Improved Corrosion Resistance of Magnesium Alloy AZ31 in Ringer Lactate by Bilayer Anodic Film/Beeswax–Colophony
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
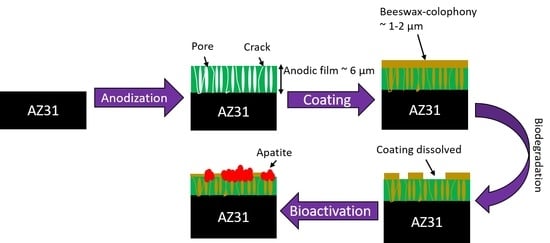
2.2. Anodization
2.3. Coating Preparation
2.4. Characterization
2.5. Electrochemical Tests
2.6. Weight Loss Test
3. Results and Discussion
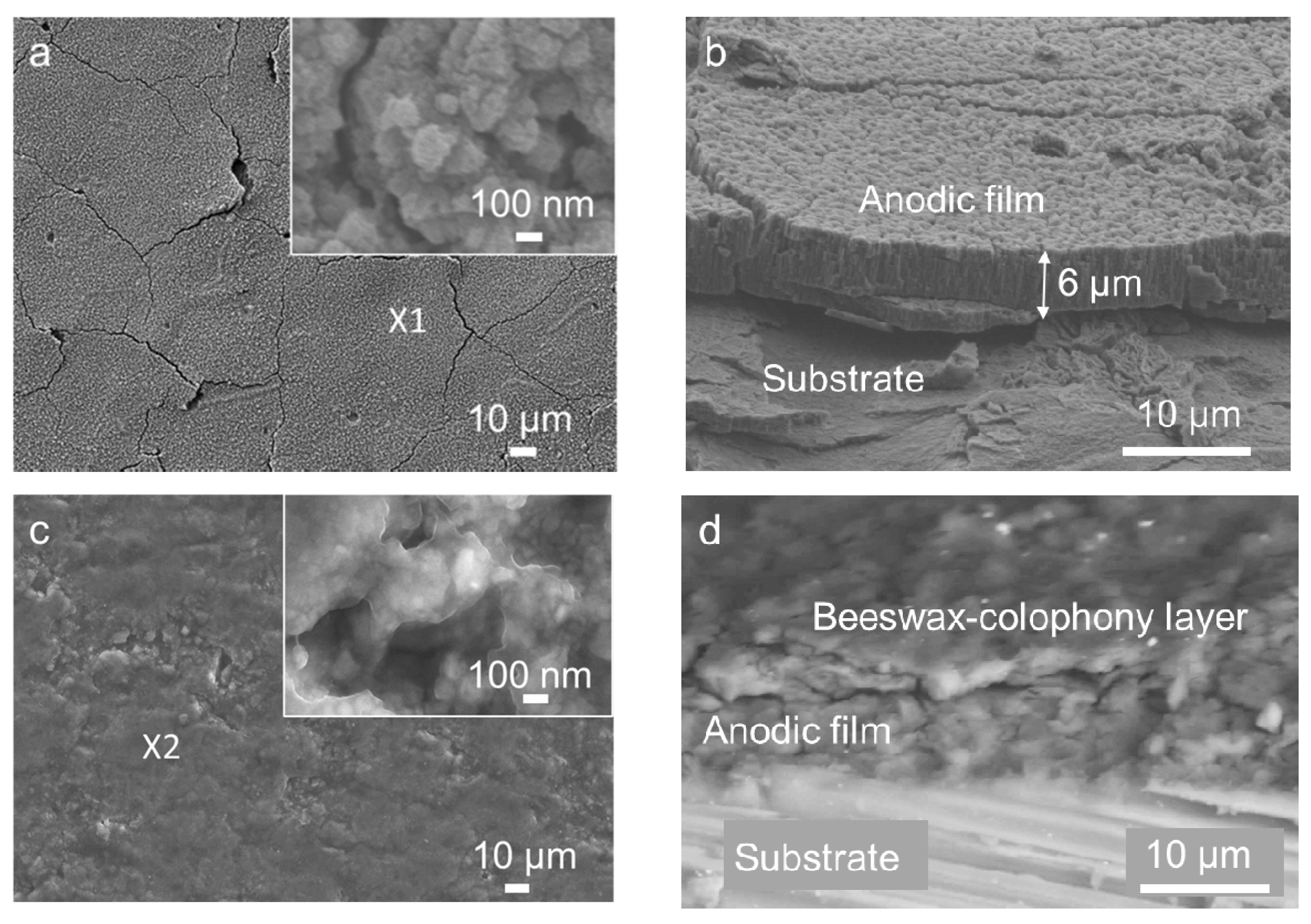
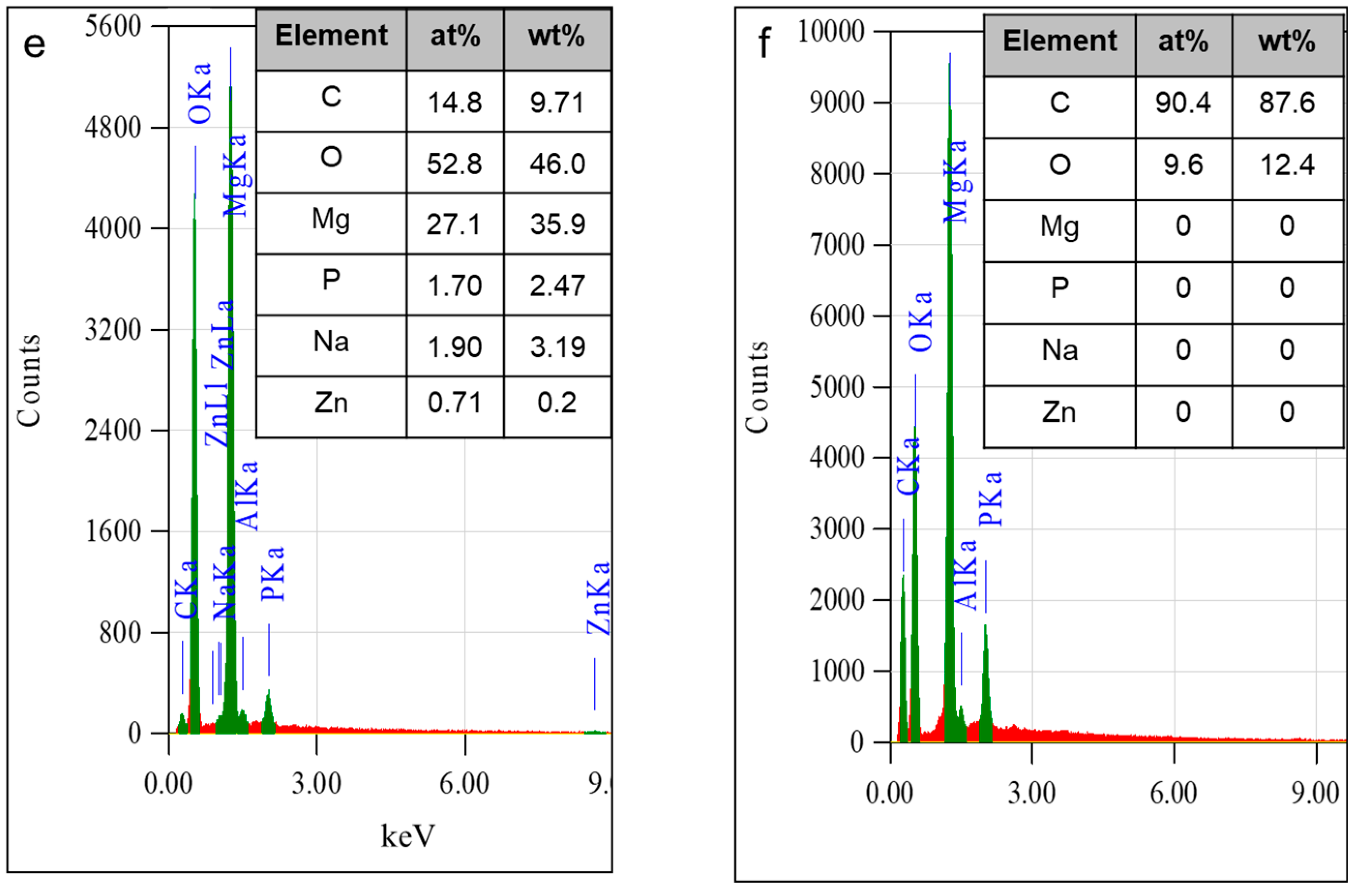
