Bacterial Adhesion Capacity of Uropathogenic Escherichia coli to Polyelectrolyte Multilayer Coated Urinary Catheter Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
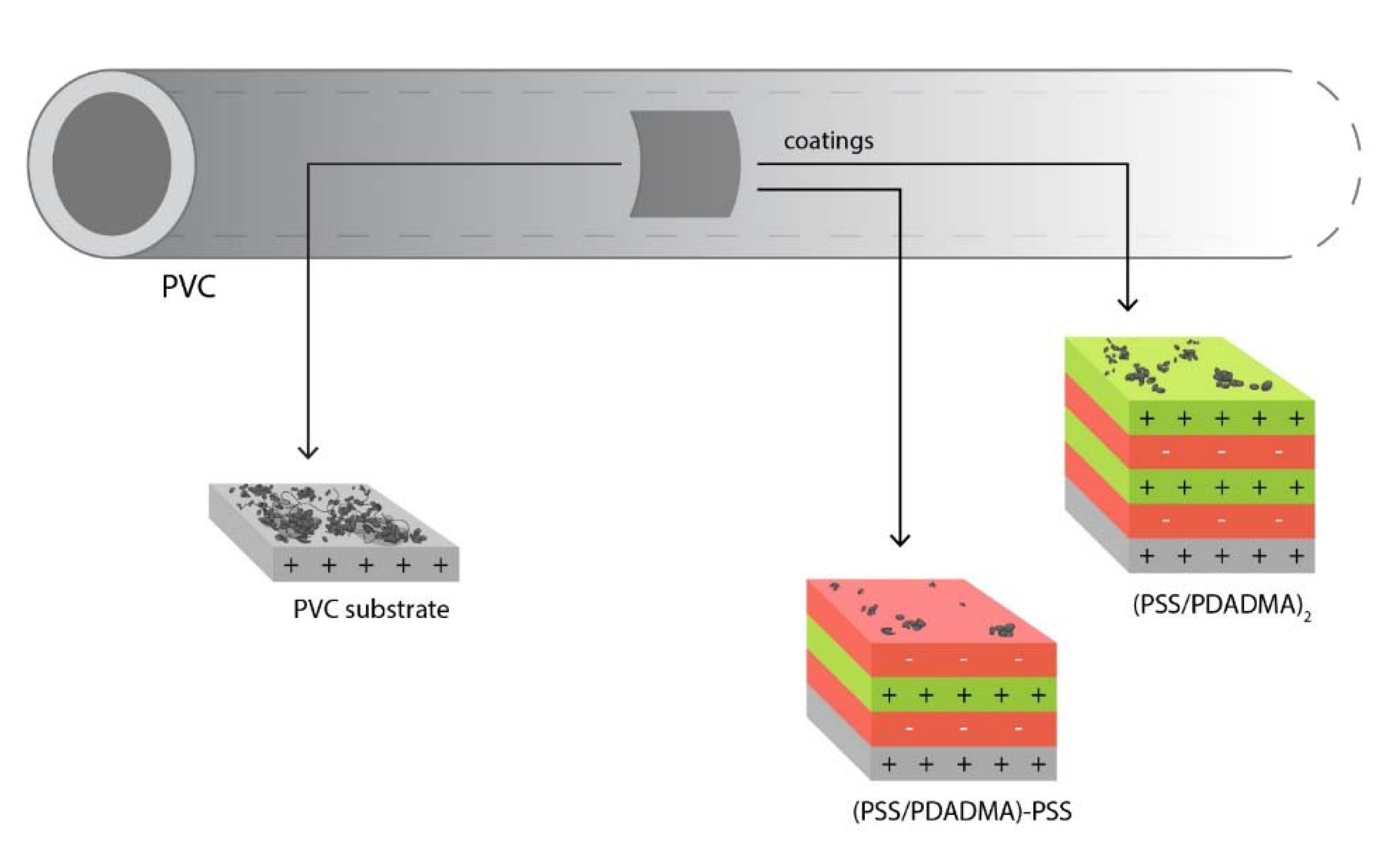
2.1.1. Substrate
2.1.2. Polyelectrolytes
2.1.3. Bacteria
2.2. Methods
2.2.1. Polyelectrolyte Multilayer Preparation
2.2.2. Surface Morphology and Roughness
2.2.3. Zeta Potential Measurements
2.2.4. Contact Angle Measurements
2.2.5. Monitoring of Bacterial Adhesion on Flat Catheter Surfaces
2.2.6. Monitoring of Bacterial Adhesion on Cylindrical Catheter Surfaces
2.2.7. Statistical Analysis
3. Results
3.1. Surface Topography and Roughness
3.2. Zeta Potential
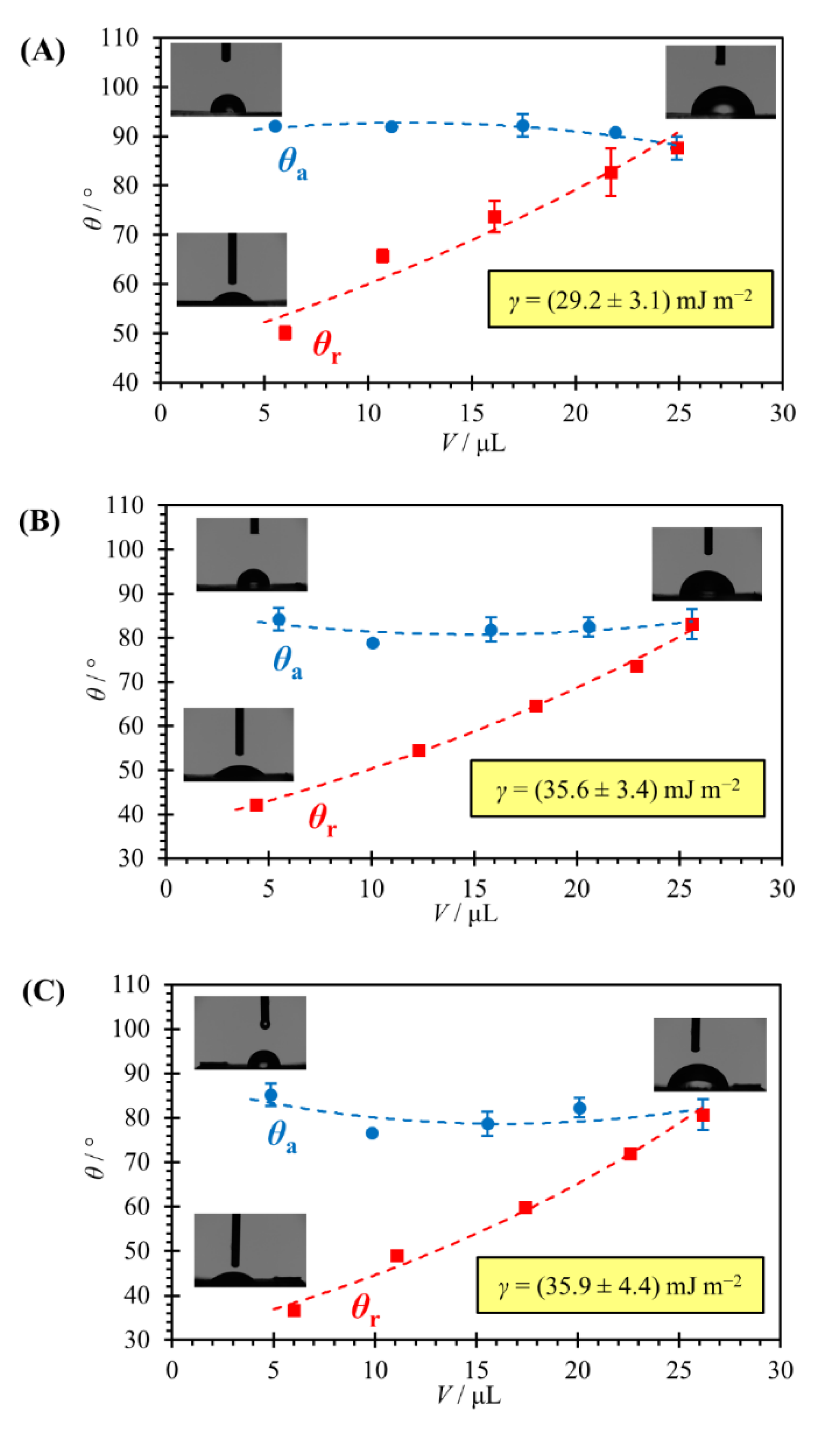
3.3. Contact Angle Measurements
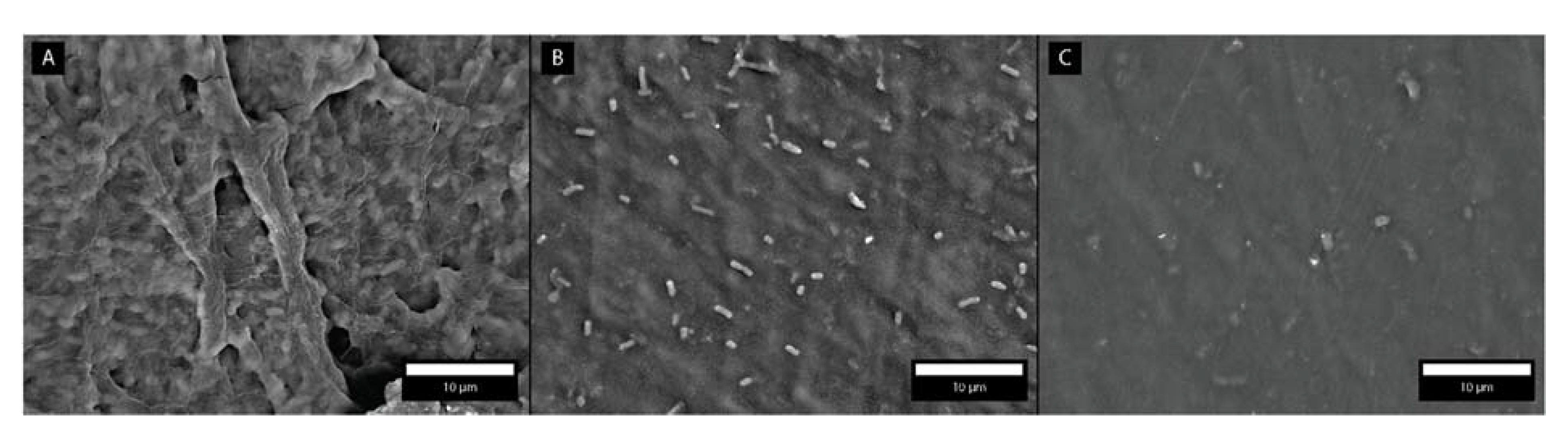
3.4. Bacterial Adhesion Extent
3.5. Bacterial Adhesion on PVC Catheter under a Liquid Flow
4. Discussion
5. Conclusions
- due to its positive potential, the bar PVC catheter surfaces attract E. coli very strongly and the biofilm starts to grow,
- the polyelectrolyte coating substantially decreases the bacterial adhesion extent and,
- among polyelectrolytes, the negatively charged polyelectrolytes are due to the strong electrostatic repulsion with negatively charged E. coli, the most appropriate surfaces regarding bacterial adhesion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kranz, J.; Schmidt, S.; Wagenlehner, F.; Schneidewind, L. Catheter-associated urinary tract infections in adult patients—Preventive strategies and treatment options. Dtsch Arztebl. Int. 2020, 117, 83–88. [Google Scholar] [CrossRef]
