang, C.; et al. Springtail-Inspired Superamphiphobic Ordered Nanohoodoo Arrays with Quasi-Doubly Reentrant Structures. Small 2020, 16. [Google Scholar] [CrossRef]
- Stefelová, J.; Slovák, V.; Siqueira, G.; Olsson, R.T.; Tingaut, P.; Zimmermann, T.; Sehaqui, H. Drying and Pyrolysis of Cellulose Nanofibers from Wood, Bacteria, and Algae for Char Application in Oil Absorption and Dye Adsorption. ACS Sustain. Chem. Eng. 2017, 5, 2679–2692. [Google Scholar] [CrossRef]
- Yin, K.; Dong, X.; Zhang, F.; Wang, C.; Duan, J.A. Superamphiphobic miniature boat fabricated by laser micromachining. Appl. Phys. Lett. 2017, 110. [Google Scholar] [CrossRef]
- Wan, Y.L.; Chuan, W.X.; Liu, Z.G.; Yu, H.D.; Li, J. Preparation of superamphiphobic aluminium alloy surface based on laser-EDM method. Micro Nano Lett. 2018, 13, 281–283. [Google Scholar] [CrossRef]
- Yin, K.; Du, H.F.; Luo, Z.; Dong, X.R.; Duan, J.A. Multifunctional micro/nano-patterned PTFE near-superamphiphobic surfaces achieved by a femtosecond laser. Surf. Coat. Technol. 2018, 345, 53–60. [Google Scholar] [CrossRef]
- Sun, Y.W.; Wang, L.L.; Gao, Y.Z.; Guo, D.M. Preparation of stable superamphiphobic surfaces on Ti-6Al-4V substrates by one-step anodization. Appl. Surf. Sci. 2015, 324, 825–830. [Google Scholar] [CrossRef]
- Barthwal, S.; Kim, Y.S.; Lim, S.-H. Fabrication of amphiphobic surface by using titanium anodization for large-area three-dimensional substrates. J. Colloid Interf. Sci. 2013, 400, 123–129. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, B.; Wei, Q.; Li, B.; Li, L.; Yang, Y. Highly transparent superamphiphobic surfaces by elaborate microstructure regulation. J. Colloid Interf. Sci. 2019, 554, 250–259. [Google Scholar] [CrossRef]
- Ellinas, K.; Pujari, S.P.; Dragatogiannis, D.A.; Charitidis, C.A.; Tserepi, A.; Zuilhof, H.; Gogolides, E. Plasma Micro-Nanotextured, Scratch, Water and Hexadecane Resistant, Superhydrophobic, and Superamphiphobic Polymeric Surfaces with Perfluorinated Monolayers. ACS Appl. Mater. Inter. 2014, 6, 6510–6524. [Google Scholar] [CrossRef]
- Cai, J.; Wang, T.; Hao, W.; Ling, H.; Hang, T.; Chung, Y.-W.; Li, M. Fabrication of superamphiphobic Cu surfaces using hierarchical surface morphology and fluorocarbon attachment facilitated by plasma activation. Appl. Surf. Sci. 2019, 464, 140–145. [Google Scholar] [CrossRef]
- Ellinas, K.; Tserepi, A.; Gogolides, E. Superhydrophobic Fabrics with Mechanical Durability Prepared by a Two-Step Plasma Processing Method. Coatings 2018, 8, 351. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Wang, E.; Zhang, X.; Yuan, R.; Wu, S.; Zhu, Y. Facile preparation of superamphiphobic epoxy resin/modified poly(vinylidene fluoride)/fluorinated ethylene propylene composite coating with corrosion/wear-resistance. Appl. Surf. Sci. 2015, 357, 229–235. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Huang, K.; Zou, H.; Yu, D.; Li, Y.; Qiu, B.; Wang, X. Durable superamphiphobic nano-silica/epoxy composite coating via coaxial electrospraying method. Appl. Surf. Sci. 2018, 436, 283–292. [Google Scholar] [CrossRef]
- Yousefi, E.; Ghadimi, M.R.; Amirpoor, S.; Dolad, A. Preparation of new superhydrophobic and highly oleophobic polyurethane coating with enhanced mechanical durability. Appl. Surf. Sci. 2018, 454, 201–209. [Google Scholar] [CrossRef]
- Huang, C.; Wang, F.; Wang, D.; Guo, Z. Wear-resistant and robust superamphiphobic coatings with hierarchical TiO2/SiO2 composite particles and inorganic adhesives. New J. Chem. 2020, 44, 1194–1203. [Google Scholar] [CrossRef]
- Xiong, D.; Liu, G.; Duncan, E.J.S. Robust amphiphobic coatings from bi-functional silica particles on flat substrates. Polymer 2013, 54, 3008–3016. [Google Scholar] [CrossRef]
- Su, C. A simple and cost-effective method for fabricating lotus-effect composite coatings. J. Coat. Technol. Res. 2012, 9, 135–141. [Google Scholar] [CrossRef]
- Zhang, F.; Qian, H.; Wang, L.; Wang, Z.; Du, C.; Li, X.; Zhang, D. Superhydrophobic carbon nanotubes/epoxy nanocomposite coating by facile one-step spraying. Surf. Coat. Technol. 2018, 341, 15–23. [Google Scholar] [CrossRef]
