Abstract
In this work, biomass obtained from seeds (S-MO) and leaves (L-MO) of the Moringa oleifera plant were used as low-cost biosorbents to remove the Pb(II), Cd(II), Co(II), and Ni(II) from aqueous solutions. The biosorption of the heavy metal ions was done using the batch technique. The effects of contact time (30–1440 min), biosorbent dosage (10–50 g/L) (0.1–0.5 g), and initial concentration of metals (10–500 mg/L) on the sorption capacity of metal ions were investigated. The S-MO and L-MO samples’ characterization was performed using pHpzc, and Fourier Transform Infrared Spectroscopy (FTIR). It was found that the pHpzc was notably different between the seeds and leave-derived biosorbents. The removal process’s experimental kinetic data for both S-MO and L-MO were best described by the pseudo-second-order model for all metal ions, with R2 above 0.997 in all cases. Langmuir and Freundlich’s models were also used to analyze the isotherms parameters. Based on the Langmuir model, the maximum sorption capacities (Qm) for L-MO were found as follows: L-MO-Pb > L-MO-Cd > L-MO-Co ≥ L-MO-Ni, and for S-MO, the values of Qm values presented the following order: S-MO-Pb > S-MO-Co > S-MO-Cd > S-MO-Ni.
1. Introduction
Heavy metals, like Pb, Cd, Co, and Ni, are considered a primary source of contamination in aquatic ecosystems and a major environmental problem due to their elevated toxicity and their persistence in the natural waters. Beyond even a low concentration, their presence in aquatic and soil matrixes can cause undesirable effects on human and animal health. Therefore, the United States Environmental Protection Agency (US EPA) established that the maximum concentration of the aforementioned heavy metal ions in wastewater must be 1.7 μg/L for Co(II), 5 μg/L for Cd(II), 15 μg/L for Pb(II), and 20 μg/L for Ni(II) [1,2]. However, concentration values up to 200 μg/L, 20 μg/L, 900 μg/L, and 670 μg/L of Pb(II), Cd(II), Co(II), and Ni(II), respectively, were reported in different effluents due to the uncontrolled discharge of these pollutants in the wastewater [1,2].
The main sources of heavy metal contamination are mining, textile manufacturing, the cement industry, conservation of the wood, dyes, tannery, steel production, energy production, photographic material, corrosive paints, and water-cooling, among others [3,4]. Therefore, the adequate treatment of these industrial effluents contaminated with these substances has become a significant environmental concern by the scientific community.
Numerous chemical or physicochemical techniques such as advanced oxidation, membrane technologies, ozonation, coagulation/flocculation, and electrochemical treatment have been explored and used for heavy metals’ removal from wastewater to overcome this challenge [5,6]. However, some disadvantages of these techniques are the high cost and inefficiency, especially when the metal concentration is very low. Additionally, the formation of sludge and the production of a large volume of solid wastes produced during the processes are additional problems that restrict the application of the technologies as mentioned earlier in real-life [4,7,8].
Biosorption has recently become an alternative and prosperous remediation technique that can remove organic and inorganic substances from waste streams and effluents. A key advantage is the potential of utilizing a plethora of biological materials/media, chiefly bacteria, algae, fungi, and inactive waste biomass [9,10]. The biosorbent materials showed high efficiency for metal removal since they can contain a wide range of high-density surface functional groups, including carboxyl, hydroxyl, carbonyl, phosphate, sulfhydryl, phenolic, and amidic. These surface functionalities promote the metal ions removal by direct binding on the surface of the biosorbent [9,10,11]. Moreover, it is crucial to consider that the low cost and the immense abundance of biosorbent materials and their easy operational wastewater treatment make biosorption an exciting technique for heavy metals removal [12,13].
More research is focused on the application of plant-derived biomass for heavy metal removal [12,14]. Recently, different types of biomass have been used as adsorbents for the removal Pb(II), Cd(II), Co(II) and Ni(II) from aqueous solutions. Among these materials, we can name Prosopis juliflora [15], Pinus eldarica [16], Strychnos potatorum L [15], Polyalthia longifolia seeds [14], Posidonia oceanica [17], Diplotaxis harra [18], Glebionis coronaria L [18], Cassia fistula [19], Pinus eldarica [16], Ulva lactuca [20]. The effectivity of these plants’ biomass on the removal of heavy metals is attributed to the biochemical constitution of their cell wall which contains the amino, carboxyl and hydroxyl functional groups that have a significant role in the adsorption process [20]. This approach has an additional advantage compared with the utilization of other types of biomaterials, such as bacteria, since it does not need a previous pre-treatment, which can further save operational costs, while the production of side wastes is minimal [21,22].
The Moringa oilefera (MO) plant has received much attention within the scientific community due to its impressive medicinal properties and environmental applications [23,24]. This plant is native to South Asia and nowadays is cultivated in many parts of the world since it grows in arid, tropical, and semi-tropical areas [25]. The different parts of MO, including leaves, seeds, flowers, and fruits, contain a great variety of chemical compounds responsible for this species’ properties [25].
