Abstract
This review article was developed based on the scientometric analysis of the evaluated studies conducted on titanium−zirconium (TixZr) alloys from 2000 to the present. The scientometric data obtained helped us to identify the most researched topics and these topics were further analyzed and discussed. An increasing number of researchers are considering TixZr alloys as opposed to the traditional ones because these alloys present improved mechanical properties and in some cases improved corrosion resistance and biocompatibility. Due to the natural layer of oxides formed on these alloys, multiple surface modification methods can be applied to solve some of the challenges faced in the field of implantable materials. A significant number of studies are now focusing on surface modifications at the nanometer scale or various coatings for improved corrosion resistance and biological interactions. Although not yet commercially available, a TiZr alloy with a nanostructured surface and embedded biologically active substances, such as antibiotics or coated with hydroxyapatite, may become a future option.
1. Introduction
Due to the good biocompatibility of Ti, research on Ti alloys used as biomaterials is on a continuous upward trend [1,2]. A radical increase in research related to zirconium dioxide (ZrO2) has also been observed due to increased mechanical strength, biocompatibility, and aesthetic properties in the case of dental work [1,3]. In this context, the titanium−zirconium (TixZr) alloys have been intensively investigated in the last decade.
The mechanical properties, porosity, surface morphology, and implant design are the determining factors for the evolution of osseointegration and longevity of an implant [4,5].
Implantable materials must have mechanical properties such as a modulus of elasticity, yield strength, and ultimate tensile strength that can withstand various biomechanical forces. Besides, biomaterials must be biocompatible, have low density and increased resistance to corrosion and wear [6].
Commercially pure titanium (cpTi) was the most attractive alternative for implants [7,8]. However, the manufacture of implants with small diameters (≤3.5 mm) from cp-Ti is associated with an increased risk of fracture due to insufficient mechanical strength. The aim is to develop new alloys and coatings to solve this problem [8,9,10,11,12,13,14].
Zr as Ti is situated in Group IV of the periodic table of elements, therefore the two elements have similar chemical properties and biocompatibilities [15,16]. Thus, TixZr type alloys began to be studied more and more frequently, with the possibility to solve these problems, as both have satisfactory mechanical properties and good biocompatibility [10,17,18,19,20].
It is known that a new alloying strategy involving the combination of several principal elements in high concentrations has been in development in the few years. The goal is to elaborate new materials named high entropy alloys with remarkable properties exceeding those of conventional alloys. Some of them contain Ti and Zr [21,22,23] and have potential implant applications [24].
Compared to other traditional biomaterials, some of the binary TixZr alloys have a higher strength/weight ratio, lower modulus of elasticity and better corrosion resistance [25,26,27]. Moreover, on the surface of alloys containing titanium and zirconium, a layer of apatite is formed that is structurally similar to bone tissue, which improves the bioactivity of these materials [28,29].
The present manuscript has a novel approach being a combined scientometric and critical one in reviewing the behavior of TiZr alloys based on their composition, surface and interface.
2. Methods
A bibliographic search was conducted in the ScienceDirect database using the terms “titanium zirconium implants” and “TiZr implants”, choosing only the “research” and “review” articles published in English from 2000 to the present (5 February 2021). Additionally, to ensure that most of the relevant studies regarding the chosen topic were taken into consideration, multiple articles were selected manually.
The selected articles were carefully analyzed considering the purpose of this review article. The inclusion criteria involved studies that examined binary TixZr alloys or those that studied relevant characteristics related to the surface of this type of alloy. The aspects monitored and evaluated were those related to changes at the micro and nanometric scale, the methods used, mechanical properties, corrosion resistance and biological interactions.
The VOSviewer software (version: 1.6.16) was used for the scientometric analysis. The evaluated aspects are co-authorship (unit of analysis: authors), co-occurrence of keywords and terms from titles and abstracts. In all three cases, the maps created from the network analysis were based on the selected bibliographic data.
For the authors to be included in the co-authorship analysis, a minimum number of 2 articles was selected for the keywords to be included in the co-occurrence analysis, a minimum number of 2 occurrences was selected and for the terms from titles and abstracts, a minimum number of 10 occurrences was selected.
3. Results and Discussions
The search identified 2508 articles in the ScienceDirect database. After reading the titles and abstracts, 2344 were excluded. The remaining 164 articles were further evaluated, from which 128 articles were selected based on the inclusion criteria. Another 35 articles were selected manually. The selection process is illustrated in Figure 1.

Figure 1.
Evaluation process.
3.1. Scientometric Analysis
For the scientometric analysis we decided upon visual representations coupled with some quantitative data. In the networks constructed, the link between two authors indicates that they have co-authored publications, while the strength of a link indicates the number of co-authored publications. In the case of terms used (keywords or other terms), the link indicates that the terms co-occur in the same publication, while the strength of the link indicates the number of publications in which the terms co-occur.
3.1.1. Co-Authorship
From the total of 858 authors, 100 meet the threshold. The map is presented in Figure 2. The highest number of publications for the analyzed data was 20 with a corresponding total link strength of 70, while the lowest was two with zero total link strength.