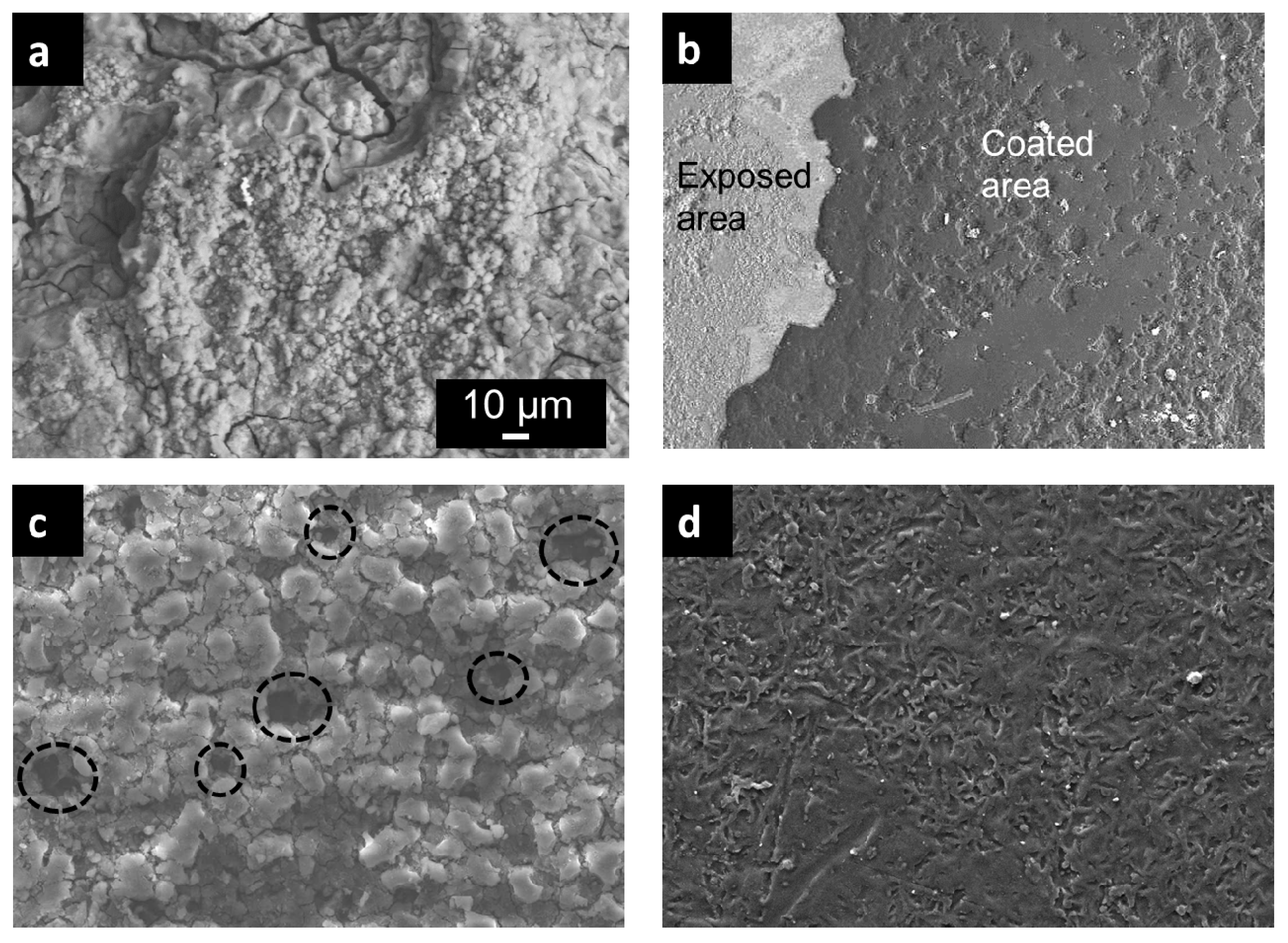
3.1. Bilayer Morphology and Composition
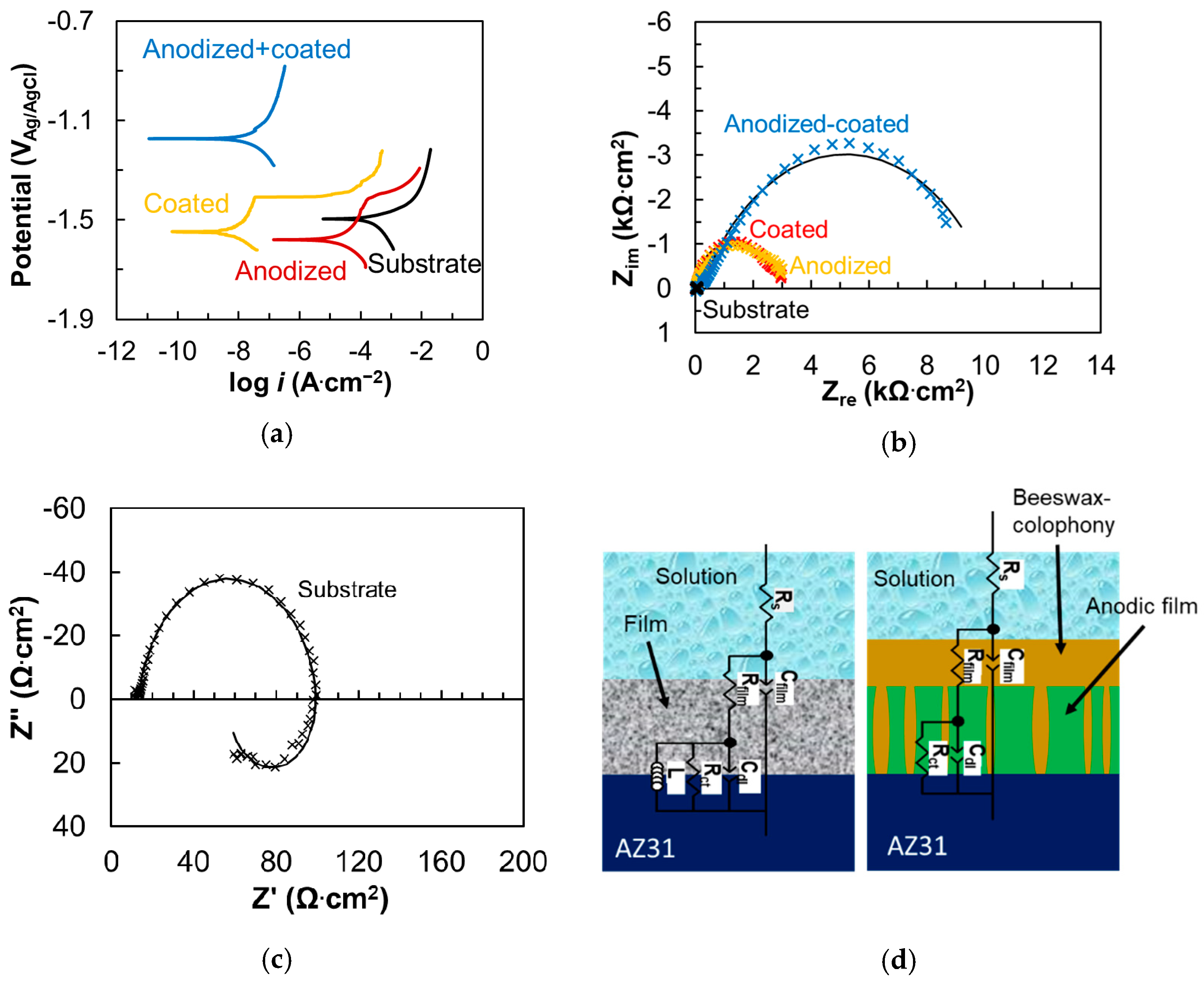
3.2. Electrochemical Corrosion
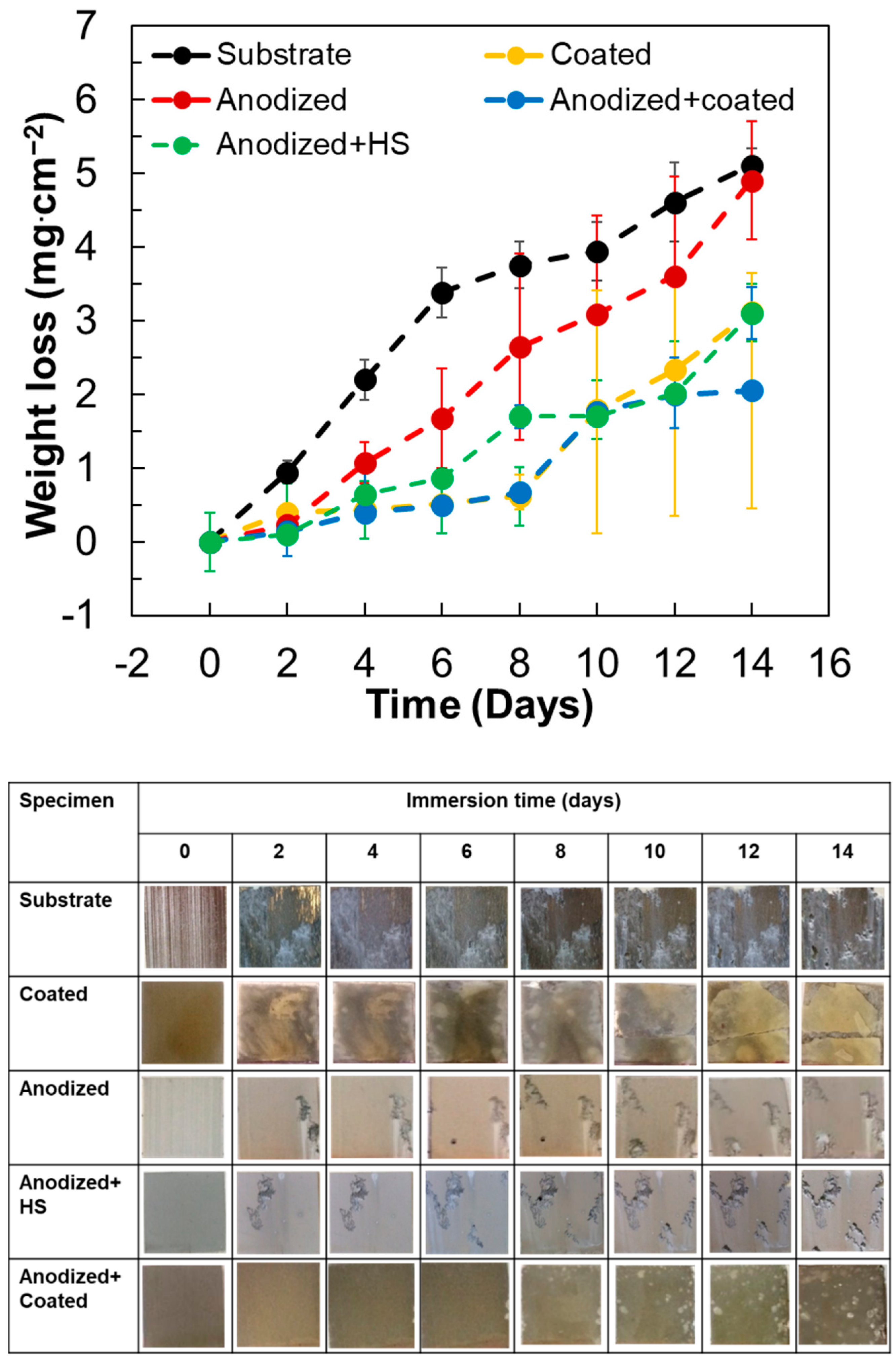
3.3. Weight Loss Behavior
4. Conclusions
- The electrochemical test showed that the bilayer, which consisted of anodic film and beeswax–colophony, enhanced the corrosion resistance of the AZ31 alloy substantially. The polarization test demonstrated four orders of magnitude lower current density and the EIS analysis revealed four orders of magnitude higher impedance of the bilayer coating than that of the substrate.