Previous studies indicated that natural or modified MO seeds are efficient for removing toxic metallic ions from water effluents [26]. The removal efficiency against Cu, Pb, Cd, Zn, As(V) and As(III) ions from aqueous media by natural MO seeds reached values of up to 90%, 80%, 60%, 50%, 85%, and 60%, respectively [27,28]. Bhatti et al. reported the adsorption of Zn(II) metallic ions from aqueous solutions using raw and pretreated MO seeds, with the maximum reported uptake of Zn(II) ions by MO treated by NaOH to reach 45.76 mg/g [29]. Other parts of the Moringa plant such as pods (MO-pods) and bark (MO-barks) were used for the removal of Cu(II), Ni(II), Cr(III), Zn(II), and Ni(II) ions from the aqueous system [30,31]. The results show that for MO-pods, the removal percentages for Cr, Cu, and Ni ions were 91%, 90%, and 60%, respectively. This biosorbent did not work well for the removal of Zn ions. Ni ions’ maximum biosorption capacity by MO-barks from aqueous solutions (pH 6.0) was 30.38 mg g−1.
It can be concluded that the parts of MO biomass have different adsorption capacity in removing heavy metals from aqueous solutions considering all the aforementioned. Therefore, this work has used the seed and leaves of Moringa oleifera to describe and compare their removal capacity for Pb(II), Cd(II), Co(II), and Ni(II) from aqueous solutions. The effects of contact time, the dosage of biosorbent, and heavy metal ions concentration were also investigated.
2. Materials and Methods
2.1. Biosorbents
The seeds and leaves of Moringa Oilefera (named S-MO and L-MO, respectively) were collected from Ciudad del Carmen, Campeche State, Mexico. Seeds shells were removed manually. The S-MO and L-MO samples were washed by deionized water for 5 min to remove any suspended materials on their surface and dried in an oven at 70 °C for 72 h. The dried samples were ground using a domestic blender and sieved through a 200 µm stainless steel sieve. The biosorbent samples were stored in glass bottles for further studies.
2.2. Preparation of Metallic Ions Solutions
The stock solutions of Pb(II), Cd(II), Co(II), and Ni(II) of 1000 mg/L concentration were prepared by dissolving the appropriate mass of PbCl2 (98%, Merck, Merck, Germany), CdCl2·2.5H2O (98%, Sigma-Aldrich, St. Louis, MO, USA), CoCl2·6H2O (99.7%, J.T. Baker, Phillipsburg, NJ, USA), and NiCl2·6H2O (97%, Riedel de Haën, Seelze, Germany) in deionized water (resistivity, 18.2 MΩ cm), respectively. Deionized water was used for the preparation of these solutions as well as any other purpose.
2.3. Characterization Techniques
The as-received and dried samples of seeds and leaves of Moringa oilefera were characterized by Fourier transform infrared spectroscopy (Nicolet Nexus 670 FTIR, Waltham, MA, USA) within the range of 4000–400 cm–1 using the KBr standard method. Spectra were collected for the pristine materials and the exposed ones.
2.4. Point of Zero Charge (pHPZC)
The following experiment process determined the pHpzc values of L-MO and S-MO biomass: an amount of 0.1 g of each biomass was added to a 50 mL of 0.01M NaCl solution at various initial pH values from 2 to 12. The mixtures were agitated for 72 h at 25 °C, followed, the phases were separated by decantation, and each solution’s pH was measured using a pH-meter (Thermo Scientific ORION 3 star pH Benchtop, Waltham, MA, USA). The pHpzc value of the biosorbent is obtained by the intersection of the initial experimental pH vs. the final pH graph with the theoretical curve (pHinitial = pHinitial) [32].
2.5. Sorption Experiments
In this work, the sorption experiments, such as the effects of contact time, biosorbent dosages, and the initial concentration of metallic ions, were performed by the batch system. For the kinetic study, a level of 0.10 g of each biomass (L-MO and S-MO) was added in conic tubes to 10 mL of each metallic ion with an initial concentration of 100 mg/L and at pH 6 in order to avoid metal precipitation. The mixtures were shaken at 25 °C in a rotary shaker at 150 rpm. The samples were centrifuged for 5 min at 3500 rpm to separate the solid and liquid phases after finalizing each adsorption experiment. The concentrations of Pb, Co, Cd, and Ni ions were determined by using the FAAS technique (Thermo Scientific).
Equation (1) gives the amount of metallic ions sorbed per gram of adsorbent at each contact time t, by L-MO and S-MO:
where is the concentration of adsorbate at time , is the initial adsorbate concentration, is the volume of the adsorbate solution, and is the adsorbent’s mass. In this case, and .
The effect of biosorbent dosage in removing metallic ions was done by the variation of biosorbent mass from 0.1 g to 0.5 g/10 mL, keeping the other parameters constant (contact time at 300 min, solution pH at 6, and initial concentration of metal ions at 100 mg/L). The effect of the initial concentration of metal ions on the sorption capacity of the biosorbents was carried out by varying from 10 to 500 mg/L. Throughout these experiments, the contact time, solution pH, and mass biosorbents were fixed at 300 min, 5.5, and 0.1 g, respectively. All experiments were conducted in duplicate to assure the repeatability of the results.
The description sorption process was investigated by fitting the experimental data to the pseudo-first-order and pseudo-second-order models. These models are described by the following equations (Equations (2) and (3)), respectively:
where qt (mg·g−1) and qe (mg·g−1) are the sorption capacities at time t and equilibrium, respectively; k1 (min−1) is the pseudo-first-order, and k2 (g mg−1·min−1) is the pseudo-second-order adsorption rate constant.
