Corrosion Behavior of AA 1100 Anodized in Gallic-Sulfuric Acid Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Anodization of AA 1100 Samples
2.3. Sample Imaging and Corrosion Studies
3. Results and Discussion
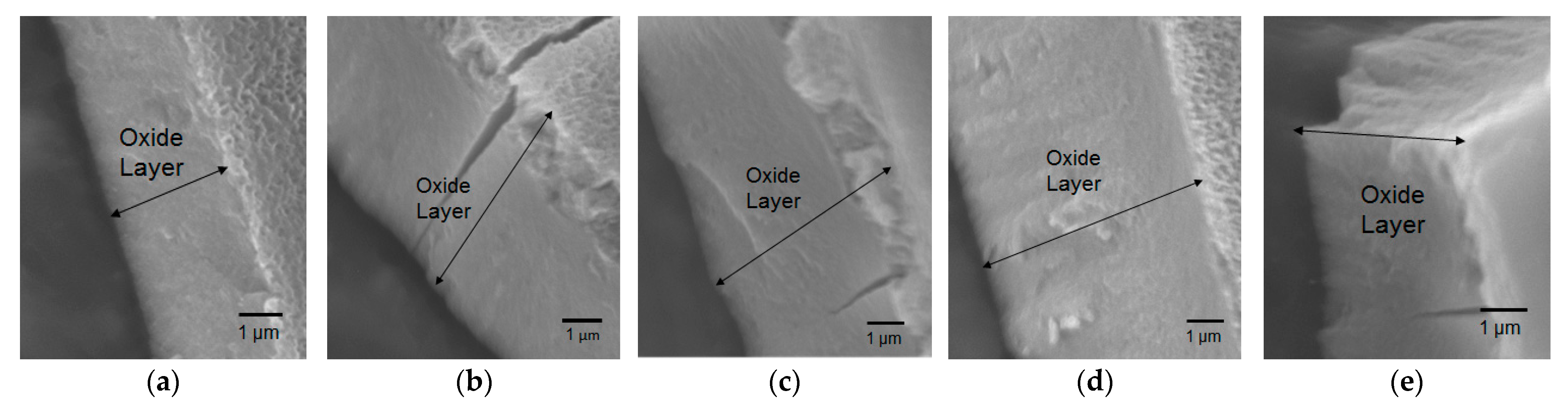
3.1. Imaging of Anodized Aluminum Oxide Samples
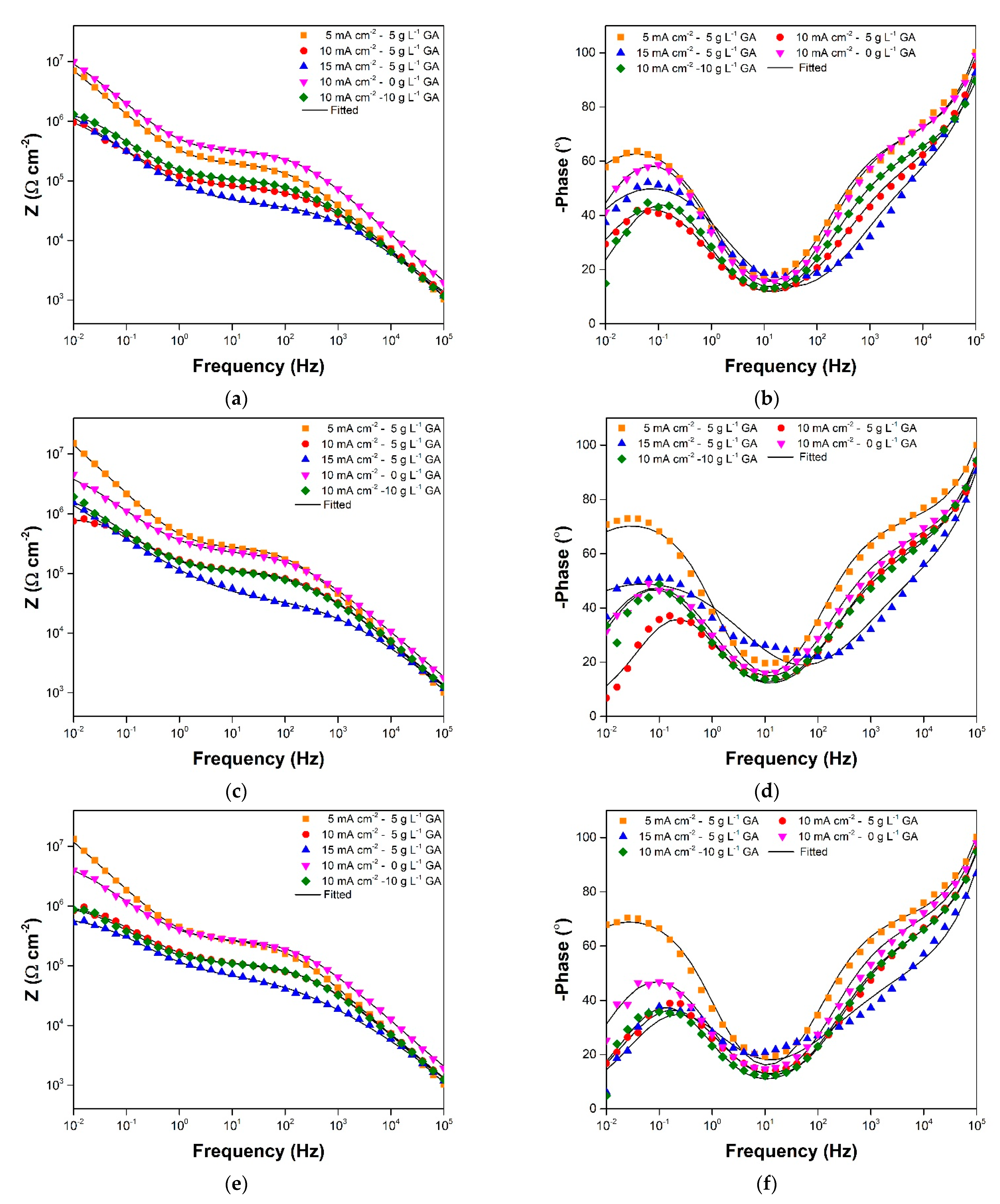
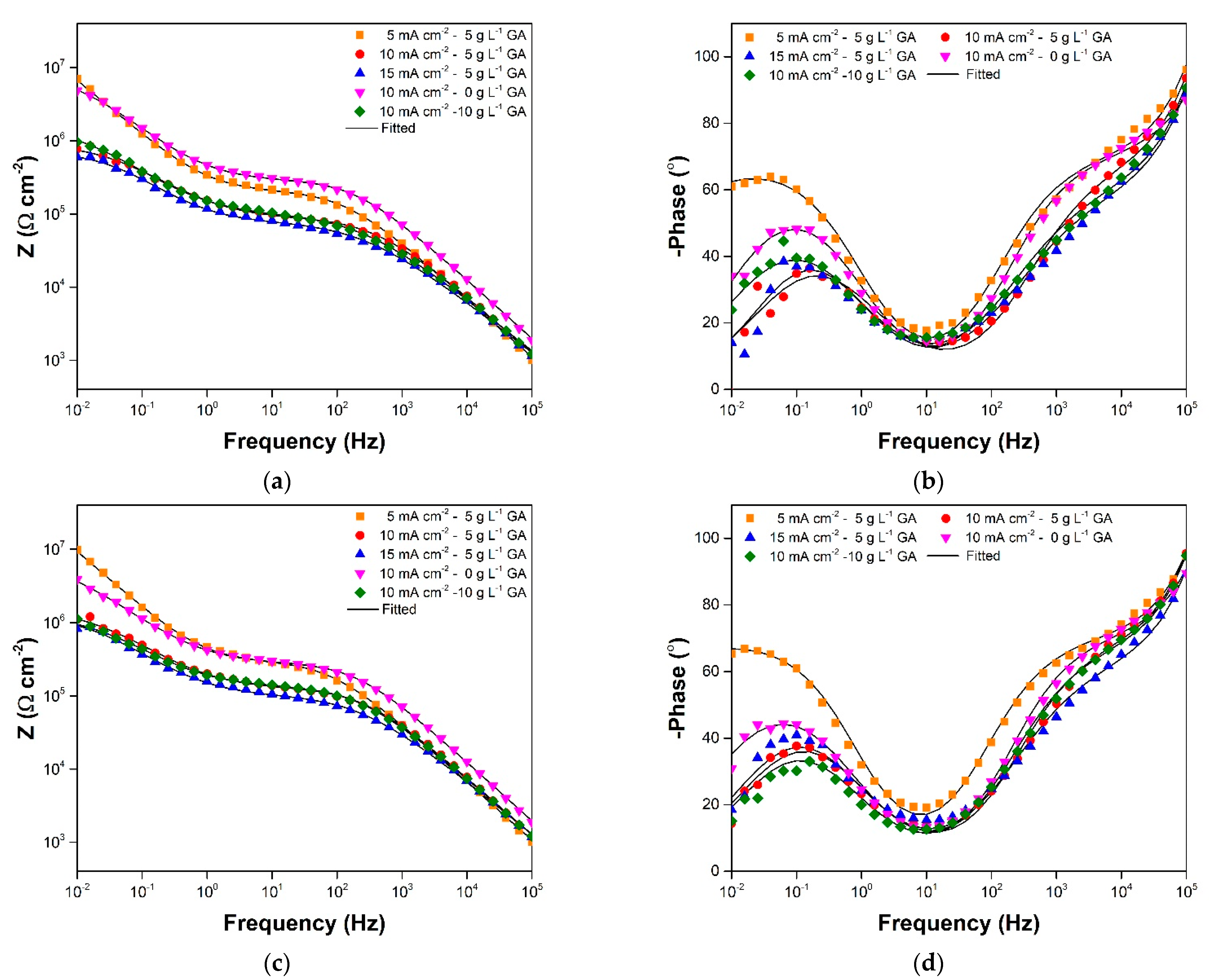
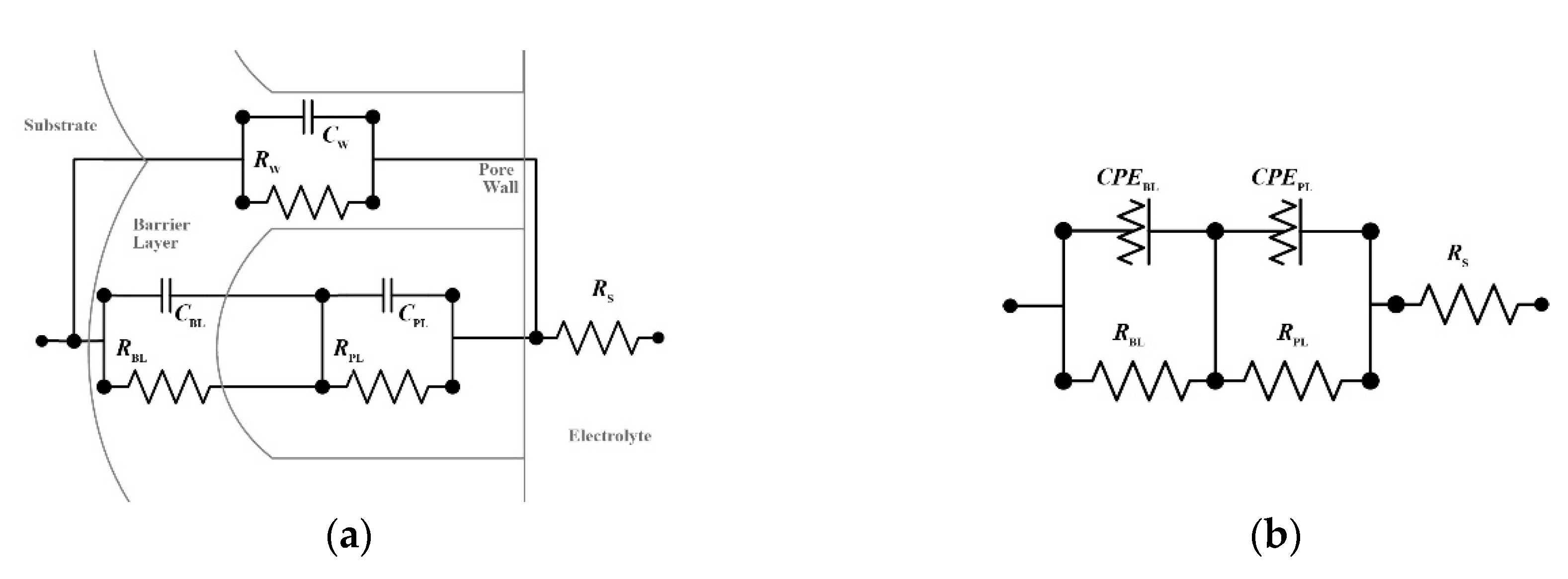
3.2. Electrochemical Impedance Spectroscopy
3.3. Linear Polarization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varshney, D.; Kumar, K. Application and use of different aluminium alloys with respect to workability, strength and welding parameter optimization. Ain Shams Eng. J. 2021, 12, 1143–1152. (in press). [CrossRef]
- Liu, G.; Müller, D.B. Addressing sustainability in the aluminum industry: A critical review of life cycle assessments. J. Clean. Prod. 2012, 35, 108–117. [Google Scholar] [CrossRef]
- Raj, V.; Mumjitha, M. Comparative study of formation and corrosion performance of porous alumina and ceramic nanorods formed in different electrolytes by anodization. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2014, 179, 25–35. [Google Scholar] [CrossRef]
- Cerchier, P.; Pezzato, L.; Gennari, C.; Moschin, E.; Moro, I.; Dabalà, M. PEO coating containing copper: A promising anticorrosive and antifouling coating for seawater application of AA 7075. Surf. Coat. Technol. 2020, 393, 125774. [Google Scholar] [CrossRef]