- Campos Mota, É.; Oliveira, A.C. Biofilm in indwelling urinary catheter and patient safety: A literature review. Vigil. Sanit. Debate 2017, 5, 116–122. [Google Scholar]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Di Ilio, C. Escherichia coli in Europe: An overview. Int. J. Environ. Res. Public Health 2013, 10, 6235–6254. [Google Scholar] [CrossRef]
- Katsikogianni, M.; Missirlis, Y.F. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cell. Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Ofek, I.; Doyle, R.J. Methods, models, and analysis of bacterial adhesion. In Bacterial Adhesion to Cells and Tissues; Springer: Boston, MA, USA, 1994. [Google Scholar]
- Sukhishvili, S.A. Responsive polymer films and capsules via layer-by-layer assembly. Curr. Opin. Colloid Interface Sci. 2005, 10, 37–44. [Google Scholar] [CrossRef]
- Ariga, K.; Yamauchi, Y.; Rydzek, G.; Ji, Q.; Yonamine, Y.; Wu, K.C.-W.; Hill, J.P. Layer-by-layer nanoarchitectonics: Invention, innovation, and evolution. Chem. Lett. 2014, 43, 36–68. [Google Scholar] [CrossRef]
- Borges, J.; Mano, J.F. Molecular interactions driving the layer-by-layer assembly of multilayers. Chem. Rev. 2014, 114, 8883–8942. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, D.; van der Burgh, S.; de Keizer, A.; Cohen Stuart, M.A. Kinetics of formation and dissolution of weak polyelectrolyte multilayers: Role of salt and free polyions. Langmuir 2002, 18, 5607–5612. [Google Scholar] [CrossRef]
