Synthesis and Properties of p-Si/n-Cd1−xAgxO Heterostructure for Transparent Photodiode Devices
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structural Analysis
3.2. Surface Morphology Analysis
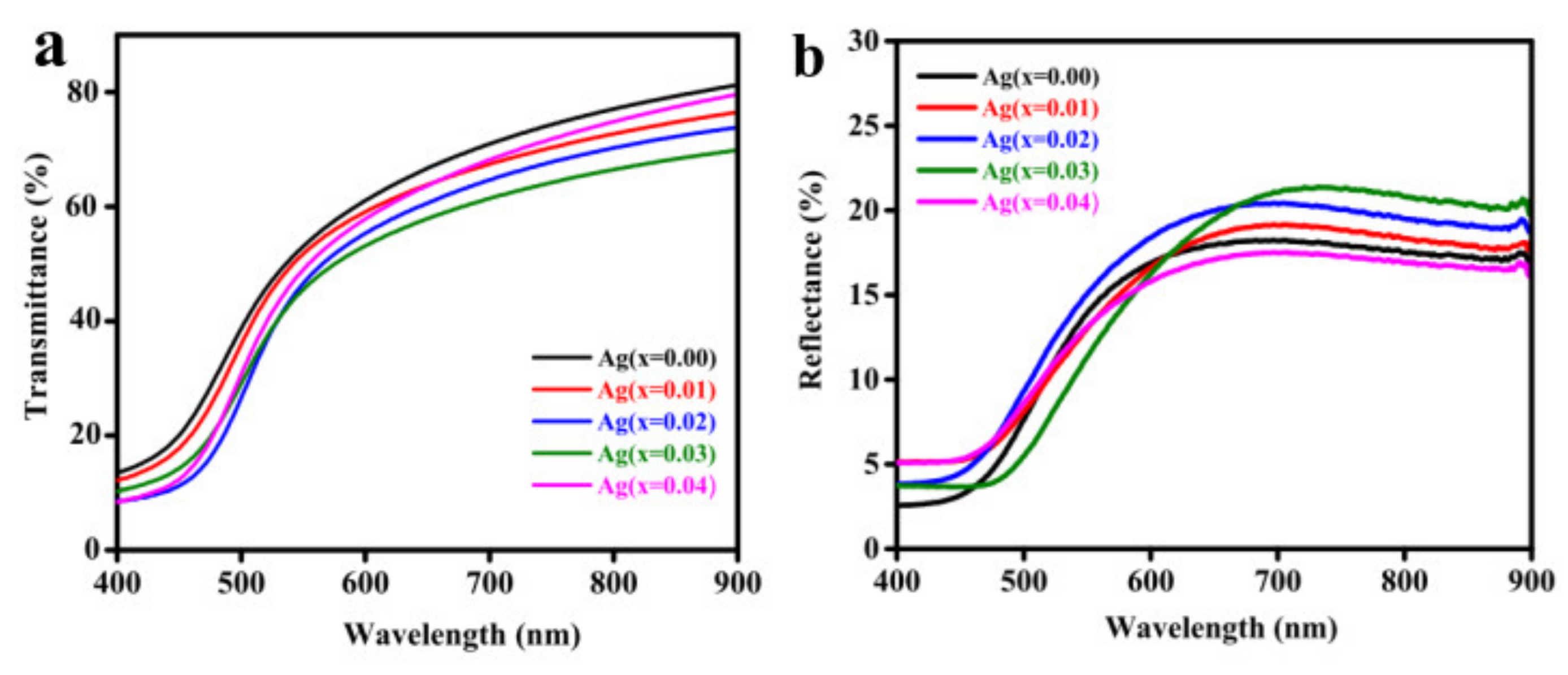
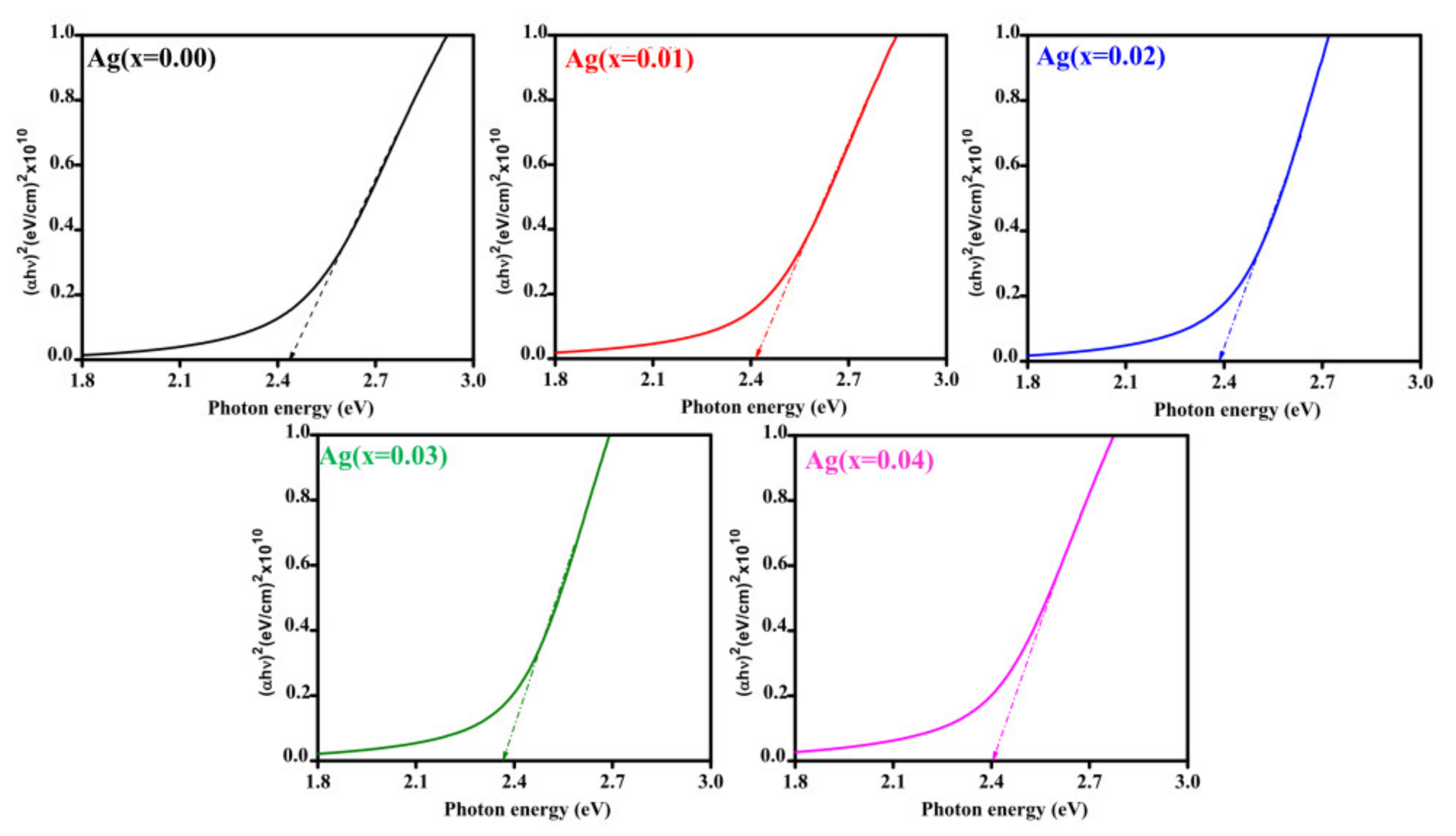
3.3. Optical Analysis
3.4. Photoluminescence Analysis
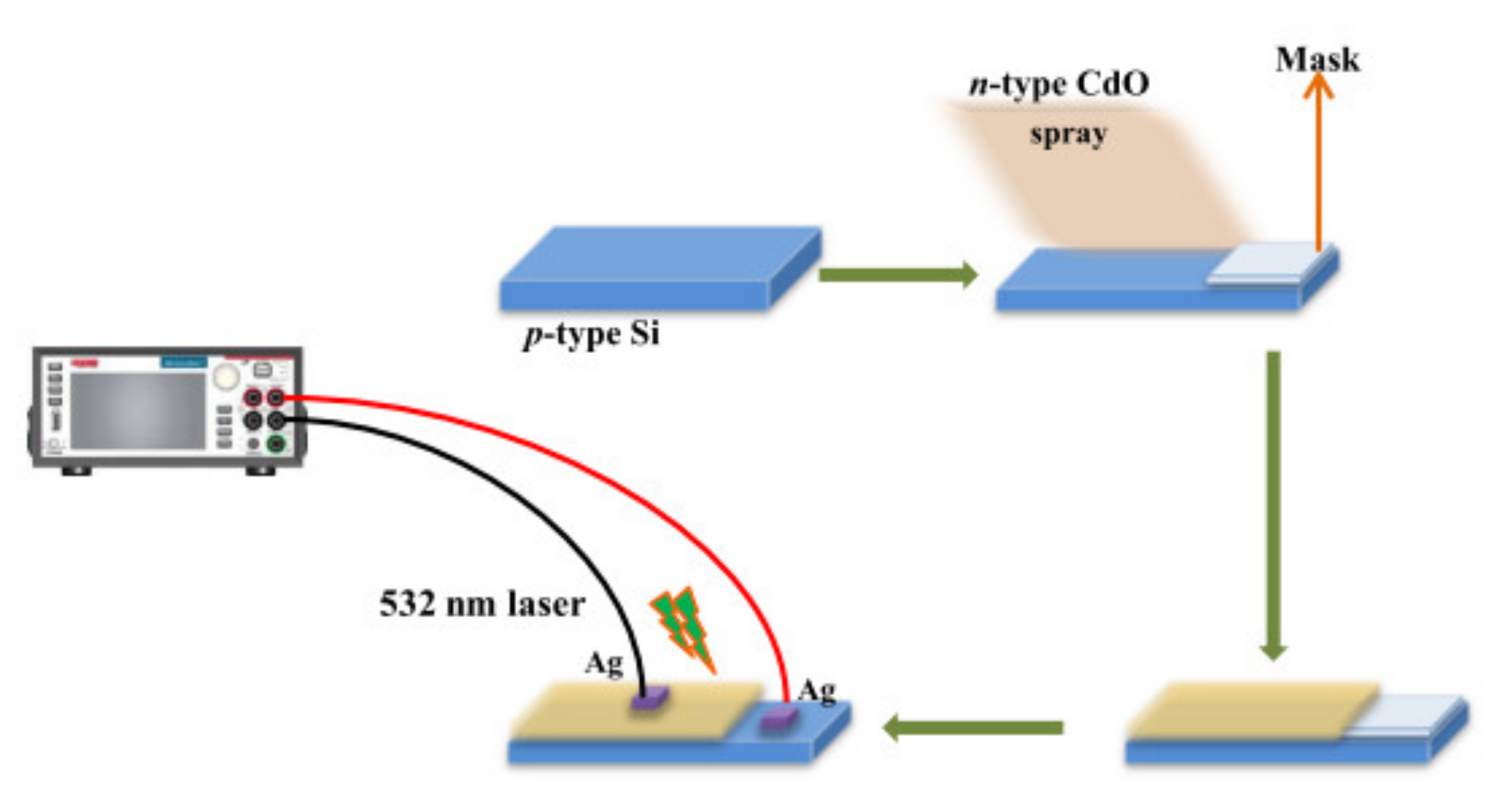
3.5. Photodiode Preparation and Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez-Serrano, A.; Muñoz-Olivas, R.; Sanz-Landaluze, J.; Olasagasti, M.; Rainieri, S.; Camara, C. Comparison of bioconcentration of ionic silver and silver nanoparticles in zebrafish eleutheroembryos. Environ. Pollut. 2014, 191, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Auffan, M.; Rose, J.; Bottero, J.-Y.; Lowry, G.V.; Jolivet, J.-P.; Wiesner, M.R. Towards a definition of isnorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, S.; Venkatesan, A.; Soundhirarajan, P.; Khatiwada, C.P. Synthesis, characterizations and anti-bacterial activities of pure and Ag doped CdO nanoparticles by chemical precipitation method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 1751–1759. [Google Scholar] [CrossRef]
- Zheng, Z.; Gan, L.; Li, H.; Ma, Y.; Bando, Y.; Golberg, D.; Zhai, T. A Fully Transparent and Flexible Ultraviolet–Visible Photodetector Based on Controlled Electrospun ZnO-CdO Heterojunction Nanofiber Arrays. Adv. Funct. Mater 2015, 25, 5885–5894. [Google Scholar] [CrossRef]
- Rajput, J.K.; Pathak, T.K.; Swart, H.C.; Purohit, L.P. Synthesis of CdO Nanoflowers by Sol-Gel Method on Different Substrates with Photodetection Application. Phys. Status Solidi 2019, 216, 1900093. [Google Scholar] [CrossRef]