- Martínez-Viademonte, M.P.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A review on anodizing of aerospace aluminum alloys for corrosion protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Osborne, J.H. Observations on chromate conversion coatings from a sol-gel perspective. Prog. Org. Coat. 2001, 41, 280–286. [Google Scholar] [CrossRef]
- Xia, L.; McCreery, R.L. Chemistry of a chromate conversion coating on aluminum alloy AA2024-T3 probed by vibrational spectroscopy. J. Electrochem. Soc. 1998, 145, 3083–3089. [Google Scholar] [CrossRef]
- Cabral, J.; Gaona, C.; Estupinán, F.; Lara, M.; Zambrano, P.; Nieves, D.; Maldonado, E.; Chacón, J.; Almeraya, F. Corrosion resistance of hard coat anodized AA 6061 in citric-sulfuric solutions. Coatings 2020, 10, 601. [Google Scholar] [CrossRef]
- Boisier, G.; Pébère, N.; Druez, C.; Villatte, M.; Suel, S. FESEM and EIS study of sealed AA2024 T3 anodized in sulfuric acid electrolytes: Influence of tartaric acid. J. Electrochem. Soc. 2008, 155, C521. [Google Scholar] [CrossRef]
- Vignoli Machado, T.; Atz Dick, P.; Knörnschild, G.H.; Dick, L.F.P. The effect of different carboxylic acids on the sulfuric acid anodizing of AA2024. Surf. Coat. Technol. 2020, 383, 125283. [Google Scholar] [CrossRef]
- Stepniowski, W.J.; Michalska, M.; Norek, M.; Twardosz, E.; Florkiewicz, W.; Polkowski, W.; Zasada, D.; Bojar, Z. Anodization of cold deformed technical purity aluminum (AA1050) in oxalic acid. Surf. Coat. Technol. 2014, 258, 268–274. [Google Scholar] [CrossRef]
