Corrosion Resistance of AISI 304 Stainless Steel Modified Both Femto- and Nanosecond Lasers
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
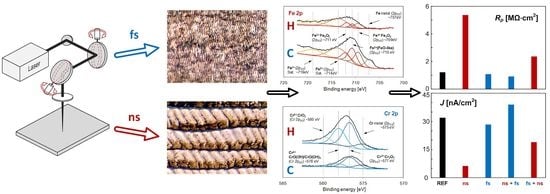
2.2. Laser Treatment
- Reference—hereinafter referred to as REF,
- irradiated using only nanosecond laser—hereinafter referred to as ‘ns’,
- irradiated using only femtosecond laser—hereinafter referred to as ‘fs’,
- irradiated using femtosecond and then nanosecond laser—hereinafter referred to as ‘fs + ns’,
- irradiated using nanosecond and then femtosecond laser—hereinafter referred to as ‘ns + fs’.
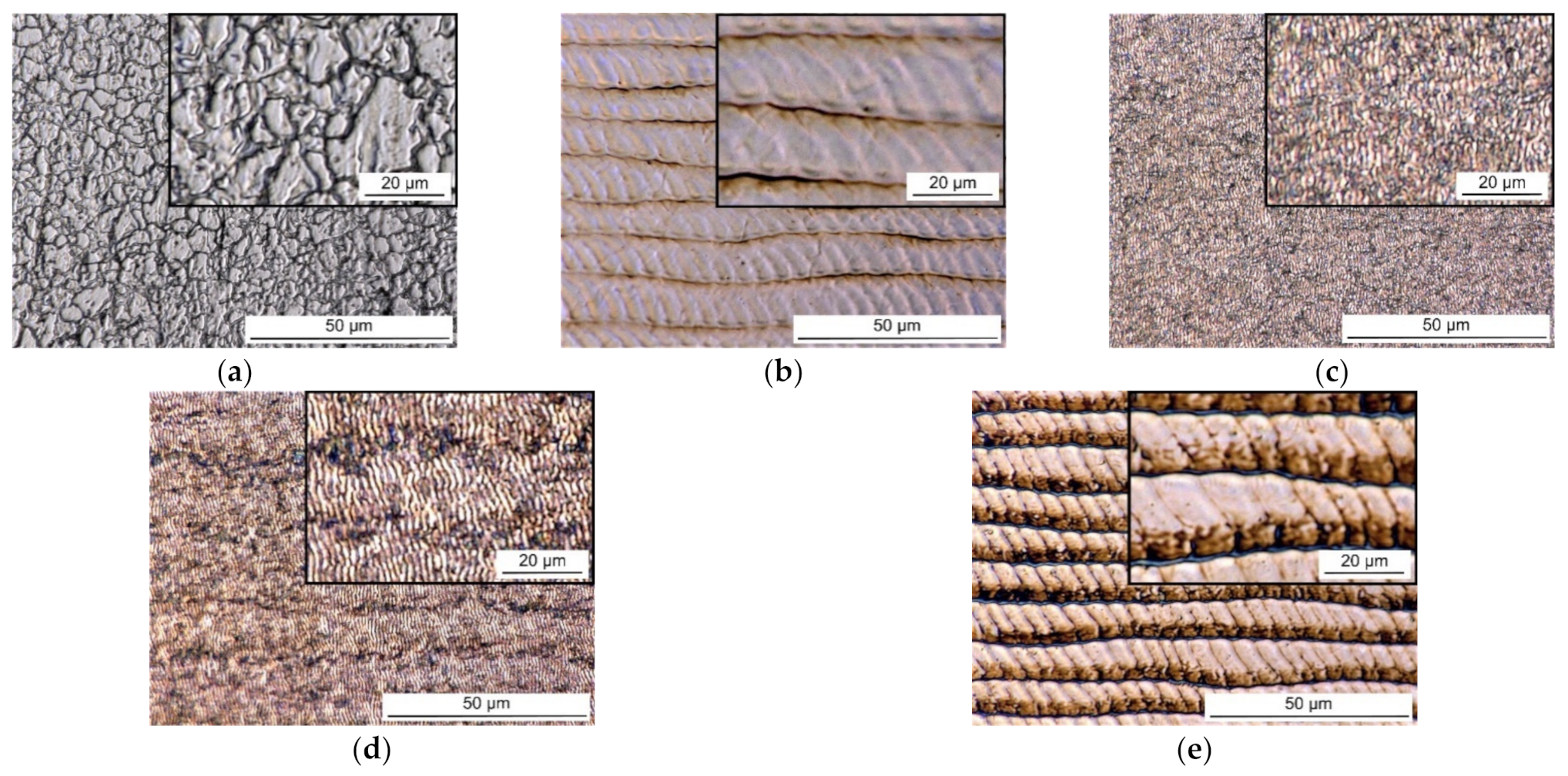
2.3. The Morphology and Surface Roughness of the Samples
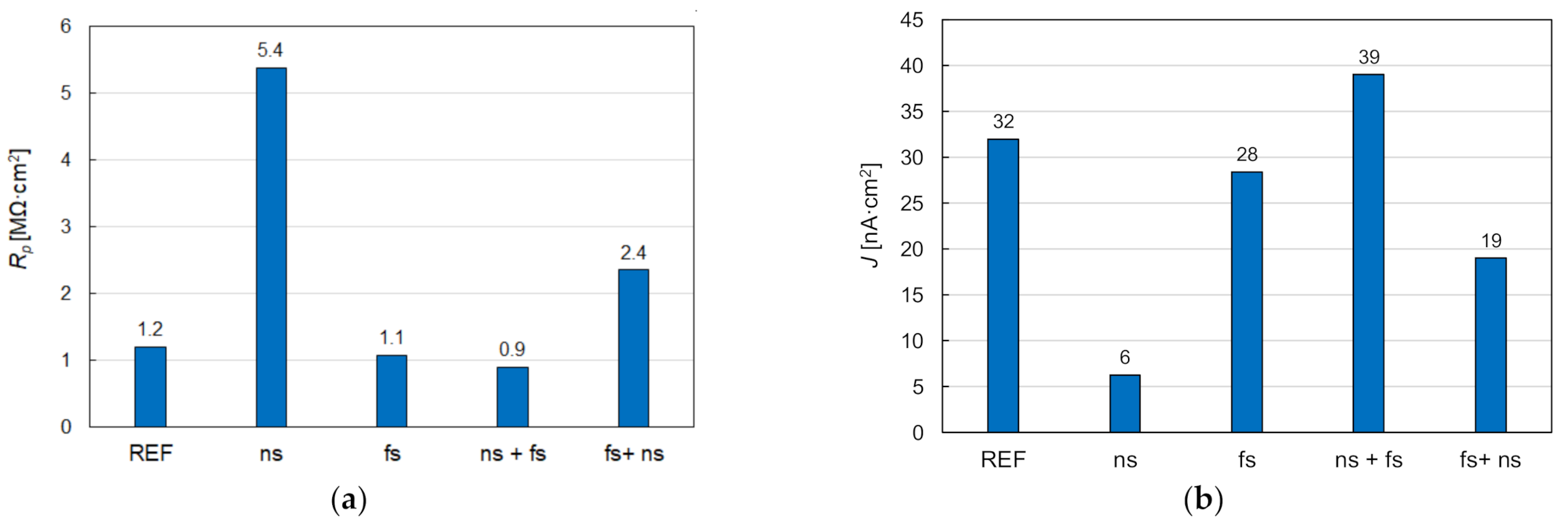
2.4. Electrochemical Corrosion Tests
2.5. Chemical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veiko, V.; Odintsova, G.; Ageev, E.; Karlagina, Y.; Loginov, A.; Skuratova, A.; Gorbunova, E. Controlled oxide films formation by nanosecond laser pulses for color marking. Opt. Express 2014, 22, 24342. [Google Scholar] [CrossRef]
- Pacquentin, W.; Caron, N.; Oltra, R. Nanosecond laser surface modification of AISI 304L stainless steel: Influence the beam overlap on pitting corrosion resistance. Appl. Surf. Sci. 2014, 288, 34–39. [Google Scholar] [CrossRef]
- Łȩcka, K.M.; Antończak, A.J.; Kowalewski, P.; Trzcinski, M. Wear resistance of laser-induced annealing of AISI 316 (EN 1.4401) stainless steel. Laser Phys. 2018, 28, 096005. [Google Scholar] [CrossRef]