- Hartmann, H.; Krastev, R. Biofunctionalization of surfaces using polyelectrolyte multilayers. BioNanoMaterials 2017, 18, 20160015. [Google Scholar] [CrossRef]
- Séon, L.; Lavalle, P.; Schaaf, P.; Boulmedais, F. Polyelectrolyte multilayers: A versatile tool for preparing antimicrobial coatings. Langmuir 2015, 31, 12856–12872. [Google Scholar] [CrossRef] [PubMed]
- Keum, H.; Kim, J.Y.; Yu, B.; Yu, S.J.; Kim, J.; Jeon, H.; Lee, D.Y.; Im, S.G.; Jon, S. Prevention of bacterial colonization on catheters by a one-step coating process involving an antibiofouling polymer in water. ACS Appl. Mater. Interfaces 2017, 9, 19736–19745. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, D.; Pratnekar, R.; Godič Torkar, K.; Salopek, J.; Dražić, G.; Abram, A.; Bohinc, K. Influence of polyelectrolyte multilayer properties on bacterial adhesion capacity. Polymers 2016, 8, 345. [Google Scholar] [CrossRef]
- Bohinc, K.; Bajuk, J.; Jukić, J.; Abram, A.; Oder, M.; Godič Torkar, K.; Raspor, P.; Kovačević, D. Bacterial adhesion capacity of protein-terminating polyelectrolyte multilayers. Int. J. Adhes. Adhes. 2020, 103, 102687. [Google Scholar] [CrossRef]
- Ghostine, R.A.; Markarian, M.Z.; Schlenoff, J.B. Asymmetric growth in polyelectrolyte multilayers. J. Am. Chem. Soc. 2013, 135, 7636–7646. [Google Scholar] [CrossRef]