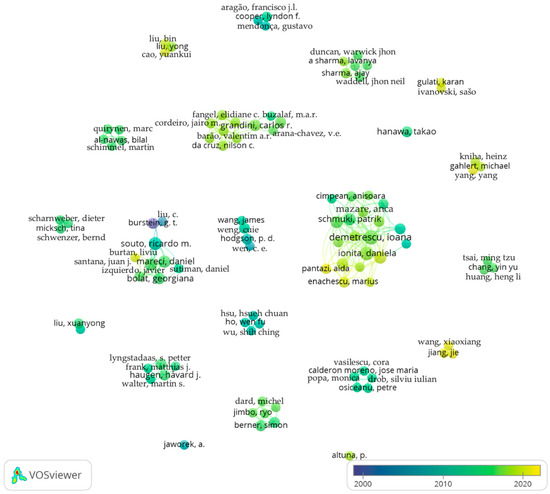
Figure 2.
Co-authorship network.
It can be observed from the obtained data that multiple, groups of authors are studying metallic biomaterials and in particular titanium alloys, such as TixZr. Additionally, an increasing trend can be observed in the last few years, compared with the starting period considered.
3.1.2. Co-Occurrence
From the total of 586 keywords, 121 meet the threshold. The map is presented in Figure 3, while the top 10 keywords based on total link strength are presented in Table 1.

Figure 3.
Co-occurrence of keywords.

Table 1.
Top 10 keywords based on total link strength from the selected bibliography.
Some limitations regarding the quantitative data come from the different name attributed to the same subject. As it can be seen in the visual representation, TixZr alloys have been named “ti-zr alloy”, “tizr”, “tizr alloy”, which indicate a much higher value for occurrences and the total link strength in this case.
The larger, central nodes indicate that the keywords were more frequently found, which also implies that the research on that particular subject began a long time ago (e.g., titanium, dental implants, osseointegration). In contrast, the marginal nodes indicate either that the topics have started to be studied more recently (e.g., antibacterial activity, bacterial adhesion, bone-implant interface, nanostructures) or that the topics were studied in the past. These aspects are also visible in the color of the nodes, which is based on the year of publishing. Moreover, the links between the nodes highlight the correlation between the subjects.
Overall, the combined data suggest that the TixZr type of alloys are intensively studied as an alternative for the conventional ones. Additionally, a trend is observed in the direction of nanostructured surface modifications with antibacterial properties.
3.1.3. Co-Occurrence (Terms from Titles and Abstracts)
From the total of 4947 terms, 128 meet the threshold. For each of the 128 terms the software calculates a relevance score to eliminate the general terms that have no useful information. Based on this score, 60% most relevant terms were selected, resulting in seven terms. Even so, some usual terms such as “day”, “contact”, “week” etc. were included. Therefore, these terms were excluded manually to obtain only the relevant terms. Finally, several two terms were selected. The map is presented in Figure 4, while the top 15 terms based on occurrences are presented in Table 2.
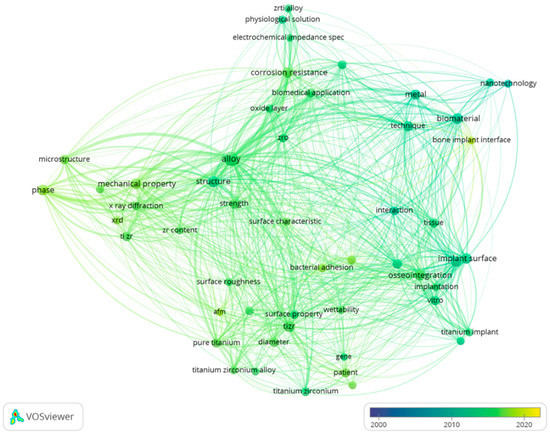
Figure 4.
Co-occurrence of terms from titles and abstracts.

Table 2.
Top 15 terms from titles and abstracts based on occurrences from the selected bibliography.
The co-occurrence of terms from titles and abstracts indicates furthermore the main subjects of interest. By analyzing the data provided in the visual representation through the size of nodes, the links between them and their color it can be concluded that different alloys are increasingly studied as biomaterials. The mechanical properties and the corrosion resistance of these alloys appear to be mentioned more throughout time, but an interest regarding surface modifications in relation to different biological aspects seems to emerge in more recent studies.
The top terms combined with the visual representation helped us to establish the most studied topics that are followed throughout this article such as type of alloy, mechanical properties, surface modifications and biocompatibility.
3.2. Properties Related to the Composition of TixZr Alloys
Bone tissue is a type of connective tissue made up of cells and fibers. From the perspective of mechanical properties, two types of bone tissue are differentiated, namely cortical and trabecular. Cortical bone has a higher density and low porosity (10%), having a modulus of elasticity between 4–30 GPa and a compressive strength between 20–193 MPa. The trabecular bone has a low density, being composed in a proportion of 50–90% from pores. This is also reflected in the modulus of elasticity which is between 0.2–2 GPa and the compressive strength which is between 2–80 MPa [30]. The modulus of elasticity of biomaterials should be close to that of the bones. If the biomaterial presents a significantly higher modulus of elasticity, a phenomenon known as stress shield may occur, which is characterized by a reduction in bone density (osteopenia) [28].