Equations (4) and (5) describe the Langmuir and Freundlich models, respectively:
where Qm is the maximum adsorption capacity (mg/g) and KL is the Langmuir constant (L/mg),
qe is the amount of each heavy metal sorbed per unit mass of biosorbents, and Ce is the amount of the heavy metals in solution at the equilibrium. KF (mg·g−1·(mg·L−1)−1/n) and n are Freundich constants.
3. Results and Discussions
3.1. Point of Zero Charge (pHPZC)
The plot of pHpzc of L-MO and S-MO is depicted in Figure 1. The pHPZC of leaves and seeds from Moringa-derived biosorbent was 6.4 and 4.8, respectively. Thus, at pH ˃ pHPZC, the heavy metal ions are attracted by the negative surface charge of biosorbents, whereas at pH < pHPZC, the adsorption of metal ions is not favored since the charge in the biosorbent surface is positive, which caused an electrostatic repulsion with metal ions [33]. This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
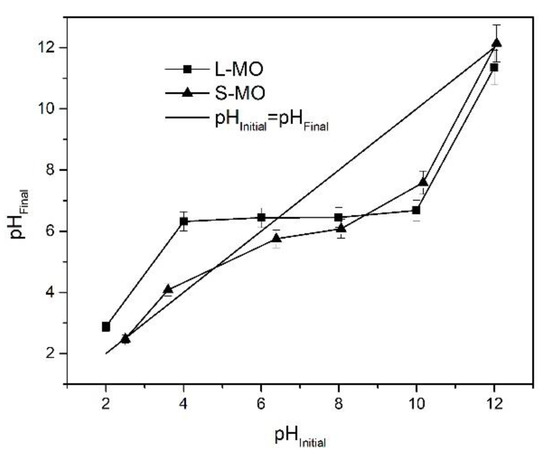
Figure 1.
pHPZC of L-MO and S-MO biosorbents.
3.2. FTIR Spectra
As shown in Figure 2a,b, the FTIR spectra of L-MO and S-MO before and after heavy metals sorption do not change significantly. The FTIR spectrum of Moringa leaves-derived material shows a broad band centered at 3281 cm−1, attributed to the presence of hydroxyl (–OH) [30]. This band was also observed in the IR spectrum of Moringa seeds-derived material (Figure 2b), indicating the presence of OH groups of lignocellulosic structures in the leaves and seeds of Moringa [30,34]. The band at 2917 and 2848 cm−1 (Figure 2a), as well as 2923 and 2852 cm−1 (Figure 2b), are due to the asymmetrical and symmetrical stretching vibrations of the C–H group present in lignocellulosic materials and/or proteins, respectively [34]. The presence of a vibrational band around 1538 cm−1 (Figure 2b) is attributed to the amidic group characteristic of Moringa seeds’ proteins [35]. The bands at 1611 cm−1 (Figure 2a) and 1649 cm−1 (Figure 2b) are associated with the vibration of the C=O bond of the fatty acid and protein structures present in the leaves and seeds from Moringa. The region between 1420 (carboxylate) and 1236 cm−1 (C–N) is attributed to carboxylic acids and amides in the Moringa leaves, respectively [36], whereas the band around 1050 cm−1 is attributed to the C–O vibrations, which are characteristic of the lignin structure of the L-MO and S-MO [36]. Overall, the presence of the difference confirms that the amino acids, alkaloids, flavonoids, and phenolics are the dominant surface functional groups in the seeds and leaves of this plant [34,35,36]. As observed in Figure 2, the band-shift after the metallic ions’ adsorption was not remarkable, indicating that the complex’s possible formation with the metallic ions should not have occurred. Therefore, the metallic ions could have some ion exchange with the functional groups of L-MO and S-MO biosorbents.
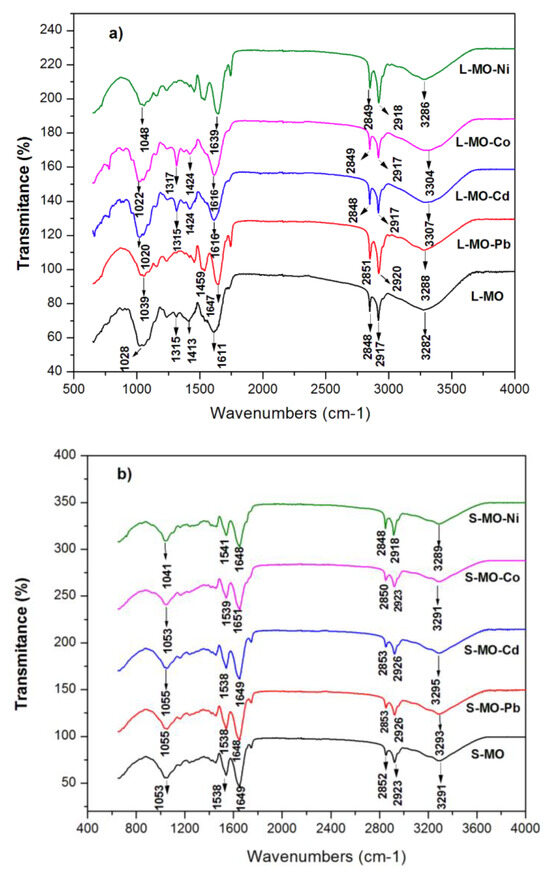
Figure 2.