- Sulka, G.D.; Stepniowski, W.J. Structural features of self-organized nanopore arrays formed by anodization of aluminum in oxalic acid at relatively high temperatures. Electrochim. Acta 2009, 54, 3683–3691. [Google Scholar] [CrossRef]
- Lu, J.; Wei, G.; Yu, Y.; Guo, C.; Jiang, L. Aluminum alloy AA2024 anodized from the mixed acid system with enhanced mechanical properties. Surf. Interfaces 2018, 13, 46–50. [Google Scholar] [CrossRef]
- Nayeem, N.; Smb, A.; Salem, H.; Ahel-Alfqy, S. Gallic acid: A promising lead molecule for drug development. J Appl. Pharm. 2016, 8, 1–4. [Google Scholar] [CrossRef]
- Friedemann, W.; Germscheid, H.G. Sealing anodized aluminum. U.S. Patent US3838023A, 24 September 1974. [Google Scholar]
- Schaedel, F.C. Aerospace Anodizing Total Quality Improvement (TQI) Activation-Protection-Promotion with the Complete Spectrum Plus System. In Proceedings of the 2015 Anodizing Conference, San Diego, CA, USA, 15–17 September 2015. [Google Scholar]
- Ali, E.H.; Himdan, T.A.; Ahmed, Z.W. Gallic acid as corrosion inhibitor for aluminum 6061 in alkali solutions. Ibn AL-Haitham J. Pure Appl. Sci. 2019, 32, 16. [Google Scholar] [CrossRef]
- Keny, S.J.; Kumbhar, A.G.; Thinaharan, C.; Venkateswaran, G. Gallic acid as a corrosion inhibitor of carbon steel in chemical decontamination formulation. Corros. Sci. 2008, 50, 411–419. [Google Scholar] [CrossRef]
- Obot, I.B.; Madhankumar, A. Enhanced corrosion inhibition effect of tannic acid in the presence of gallic acid at mild steel/HCl acid solution interface. J. Ind. Eng. Chem. 2015, 25, 105–111. [Google Scholar] [CrossRef]