- Chopra, S.R. Thin Film Solar Cells; Plenum Press: New York, NY, USA, 1993. [Google Scholar]
- Minami, T.; Ida, S.; Miyata, T. High rate deposition of transparent conducting oxide thin films by vacuum arc plasma evaporation. Thin Solid Film. 2002, 416, 92–96. [Google Scholar] [CrossRef]
- Pan, L.L.; Li, G.Y.; Lian, J.S. Structural, optical and electrical properties of cerium and gadolinium doped CdO thin films. Appl. Surf. Sci. 2013, 274, 365–370. [Google Scholar] [CrossRef]
- Aydın, C.; El-Nasser, H.M.; Yakuphanoglu, F.; Yahia, I.S.; Aksoy, M. Nanopowder synthesis of aluminum doped cadmium oxide via sol–gel calcination processing. J. Alloy Compd. 2011, 509, 854–858. [Google Scholar] [CrossRef]
- Wongcharoen, N.; Gaewdang, T.; Wongcharoen, T. Electrical Properties of Al-Doped CdO Thin Films Prepared by Thermal Evaporation in Vacuum. Energy Procedia 2012, 15, 361–370. [Google Scholar] [CrossRef]
- Gao, W.; Yang, S.; Yang, S.; Lv, L.; Du, Y. Synthesis and magnetic properties of Mn doped CuO nanowires. Phys. Lett. A 2010, 375, 180–182. [Google Scholar] [CrossRef]
- Thambidurai, M.; Muthukumarasamy, N.; Ranjitha, A.; Velauthapillai, D. Structural and optical properties of Ga-doped CdO nanocrystalline thin films. Superlattices Microstruct. 2015, 86, 559–563. [Google Scholar] [CrossRef]
- Zheng, B.; Hu, W. Influence of substrate temperature on the structural and properties of In-doped CdO films prepared by PLD. J. Semicond. 2013, 34, 053003. [Google Scholar] [CrossRef]