The interstitial elements, carbon (C), oxygen (O), nitrogen (N) and hydrogen (H) have a hardening effect on transition metals due to the high suppression of dislocation movements and network distortion [31]. Of these, O has the highest solubility in Ti and a considerable hardening effect. In TixZr alloys, an increase in hardness has been reported with an increase in O content [31,32]. Additionally, increasing the concentration of Zr leads to finer grains, which together with the strengthening of the solid solution leads to increased hardness, strength and plasticity. At the same time, an increased performance regarding osteoinduction is obtained due to better adhesion of proteins [10].
Zirconium is considered an isomorphic stabilizer being completely soluble in Ti, it can exist in both pure α and β phase [33]. In solid solution with another β stabilizer, Zr can also act as a β stabilizer [34]. The α and α−β mix phases have good strength and creep resistance, but β alloys have a better fatigue strength and lower Young’s modulus [33]. The Zr content in the allotropic form α′ (hexagonal martensite) increases the modulus of elasticity [35]. While the α phase has small benefits at the temperature and stress associated with implants, the β phase is especially beneficial for orthopedic implants and any long-term implant [33,36].
As high entropy alloys (HEAs) have exceptional mechanical and corrosion behavior new phases derived from the AlCoFeNiSmTiVZr [37] and from Ti−Nb−Ta−Zr after Al addition have also been investigated [38] establishing the formation of the intermetallic phase after annealing at 600–1200 °C.
A study performed on TixZr alloys (x = 10, 20, 30, 40 wt.%) shows that the microhardness values, the bending strengths and the elastic recovery angles increase with the content of Zr, being much higher compared to cpTi [2]. In the case of increasing the Zr content, in ZrxTi alloys (x = 10, 20, 30, 40 wt.%) the properties no longer vary constantly with the Ti content. Among the studied alloys, the best values of microhardness and bending modulus were reported for Ti-40Zr wt.% [39]. TixZr binary alloys with high Zr content also present high strength [40]. The hardness of Ti50Zr alloy (at.%) is 2.5 times higher than pure Ti or Zr [28,41].
Current information shows that TiZr alloys can also be manufactured to have a porous structure and mechanical properties similar to those of bone tissue [29,42,43]. Wen et al. report the manufacture of TiZr alloy foams with a density of approximately 0.3 and pores of 200–500 μm. The Young’s modulus for these is between 78.4 MPa and 15.3 GPa [29]. Porous bulk metallic glass based on Ti-Zr has also been studied as an alternative to conventional materials [44,45].
Comparative analysis regarding the mechanical properties between different studies was difficult to perform for several reasons. On the one hand, different alloy manufacturing techniques affect their structure and thus their mechanical properties, even if the composition of the alloys is the same. On the other hand, the evaluated characteristics and the evaluation methods differ, which induces a greater variability. However, an attempt was made to summarize the data and draw some conclusions. The observations are found in Table 3.

Table 3.
Comparative aspects Ti−TixZr−Zr.
It can be seen that TixZr type alloys and especially Ti50Zr have improved mechanical properties compared to the constituent metals.
In other studies, it is also remarked that the mechanical properties of Ti are superior to Zr, as well as those of osseointegration, but Zr is superior to Ti regarding bacterial adhesion [46]. The addition of Zr in Ti increases the hardness due to the transformation of the structure from α (hexagonal) to α′ (orthorhombic) [47]. The microhardness, mechanical strength and plasticity also increase with increasing Zr content [48]. Ti50Zr presents significantly increased hardness due to solid solution strengthening, an effect caused by the differences between the atomic radii of Ti and Zr [41] and increased corrosion resistance compared to the individual metals (Zr or Ti) [49]. It is noted as well that the addition of Zr significantly increases the corrosion resistance, but up to a maximum content of 50% Zr [50]. In terms of biocompatibility, TixZr alloys present similar properties compared to Ti [51] or even better [12].
3.3. Surface of TixZr Alloys
Material surface is of particular importance regarding the interaction with the body. This should stimulate the integration with the surrounding tissue and prevent bacterial adhesion and colonization. Obtaining these characteristics is a difficult task because the surfaces of biomaterials that facilitate cell adhesion are also favorable for the adhesion of bacteria, the mechanisms being similar [56,57].
The physicochemical properties of the implant surface directly influence the formation of new bone tissue. This phenomenon is known as contact osteogenesis. Thus, over time, several types of implants have been studied, from solid and macroporous to modified surfaces at the micrometric, submicrometric and more recently, nanometric scale [58].
Surface characteristics are generally separated into two categories, topographic and chemical. Topographic characteristics refer to the surface roughness, which can be evaluated by several parameters, of which the most commonly used is Ra, the arithmetic mean of deviations from the mean line. Three-dimensional parameters are also used, such as Sa, which represents the arithmetic mean of deviations from the mean plane of analysis. A problem in evaluating this parameter is that these measurement techniques are developed for flat surfaces and are less suitable for threaded implants, such as screws [59].