- Salopek, J.; Sadžak, A.; Kuzman, D.; Požar, J.; Kovačević, D. Polyelectrolyte multilayers on silica surfaces: Effect of ionic strength and sodium salt type. Croat. Chem. Acta 2017, 90, 281–287. [Google Scholar] [CrossRef]
- Elshof, M.G.; de Vos, W.M.; de Grooth, J.; Benes, N.E. On the long-term pH stability of polyelectrolyte multilayer nanofiltration membranes. J. Membr. Sci. 2020, 615, 118532. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Bohinc, K.; Dražić, G.; Abram, A.; Jevšnik, M.; Jeršek, B.; Nipič, D.; Kurinčič, M.; Raspor, P. Metal surface characteristics dictate bacterial adhesion capacity. Int. J. Adhes. Adhes. 2016, 68, 39–46. [Google Scholar] [CrossRef]
- Bohinc, K.; Dražić, G.; Fink, R.; Oder, M.; Jevšnik, M.; Nipič, D.; Godič-Torkar, K.; Raspor, P. Available surface dictates microbial adhesion capacity. Int. J. Adhes. Adhes. 2014, 50, 265–272. [Google Scholar] [CrossRef]
- Zore, A.; Bezek, K.; Jevšnik, M.; Abram, A.; Runko, V.; Slišković, I.; Raspor, P.; Kovačević, D.; Bohinc, K. Bacterial adhesion rate on food grade ceramics and Teflon as kitchen worktop surfaces. Int. J. Food Microbiol. 2020, 332, 108764. [Google Scholar] [CrossRef]
- Neoh, K.G.; Li, M.; Kang, E.-T.; Chiong, E.; Anantharajah Tambyah, P. Surface modification strategies for combating catheter-related complications: Recent advances and challenges. J. Mater. Chem. B 2017, 5, 2045–2067. [Google Scholar] [CrossRef] [PubMed]