- Long, J.; Borissova, A.; Wilson, A.D.; Avelar-Batista Wilson, J.C. Sample preparation of anodised aluminium oxide coatings for scanning electron microscopy. Micron 2017, 101, 87–94. [Google Scholar] [CrossRef][Green Version]
- Chung, I.C.; Chung, C.K.; Su, Y.K. Effect of current density and concentration on microstructure and corrosion behavior of 6061 Al alloy in sulfuric acid. Surf. Coat. Technol. 2017, 313, 299–306. [Google Scholar] [CrossRef]
- Ono, S.; Asoh, H. Mechanism of hot water sealing of anodic films formed on aluminum. Corros. Sci. 2021, 181, 109221. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Ali, N.; Fawcett, D. Progress in nano-engineered anodic aluminum oxide membrane development. Materials 2011, 4, 487–526. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kawahara, K.; Kikuchi, T.; Suzuki, R.O.; Natsui, S. Corrosion-resistant porous alumina formed via anodizing aluminum in etidronic acid and its pore-sealing behavior in boiling water. J. Electrochem. Soc. 2019, 166, C261–C269. [Google Scholar] [CrossRef]
- Vrublevsky, I.; Parkoun, V.; Sokol, V.; Schreckenbach, J. Study of chemical dissolution of the barrier oxide layer of porous alumina films formed in oxalic acid using a re-anodizing technique. Appl. Surf. Sci. 2004, 236, 270–277. [Google Scholar] [CrossRef]
- Usman, B.J.; Scenini, F.; Curioni, M. Corrosion testing of anodized aerospace alloys: Comparison between immersion and salt spray testing using electrochemical impedance spectroscopy. J. Electrochem. Soc. 2020, 167, 041505. [Google Scholar] [CrossRef]