- Khalfallah, I.Y.; Rahoma, M.N.; Abboud, J.H.; Benyounis, K.Y. Microstructure and corrosion behavior of austenitic stainless steel treated with laser. Opt. Laser Technol. 2011, 43, 806–813. [Google Scholar] [CrossRef]
- Bonse, J.; Hohm, S.; Kirner, S.V.; Rosenfeld, A.; Kruger, J. Laser-induced periodic surface structures—A scientific evergreen. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 9000615. [Google Scholar] [CrossRef]
- Valette, S.; Steyer, P.; Richard, L.; Forest, B.; Donnet, C.; Audouard, E. Influence of femtosecond laser marking on the corrosion resistance of stainless steels. Appl. Surf. Sci. 2006, 252, 4696–4701. [Google Scholar] [CrossRef]
- Bonse, J.; Koter, R.; Hartelt, M.; Spaltmann, D.; Pantzien, S.; Hohm, S.; Rosenfeld, A.; Kruger, J. Femtosecond laser-induced periodic surface structures on steel and titanium alloy for tribological applications. Appl. Phys. A Mater. Sci. Process. 2014, 117, 103–110. [Google Scholar] [CrossRef]
- Dzienny, P.; Stępak, B.; Budnicki, A.; Antończak, A.J. The influence of laser pulse duration on periodic surface structures on stainless steel. J. Laser Micro. Nanoeng. 2020, 15, 4–7. [Google Scholar]
- Łęcka, K.M.; Antończak, A.J.; Szubzda, B.; Wójcik, M.R.; Stępak, B.D.; Szymczyk, P.; Trzcinski, M.; Ozimek, M.; Abramski, K.M. Effects of laser-induced oxidation on the corrosion resistance of AISI 304 stainless steel. J. Laser Appl. 2016, 28, 032009. [Google Scholar] [CrossRef]