- Ravichandran, A.T.; Robert Xavier, A.; Pushpanathan, K.; Nagabhushana, B.M.; Chandramohan, R. Structural and optical properties of Zn doped CdO nanoparticles synthesized by chemical precipitation method. J. Mater. Sci. Mater. Electron. 2016, 27, 2693–2700. [Google Scholar] [CrossRef]
- Yüksel, M.; Şahin, B.; Bayansal, F. Nano structured CdO films grown by the SILAR method: Influence of silver-doping on the morphological, structural and optical properties. Ceram. Int. 2016, 42, 6010–6014. [Google Scholar] [CrossRef]
- Malachová, K.; Praus, P.; Rybková, Z.; Kozák, O. Antibacterial and antifungal activities of silver, copper and zinc montmorillonites. Appl. Clay Sci. 2011, 53, 642–645. [Google Scholar] [CrossRef]
- Salem, A. Silver-doped cadmium oxide nanoparticles: Synthesis, structural and optical properties. Eur. Phys. J. Plus 2014, 129, 263. [Google Scholar] [CrossRef]
- Majid, A.; Afzal, Z.; Murtaza, S.; Nabi, G.; Ahmad, N. Synthesis and Characterization of Silver Doped Cadmium Oxide Nanoparticles. J. Adv. Phys. 2013, 2, 116–118. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Muthuraj, V.; Jeyaraj, M. The design of novel visible light driven Ag/CdO as smart nanocomposite for photodegradation of different dye contaminants. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 188, 291–300. [Google Scholar] [CrossRef]
- Deva Arun Kumar, K.; Mele, P.; Ponraj, J.S.; Haunsbhavi, K.; Varadharajaperumal, S.; Alagarasan, D.; Algarni, H.; Angadi, B.; Murahari, P.; Ramesh, K. Methanol solvent effect on photosensing performance of AZO thin films grown by nebulizer spray pyrolysis. Semicond. Sci. Technol. 2020, 35, 085013. [Google Scholar] [CrossRef]