- Huang, Y.; Shih, H.; Huang, H.; Daugherty, J.; Wu, S.; Ramanathan, S.; Chang, C.; Mansfeld, F. Evaluation of the corrosion resistance of anodized aluminum 6061 using electrochemical impedance spectroscopy (EIS). Corros. Sci. 2008, 50, 3569–3575. [Google Scholar] [CrossRef]
- Moutarlier, V.; Gigandet, M.P.; Pagetti, J.; Ricq, L. Molybdate/sulfuric acid anodising of 2024-aluminium alloy: Influence of inhibitor concentration on film growth and on corrosion resistance. Surf. Coat. Technol. 2003, 173, 87–95. [Google Scholar] [CrossRef]
- Bouchama, L.; Azzouz, N.; Boukmouche, N.; Chopart, J.P.; Daltin, A.L.; Bouznit, Y. Enhancing aluminum corrosion resistance by two-step anodizing process. Surf. Coat. Technol. 2013, 235, 676–684. [Google Scholar] [CrossRef]
- González-Rovira, L.; González-Souto, L.; Astola, P.J.; Bravo-Benítez, C.; Botana, F.J. Assessment of the corrosion resistance of self-ordered anodic aluminum oxide (AAO) obtained in tartaric-sulfuric acid (TSA). Surf. Coat. Technol. 2020, 399, 126131. [Google Scholar] [CrossRef]
- Zhao, X.H.; Zuo, Y.; Zhao, J.M.; Xiong, J.P.; Tang, Y.M. A study on the self-sealing process of anodic films on aluminum by EIS. Surf. Coat. Technol. 2006, 200, 6846–6853. [Google Scholar] [CrossRef]
- Suay, J.J.; Giménez, E.; Rodríguez, T.; Habbib, K.; Saura, J.J. Characterization of anodized and sealed aluminium by EIS. Corros. Sci. 2003, 45, 611–624. [Google Scholar] [CrossRef]