- Antończak, A.J.; Kocoń, D.; Nowak, M.; Kozioł, P.; Abramski, K.M. Laser-induced colour marking—Sensitivity scaling for a stainless steel. Appl. Surf. Sci. 2013, 264, 229–236. [Google Scholar] [CrossRef]
- Rawlins, J.W.; James, W.; Storey, R.F. The waterborne symposium. In Proceedings of the Thirty-Ninth Annual International Waterborne, High-Solids, and Powder Coatings Symposium, New Orleans, LA, USA, 13–17 February 2021; Volume 39. [Google Scholar]
- Alwan, A.S.; Khalid, E.A.; Jaddoa, A.A. Improvement the corrosion behavior and wear characteristics of AISI 304 stainless steel by using Nd:YAG laser surface treatment. J. Mech. Eng. Res. Dev. 2020, 43, 50–59. [Google Scholar]
- Ruzankina, J.S.; Vasiliev, O.S. Study on possibility for the improvement of corrosion resistance of metals using laser-formed oxide surface structure. J. Phys. Conf. Ser. 2016, 735, 012050. [Google Scholar] [CrossRef]
- Krawczyk, B.; Heine, B.; Engelberg, D.L. Performance optimization of cold rolled Type 316L stainless steel by sand blasting and surface linishing treatment. J. Mater. Eng. Perform. 2016, 25, 884–893. [Google Scholar] [CrossRef]
- Pieretti, E.F.; Pessine, E.J.; Correa, O.V.; de Rossi, W.; das Neves, M.D.M. Effect of laser parameters on the corrosion resistance of the ASTM F139 stainless steel. Int. J. Electrochem. Sci. 2015, 10, 1221–1232. [Google Scholar]
- Conde, A.; García, I.; de Damborenea, J.J. Pitting corrosion of 304 stainless steel after laser surface melting in argon and nitrogen atmospheres. Corros. Sci. 2001, 43, 817–828. [Google Scholar] [CrossRef]