As the surface is the only region that comes into contact with bone tissue, much of the studies have focused on modifying certain properties such as chemical composition and roughness to promote osseointegration and mechanical fixation. The increase in roughness leads to a larger contact surface for proteins and cells to interact with the material [60]. In contrast, the metallic biomaterials that are designed to come into direct contact with whole blood (heart valves, heart aid devices, heart pumps) must have low thrombogenicity [61] and high surface roughness, as well as high surface energy, which is associated with high thrombogenicity [9,62]. Thus, when designing the biomaterial surfaces multiple aspects must be considered.
Surface chemistry is relevant through composition, biocompatible metals such as Ti and Zr, being valve metals, form a natural layer of oxides that represent a barrier in the corrosion process, but also through other properties such as surface energy or surface charge. It is important to note that changes in topography often induce changes in chemistry, or vice versa [59,63,64]. TixZr type alloys have a better wettability than cpTi, which leads to a different biological behavior [17]. Studies suggest that surfaces with higher hydrophilicity lead to better tissue integration, osseointegration and faster healing [65].
Surface modifications of metallic biomaterials mainly aim at increasing corrosion resistance and improving bioactivity [30,66,67]. Additionaaly, because after surgery there is an increased risk of developing a bacterial infection and oral antibiotics are generally given, a more effective alternative could be to load implants with active substances that act locally, over time [7,12,68,69,70].
The techniques used can be mechanical, chemical or physical. Mechanical methods include grinding, polishing and blasting. Chemical methods include treatments with acids, hydrogen peroxide, alkaline solutions, sol-gel techniques, anodic oxidation, micro-arc oxidation (MAO) and chemical vapor deposition. Physical methods include physical vapor deposition, femtosecond laser ablation in solution (FLAS) and several types of thermal spraying methods [71,72,73,74,75,76,77].
The naturally formed oxide film on the surface of Ti is composed of several layers, namely TiO (in contact with the metal), Ti2O3 (intermediate layer) and TiO2 (outer layer). Oxidation processes can increase the concentration of TiO2 quite a lot so that the layers of suboxides can no longer be detected, but the organization exists [74].
Oxidic compounds formed by the natural phenomenon of passivation may result from the following oxidation reactions [25]:
The Gibbs free energy for Zr oxidation (−1117.8 kJ/mol) is greater than that for Ti oxidation (−890.5 kJ/mol), which suggests that Zr is preferentially oxidized [78]. However, a study performed on the oxide film developed by Ti50Zr alloys (at.%) through anodization (9 V) in different solutions shows that the mobility of Ti4+ ions is higher than that of Zr4+ ions, the outer layer being composed mostly of TiO2. The average reported composition was 76% TiO2 and 24% ZrO2 [79]. This aspect is revealed in another study on TixZr alloys (x = 23, 42, 62.5 at.%), showing that the outer layer is composed of TiO2 and that it decreases in thickness with increasing Zr content. The reported composition of the inner layers was (Ti0.74Zr0.26)O2, (Ti0.56Zr0.44)O2, and (Ti0.365Zr0.635)O2 [80].
The methods used to modify the surfaces of TixZr alloys mentioned in the literature, as well as some relevant aspects are summarized in Table 4.

Table 4.
Methods for surface modifications.
3.3.1. Surface Chemistry
Even biomaterials such as TixZr, considered “inert”, degrade to some extent over time, resulting in increased concentrations of metal ions. The release of metal ions from the implant takes place through the dissolution of the passive layer, mechanical wear and electrochemical corrosion. Metal ions can exist as such, but also in organometallic complexes, metal oxides or even nanoparticles [96]. Zirconium is considered an anodic alloying element for Ti, which reduces anodic activity [97].
Implant corrosion leads to a decrease in their resistance and the release of metal ions in the surrounding tissues [98]. Valve metals, which develop a layer of oxide on the surface through exposure to air, have good corrosion resistance [30,99]. If the oxide layer is affected, the metal ions are released continuously until repassivation takes place [30].
Despite the fact that the passive layer functions as a physical barrier responsible for corrosion resistance that significantly hinders the release of metal ions into the surrounding tissues, small amounts of metal ions are still released from the metallic material. This process is a part of transient breakdown and reforming events of this film and to metal debris with acidification as a detriment to the stability of the passive regime. The breakdown of the titanium oxide layer by nucleation of corrosion pits occurs in bioliquids such as Ringer’s solution at electrode potentials, well below the pitting potential. The frequency of breakdown increases significantly with pH value decrease, with temperature increase and in particular in the presence of chloride anions [100,101,102,103].
A study evaluating the corrosion resistance of Ti, TixZr (x = 30, 50, 70%) and Zr in a lactic acid + NaCl solution and artificial saliva solution shows that the addition of Zr significantly increases the corrosion resistance, but up to a maximum content of 50% [50].
Although the oxide layer forms naturally on the surface of TixZr alloys, it is thin and weak. To improve Zr20Ti and Zr40Ti implants, oxidation at temperature (500 °C, 2 h) was proposed, which led to the formation of an oxide layer of ZrO2 and ZrTiO4 with a thickness of 11–14 μm. The oxide layer formed had both increased corrosion resistance and better wear resistance, the wear rate being almost 10 times lower in the case of Zr20Ti and almost 20 times in the case of Zr40Ti [18].