FTIR spectra of (a) L-MO, L-MO-Pb, L-MO-Cd, L-MO-Co, and L-MO-Ni; and (b) S-MO, S-MO-Pb, S-MO-Cd, S-MO-Co, and S-MO-Ni.
Based on FTIR results, the mechanism of adsorption following Scheme 1 is proposed.
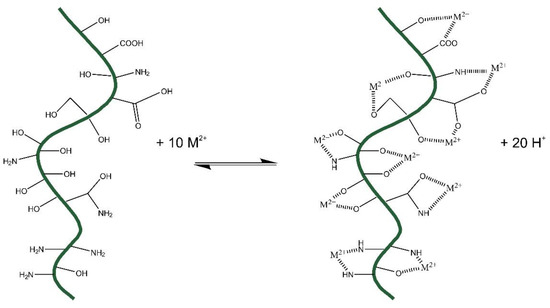
Scheme 1.
Ion exchange mechanism of S-MO and L-MO with M2+ metallic cations (Cd2+, Pb2+, Cu2+, Ni2+).
3.3. Effect of the Dosage
The mass effect of the leaves and seeds of Moringa oilefera-derived biosorbent on the biosorption capacity for Pb(II), Cd(II), Ni(II), and Co(II) is shown in Figure 3a–d. The removal percentage of all metallic ions increases with the augmentation of biosorbent mass from 0.1 to 0.5 g. This sentence should be changed by the following: This effect was more notable for S-MO than L-MO: for instance, Cd(II), Ni(II) and Co(II) were removed at 40%, 30% and 30%, respectively, whereas this was not significant for Pb(II) (1.59 %). The increase in the percentage removal of Cd(II) and Pb(II) with increasing L-MO mass was insignificant (1.94% and 6.59%, respectively), whereas a slight increase presented in the case of Co(II) (14.37%) and a significant increase presented for Ni(II) (31.83%). In various investigations, similar behavior was observed and attributed to the great accessibility of active adsorption sites due to the biosorbent mass increase [37,38]. Furthermore, the differences observed also depend on the chemical nature of the heavy metals involved and their affinity to the biosorbents.
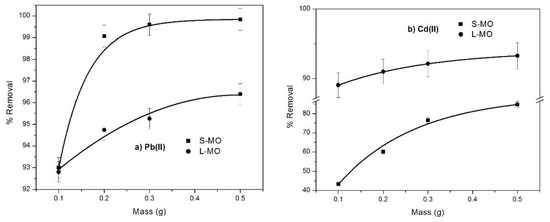
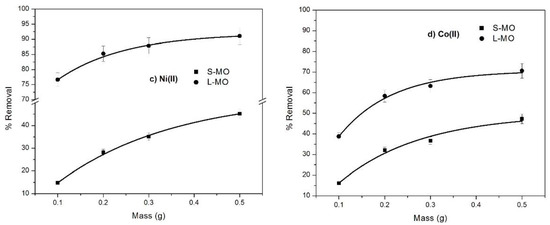
Figure 3.
The effect of adsorbent dosage of S-MO and L-MO on the removal percentage of (a) Pb(II), (b) Cd(II), (c) Ni (II) and (d) Co(II) metallic ions at the following conditions: Ci = 100 mg/L; pH=6; and contact time = 300 min.
3.4. Kinetic
Figure 4a–d show the amounts of Pb(II), Cd(II), Ni(II), and Co(II) sorbed from aqueous solutions onto L-MO and S-MO at different contact times. It is observed that, for both Moringa components (seeds and leaves), the biosorption process is mainly carried out in two stages. In the first step, approximately 90% of all metal ions’ maximum quantity removal occurred within the first 60 min. This fast removal can be related to the various surface chemical functionalities acting as available active sites on the L-MO and S-MO surfaces. After 60 min, the biosorption process becomes almost invariable due to the few available sites on the biosorbent surfaces [20]. The time to attain the equilibrium by using Moringa leaves and seeds is between 120 and 240 min for all metal ions.
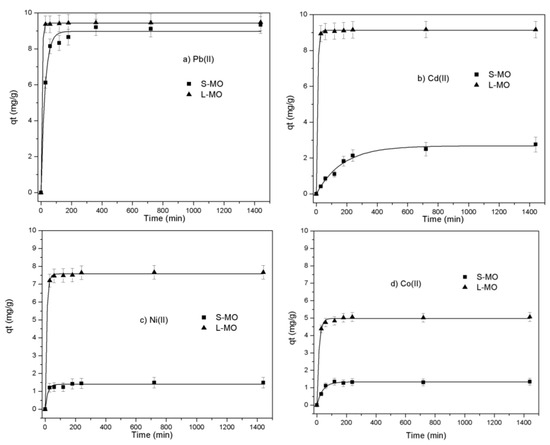
Figure 4.
Heavy metal uptakes (a) Pb(II), (b) Cd(II), (c)Ni(II) and (d) Co(II)) as a function of contact time at the following conditions: m =0.1 g; Ci = 100 mg/L; and pH = 6.