- Tafel, J.; Naumann, K. Beziehungen zwischen Kathodenpotential und elektrolytischer Reduktionswirkung. Z. Phys. Chem. 1905, 50, 713–752. [Google Scholar] [CrossRef]
- Tafel, J. Über die Polarisation bei kathodischer Wasserstoffentwicklung. Z. Phys. Chem. 1905, 50, 641–712. [Google Scholar] [CrossRef]
- Abbass, M.K. Effect of Nd-YAG laser treatment on corrosion behavior of AISI316L stainless steel in artificial saliva solution. IOP Conf. Ser. Mater. Sci. Eng. 2019, 518, 032006. [Google Scholar] [CrossRef]
- Westin, E.M.; Laakso, P.; Oliver, J.; Penttilä, R. Effect of laser color marking on the corrosion performance of stainless steel. Icaleo 2009, M1009, 1245–1250. [Google Scholar]
- Szubzda, B.; Antończak, A.; Kozioł, P.; Łazarek, Ł.; Stępak, B.; Łęcka, K.; Szmaja, A.; Ozimek, M. Corrosion resistance of the AISI 304, 316 and 321 stainless steel surfaces modified by laser. IOP Conf. Ser. Mater. Sci. Eng. 2016, 113, 012017. [Google Scholar] [CrossRef]
- Huang, J.; Li, Z.; Liaw, B.Y.; Zhang, J. Graphical analysis of electrochemical impedance spectroscopy data in Bode and Nyquist representations. J. Power Sources 2016, 309, 82–98. [Google Scholar] [CrossRef]
- Lv, J.; Luo, H. Comparison of corrosion behavior between coarse grained and nano/ultrafine grained 304 stainless steel by EWF, XPS and EIS. J. Nucl. Mater. 2014, 452, 469–473. [Google Scholar] [CrossRef]