Regarding the electrochemical stability of TixZr implants, it was observed that the presence of fluoride anions can have a negative impact, favoring corrosion. Given that these implants are frequently used as dental implants and that fluoride is introduced into toothpaste and mouthwash to prevent cavities, solutions must be found. It has been observed that the oxide film reacts with these anions, resulting in titanium fluoride or sodium titanium fluoride. The thermal oxidation of TixZr alloys in air at 500 °C for two hours appears to significantly reduce the effect of fluoride [104] (pp. 7–8). Another study regarding the corrosion resistance of Zr−Ti alloys shows too that the thermal oxidation in air at 500 °C does improve the corrosion resistance and suggests that prior to the use of such alloys as implant materials, a treatment consisting of thermal oxidation in air followed by exposure to a physiological solution should be applied [105].
The fluoride effect was tested on Zr5Ti, Zr25Ti and Zr45Ti alloys in acidic artificial saliva, Ph = 3 with a NaF concentration of 0.2 wt.%, 0.5 wt.% and 1 wt.%. The alloys were tested as such and after thermal oxidation in air at 500 °C for two hours. The results showed that although acidic artificial saliva with fluoride is a very aggressive medium for ZrxTi alloys, thermal oxidation is an effective method of surface treatment. The oxide layers formed by TiO2 and ZrO2 provided a good corrosion resistance [106].
Another study performed on Zr5Ti, Zr25Ti and Zr45Ti in artificial saliva solution, in the presence of NaF (0.05 wt.%, 0.1 wt.% and 0.2 wt.%) and albumin (0.6 wt.%) showed that Zr45Ti has better corrosion resistance [107]. The same alloys were tested in Ringer’s solution (pH = 6.8) [108] and physiologically acidified Ringer’s solution at pH = 3, also evidencing that Zr45Ti has the highest corrosion resistance. Moreover, it was observed that after thermal oxidation at 500 °C, the oxide layers became thicker and more stable [81]. The higher Ti content (45%) seems to lead to the suppression of pitting corrosion [109].
The evaluation of the oxide layer and its corrosion resistance on Ti20Zr alloy showed that the passivation takes place more easily and that the oxide layer has better corrosion resistance in Ringer’s solution at acidic, neutral and alkaline pH compared to Ti. It was also observed that at acidic pH, calcium and phosphate ions were deposited on the surface, and at neutral and alkaline pH a protective layer of hydroxyapatite (HAp) was formed [110].
The surface degradation of commercial Ti, Zr, and ZrO2 caused by bacterial adhesion (in particular, the Streptococcus species) has been discussed in several papers in the literature [111,112,113] concluding more recently that dental implant surfaces of TiZr and ZrO2 are not more susceptible to colonization than commercially pure Ti implants [114,115].
In addition to surface composition, the internal characteristics of metallic biomaterials, such as allotropic phase and defects, also influence the initiation and propagation of corrosion [116].
3.3.2. Surface Topography
Comparing the results reported on the surface roughness of biomaterials is difficult to achieve because there are variations regarding the analytical techniques and how the results are reported. Moreover, the terminology used is not harmonized and the same terms may refer to different procedures. However, it is generally accepted that surfaces with higher roughness favor osseointegration compared to smoother ones [117,118].
In addition to the modification techniques, which determine the properties of the created surfaces, the methods of investigating them are also of major importance. Techniques such as nanoindenting and scratching techniques are used to characterize submicrometric surfaces. Nanoindentation allows for the evaluation of hardness and Young’s modulus. The scratch test allows for the evaluation of coating adhesion to the substrate [19,66].
Rough surfaces can be differentiated into two categories: modified surfaces by coating (e.g., HAp) or without coating (e.g., sandblasting, acid etching). Electrochemical changes, such as anodic oxidation or NaOH treatment lead to the formation of biomimetic surfaces by promoting the formation of apatite [119].
Obtaining structured surfaces on a micrometric scale is generally carried out by acid etching, sandblasting (SB) or oxidation. Sandblasting involves the use of abrasive particles (Al2O3, TiO2, SiO2) at high pressure. This technique can lead to the incorporation of the particles used in the material and thus to the modification of chemical properties and reduced corrosion resistance. To solve this problem, a subsequent acid etching step is generally used to dissolve at least some of the projected particles. Acid etching produces similar results to sandblasting. The results obtained vary depending on the exposure time, type of acids used and temperature [117].
There is a TixZr alloy marketed under the name Roxolid-SLA® (Straumann, Basel, Switzerland) [11,13,120,121,122,123]. This alloy contains 13–17% zirconium and is beginning to be considered the first choice in medical procedures [124]. The smaller grains of 1–2 μm, compared to 20–30 μm in cpTi lead to increased overall strength [125]. Another variant also produced by Straumann is RXD-SLActive® this implant is hydrophilic and has a nanostructured surface compared to Roxolid-SLA® [126].
The surface named SLA® is obtained by sandblasting with corundum (particle size 250–500 μm) followed by etching with a concentrated mixture of hydrochloric acid and sulfuric acid. The implant is then cleaned with nitric acid, rinsed with deionized water and dried in an ambient atmosphere. For the SLActive® surface, the difference lies in rinsing with an NaCl solution in a nitrogen atmosphere and storage in 0.15 M saline solution. This change, apparently minor, leads to a hydrophilic surface with better properties in terms of osseointegration. The formation of nanostructures was also observed on the SLActive® surface [127].