As seen in Figure 4a–d, the Moringa leaves-derived biosorbent showed a better removal performance than the one obtained from the seeds. The amounts of Pb(II), Cd(II), Ni(II), and Co(II) sorbed on L-MO at equilibrium reached 9.46, 9.17, 7.74, and 5.07 mg/g, respectively. In S-MO’s case, the metals’ sorption revealed the same order, with the capacities to be 9.34, 2.76, 1.48, and 1.34 mg/g, respectively. These results can be assigned to the interactions of these metallic ions with the functional groups from L-MO and S-MO, as shown in the FTIR spectra.
The differences in the adsorption capacities between leaves and seeds could be due to their chemical composition. It has been reported that M. oleifera seeds contain a higher amount of 4-(a-L-rhamnopyranosyloxy)-benzylglucosinolate than the leaves, and the leaves also contain three monoacetyl isomers of that glucosinolate [39]. Therefore, the functional groups of those compounds represent adsorption sites available for interaction with the metallic species. The preference in the removal efficiency exhibited by the biosorbent for Pb2+ over Cd2+, Co2+ and Ni2+ may be attributed to Pb2+ smaller hydrated radius (Pb2+ = 0.401 nm; Cd2+ = 0.426 nm; Co2+ = 0.423 nm; Ni2+ = 0.404 nm) as well as hydration energy (Pb2+ = −1481 kJ/mol; Cd2+ = −1807 kJ/mol; Co2+ = −1996 kJ/mol; Ni2+ = −2106 kJ/mol) [40]. Cd2+ showed higher retention capacity in comparison with Co2+ and Ni2+, which could be more associated with hydration energy and electrostatic factors than with hydrated radius and steric hindrance.
The pHpzc of the surface of the biomasses from leaves and seeds (6.4 and 4.8, respectively) can also exert a vital role in the adsorption process as well as each metal’s chemical species in the solution. The predominant species that could be found in aqueous solution considering 100 mg/L of each metal at pH 6 are Pb2+ (50%) and Pb(OH)2 (50%); Cd2+; Ni2+; and Co2+ [41]. Based on the results presented in Figure 4, the metallic cationic species were efficiently adsorbed by the leaves biomass of M. oilefera, which has a pHpzc near to 7 compared to the acid surface of seeds biomass. It is important to mention that in the case of Pb, the cationic exchange and the precipitation of the lead species on the surface of the biomasses could be the mechanisms to remove this heavy metal from aqueous solutions.
The values of the kinetic parameters were obtained from the slope and the intercept by plotting of and graphs. The results of the linear graphs are presented in Table 1. As observed, the experimental values of sorption capacities of Pb(II), Cd(II), Ni (II), and Co(II) sorbed at equilibrium (qe,exp) are very close to those calculated from the pseudo-second-order (qe,cal). Moreover, as seen in Table 1, the determination coefficient (R2) values of the pseudo-second-order are between 0.999 and 1.0, whereas for the pseudo-first-order model, the R2 values are between 0.249 to 0.973. Therefore, the experimental kinetics data of metal ions biosorption onto L-MO and S-MO are better expressed by the pseudo-second-order model than the pseudo-first-order model.

Table 1.
Kinetic parameters for the sorption of Pb(II), Cd(II), Ni(II), and Co(II) by L-MO and S-MO.
The k2 constant depends on the plant’s part (leaves or seeds) used as biosorbent and the heavy metal’s chemical nature. The k2Pb(II) and k2Cd(II) are 17 and 127 times higher for L-MO than for S-MO, respectively. However, k2Ni(II) and k2Co(II) are slightly different between L-MO and S-MO (1.8% and 22 %, respectively).
3.5. Isotherms
Figure 5a–d show the sorption isotherms for the two biomasses (L-MO and S-MO). The experimental data were fitted to Langmuir and Freundlich isotherms to describe the biosorption behavior.
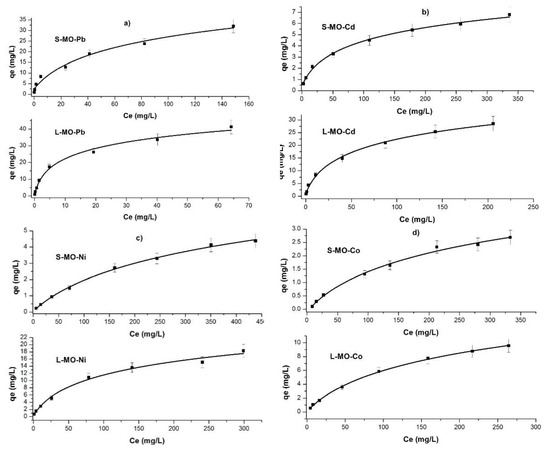
Figure 5.
Sorption isotherms of (a) Pb(II); (b) Cd(II); (c) Ni(II); and (d) Co(II) onto L-MO and S-MO.
Both models well described the biosorption behavior of the heavy metals by the biosorbents according to the determination coefficients (R2) presented in Table 2.

Table 2.
Freundlich and Langmuir parameters for the sorption of Pb(II), Cd(II), Ni(II), and Co(II) by Leaves and seeds of Moringa oilefera.
The equilibrium constant of sorption values KL (L/mg)) obtained from the Langmuir isotherm for L-MO and S-MO were highest for Pb (15.89 × 10−2 and 55.01 × 10−2) and lowest for Co (1.23 × 10−2 and 0.33 × 10−2). According to the Qm value observed here, the biomasses adsorbed the heavy metal ions in the order: Pb(II) > Cd(II) > Ni(II) > Co(II). These results are under Zamzow et al., who investigated the adsorption onto zeolites [23].