- Deva Arun Kumar, K.; Valanarasu, S.; Tamilnayagam, V.; Amalraj, L. Structural, morphological and optical properties of SnS2 thin films by nebulized spray pyrolysis technique. J. Mater. Sci. Mater. Electron. 2017, 28, 14209–14216. [Google Scholar] [CrossRef]
- Kathalingam, A.; Kesavan, K.; Rana, A.U.H.S.; Jeon, J.; Kim, H.-S. Analysis of Sn Concentration Effect on Morphological, Optical, Electrical and Photonic Properties of Spray-Coated Sn-Doped CdO Thin Films. Coatings 2018, 8, 167. [Google Scholar] [CrossRef]
- Velusamy, P.; Babu, R.R.; Ramamurthi, K.; Dahlem, M.S.; Elangovan, E. Highly transparent conducting cerium incorporated CdO thin films deposited by a spray pyrolytic technique. RSC Adv. 2015, 5, 102741–102749. [Google Scholar] [CrossRef]
- Rosario, S.R.; Kulandaisamy, I.; Kumar, K.D.A.; Ramesh, K.; Ibrahium, H.A.; Awwad, N.S. Ag-doped PbS thin films by nebulizer spray pyrolysis for solar cells. Int. J. Energy Res. 2020, 44, 4505–4515. [Google Scholar] [CrossRef]
- Velusamy, P.; Babu, R.R.; Ramamurthi, K.; Elangovan, E.; Viegas, J.; Dahlem, M.S.; Arivanandhan, M. Characterization of spray pyrolytically deposited high mobility praseodymium doped CdO thin films. Ceram. Int. 2016, 42, 12675–12685. [Google Scholar] [CrossRef]
- Anitha, M.; Saravanakumar, K.; Anitha, N.; Amalraj, L. Influence of a novel co-doping (Zn + F) on the physical properties of nano structured (1 1 1) oriented CdO thin films applicable for window layer of solar cell. Appl. Surf. Sci. 2018, 443, 55–67. [Google Scholar] [CrossRef]
- Haunsbhavi, K.; Deva Arun Kumar, K.; Mele, P.; Aldossary, O.M.; Ubaidullah, M.; Mahesh, H.M.; Murahari, P.; Angadi, B. Pseudo n-type behaviour of nickel oxide thin film at room temperature towards ammonia sensing. Ceram. Int. 2021. [Google Scholar] [CrossRef]