- Pieretti, E.F.; Costa, I. Surface characterisation of ASTM F139 stainless steel marked by laser and mechanical techniques. Electrochim. Acta 2012, 114, 838–843. [Google Scholar] [CrossRef]
- Jinlong, L.; Tongxiang, L.; Chen, W. Surface enriched molybdenum enhancing the corrosion resistance of 316L stainless steel. Mater. Lett. 2016, 171, 38–41. [Google Scholar] [CrossRef]
- Trethewey, K.R.; Paton, M. Electrochemical impedance behaviour of type 304L stainless steel under tensile loading. Mater. Lett. 2004, 58, 3381–3384. [Google Scholar] [CrossRef]
- Bret, C. Electrochemistry-Principles, Methods and Applications; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Gabrielli, C. Identification of Electrochemical Processes by Frequency Response; Schlumberger Instruments: Farnborough, UK, 1984; p. 15. [Google Scholar]
- Bösing, I.; Cramer, L.; Steinbacher, M.; Zoch, H.W.; Thöming, J.; Baune, M. Influence of heat treatment on the microstructure and corrosion resistance of martensitic stainless steel. AIP Adv. 2019, 9, 065317. [Google Scholar] [CrossRef]
- Ribeiro, D.V.; Abrantes, J.C.C. Application of electrochemical impedance spectroscopy (EIS) to monitor the corrosion of reinforced concrete: A new approach. Constr. Build. Mater. 2016, 111, 98–104. [Google Scholar] [CrossRef]
- Jafari, H.; Idris, M.H.; Ourdjini, A.; Rahimi, H.; Ghobadian, B. EIS study of corrosion behavior of metallic materials in ethanol blended gasoline containing water as a contaminant. Fuel 2011, 90, 1181–1187. [Google Scholar] [CrossRef]