The 1/n value was lowest for Pb by L-MO and S-MO (0.56 and 0.48), and it was highest for Co for both biosorbents (0.68 and 0.77, respectively). It is important to note that L-MO and S-MO presented a higher maximum adsorption capacity for Pb(II) than the Cd(II), Ni(II), and Co(II), respectively, in this order. In general, the biosorbent L-MO is more efficient in removing the heavy metals in solution than S-MO. According to the Qm value obtained based on the Langmuir model, the maximum adsorption capacities of each heavy metal follow the order:
L-MO-Pb > L-MO-Cd > S-MO-Pb > L-MO-Co ≥ L-MO-Ni > S-MO-Co > S-MO-Cd > S-MO-Ni.
In Table 3, plenty of reported removal capacities for other biosorbents are collected for the sake of comparison with the herein studied materials. It can be seen that the maximum uptake capacities of L-MO and S-MO (Qm) with other reported biosorbents under similar experimental conditions. It can be noted that the sorption capacities depend on the physicochemical characteristics of the biosorbents and the heavy metal (pKa, solubility, chemical speciation, among others). Additionally, the leaves-derived sample exhibits a higher efficiency than the seeds-derived sample for the removal of Pb(II), Cd(II), Ni(II), and Co(II) from aqueous solutions. The values showed in Table 3 indicate that the adsorption capacities of heavy metals on the leaves and seeds of Moringa are within the range of values reported for other biosorbents. The outperformance most worth highlighting was for a Moringa oilefera leaves-derived sample against Pb(II), with a value that, to the best of our knowledge, was the highest among the plant biomass-derived biosorbents.

Table 3.
Comparison of L-MO and S-MO with other biosorbents to remove Pb(II), Cd(II), Co(II), and Ni(II) from aqueous solutions.
4. Conclusions
In this study, the remediation efficiency of two biosorbents derived from Moringa oilefera leaves or seeds was explored against four heavy metals. It was found that the sorption capacity of Pb(II), Cd(II), Co(II), and Ni(II) from aqueous solution by Moringa leaves-derived sample (L-MO) is higher than that for the seeds-derived biosorbent (S-MO). Both L-MO and S-MO biosorbents presented the following removal efficiency order: Pb(II) >Cd(II) >Co(II) >Ni(II). In all cases, the metal ions’ sorption capacity was dependent on the contact time, the dosage of biosorbent, and the initial metal concentration. The effect of contact time on the sorption capacity indicates the very fast metal-binding for the L-MO and S-MO biosorbent, since the equilibrium time was obtained within the first 60 min. Based on the values of correlation coefficients (R2 values close or equal to 1), sorption kinetics was well described by the pseudo-second-order kinetic model. The Langmuir and Freundlich models were also fitted well on the sorption isotherms. The R² values for both isotherms are close to 1, which suggests that the sorption process of the metal ions on L-MO and S-MO satisfied the Langmuir and Freundlich assumptions. The maximum sorption capacities were found in the following order: L-MO-Pb > L-MO-Cd > S-MO-Pb > L-MO-Co ≥ L-MO-Ni > S-MO-Co > S-MO-Cd > S-MO-Ni. Finally, but no less importantly, it is worthy to point out that the Moringa oilefera leaves-derived sample presented the highest remediation performance against Pb(II) compared to various other plant biomass-derived biosorbents reported in the literature.
Author Contributions
M.A. and M.T.O. conceived and planned the experiments; M.A. wrote the manuscript with input from all authors. I.A., D.A.G., E.C.L., M.T.O., J.V. and C.A. contributed to the characterization of the samples and the interpretation of the results. All authors provided critical feedback and helped shape the research, analysis and manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors are grateful to Adriana Tejeda Cruz and Eliezer Hernández Mecinas for their assistance in FTIR measurements
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, Y.; Lei, J.; Zhang, Y.; Zhu, J.; Lu, Y.; Wu, X.; Fang, H. Determining discharge characteristics and limits of heavy metals and metalloids for wastewater treatment plants (WWTPs) in China based on statistical methods. Water 2018, 10, 1248. [Google Scholar] [CrossRef]
- Obasi, P.N.; Akudinobi, B.B. Potential health risk and levels of heavy metals in water resources of lead–zinc mining communities of Abakaliki, southeast Nigeria. Appl. Water Sci. 2020, 10, 1–23. [Google Scholar] [CrossRef]