- Moutarlier, V.; Gigandet, M.P.; Normand, B.; Pagetti, J. EIS characterisation of anodic films formed on 2024 aluminium alloy, in sulphuric acid containing molybdate or permanganate species. Corros. Sci. 2005, 47, 937–951. [Google Scholar] [CrossRef]
- Vrublevsky, I.; Parkoun, V.; Sokol, V.; Schreckenbach, J.; Marx, G. The study of the volume expansion of aluminum during porous oxide formation at galvanostatic regime. Appl. Surf. Sci. 2004, 222, 215–225. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Liao, Y.; Chen, X.; Zhang, C.; Wu, H.; Wang, Z.; Huang, W. Effect of anodizing parameters on film morphology and corrosion resistance of AA2099 aluminum-lithium alloy. J. Electrochem. Soc. 2016, 163, C369–C376. [Google Scholar] [CrossRef]
- Ó’Coinceanainn, M.; Hynes, M.J. The kinetics and mechanisms of the reactions of aluminium(III) with gallic acid, gallic acid methyl ester and adrenaline. J. Inorg. Biochem. 2001, 84, 1–12. [Google Scholar] [CrossRef]
- García-Rubio, M.; de Lara, M.P.; Ocón, P.; Diekhoff, S.; Beneke, M.; Lavía, A.; García, I. Effect of postreatment on the corrosion behaviour of tartaric-sulphuric anodic films. Electrochim. Acta 2009, 54, 4789–4800. [Google Scholar] [CrossRef]
- Ezuber, H.; El-Houd, A.; El-Shawesh, F. A study on the corrosion behavior of aluminum alloys in seawater. Mater. Des. 2008, 29, 801–805. [Google Scholar] [CrossRef]