- Skale, S.; Doleček, V.; Slemnik, M. Substitution of the constant phase element by Warburg impedance for protective coatings. Corros. Sci. 2007, 49, 1045–1055. [Google Scholar] [CrossRef]
- Verdian, M.M.; Raeissi, K.; Salehi, M. Electrochemical impedance spectroscopy of HVOF-sprayed NiTi intermetallic coatings deposited on AISI 1045 steel. J. Alloys Compd. 2010, 507, 42–46. [Google Scholar] [CrossRef]
- Li, R.; Li, Z.; Zhu, Y.; Qi, K. Structure and corrosion resistance properties of Ni-Fe-B-Si-Nb amorphous composite coatings fabricated by laser processing. J. Alloys Compd. 2013, 580, 327–331. [Google Scholar] [CrossRef]
- Li, Z.L.; Zheng, H.Y.; Teh, K.M.; Liu, Y.C.; Lim, G.C.; Seng, H.L.; Yakovlev, N.L. Analysis of oxide formation induced by UV laser coloration of stainless steel. Appl. Surf. Sci. 2009, 256, 1582–1588. [Google Scholar] [CrossRef]
- Allen, G.C.; Dyke, J.M.; Harris, S.J.; Morris, A. A surface study of the oxidation of type 304L stainless steel at 600 K in air. Oxid. Met. 1988, 29, 391–408. [Google Scholar] [CrossRef]



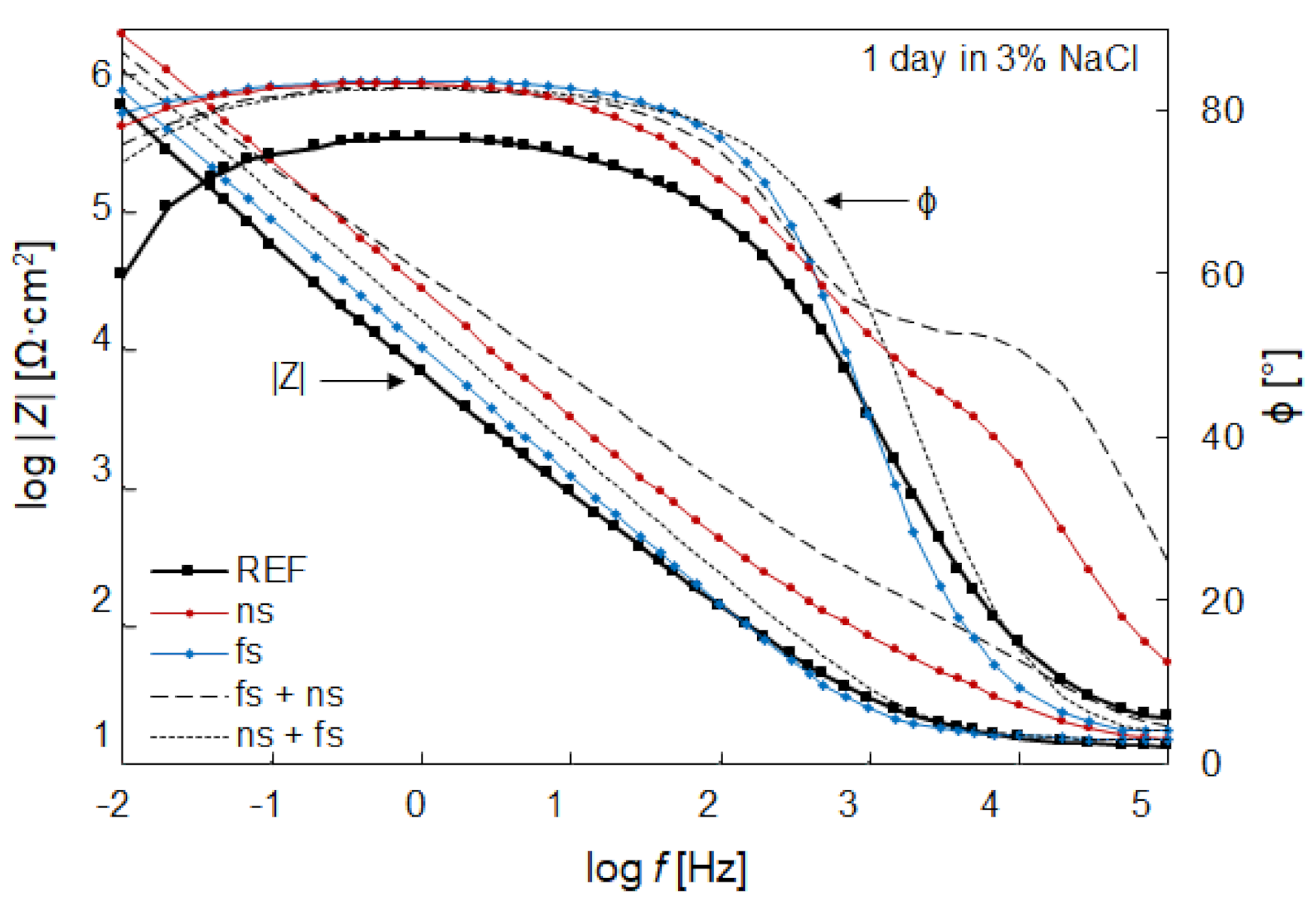

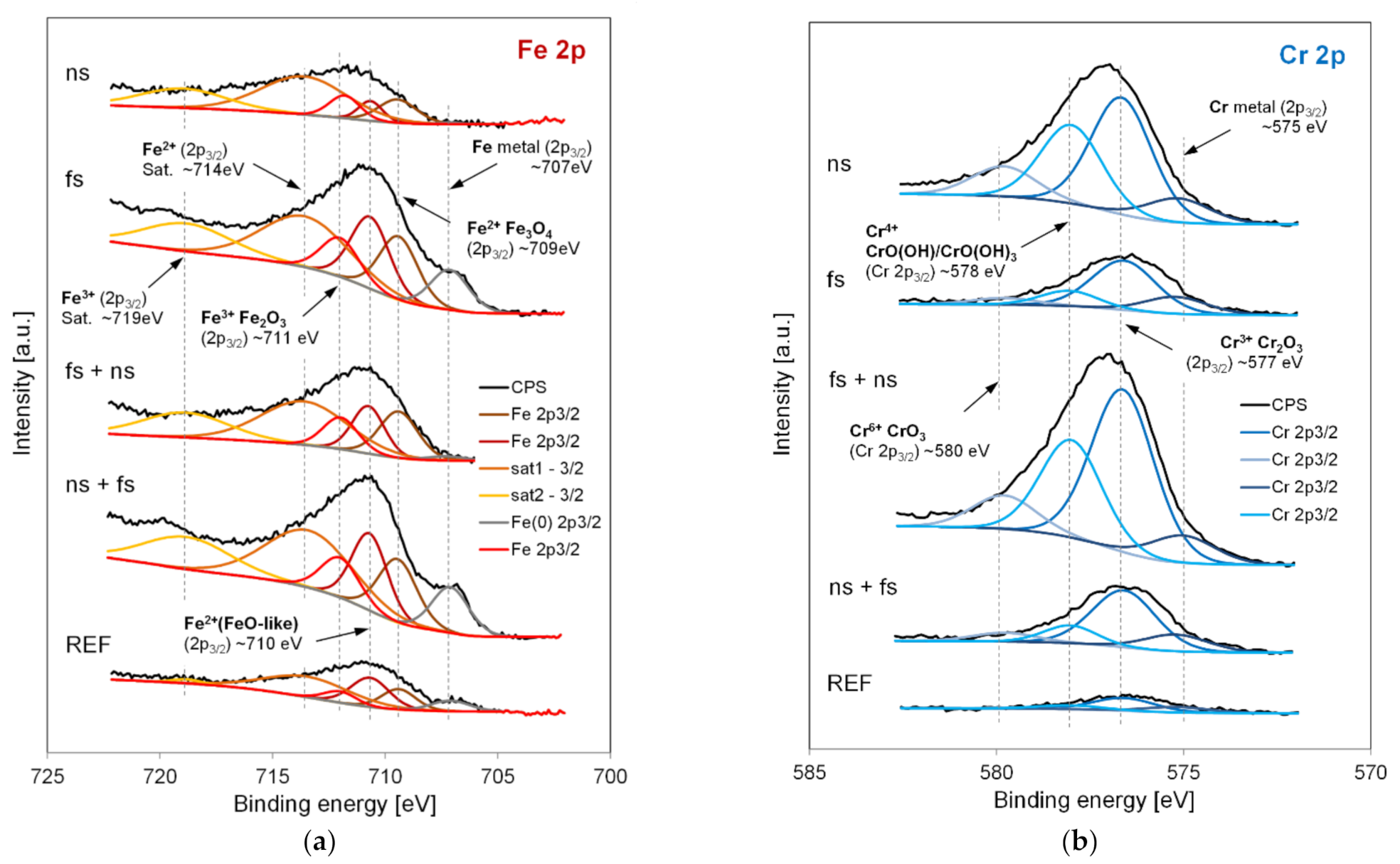

| Sample | Model | Rs (Ω∙cm2) | Y1 (μS/cm2) | N1 | R1 (MΩ∙cm2) | Y2 (μS/cm2) | N2 | R2 (Ω∙cm2) | Wo (kS/cm2) |
|---|---|---|---|---|---|---|---|---|---|
| REF | 1 | 0.47 | 0.97 | 0.85 | 24.5 | - | - | - | - |
| ns | 2 | 0.54 | 1.50 | 0.90 | - | 1.48 | 0.92 | 25.3 | 0.883 |
| fs | 1 | 0.15 | 0.17 | 0.93 | 17.3 | - | - | - | - |