- Correa, M.L.; Velasquez, J.A.; Quintana, G.C. Uncommon Crop Residues as Ni (II) and Cd (II) Biosorbents. Ind. Eng. Chem. Res. 2012, 51, 12456–12462. [Google Scholar] [CrossRef]
- Marín, A.P.; Aguilar, M.I.; Meseguer, V.F.; Ortuno, J.F.; Sáez, J.; Lloréns, M. Biosorption of chromium (III) by orange (Citrus cinensis) waste: Batch and continuous studies. Chem. Eng. J. 2009, 155, 199–206. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K.; Witek-Krowiak, A. State of the art for the biosorption process—A Review. Appl. Biochem. Biotechnol. 2013, 170, 1389–1416. [Google Scholar] [CrossRef]
- Basso, M.C.; Cerrella, E.G.; Cukierman, A.L. Lignocellulosic materials as potential biosorbents of trace toxic metals from wastewater. Ind. Eng. Chem. Res. 2002, 41, 3580–3585. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. A new strategy for heavy metal polluted environments: A review of microbial biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Ngah, W.W.; Hanafiah, M.M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour. Technol. 2008, 99, 3935–3948. [Google Scholar] [CrossRef]
- Gutha, Y.; Munagapati, V.S.; Alla, S.R.; Abburi, K. Biosorptive removal of Ni (II) from aqueous solution by caesalpinia bonducella seed powder biosorptive removal of Ni (II) from aqueous solution by Caesalpinia bonducella seed powder. Sep. Sci. Technol. 2011, 46, 2291–2297. [Google Scholar] [CrossRef]
- Fiorentin, L.D.; Trigueros, D.E.; Módenes, A.N.; Espinoza-Quiñones, F.R.; Pereira, N.C.; Barros, S.T.; Santos, O.A. Biosorption of reactive blue 5G dye onto drying orange bagasse in batch system: Kinetic and equilibrium modeling. Chem. Eng. J. 2010, 163, 68–77. [Google Scholar] [CrossRef]
- Rao, R.A.K.; Rehman, F. Use of Polyalthia longifolia Seeds (Seeds of Indian Mast Tree) as Adsorbent for the Removal of Cd (II) from Aqueous Solution Use of Polyalthia longifolia Seeds (Seeds of Indian Mast Tree) as Adsorbent for the Removal of Cd (II) from Aqueous Soluti. J. Dispers. Sci. Technol. 2012, 33, 37–41. [Google Scholar] [CrossRef]
- Jain, C.K.; Malik, D.S.; Yadav, A.K. Applicability of plant based biosorbents in the removal of heavy metals: A review. Environ. Process. 2016, 3, 495–523. [Google Scholar] [CrossRef]
- Asgarzadeh, S.; Rostamian, R.; Faez, E.; Maleki, A.; Daraei, H. Biosorption of Pb (II), Cu (II), and Ni (II) ions onto novel lowcost P. eldarica leaves-based biosorbent: Isotherm, kinetics, and operational parameters investigation. Desalin. Water Treat. 2015, 57, 14544–14551. [Google Scholar] [CrossRef]
- Meseguer, V.F.; Ortuño, J.F.; Aguilar, M.I.; Pinzón-Bedoya, M.L.; Lloréns, M.; Sáez, J.; Pérez-Marín, A.B. Biosorption of cadmium (II) from aqueous solutions by natural and modified non-living leaves of Posidonia oceanica. Environ. Sci. Pollut. Res. 2016, 32, 24032–24046. [Google Scholar] [CrossRef]
- Ounsadi, H.; Khalidi, A.; Abdennouri, M.; Barka, N. Biosorption potential of Diplotaxis harra and Glebionis coronaria L. biomasses for the removal of Cd(II) and Co(II) from aqueous solutions. J. Environ. Chem. Eng. 2015, 3, 822–830. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nadeem, R.; Bhatti, H.N.; Ahmad, N.R.; Ansari, T.M. Ni(II) biosorption by Cassia fistula (Golden Shower) biomass. J. Hazard. Mater. 2007, 139, 345–355. [Google Scholar] [CrossRef]
- Long, J.; Huang, X.; Fan, X.; Peng, Y.; Xia, J. Effective adsorption of nickel (II) with Ulva lactuca dried biomass: Isotherms, kinetics and mechanisms Jianyou Long, Xiaona Huang, Xiaoli Fan, Yan Peng and Jianrong Xia. Water Sci. Technol. 2018, 78, 156–164. [Google Scholar] [CrossRef]
- Aksu, Z. Application of biosorption for the removal of organic pollutants: A review. Process Biochem. 2005, 40, 997–1026. [Google Scholar] [CrossRef]
- Wang, M.X.; Zhang, Q.L.; Yao, S.J. A novel biosorbent formed of marine-derived Penicillium janthinellum mycelial pellets for removing dyes from dye-containing wastewater. Chem. Eng. J. 2015, 259, 837–844. [Google Scholar] [CrossRef]
- Zamzow, M.J.; Eichbaum, B.R.; Sandgren, K.R.; Shanks, D.E. Removal of heavy metals and other cations from wastewater using zeolites. Sep. Sci. Technol. 1990, 25, 1555–1569. [Google Scholar] [CrossRef]
- Ghebremichael, K.; Gebremedhin, N.; Amy, G. Performance of Moringa oliefera as a biosorbent for chromium removal. Water Sci. Technol. 2010, 62, 1106–1111. [Google Scholar] [CrossRef]
- Pandey, V.N.; Chauhan, V.; Pandey, V.S.; Upadhyaya, P.P.; Kopp, O.R. Moringa oleifera Lam: A Biofunctional Edible Plant from India, Phytochemistry and Medicinal Properties. J. Plant Stud. 2019, 8, 10–19. [Google Scholar] [CrossRef][Green Version]