- Bagheri Khatibani, A.; Hallaj, Z.A.; Rozati, S.M. Some physical properties of CdO:F thin films prepared by spray pyrolysis. Eur. Phys. J. Plus 2015, 130, 254. [Google Scholar] [CrossRef]
- Thirumoorthi, M.; Prakash, J.T.J. A study of Tin doping effects on physical properties of CdO thin films prepared by sol–gel spin coating method. J. Asian Ceram. Soc. 2016, 4, 39–45. [Google Scholar] [CrossRef]
- Dakhel, A.A. Correlated transport and optical phenomena in Ga-doped CdO films. Sol. Energy 2008, 82, 513–519. [Google Scholar] [CrossRef]
- Usharani, K.; Balu, A.R.; Nagarethinam, V.S.; Suganya, M. Characteristic analysis on the physical properties of nanostructured Mg-doped CdO thin films—Doping concentration effect. Prog. Nat. Sci. Mater. Int. 2015, 25, 251–257. [Google Scholar] [CrossRef]
- Anand, V.; Sakthivelu, A.; Karuppiah, D.A.K.; Valanarasu, S.; Ganesh, V.; Shkir, M.; AlFaify, S.; Algarni, H. Rare earth Eu3+ co-doped AZO thin films prepared by nebulizer spray pyrolysis technique for optoelectronics. J. Sol-Gel Sci. Technol. 2018, 86, 293–304. [Google Scholar] [CrossRef]
- Balachandran, S.; Praveen, S.G.; Velmurugan, R.; Swaminathan, M. Facile fabrication of highly efficient, reusable heterostructured Ag–ZnO–CdO and its twin applications of dye degradation under natural sunlight and self-cleaning. RSC Adv. 2014, 4, 4353–4362. [Google Scholar] [CrossRef]
- Farhat, O.F.; Halim, M.M.; Ahmed, N.M.; Qaeed, M.A. ZnO nanofiber (NFs) growth from ZnO nanowires (NWs) by controlling growth temperature on flexible Teflon substrate by CBD technique for UV photodetector, Superlattice. Microst 2016, 100, 1120. [Google Scholar] [CrossRef]