To highlight the differences between mechanical processing and acid etching, the properties of TixZr alloys (x = 5, 10, 15 wt.%) with these two types of surface treatments were evaluated. The mechanically prepared samples were polished with an automatic polisher, and the etched ones were immersed in a mixture of sulfuric and hydrochloric acid for 1 h and 50 min. Acid etching treatment produced a rougher surface and better overall properties [82].
The comparative study of the surface Ti, TixZr after sandblasting and acid etching respectively, showed that in the case of TixZr, the hydrogen concentration is 1.9 times higher. The formation of hydrides favored by the presence of Zr increases the roughness by the appearance of nanostructures, which in the case of the Ti surface was not observed. It was also observed that the hydride layer is below the oxide layer, which can be explained in the case of sandblasting-acid etching (SBAE) by the fact that the oxide layer is formed after the formation of hydride, but also by the fact that oxygen could replace hydrogen in the outer layer [128].
3.4. Coatings with TiZr
To obtain the advantages of TixZr alloys, several studies have been developed that aimed to cover other substrates with these metals.
Through the plasma surface alloying technology, Zr ions were implanted in the surface of a Ti material, thus creating a TixZr alloy only in the surface layer. The alloys thus created had a Zr content of 1.09 at.%, 1.77 at.% and 3.68 at.%. The surface hardness and corrosion resistance proved to be better than that of cpTi. Good biocompatibility was also observed [17].
A TixZr surface was also obtained on Ti alloy by ion-assisted arc-plasma deposition in vacuum. The deposition was made by using two cathodes, one of Ti and one of Zr, which were arc evaporated in Ar atmosphere, at 0.2 Pa. The thickness of the obtained layer was 5 μm, with a Zr content of 11 wt.% and 22 wt.%. The material thus obtained showed a low modulus of elasticity, of 77–98 GPa, compared with the Ti substrate which had 110 GPa. Moreover, nanoindentation showed an increase in resistance to plastic deformation with increasing Zr content from 11 wt.% to 22 wt.% [129].
The use of a layer of TixZr deposited on a steel material, SS304, also led to a significant improvement in corrosion resistance [130].
The explosive spraying of Ti−Zr coating could help as well by reducing the Young’s modulus of the materials used as substrate (cpTi or Ti6Al4V), therefore reducing stress shielding [131].
Ti−Zr films were also deposited on Ti substrates by MAO, obtaining a porous crystalline layer of titanium and zirconium oxides. It has been observed that this approach leads to increased biocompatibility and that cell development increases proportionally with Zr content [132].
The incorporation of zirconium oxide into the oxide layer of Ti by plasma electrolytic oxidation (PEO) is another possible approach. A rougher surface was thus obtained, which by immersion in simulated body fluid (SBF) led to the formation of an apatite layer [133]. It was also shown that through this approach, the oxide layer formed favored osteoblast adhesion and could even decrease the bacterial adhesion [134]. Besides this, it was shown that nano-ZnO have antibacterial properties [135].
A possible coating that could lead to improved properties would be that with ZrTiO4 by the sol-gel method. The synthesis method has been developed but has not yet been applied to biomaterials [136].
3.5. Nanostructured Coatings
The design of nanometer-scale biomaterial surfaces is a subject of continuous research [137,138,139,140]. Nanostructured surfaces influence the chemical reactivity of materials and implicitly the biomolecular interactions [141,142]. It has been observed that nanoscale changes promote osteoinduction and biomaterial–tissue interaction and that implants with nanotubular surfaces show a significant improvement in bone creation and gene expression compared to implants without nanostructured surfaces [143,144,145,146,147].
3.5.1. Nanotubes
Anodizing may lead to self-ordered nanotubes, with certain dimensions (diameter, length) by varying the anodizing conditions. Nanostructures created in this way may have a partially crystalline structure. Their morphology influences electrochemical stability, wettability and biocompatibility [124].
The formation of nanotubes on TiZr substrate can be accomplished by a two-step anodizing procedure. They can be annealed in air or reduced in the atmosphere by Ar/H2. By annealing, the mixed oxide tubes are converted to zirconium titanate (ZrTiO4), and by reduction to (Zr0.333Ti0.666)O2 [124].
Nanotubes were reported to be obtained by two-step anodizing using an electrolyte solution with glycerin, 15% water and 0.2 M NH4F. The first anodization was performed at 55 V for 4 h, and then the samples were ultrasonicated to remove the formed structures. The second anodization was performed at 75 V for 1 h. The formation of nanotubes is the result of a competition between the electrochemical formation of oxides and the chemical dissolution of oxides by the fluoride anion. Reactions can be described as follows [68]:
In the case of nanotubes formed on TiZr type alloys, the diameter of the nanotubes decreases with increasing Zr content. Nanotubes with a diameter of approximately 50 nm and a length of 17 nm were obtained. Regarding the heat treatment, fluorine can be removed at 300 °C, and at 800 °C crystallization occurs, but also the collapse of nanotubes [148].