- The top organic coating acted as an excellent barrier for the metal surface as the underlying porous structure of the anodic film allowed strong anchorage of the beeswax–colophony layer. The anodic film itself was not able to inhibit the solution penetration leading to a localized type of corrosion, as revealed from the corrosion morphology. Similarly, direct application of the beeswax–colophony on the metal surface was not able to withstand undermining corrosion, which yielded to local coating detachment. The molten beeswax–colophony effectively blocked the pores and defects in the anodic film when applied as the overcoating, resulting in a remarkable improvement of corrosion resistance of the AZ31 alloy. The bilayer mostly remained attached on the surface except at a few localized blisters after 14 days exposure in Ringer lactate.
- Surface investigation and EDS analysis indicated the bilayer exhibited high apatite-forming ability, particularly the exposed anodic film at which the beeswax–colophony layer was peeled off. EDS detected a Ca–P compound with a Ca/P ratio of 1.68 on the exposed anodic film. The remarkable improvement of corrosion resistance and the high apatite-forming ability of the bilayer shows promise as a coating for biomedical implant application.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, Y.-K.; Jang, Y.-S.; Kim, S.-Y.; Lee, M.-H. Functions achieved by the hyaluronic acid derivatives coating and hydroxide film on bio-absorbed Mg. Appl. Surf. Sci. 2019, 473, 31–39. [Google Scholar] [CrossRef]
- Santos, L.R.; Marino, C.E.; Riegel-Vidotti, I.C. Silica/chitosan hybrid particles for smart release of the corrosion inhibitor benzotriazole. Eur. Polym. J. 2019, 115, 86–98. [Google Scholar] [CrossRef]
- Vahabi, H.; Rad, E.R.; Parpaite, T.; Langlois, V.; Saeb, M.R. Biodegradable polyester thin films and coatings in the line of fire: The time of polyhydroxyalkanoate (PHA)? Prog. Org. Coat. 2019, 133, 85–89. [Google Scholar] [CrossRef]
- Saji, V.S. Organic conversion coatings for magnesium and its alloys. J. Ind. Eng. Chem. 2019, 75, 20–37. [Google Scholar] [CrossRef]
- Battegazzore, D.; Frache, A.; Carosio, F. Sustainable and high performing biocomposites with chitosan/sepiolite layer-by-layer nanoengineered interphases. ACS Sustain. Chem. Eng. 2018, 6, 9601–9605. [Google Scholar] [CrossRef]
- Castro, Y.; Durán, A. Control of degradation rate of Mg alloys using silica sol–gel coatings for biodegradable implant materials. J. Sol-Gel Sci. Technol. 2018, 90, 198–208. [Google Scholar] [CrossRef]
- Chang, W.; Qu, B.; Liao, A.; Zhang, S.; Zhang, R.; Xiang, J. In vitro biocompatibility and antibacterial behavior of anodic coatings fabricated in an organic phosphate containing solution on Mg–1.0Ca alloys. Surf. Coat. Technol. 2016, 289, 75–84. [Google Scholar] [CrossRef]
- Wu, H.; Shi, Z.; Zhang, X.; Qasim, A.M.; Xiao, S.; Zhang, F.; Wu, Z.; Wu, G.; Ding, K.; Chu, P.K. Achieving an acid resistant surface on magnesium alloy via bio-inspired design. Appl. Surf. Sci. 2019, 478, 150–161. [Google Scholar] [CrossRef]
- Hong, L.; Shen, M.; Fang, J.; Wang, Y.; Bao, Z.; Bu, S.; Zhu, Y. Hyaluronic acid (HA)-based hydrogels for full-thickness wound repairing and skin regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 150. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Waksman, R.; Erbel, R.; Di Mario, C.; Bartunek, J.; de Bruyne, B.; Eberli, F.R.; Erne, P.; Haude, M.; Horrigan, M.; Ilsley, C.; et al. Early- and long-term intravascular ultrasound and angiographic findings after bioabsorbable magnesium stent implantation in human coronary arteries. JACC Cardiovasc. Interv. 2009, 2, 312–320. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg–Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Anawati; Asoh, H.; Ono, S. Enhanced uniformity of apatite coating on a PEO film formed on AZ31 Mg alloy by an alkali pretreatment. Surf. Coat. Technol. 2015, 272, 182–189. [Google Scholar] [CrossRef]