- Poul Raj, I.L.; Valanarasu, S.; Hariprasad, K.; Ponraj, J.S.; Chidhambaram, N.; Ganesh, V.; Ali, H.E.; Khairy, Y. Enhancement of optoelectronic parameters of Nd-doped ZnO nanowires for photodetector applications. Opt. Mater. 2020, 109, 110396. [Google Scholar] [CrossRef]
- Shkir, M.; Ashraf, I.M.; Khan, A.; Khan, M.T.; El-Toni, A.M.; AlFaify, S. A facile spray pyrolysis fabrication of Sm:CdS thin films for high-performance photodetector applications. Sens. Actuators A Phys. 2020, 306, 111952. [Google Scholar] [CrossRef]
- Ravikumar, M.; Chandramohan, R.; Kumar, K.D.A.; Valanarasu, S.; Ganesh, V.; Shkir, M.; Alfaify, S.; Kathalingam, A. Effect of Nd doping on structural and opto-electronic properties of CdO thin films fabricated by a perfume atomizer spray method. Bull. Mater. Sci. 2019, 42, 8. [Google Scholar] [CrossRef]
- Najm, N.I.; Hassun, H.K.; al-Maiyaly, B.K.H.; Hussein, B.H.; Shaban, A.H. Highly selective CdS:Ag heterojunction for photodetector applications. Aip Conf. Proc. 2019, 2123, 020031. [Google Scholar] [CrossRef]
- Hanna, B.; Surendran, K.P.; Narayanan Unni, K.N. Low temperature-processed ZnO thin films for p–n junction-based visible-blind ultraviolet photodetectors. RSC Adv. 2018, 8, 37365–37374. [Google Scholar] [CrossRef]
- Gong, X.; Tong, M.; Xia, Y.; Cai, W.; Moon, J.S.; Cao, Y.; Yu, G.; Shieh, C.-L.; Nilsson, B.; Heeger, A.J. High-Detectivity Polymer Photodetectors with Spectral Response from 300 nm to 1450 nm. Science 2009, 325, 1665–1667. [Google Scholar] [CrossRef]