| ns + fs | 1 | 1.27 | 0.09 | 0.91 | 5.19 | - | - | - | - |
| fs + ns | 2 | 0.25 | 0.16 | 0.74 | - | 0.15 | 0.92 | 0.003 | 2.61 |
| Sample | % Fe | % Mn | % Cr | % Si | % C | % O |
|---|---|---|---|---|---|---|
| REF | 2.60 | 0.10 | 1.23 | 0.00 | 64.73 | 31.34 |
| ns | 2.05 | 4.25 | 13.35 | 7.86 | 12.57 | 59.92 |
| fs | 10.37 | 2.86 | 5.99 | 1.41 | 27.76 | 51.61 |
| fs + ns | 4.21 | 3.68 | 15.88 | 5.03 | 11.96 | 59.24 |
| ns + fs | 9.82 | 3.17 | 6.06 | 1.52 | 25.58 | 53.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mroczkowska, K.M.; Dzienny, P.; Budnicki, A.; Antończak, A.J. Corrosion Resistance of AISI 304 Stainless Steel Modified Both Femto- and Nanosecond Lasers. Coatings 2021, 11, 592. https://doi.org/10.3390/coatings11050592
Mroczkowska KM, Dzienny P, Budnicki A, Antończak AJ. Corrosion Resistance of AISI 304 Stainless Steel Modified Both Femto- and Nanosecond Lasers. Coatings. 2021; 11(5):592. https://doi.org/10.3390/coatings11050592
Chicago/Turabian StyleMroczkowska, Katarzyna M., Paulina Dzienny, Aleksander Budnicki, and Arkadiusz J. Antończak. 2021. "Corrosion Resistance of AISI 304 Stainless Steel Modified Both Femto- and Nanosecond Lasers" Coatings 11, no. 5: 592. https://doi.org/10.3390/coatings11050592
APA StyleMroczkowska, K. M., Dzienny, P., Budnicki, A., & Antończak, A. J. (2021). Corrosion Resistance of AISI 304 Stainless Steel Modified Both Femto- and Nanosecond Lasers. Coatings, 11(5), 592. https://doi.org/10.3390/coatings11050592