- Brady, M.P.; Fayek, M.; Leonard, D.N.; Meyer, H.M.; Thomson, J.K.; Anovitz, L.M.; Rother, G.; Song, G.-L.; Davis, B. Tracer Film Growth Study of the Corrosion of Magnesium Alloys AZ31B and ZE10A in 0.01% NaCl Solution. J. Electrochem. Soc. 2017, 164, C367–C375. [Google Scholar] [CrossRef]
- Srinivasan, P.B.; Liang, J.; Blawert, C.; Störmer, M.; Dietzel, W. Characterization of calcium containing plasma electrolytic oxidation coatings on AM50 magnesium alloy. Appl. Surf. Sci. 2010, 256, 4017–4022. [Google Scholar] [CrossRef]
- Gao, Y.; Yerokhin, A.; Matthews, A. Deposition and evaluation of duplex hydroxyapatite and plasma electrolytic oxidation coatings on magnesium. Surf. Coat. Technol. 2015, 269, 170–182. [Google Scholar] [CrossRef]
- Pekguleryuz, M.; Kainer, K.; Kaya, A. Fundamentals of Magnesium Alloy; Woodhead Publishing Ltd.: Cambridge, UK, 2013. [Google Scholar]
- Wang, C.; Yi, Z.; Sheng, Y.; Tian, L.; Qin, L.; Ngai, T.; Lin, W. Development of a novel biodegradable and anti-bacterial polyurethane coating for biomedical magnesium rods. Mater. Sci. Eng. C 2019, 99, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, J.; Wang, Y. Evaluation of cyto-toxicity and corrosion behavior of alkali-heat-treated magnesium in simulated body fluid. Surf. Coat. Technol. 2004, 185, 92–98. [Google Scholar] [CrossRef]
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Beeswax: A minireview of its antimicrobial activity and its application in medicine. Asian Pac. J. Trop. Med. 2016, 9, 839–843. [Google Scholar] [CrossRef]
- Sudmann, B.; Bang, G.; Sudmann, E. Histologically verified bone wax (beeswax) granuloma after median sternotomy in 17 of 18 autopsy cases. Pathology 2006, 38, 138–141. [Google Scholar] [CrossRef]
- Yadav, B.K.; Gidwani, B.; Vyas, A. Rosin: Recent advances and potential applications in novel drug delivery system. J. Bioact. Compat. Polym. 2015, 31, 111–126. [Google Scholar] [CrossRef]
- Gumelar, M.D.; Putri, N.A.; Anggaravidya, M.; Anawati, A. Corrosion behavior of biodegradable material AZ31 coated with beeswax-colophony resin. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; Volume 1964. [Google Scholar] [CrossRef]
- Fitriana, M.F.; Anawati, A. Remarkable improvement in corrosion resistance of anodized AZ31 alloy by sealing with beeswax-colophony resin. J. Phys. Conf. Ser. 2019, 1191, 012032. [Google Scholar] [CrossRef]
- Abdikheibari, S.; Parvizi, R.; Moayed, M.H.; Zebarjad, S.M.; Sajjadi, S.A. Beeswax-colophony blend: A novel green organic coating for protection of steel drinking water storage tanks. Metals 2015, 5, 1645–1664. [Google Scholar] [CrossRef]
- Song, G.-L.; Shi, Z. Corrosion mechanism and evaluation of anodized magnesium alloys. Corros. Sci. 2014, 85, 126–140. [Google Scholar] [CrossRef]
- Murakami, K.; Hino, M.; Nakai, K.; Kobayashi, S.; Saijo, A.; Kanadani, T. Mechanism of corrosion protection of anodized magnesium alloys. Mater. Trans. 2008, 49, 1057–1064. [Google Scholar] [CrossRef]
- Avedesian, M.M.; Baker, H. ASM Specialty Handbook: Magnesium and Magnesium Alloys; ASM International, 1999; Available online: https://books.google.co.id/books?id=0wFMfJg57YMC (accessed on 25 April 2017).