- Xiao, X.; Liang, L.; Pei, Y.; Yu, J.; Duan, H.; Chang, T.-C.; Cao, H. Solution-processed amorphous Ga2O3:CdO TFT-type deep-UV photodetectors. Appl. Phys. Lett 2020, 116, 192102. [Google Scholar] [CrossRef]
- Rhoderick, E.H.; Williams, R.H. Metal-Semiconductor Contacts; Clarendon Press Oxford: Oxford, UK, 1978. [Google Scholar]
- Moun, M.; Kumar, M.; Garg, M.; Pathak, R.; Singh, R. Understanding of MoS(2)/GaN Heterojunction Diode and its Photodetection Properties. Sci. Rep. 2018, 8, 11799. [Google Scholar] [CrossRef] [PubMed]
- Yakuphanoglu, F.; Caglar, M.; Caglar, Y.; Ilican, S. Electrical characterization of nanocluster n-CdO/p-Si heterojunction diode. J. Alloy. Compd. 2010, 506, 188–193. [Google Scholar] [CrossRef]
- Gozeh, B.A.; Karabulut, A.; Yildiz, A.; Dere, A.; Arif, B.; Yakuphanoglu, F. SILAR Controlled CdS Nanoparticles Sensitized CdO Diode Based Photodetectors. Silicon 2020, 12, 1673–1681. [Google Scholar] [CrossRef]
- Çiçek, O.; Tecimer, H.U.; Tan, S.O.; Tecimer, H.; Altındal, Ş.; Uslu, İ. Evaluation of electrical and photovoltaic behaviours as comparative of Au/n-GaAs (MS) diodes with and without pure and graphene (Gr)-doped polyvinyl alcohol (PVA) interfacial layer under dark and illuminated conditions. Compos. Part B Eng. 2016, 98, 260–268. [Google Scholar] [CrossRef]
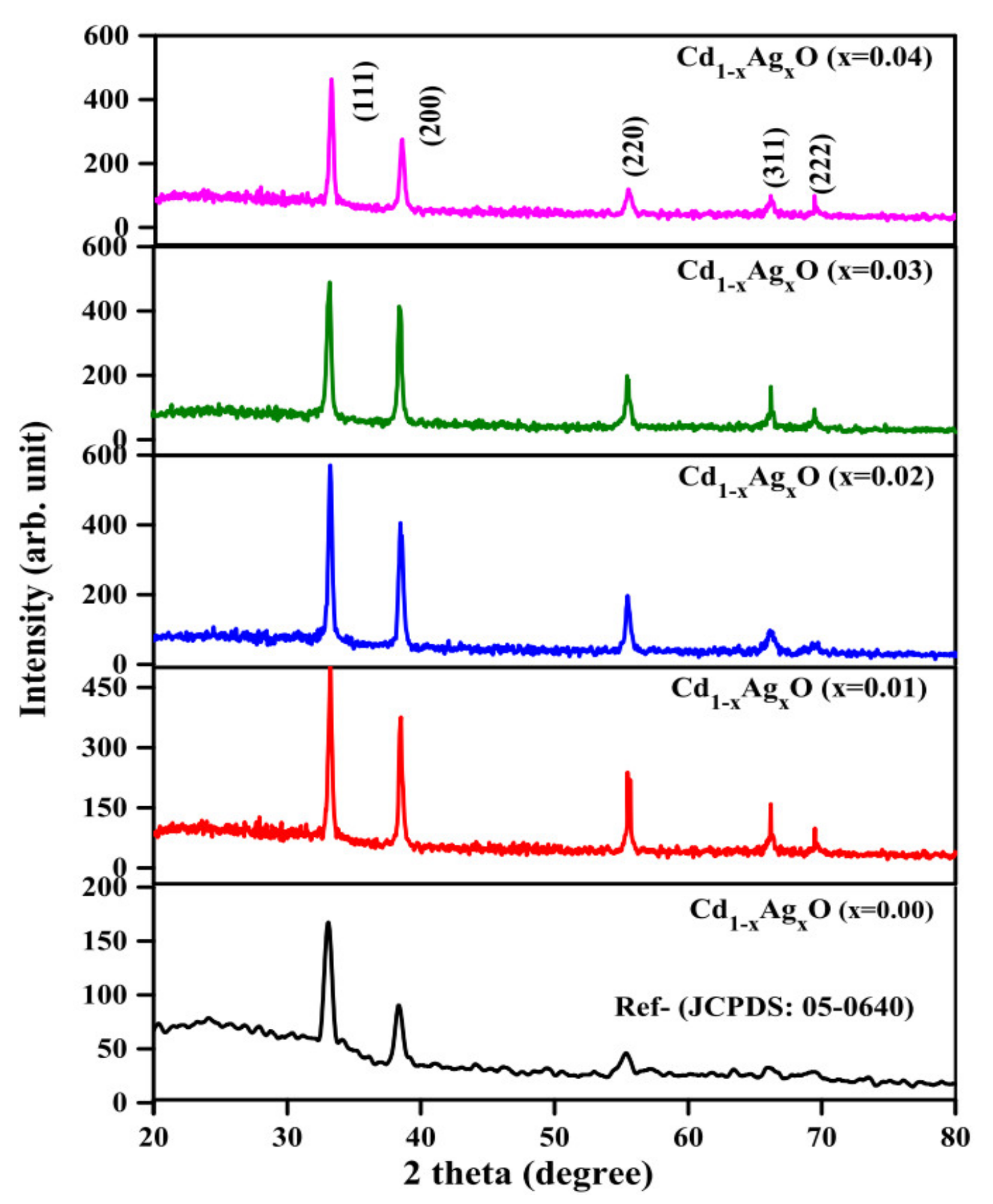

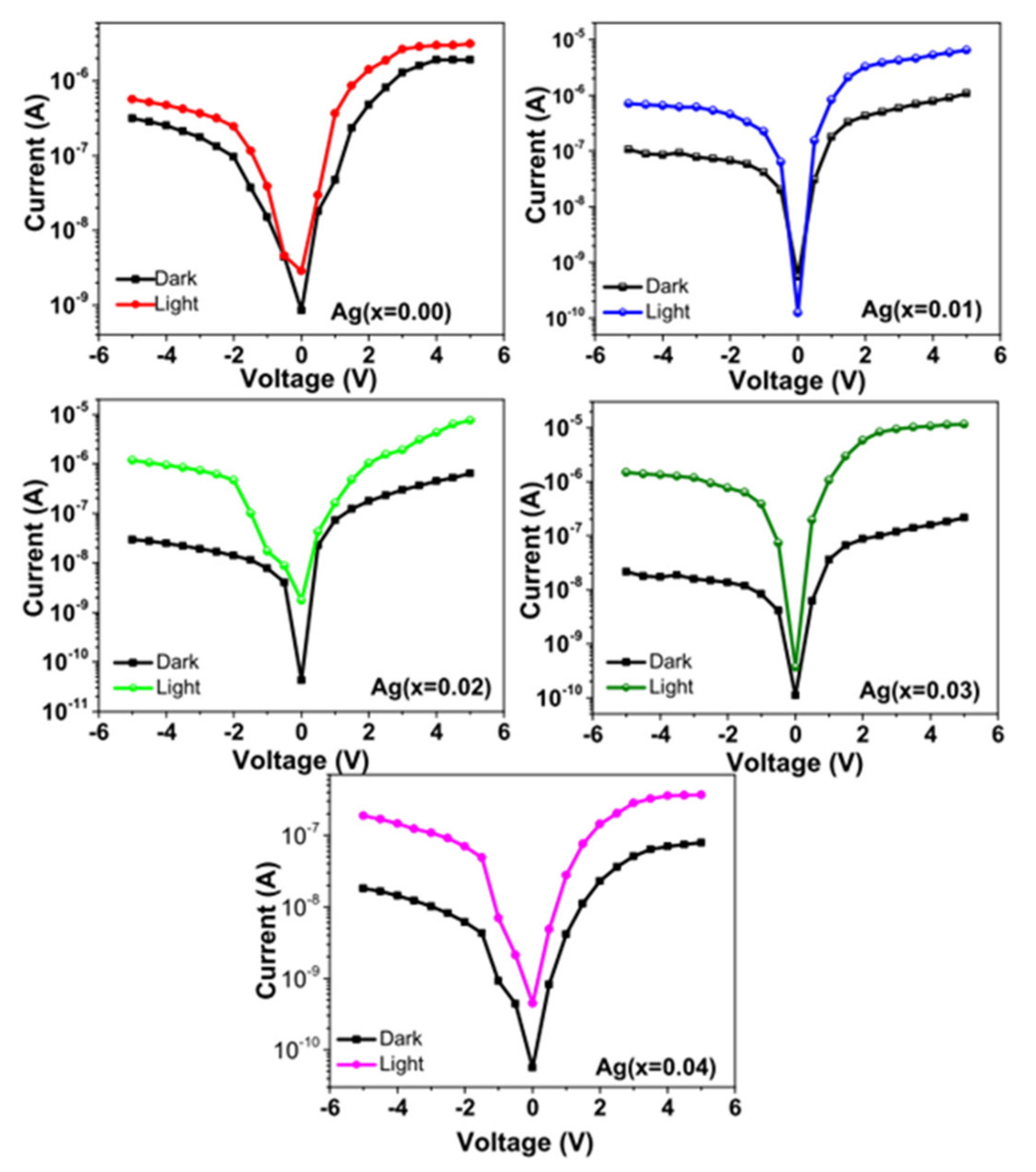
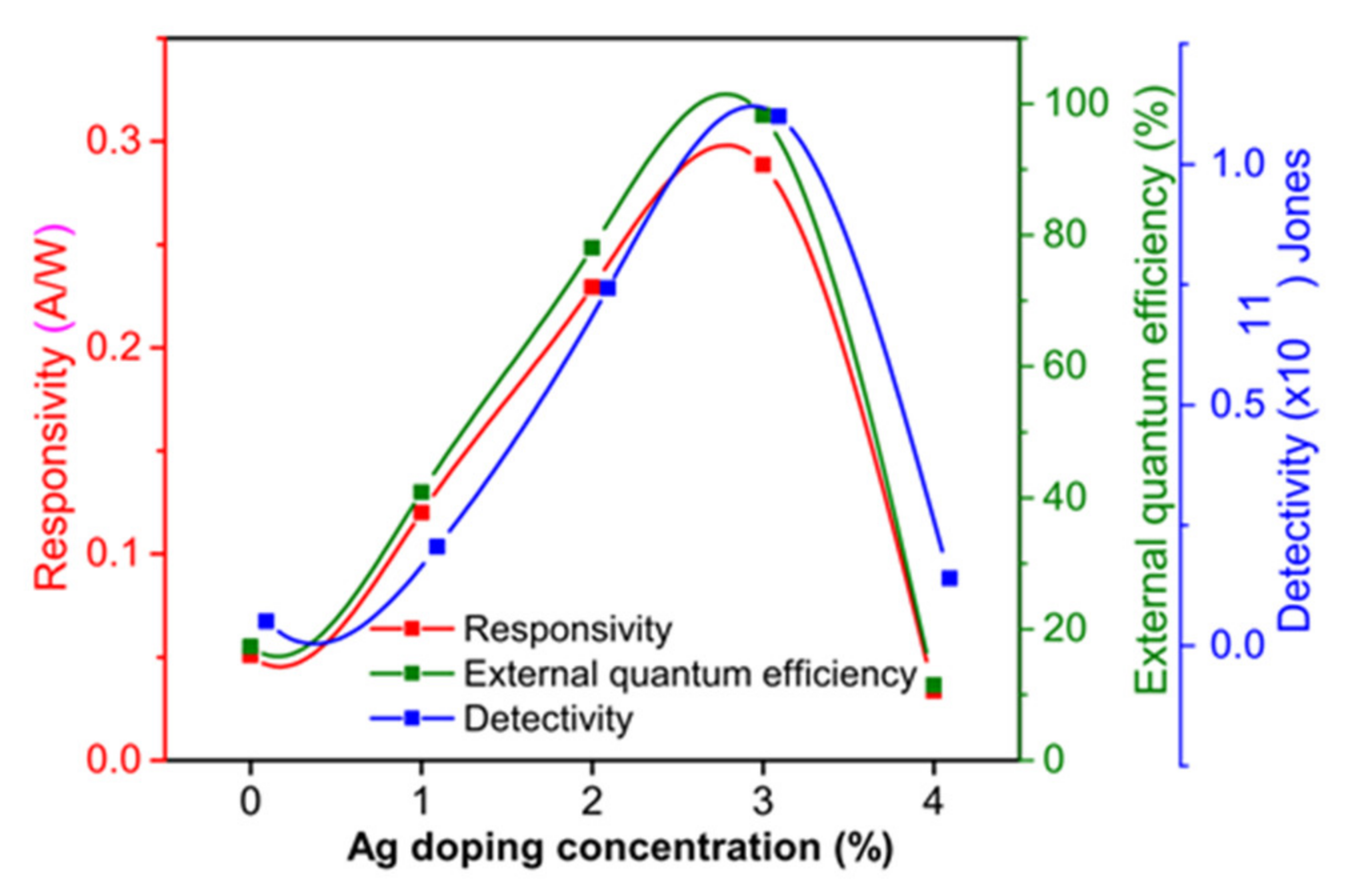

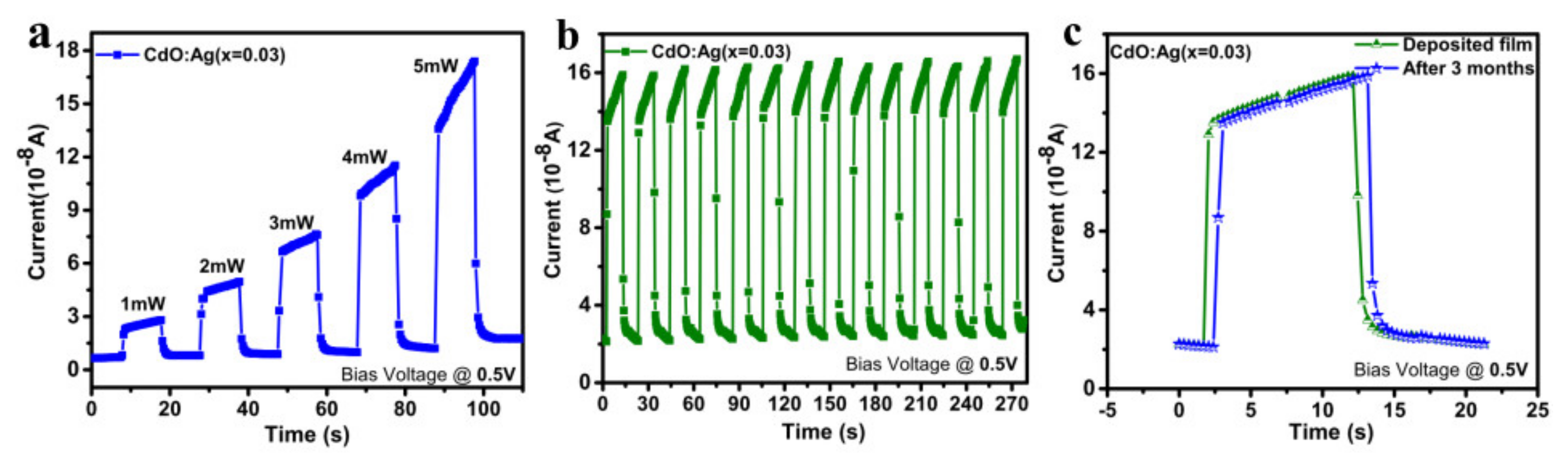
| Ag Doping Level (at.%) | Crystallite Size (nm) | Strain ε | Lattice Constant a = b = c (Å) | Unit Cell Volume (v) (Å)3 | TC |
|---|---|---|---|---|---|
| 0 | 28 | 0.0042 | 4.6828 | 102.69 | 1.76 |
| 1 | 29 | 0.0040 | 4.6842 | 102.78 | 1.93 |