The formation of TiO2 nanotubes by anodization at 10 V in 1.0 M phosphoric acid and 0.5% NaF for 2 h was also studied on TixZr alloys (x = 10, 20, 30, 40%). The nanotubes had an average diameter of 150–200 nm and a wall thickness of 20 nm. The distance between the nanotubes increased with increasing Zr content of the alloy, being 60, 70, 100 and 130 nm [87].
Nanotubes were also obtained on Ti50Zr substrate by two-step anodizing at different voltages (15, 30, 45 V) in glycol with 15% H2O and 0.2 M NH4F. The layer formed in the first anodization was removed by ultrasonic treatment, thus obtaining a prepared surface so that in the second stage of anodization nanotubular structures with a high degree of organization were obtained [149].
The zirconium titanate (ZrTiO4) nanotubes formed on such alloys have increased structural flexibility compared to pure TiO2 nanotubes. The diameter and length of the formed tubes can vary depending on the anodizing potential used without damaging the structural configuration, at higher potentials the amorphous structure predominates over the crystalline one [145,150]. Another important aspect, less studied, is that nanotubes formed in this way can have an antibacterial role [150].
3.5.2. Other Nanostructured Surfaces
It was observed that nanopores were obtained on Ti50Zr by anodizing at 5 V and 10 V in a solution of 1 M (NH4)2SO4 and 0.5 wt% NH4F. The diameter of the nanopores was increasing with the applied potential. The material was subsequently annealed at 500 °C for three hours. The surfaces thus formed had an antibacterial effect, even if smaller than in the case of nanotubes [150].
The formation of nanopores was also observed by immersing an alloy of Ti50Zr with nanotubular surface obtained by two-step anodizing in a solution of H2SO4 (37 N)/H2O2 (30%) for two hours at room temperature [68] and when using spark anodization in a solution of glycerophosphate and calcium acetate on TixZr (x = 12–15 wt.%) [83].
The surface of a Ti20Zr alloy was galvanostatically anodized in phosphoric acid solution to increase the corrosion resistance. The current density was 10 mA/cm2, the concentration of phosphoric acid was 1 M, and the anodizing time was 45 min. It has been observed that this approach results in a nanometric layer consisting mostly of crystalline TiO2 with incorporated phosphorus ions. The corrosion resistance was 10 times higher in the case of the anodized sample [86].
Anodization in a hot solution of glycerol-phosphate of a Ti50Zr alloy led to one-dimensional nanostructures in the form of channels [90,151]. As a result of this treatment, better corrosion resistance and higher hydrophilicity were obtained. Additionally, in vitro studies on RAW 264.7 macrophages showed a reduced reaction in the inflammatory response [90].
In a study on the cathodic polarization of some TiZr materials (13–17% Zr) with a surface previously processed by sandblasting with aluminum oxide particles (0.25–0.5 mm), the acid etching treatment with a mixture of hydrochloric acid and sulfuric acid at 125–130 °C for 5 min produced nano-nodules [84].
Another type of nanostructured coating was made with two biopolymers, poly(lactic acid) and polycaprolactone. The methods used were dip coating and electrospinning, respectively, thus obtaining nanofibers [93].
3.6. Biomimetic Coatings
On a Ti15Zr alloy, obtained by mechanical processing (Straumann AG), a biofunctional coating was formed by PEO. As the control, the same unmodified alloy was used, and some were SLA®-treated (sandblasting and acid etching). The samples were used as an anode, while the cathode was a steel tank equipped with a cooling system. The electrolyte solution contained calcium acetate and disodium glycerol phosphate. The samples were immersed in 500 mL of solution for 10 min, using a voltage of 290 V and a frequency of 250 Hz. During the electrochemical treatment, pores appeared in the places where the micro-discharge took place, thus obtaining molten oxides which were quickly cooled in the presence of electrolytes when the spark was extinguished. This phenomenon led to the incorporation of Ca and P, resulting in a Ca/P ratio close to that of HAp. This aspect combined with the increased surface roughness significantly influenced the biological properties of the implant. In terms of stability, the PEO-treated alloy showed the highest corrosion resistance compared to control ones [85].
Two other ways to modify the surface of a Ti50Zr at.% alloy have been proposed. One of the methods involved immersing the sample in 10.0 M NaOH at 60 °C for 24 h. The samples were dried at room temperature in air for another 24 h. They were then heat treated at 600 °C for one hour in an electric high vacuum furnace. The other method involved the hydrothermal storage in a supersaturated solution of 0.2 M calcium hydroxide in an autoclave at 200 °C for eight hours. In the first case, a 12 μm oxide layer composed of TiO2, ZrO2, Na2Ti5O11 and Na2ZrO3, and in the second case a 45 nm layer composed of TiO2, ZrO2, CaTiO3 and CaZrO3 was formed. When immersed in SBF, a layer of apatite was observed in the case of both treated surfaces, but not in the untreated ones [91].
In the case of alkaline treatment, the native layer partially dissolves in the alkaline solution, forming HTiO3-H2O anions, which combine with the cations to form a hydrogel layer. During the heat treatment, this layer is dehydrated resulting in an amorphous or crystalline form of alkali titanate [152].