- Toshev, Y.; Mandova, V.; Boshkov, N.; Stoychev, D.; Petrov, P.; Tsvetkova, N.; Raichevski, G.; Tsvetanov, C.; Gabev, A.; Velev, R.; et al. Protective coating of zinc and zinc alloys for industrial applications. In Proceedings of the 4M 2006—Second International Conference on Multi-Material Micro Manufacture; Elsevier: Amsterdam, Netherlands, 2006; pp. 323–326. [Google Scholar]


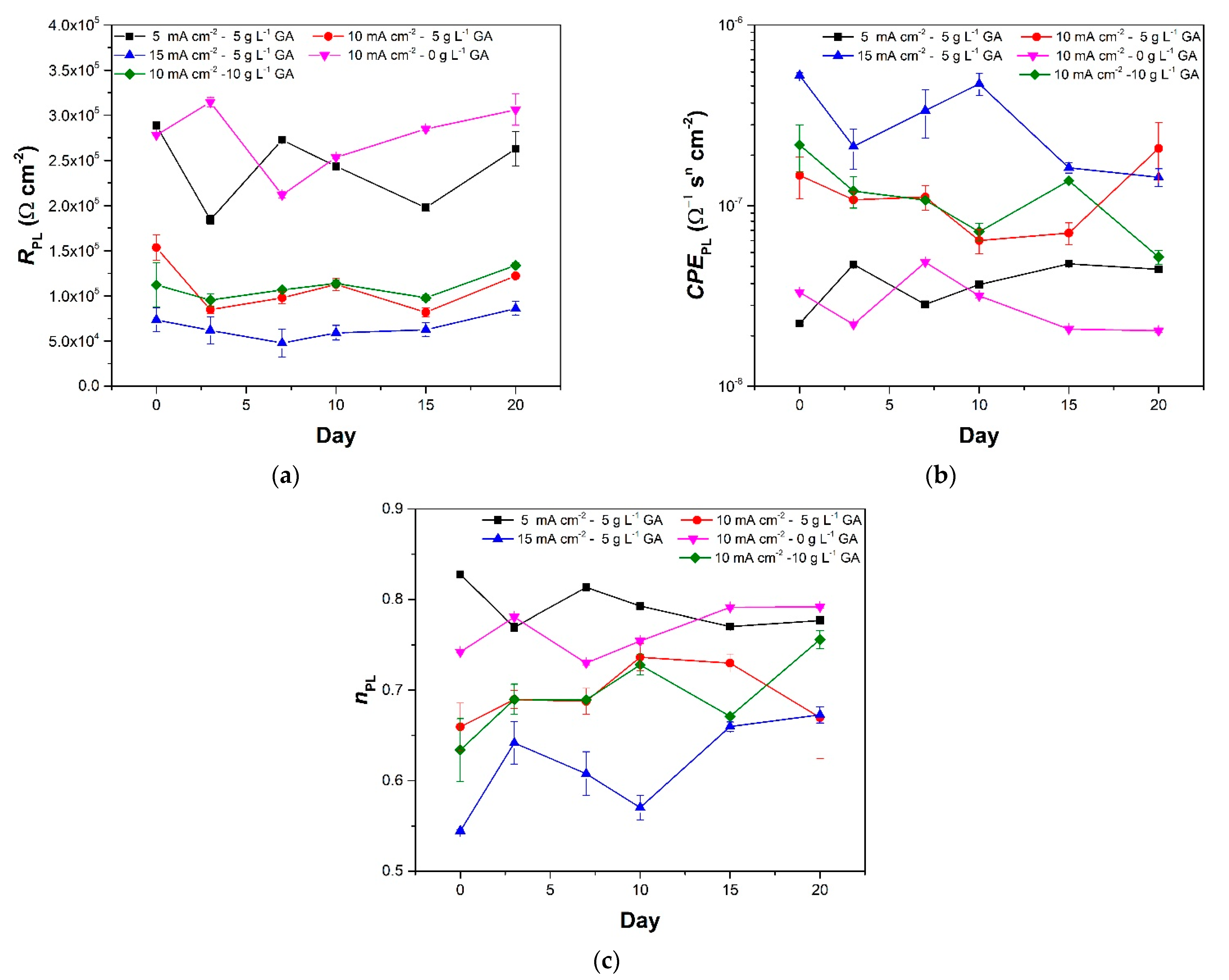

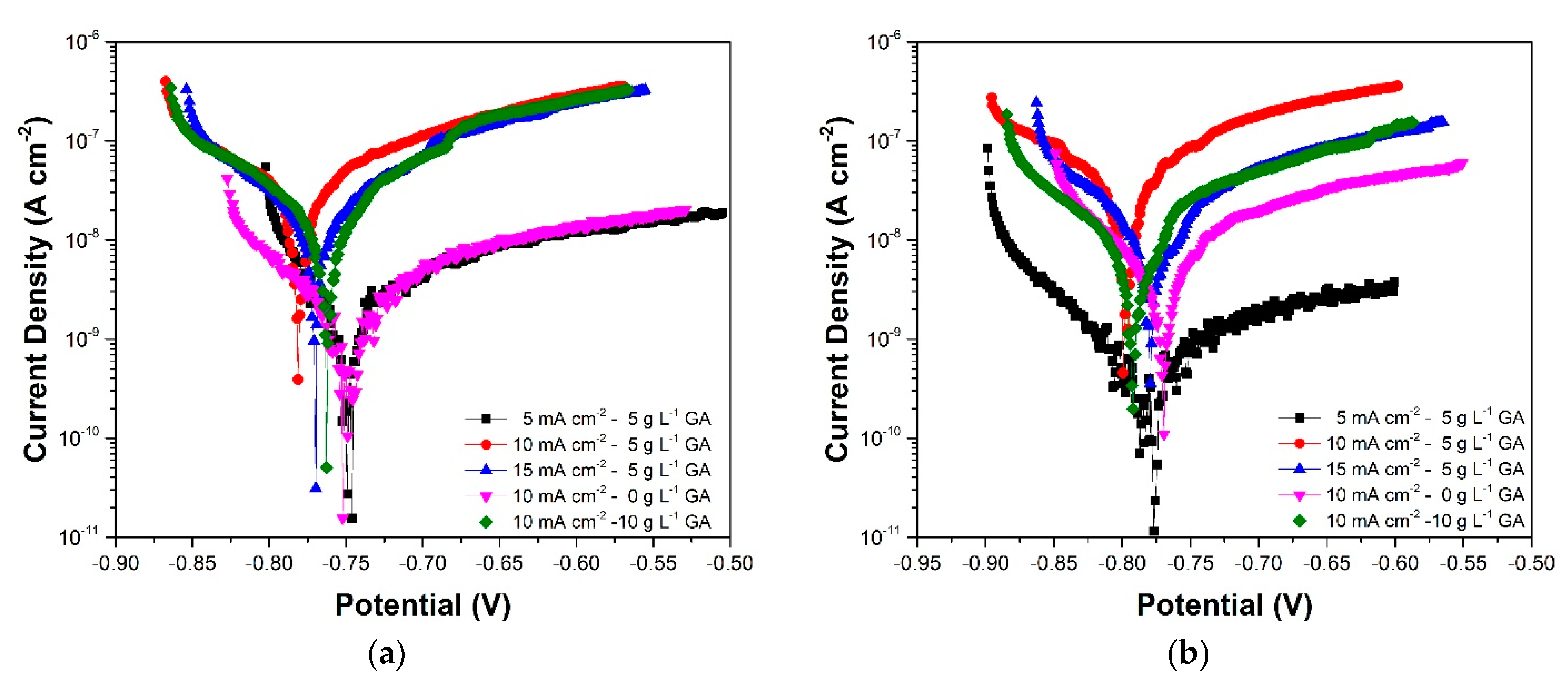
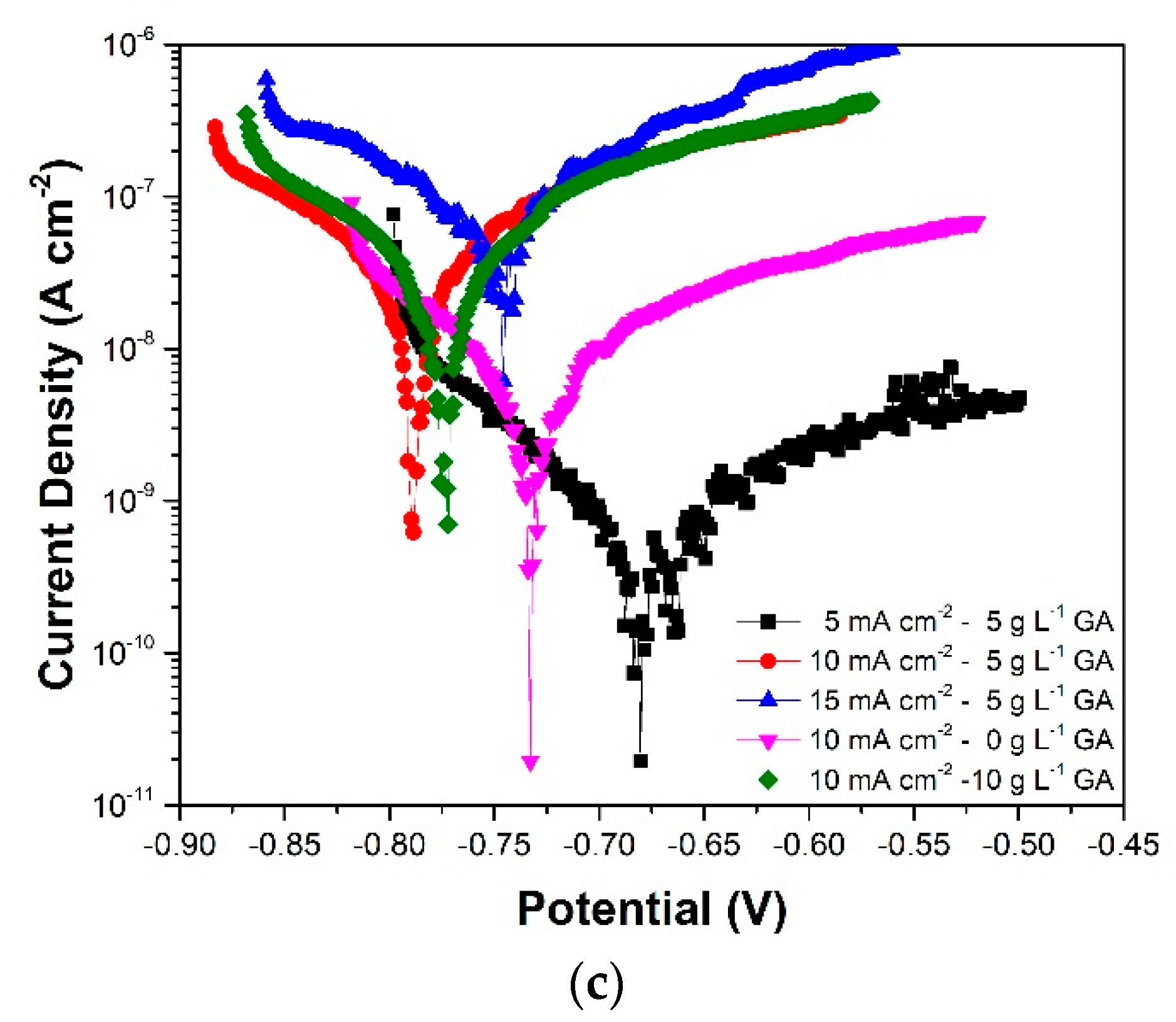
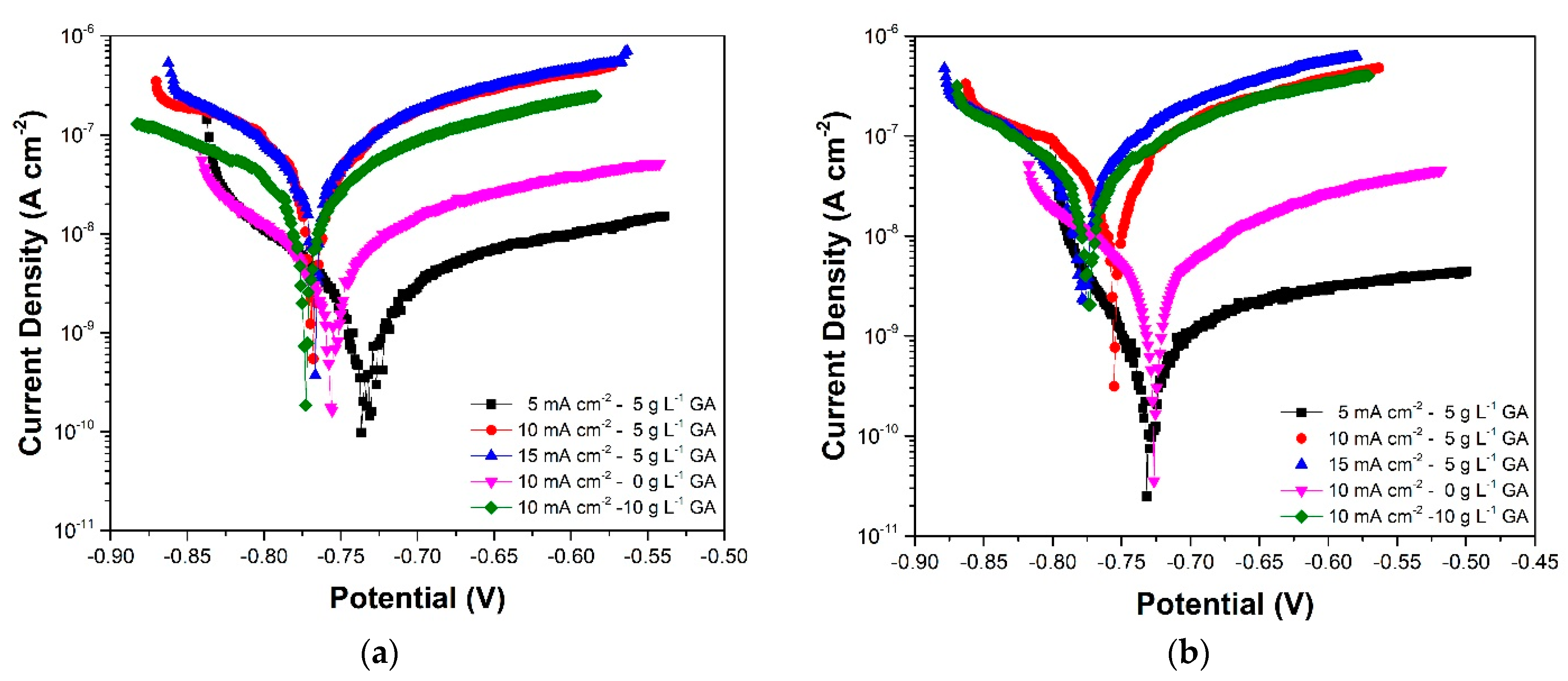
| Sample | Gallic Acid Concentration (g·L−1) | Anodization Current Density (mA·cm−2) | Sulfuric Acid Concentration (g·L−1) |
|---|---|---|---|
| 5CD-5GA | 5 | 5 | 50 |
| 10CD-5GA | 5 | 10 | 50 |
| 15CD-5GA | 5 | 15 | 50 |
| 10CD-0GA | 0 | 10 | 50 |
| 10CD-10GA | 10 | 10 | 50 |
| Sample | Ecorr (V vs. Ag/AgCl (3M)) | jcorr (A·cm−2) | RP (Ω·cm−2) | |
|---|---|---|---|---|
| 5CD-5GA | (3 days) | −0.749 | 8.99 × 10−10 | 1.38 × 107 |
| (7 days) | −0.786 | 7.88 × 10−10 | 5.88 × 107 | |
| (10 days) | −0.683 | 1.96 × 10−10 | 5.64 × 107 | |
| (15 days) | −0.733 | 1.68 × 10−9 | 1.71 × 107 | |
| (20 days) | −0.73 | 5.76 × 10−10 | 3.82 × 107 | |
| 10CD-5GA | (3 days) | −0.78 | 3.28 × 10−8 | 8.76 × 105 |
| (7 days) | −0.801 | 8.19 × 10−8 | 6.74 × 105 | |
| (10 days) | −0.791 | 5.51 × 10−8 | 1.03 × 106 | |
| (15 days) | −0.767 | 1.80 × 10−7 | 6.66 × 105 | |
| (20 days) | −0.755 | 1.36 × 10−8 | 1.02 × 106 | |
| 15CD-5GA | (3 days) | −0.771 | 6.07 × 10−8 | 1.54 × 106 |
| (7 days) | −0.775 | 9.72 × 10−9 | 2.19 × 106 | |
| (10 days) | −0.746 | 1.18 × 10−7 | 3.71 × 105 | |
| (15 days) | −0.767 | 1.28 × 10−7 | 6.01 × 105 | |