| 2 | 31 | 0.0038 | 4.6854 | 102.86 | 2.18 |
| 3 | 34 | 0.0034 | 4.6869 | 102.97 | 2.35 |
| 4 | 32 | 0.0038 | 4.6893 | 103.11 | 1.97 |
| Ag Doping Level (at.%) | For λ = Average of 600–900 nm | Bandgap Eg (eV) | ||
|---|---|---|---|---|
| T% | R% | n | ||
| 0 | 75 | 17 | 2.41 | 2.45 |
| 1 | 71 | 19 | 2.53 | 2.43 |
| 2 | 68 | 20 | 2.58 | 2.40 |
| 3 | 65 | 22 | 2.62 | 2.37 |
| 4 | 70 | 16 | 2.36 | 2.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anitha, M.; Deva Arun Kumar, K.; Mele, P.; Anitha, N.; Saravanakumar, K.; Sayed, M.A.; Ali, A.M.; Amalraj, L. Synthesis and Properties of p-Si/n-Cd1−xAgxO Heterostructure for Transparent Photodiode Devices. Coatings 2021, 11, 425. https://doi.org/10.3390/coatings11040425
Anitha M, Deva Arun Kumar K, Mele P, Anitha N, Saravanakumar K, Sayed MA, Ali AM, Amalraj L. Synthesis and Properties of p-Si/n-Cd1−xAgxO Heterostructure for Transparent Photodiode Devices. Coatings. 2021; 11(4):425. https://doi.org/10.3390/coatings11040425
Chicago/Turabian StyleAnitha, Mannarsamy, Karuppiah Deva Arun Kumar, Paolo Mele, Nagarajan Anitha, Karunamoorthy Saravanakumar, Mahmoud Ahmed Sayed, Atif Mossad Ali, and Lourdusamy Amalraj. 2021. "Synthesis and Properties of p-Si/n-Cd1−xAgxO Heterostructure for Transparent Photodiode Devices" Coatings 11, no. 4: 425. https://doi.org/10.3390/coatings11040425
APA StyleAnitha, M., Deva Arun Kumar, K., Mele, P., Anitha, N., Saravanakumar, K., Sayed, M. A., Ali, A. M., & Amalraj, L. (2021). Synthesis and Properties of p-Si/n-Cd1−xAgxO Heterostructure for Transparent Photodiode Devices. Coatings, 11(4), 425. https://doi.org/10.3390/coatings11040425