- Islas, L.; Ruiz, J.-C.; Muñoz-Muñoz, F.; Isoshima, T.; Burillo, G. Surface characterization of poly(vinyl chloride) urinary catheters functionalized with acrylic acid and poly(ethylene glycol) methacrylate using gamma-radiation. Appl. Surf. Sci. 2016, 384, 135–142. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, S.; He, T.; Jiang, S.; Jańczewski, D.; Vancso, G.J. Engineered, robust polyelectrolyte multilayers by precise control of surface potential for designer protein, cell, and bacteria adsorption. Langmuir 2016, 32, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Bohinc, K.; Kovačević, D. Bacterial adhesion on polyelectrolyte multilayers. In Fighting Antimicrobial Resistance; Budimir, A., Ed.; IAPC: Zagreb, Croatia, 2018; Chapter 14; pp. 335–350. [Google Scholar]
- Tallósy, P.; Janovák, L.; Nagy, E.; Deák, A.; Juhász, A.; Csapó, E.; Buzás, N.; Dékány, I. Adhesion and inactivation of gram-negative and gram-positive bacteria on photoreactive TiO2/polymer and Ag–TiO2/polymer nanohybrid films. Appl. Surf. Sci. 2016, 371, 139–150. [Google Scholar] [CrossRef]
- Kostler, S.; Delgado, A.V.; Ribitsch, V. Surface thermodynamic properties of polyelectrolyte multilayers. J. Coll. Inter. Sci. 2005, 286, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Tuson, H.H.; Weibel, D.B. Bacteria-surface interactions. Soft Matter 2013, 9, 4368–4380. [Google Scholar] [CrossRef] [PubMed]
- Maharubin, S.; Nayak, C.; Phatak, O.; Kurhade, A.; Singh, M.; Zhou, Y.; Tan, G. Polyvinylchloride coated with silver nanoparticles and zinc oxide nanowires for antimicrobial applications. Mater. Lett. 2019, 249, 108–111. [Google Scholar] [CrossRef]