- Xiu, Y.H.; Liu, Y.; Balu, B.; Hess, D.W.; Wong, C. Robust Superhydrophobic Surfaces Prepared with Epoxy Resin and Silica Nanoparticles. IEEE T. Comp. Pack. Man. 2012, 2, 395–401. [Google Scholar] [CrossRef]
- Han, X.; Peng, J.; Jiang, S.; Xiong, J.; Song, Y.; Gong, X. Robust Superamphiphobic Coatings Based on Raspberry-like Hollow SnO2 Composites. Langmuir 2020, 36, 11044–11053. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhao, X.; Wang, W.; Gong, X. Durable Self-Cleaning Surfaces with Superhydrophobic and Highly Oleophobic Properties. Langmuir 2019, 35, 8404–8412. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Panayiotou, C. Tuning the wetting properties of siloxane-nanoparticle coatings to induce superhydrophobicity and superoleophobicity for stone protection. Mater. Des. 2016, 108, 736–744. [Google Scholar] [CrossRef]
- Zhu, K.; Zhang, J.; Zhang, H.; Tan, H.; Zhang, W.; Liu, Y.; Zhang, H.; Zhang, Q. Fabrication of durable superhydrophobic coatings based on a novel branched fluorinated epoxy. Chem. Eng. J. 2018, 351, 569–578. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, J.; Liu, Y.; Hou, C.; Ma, Y.; Gu, J.; Zhang, B.; Zhang, H.; Zhang, Q. Design and fabrication of robust, rapid self-healable, superamphiphobic coatings by a liquid-repellent “glue plus particles” approach. Mater. Des. 2017, 135, 16–25. [Google Scholar] [CrossRef]
- Wang, K.L.; Liu, X.R.; Tan, Y.; Zhang, W.; Zhang, S.F.; Liu, J.Z.; Huang, A.M. Highly fluorinated and hierarchical HNTs/SiO2 hybrid particles for substrate-independent superamphiphobic coatings. Chem. Eng. J. 2018, 359, 626–640. [Google Scholar] [CrossRef]
- Li, Y.B.; Li, B.C.; Zhao, X.; Tian, N.; Zhang, J.P. Totally Waterborne, Nonfluorinated, Mechanically Robust, and Self-Healing Superhydrophobic Coatings for Actual Anti-Icing. ACS Appl. Mater. Inter. 2018, 10, 39391–39399. [Google Scholar] [CrossRef]






| Adhesion Level | Description | Percent (%) Area Removed |
|---|---|---|
| 0 | The edges of the square are completely smooth, none of the squares of the lattice are detached | 0% |
| 1 | Small flakes of the coating are detached at intersections, and less than 5% of the area is affected | <5% |
| 2 | Small flakes of the coating are detached along edges and at the intersections of cuts | 5–15% |
| 3 | The coating has flaked along the edges and on parts of the squares | 15–35% |
| 4 | The coating has flaked along the edges of the cuts in large ribbons and whole squares have detached | 35–65% |
| 5 | Severe flaking and detachment across entire square | >65% |
| CAs (°) | Water | Glycerol | Glycol | Diiodomethane |
|---|---|---|---|---|
| micro:nano = 1:0 | 123.4 ± 2.3 | 105.8 ± 1.4 | 127.3 ± 1.1 | 113.6 ± 1.3 |
| micro:nano = 2:1 | 135.3 ± 0.8 | 110.4 ± 1.3 | 128.6 ± 1.5 | 118.5 ± 2.1 |
| micro:nano = 1:1 | 158.6 ± 1.1 | 140.7 ± 0.9 | 152.4 ± 0.9 | 153.4 ± 1.3 |
| micro:nano = 1:2 | 125.2 ± 1.5 | 120.5 ± 1.5 | 132.7 ± 1.4 | 120.8 ± 1.8 |
| micro:nano = 0:1 | 120.7 ± 2.7 | 114.4 ± 1.8 | 118.5 ± 1.7 | 108.5 ± 2.4 |
| SAs (°) | Water | Glycerol | Glycol | Diiodomethane |
|---|---|---|---|---|
| FEP-S | 8.6 ± 1.1 | 14.6 ± 1.2 | 18.4 ± 1.5 | 17.5 ± 1.4 |
| CAs (°) | Water | Glycerol | Diiodomethane | Glycol |
|---|---|---|---|---|
| 1M HCl | 158.4 ± 0.8 | 152.5 ± 0.7 | 135.3 ± 1.1 | 147.5 ± 0.9 |
| 1M NaOH | 157.6 ± 0.7 | 153.7 ± 0.6 | 136.4 ± 0.9 | 148.6 ± 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Yu, R. Robust Superhydrophobic and Repellent Coatings Based on Micro/Nano SiO2 and Fluorinated Epoxy. Coatings 2021, 11, 663. https://doi.org/10.3390/coatings11060663
Huang X, Yu R. Robust Superhydrophobic and Repellent Coatings Based on Micro/Nano SiO2 and Fluorinated Epoxy. Coatings. 2021; 11(6):663. https://doi.org/10.3390/coatings11060663
Chicago/Turabian StyleHuang, Xiaoye, and Ruobing Yu. 2021. "Robust Superhydrophobic and Repellent Coatings Based on Micro/Nano SiO2 and Fluorinated Epoxy" Coatings 11, no. 6: 663. https://doi.org/10.3390/coatings11060663
APA StyleHuang, X., & Yu, R. (2021). Robust Superhydrophobic and Repellent Coatings Based on Micro/Nano SiO2 and Fluorinated Epoxy. Coatings, 11(6), 663. https://doi.org/10.3390/coatings11060663