| (20 days) | −0.778 | 5.26 × 10−8 | 7.69 × 105 | |
| 10CD-0GA | (3 days) | −0.753 | 9.56 × 10−10 | 1.63 × 107 |
| (7 days) | −0.77 | 1.54 × 10−8 | 4.35 × 106 | |
| (10 days) | −0.733 | 2.15 × 10−8 | 5.40 × 106 | |
| (15 d) | −0.755 | 1.17 × 10−8 | 6.13 × 106 | |
| (20 d) | −0.729 | 1.62 × 10−8 | 7.84 × 106 | |
| 10CD-10GA | (3 d) | −0.763 | 4.56 × 10−8 | 1.62 × 106 |
| (7 d) | −0.791 | 3.12 × 10−8 | 2.83 × 106 | |
| (10 d) | −0.776 | 1.51 × 10−7 | 9.96 × 105 | |
| (15 d) | −0.771 | 1.00 × 10−7 | 1.30 × 106 | |
| (20 d) | −0.774 | 1.69 × 10−7 | 8.11 × 105 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mopon, M.L., Jr.; Garcia, J.S.; Manguerra, D.M.; Narisma, C.J.C. Corrosion Behavior of AA 1100 Anodized in Gallic-Sulfuric Acid Solution. Coatings 2021, 11, 405. https://doi.org/10.3390/coatings11040405
Mopon ML Jr., Garcia JS, Manguerra DM, Narisma CJC. Corrosion Behavior of AA 1100 Anodized in Gallic-Sulfuric Acid Solution. Coatings. 2021; 11(4):405. https://doi.org/10.3390/coatings11040405
Chicago/Turabian StyleMopon, Marlon L., Jr., Jayson S. Garcia, Dexter M. Manguerra, and Cyril John C. Narisma. 2021. "Corrosion Behavior of AA 1100 Anodized in Gallic-Sulfuric Acid Solution" Coatings 11, no. 4: 405. https://doi.org/10.3390/coatings11040405
APA StyleMopon, M. L., Jr., Garcia, J. S., Manguerra, D. M., & Narisma, C. J. C. (2021). Corrosion Behavior of AA 1100 Anodized in Gallic-Sulfuric Acid Solution. Coatings, 11(4), 405. https://doi.org/10.3390/coatings11040405