- Yu, H.; Liu, L.; Yang, H.; Zhou, R.; Che, C.; Li, X.; Li, C.; Luan, S.; Yin, J.; Shi, H. Water-insoluble polymeric guanidine derivative and application in the preparation of antibacterial coating of catheter. ACS Appl. Mater. Interfaces 2018, 10, 39257–39267. [Google Scholar] [CrossRef]
- Thorarinsdottir, H.R.; Kander, T.; Holmberg, A.; Petronis, S.; Klarin, B. Biofilm formation on three different endotracheal tubes: A prospective clinical trial. Crit. Care 2020, 24, 382. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, K.; Si, Y.; Guo, X.; Xu, Y. Protein–polyelectrolyte interaction: Thermodynamic analysis based on the titration method. Polymers 2019, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Filipović, U.; Gošnak Dahmane, R.; Slaheddine, G.; Zore, A.M.; Bohinc, K. Bacterial adhesion on orthopedic implants. Adv. Colloid Interface Sci. 2020, 283, 1–12. [Google Scholar] [CrossRef] [PubMed]
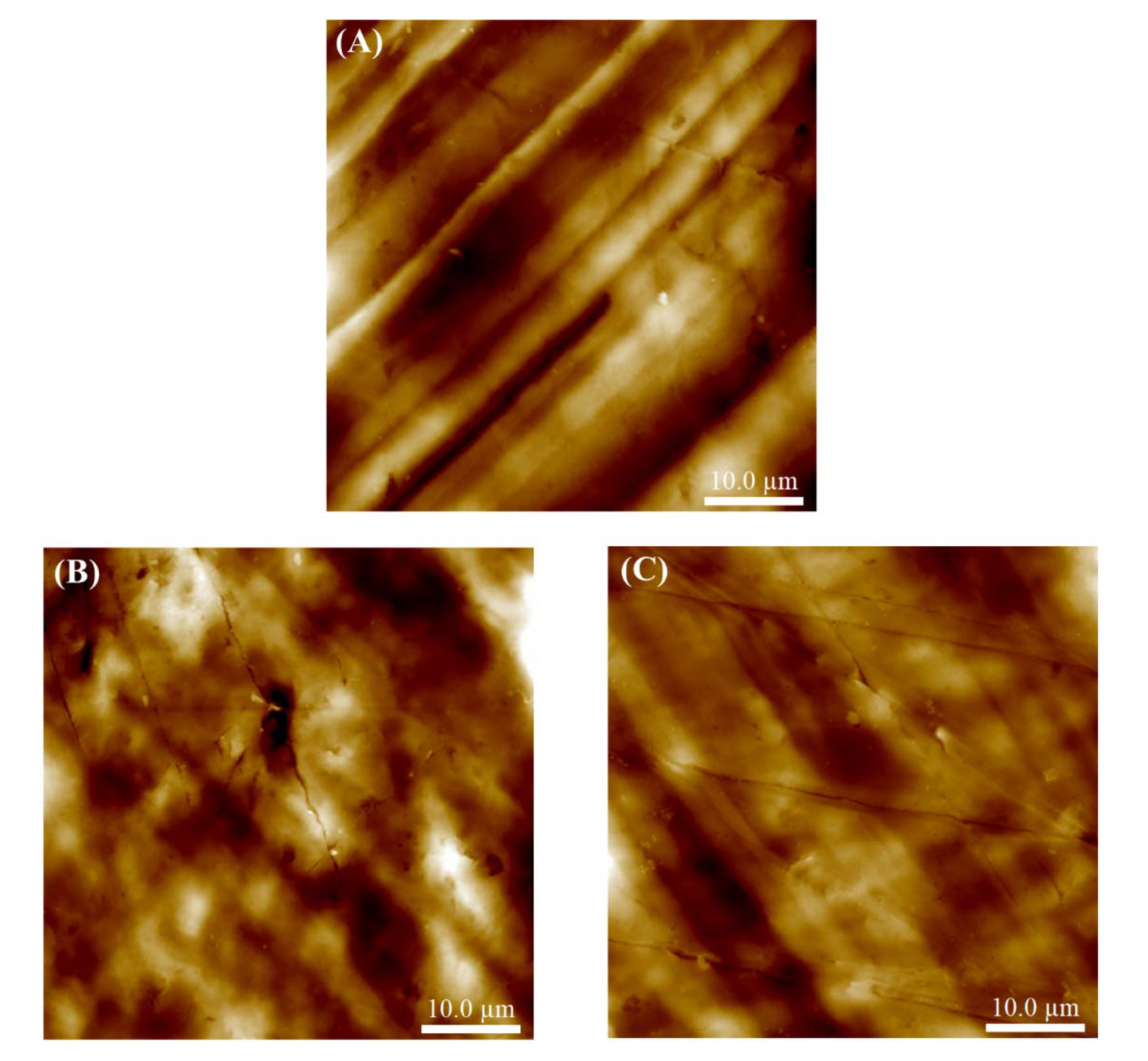
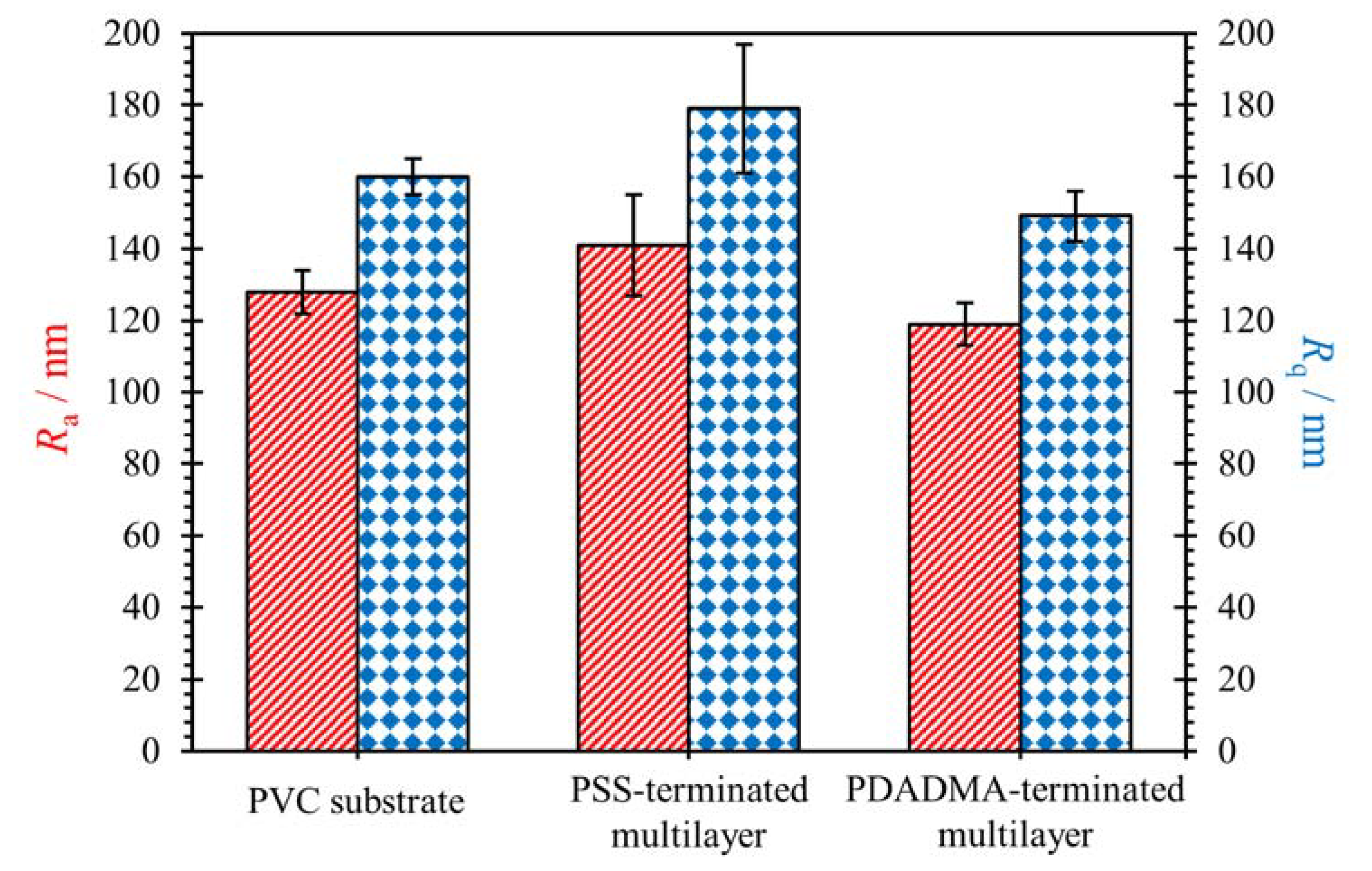

| Sample | Ra/nm | Rq/nm |
|---|---|---|
| PVC substrate | 128 ± 6 | 160 ± 5 |
| PVC substrate coated with PSS-terminated multilayer | 141 ± 14 | 179 ± 18 |
| PVC substrate coated with PDADMA-terminated multilayer | 119 ± 6 | 149 ± 7 |
| Sample | (ζ + SE)/mV |
|---|---|
| PVC substrate | +15.84 ± 2.0 |
| PVC substrate coated with PSS-terminated multilayer | −63.25 ± 0.35 |