- Sajidu, S.M.; Henry, E.M.T.; Kwamdera, G.; Mataka, L. Removal of lead, iron and cadmium ions by means of polyelectrolytes of the Moringa Oleifera whole seed kernel. WIT Trans. Ecol. Environ. 2005, 80, 251–258. [Google Scholar] [CrossRef]
- Kumari, P.; Sharma, P.; Srivastava, S.; Srivastava, M.M. Biosorption studies on shelled Moringa oleifera Lamarck seed powder: Removal and recovery of arsenic from aqueous system. Int. J. Miner. Process. 2006, 78, 131–139. [Google Scholar] [CrossRef]
- Imran, M.; Anwar, K.; Akram, M.; Shah, G.M.; Ahmad, I.; Samad Shah, N.; Khan, Z.U.H.; Rashid, M.I.; Akhtar, M.N.; Ahmad, S.; et al. Biosorption of Pb (II) from contaminated water onto Moringa oleifera biomass: Kinetics and equilibrium studies. Int. J. Phytoremed. 2019, 21, 777–789. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Mumtaz, B.; Hanif, M.A.; Nadeem, R. Removal of Zn (II) ions from aqueous solution using Moringa oleifera Lam. (horseradish tree) biomass. Process Biochem. 2007, 42, 547–553. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Ramana, D.K.V.; Seshaiah, K.; Reddy, A.V.R. Biosorption of Ni (II) from aqueous phase by Moringa oleifera bark, a low cost biosorbent. Desalination 2011, 268, 150–157. [Google Scholar] [CrossRef]
- Matouq, M.; Jildeh, N.; Qtaishat, M.; Hindiyeh, M.; Al Syouf, M.Q. The adsorption kinetics and modeling for heavy metals removal from wastewater by Moringa pods. J. Environ. Chem. Eng. 2015, 3, 775–784. [Google Scholar] [CrossRef]
- Abatal, M.; Olguin, M.T. Comparative adsorption behavior between phenol and p-nitrophenol by Na- and HDTMA-clinoptilolite-rich tuff. Environ. Earth Sci. 2013, 69. [Google Scholar] [CrossRef]
- Peng, S.; Hao, K.; Han, F.; Tang, Z.; Niu, B.; Zhang, X.; Wang, Z.; Hong, S. Enhanced removal of bisphenol-AF onto chitosan-modified zeolite by sodium cholate in aqueous solutions. Carbohydr. Polym. 2015, 130, 364–371. [Google Scholar] [CrossRef]
- Araújo, C.S.; Alves, V.N.; Rezende, H.C.; Almeida, I.L.; De Assuncao, R.; Tarley, C.R.; Segatelli, M.G.; Coelho, N.M.M. Characterization and use of Moringa oleifera seeds as biosorbent for removing metal ions from aqueous effluents. Water Sci. Technol. 2010, 62, 2198–2203. [Google Scholar] [CrossRef]
- Elumalai, K.; Velmurugan, S.; Ravi, S.; Kathiravan, V.; Ashokkumar, S. Green synthesis of Zinc oxide nanoparticles using Moringa oleifera leaf extract and evaluation of its antimicrobial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 143, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.H.K.; Seshaiah, K.; Reddy, A.V.R.; Rao, M.M.; Wang, M.C. Biosorption of Pb2+ from aqueous solutions by Moringa oleifera bark: Equilibrium and kinetic studies. J. Hazard. Mater. 2010, 174, 831–838. [Google Scholar] [CrossRef]
- Kumar, Y.P.; King, P.; Prasad, V.S.R.K. Equilibrium and kinetic studies for the biosorption system of copper (II) ion from aqueous solution using Tectona grandis L.f. leaves powder. J. Hazard. Mater. 2006, 137, 1211–1217. [Google Scholar] [CrossRef]
- Rubeš, M.; Koudelková, E.; De Oliveira Ramos, F.S.; Trachta, M.; Bludský, O.; Bulánek, R. Experimental and theoretical study of propene adsorption on K-FER zeolites: New evidence of bridged complex formation. J. Phys. Chem. C 2018, 122, 6128–6136. [Google Scholar] [CrossRef]
- Bennett, R.; Mellon, F.; Perkins, L.; Centre, J.I.; Kroon, P.A. Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (Horseradish Tree) and Moringa profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpo. J. Agric. Food Chem. 2003, 51, 3546–3553. [Google Scholar] [CrossRef]
- Cong, N.; Chen, S.; Hsu, H.; Li, C. Separation of three divalent cations (Cu2+, Co2+ and Ni2+) by NF membranes from pHs 3 to 5. DES 2013, 328, 51–57. [Google Scholar] [CrossRef]
- Puigdomenech, I. Hydra/Medusa Chemical Equilibrium Database and Plotting Software; KTH Royal Institute of Technology: Stockholm, Sweden, 2004. [Google Scholar]
- Munagapati, V.S.; Yarramuthi, V.; Nadavala, S.K.; Alla, S.R.; Abburi, K. Biosorption of Cu(II), Cd(II) and Pb(II) by Acacia leucocephala bark powder: Kinetics, equilibrium and thermodynamics. Chem. Eng. J. 2010, 157, 357–365. [Google Scholar] [CrossRef]
- Tofan, L.; Teodosiu, C.; Paduraru, C.; Wenkert, R. Cobalt (II) removal from aqueous solutions by natural hemp fibers: Batch and fixed-bed column studies. Appl. Surf. Sci. 2013, 285, 33–39. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).