- Zong, Y.; Yuan, G.; Zhang, X.; Mao, L.; Niu, J.; Ding, W. Comparison of biodegradable behaviors of AZ31 and Mg–Nd–Zn–Zr alloys in Hank’s physiological solution. Mater. Sci. Eng. B 2012, 177, 395–401. [Google Scholar] [CrossRef]
- Song, G.L. Corrosion of magnesium (Mg) alloys: Concluding remarks. In Corrosion of Magnesium Alloys; Woodhead Publishing: Cambridge, UK, 2011; pp. 615–617. [Google Scholar] [CrossRef]
- Buchwald, R.; Breed, M.D.; Greenberg, A.R. The thermal properties of beeswaxes: Unexpected findings. J. Exp. Biol. 2008, 211, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Edwards, H.; Farwell, D.; Daffner, L. Fourier-transform Raman spectroscopic study of natural waxes and resins. I. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1996, 52, 1639–1648. [Google Scholar] [CrossRef]
- Atrens, A.; Liu, M.; Abidin, N.I.Z. Corrosion mechanism applicable to biodegradable magnesium implants. Mater. Sci. Eng. B 2011, 176, 1609–1636. [Google Scholar] [CrossRef]
- Anawati, A.; Asoh, H.; Ono, S. Effects of alloying element ca on the corrosion behavior and bioactivity of anodic films formed on AM60 Mg alloys. Materials 2016, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Echeverry-Rendon, M.; Duque, V.; Quintero, D.; Robledo, S.M.; Harmsen, M.C.; Echeverria, F. Improved corrosion resistance of commercially pure magnesium after its modification by plasma electrolytic oxidation with organic additives. J. Biomater. Appl. 2018, 33, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Leng, Y. Theoretical analysis of calcium phosphate precipitation in simulated body fluid. Biomaterials 2005, 26, 1097–1108. [Google Scholar] [CrossRef] [PubMed]

| Compound | Concentration (g/L) |
|---|---|
| NaCl | 6.0 |
| KCl | 0.3 |
| CaCl2 | 0.2 |
| NaC3H5O3 | 3.1 |
| Specimen | Ecorr (VAg/AgCl) | Icorr (A·cm−2) |
|---|---|---|
| Substrate | −1.50 | 5.72 × 10−4 |
| Anodized | −1.58 | 3.40 × 10−5 |
| Coated | −1.55 | 2.54 × 10−8 |
| Anodized + Coated | −1.17 | 3.19 × 10−8 |
| Specimen | Rs (Ω cm2) | Rfilm (Ω cm2) | Cfilm (Ω−1 Sn cm−2) | n1 | Cdl (Ω−1 Sn cm−2) | n2 | Rct (Ω cm2) | L1 (H) |
|---|---|---|---|---|---|---|---|---|
| Substrate | 13.00 | 43.33 | 4.31 × 10−5 | 0.91 | 6.63 × 10−6 | 0.99 | 43.85 | 17.67 |
| Anodized | 18.74 | 2900 | 1.94 × 10−5 | 0.75 | 0.86 × 10−6 | 0.99 | 1450 | 10.97 |
| Coated | 12.28 | 4809 | 1.39 × 10−5 | 0.76 | 0.37 × 10−6 | 0.80 | 5328 | - |
| Anodized + coated | 20.00 | 995,000 | 0.02 × 10−5 | 0.70 | 0.09 × 10−6 | 0.90 | 4001 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anawati, A.; Fitriana, M.F.; Gumelar, M.D. Improved Corrosion Resistance of Magnesium Alloy AZ31 in Ringer Lactate by Bilayer Anodic Film/Beeswax–Colophony. Coatings 2021, 11, 564. https://doi.org/10.3390/coatings11050564
Anawati A, Fitriana MF, Gumelar MD. Improved Corrosion Resistance of Magnesium Alloy AZ31 in Ringer Lactate by Bilayer Anodic Film/Beeswax–Colophony. Coatings. 2021; 11(5):564. https://doi.org/10.3390/coatings11050564
Chicago/Turabian StyleAnawati, Anawati, Medio Febby Fitriana, and Muhammad Dikdik Gumelar. 2021. "Improved Corrosion Resistance of Magnesium Alloy AZ31 in Ringer Lactate by Bilayer Anodic Film/Beeswax–Colophony" Coatings 11, no. 5: 564. https://doi.org/10.3390/coatings11050564
APA StyleAnawati, A., Fitriana, M. F., & Gumelar, M. D. (2021). Improved Corrosion Resistance of Magnesium Alloy AZ31 in Ringer Lactate by Bilayer Anodic Film/Beeswax–Colophony. Coatings, 11(5), 564. https://doi.org/10.3390/coatings11050564