| PVC substrate coated with PDADMA-terminated multilayer | +4.19 ± 0.25 |
| PVC Substrate | PVC Substrate Coated with PDADMA-Terminated Multilayer | PVC Substrate Coated with PSS-Terminated Multilayer | |
|---|---|---|---|
| Number of adhered bacteria per 1700 μm2 | Biofilm formation | 50 ± 12 | 31 ± 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohinc, K.; Kukić, L.; Štukelj, R.; Zore, A.; Abram, A.; Klačić, T.; Kovačević, D. Bacterial Adhesion Capacity of Uropathogenic Escherichia coli to Polyelectrolyte Multilayer Coated Urinary Catheter Surface. Coatings 2021, 11, 630. https://doi.org/10.3390/coatings11060630
Bohinc K, Kukić L, Štukelj R, Zore A, Abram A, Klačić T, Kovačević D. Bacterial Adhesion Capacity of Uropathogenic Escherichia coli to Polyelectrolyte Multilayer Coated Urinary Catheter Surface. Coatings. 2021; 11(6):630. https://doi.org/10.3390/coatings11060630
Chicago/Turabian StyleBohinc, Klemen, Lora Kukić, Roman Štukelj, Anamarija Zore, Anže Abram, Tin Klačić, and Davor Kovačević. 2021. "Bacterial Adhesion Capacity of Uropathogenic Escherichia coli to Polyelectrolyte Multilayer Coated Urinary Catheter Surface" Coatings 11, no. 6: 630. https://doi.org/10.3390/coatings11060630
APA StyleBohinc, K., Kukić, L., Štukelj, R., Zore, A., Abram, A., Klačić, T., & Kovačević, D. (2021). Bacterial Adhesion Capacity of Uropathogenic Escherichia coli to Polyelectrolyte Multilayer Coated Urinary Catheter Surface. Coatings, 11(6), 630. https://doi.org/10.3390/coatings11060630