The deposition of a HAp/TiO2 layer on TiZr alloy has been reported by the sol-gel method at 3000 rpm for 15 s, followed by heat treatment at 600 °C for 20 min. Subsequent heat treatment at 600 °C for another 20 min resulted in HAp crystallization. The obtained layer had a total thickness of 50 μm, being formed in equal proportion of TiO2/HAp [92]. Regardless of the sol-gel method used, cracks may occur due to contractions caused by the evaporation of a large volume of solvent [153]. In the case of TiO2 coating, the formed cracks were subsequently coated with HAp. The mechanism of apatite formation in SBF can be explained by the release of Ca2+, Na+, K+, ions from the HAp deposited layer and their exchange with hydronium ions from the SBF solution, forming Ti−OH, Zr−OH groups. These functional groups can react with water molecules in the environment by inducing nucleation. Once formed, apatite nuclei can grow spontaneously by consuming ions from the SBF solution [92].
The coating of TiZr with HAp was also performed in combination with chitosan (CS), obtaining coatings with different porosities and contact angles depending on the HAp/CS ratio used [20,95,154].
Another type of biomimetic coating used on TixZr type alloys (Ti5Zr, Ti25Zr and Ti45Zr) can be the coating with HAp-ZrO2-Ag, which favors the formation of bone tissue [88,155]. Silver nanoparticles (AgNPs) are effective, in very low concentrations (0.5–1.0%), in preventing the formation of bacterial biofilm. They interact with proteoglycans on the membrane of bacterial cells and inside them. Silver ions can also interact with sulfuryl groups preventing the replication of bacterial DNA [156,157,158].
Moreover, the development of bioinductive surfaces could increase the healing capacity of bone tissue and is a solution for patients with risk factors [70,159]. The implantation of a biologically active molecule (parathyroid hormone fragment 1−34) in the natural oxide layer was performed on a TiZr implant (Straumann AG) [70].
3.7. Biological Aspects
The interactions between cells and biomaterial are determined by the surface properties [160,161]. Cells do not interact directly with the surface of the biomaterial, but with the protein layer adsorbed on the surface. The transcriptomic and proteomic technologies used to create gene and protein expression profiling can be used for a more thorough understanding and to predict the biocompatibility of the researched materials [162,163,164,165].
The interaction between human gingival fibroblasts and TixZr materials with surfaces treated in different ways shows that cell adhesion and differentiation are influenced by surface properties [166,167]. Another study that looked at the expression of several genes involved in the process of cell adhesion shows that high surface energy positively influences the adhesion of osteoblasts [168].
Microbial infections and in particular infections with multidrug resistant microbes are a major problem that must be considered in the development of all biomaterials [169,170,171]. Recent research focuses on the use of nanotechnology in solving this problem, nanometrically structured materials having characteristics far superior to conventional ones (bulk form). Additionally, the reactive oxygen species produced by the use of nanocomposite oxide metals or nanoparticles cause the inhibition of bacterial growth [170].
Bacterial adhesion can lead initially to mucositis and later to periimplantitis, which can cause implant loss. Thus this aspect is approached and studied in many studies [172,173,174]. Surface properties, such as roughness, free energy (wettability) and chemical composition are the determining factors in terms of cell adhesion and microbial colonization [173,175,176].
Surface roughness, chemical composition, wettability and surface charge also influence the structure and size of the biofilm. Although it is a subject often studied, the results obtained in vitro may differ from those obtained in vivo. The differences appear because it is difficult to imitate the biologically complex environment. In vivo studies show that bacterial adhesion increases with increasing roughness and hydrophobic surfaces promote the accumulation of proteins, which serve as a binding site for bacteria [177].
In a time with more aggressive bacteria and viruses, for the success of dental implants, besides the incorporation of efficient antibacterial agents inside implants, loading of the anti-inflammatory drugs to reduce inflammation and bone forming proteins/peptides/growth factors (bone morphogenetic protein-2/BMP-2, parathyroid hormone, Zn/Ag/Sr/Mg ions/nanoparticles) to enhance new bone formation at the implant–bone interface have been applied [178,179,180]. More recently, creating composite structures with graphene and nanotubes on the implant surface proved to have efficient dual antibacterial and osteogenic properties [181].
Another important aspect is related to the tribocorrosion of metallic materials. The released metal nanoparticles (metal wear debris) can migrate to any part of the body (except the brain), depositing in the lymph nodes, the liver, spleen and bone marrow. They also have a genotoxic potential, which can affect the genetic material, which could lead to cancer or birth defects [182].
The dissolution of the oxide layers is relatively low in vitro because the potential changes are small, but in the body, this process can be accelerated by the presence of amino acids and proteins. Another process that can accelerate this phenomenon is the generation of reactive oxygen species by macrophages. Superoxide dismutase catalyzes O2− producing H2O2 that hyperoxidize the surface of the material. The process that takes place simultaneously with the dissolution is the reprecipitation, the two being in equilibrium. Phosphate ions are adsorbed on the outer oxide layers of Ti alloys, and subsequently, calcium ions are adsorbed by them forming an apatite layer. In addition to these, proteins, sulfites and sulfates have also been observed [183,184].
3.7.1. The Influence of Surfaces In Vitro
TixZr alloys (x = 5, 10, 15, 20 wt.%) were studied, proving that they do not show cytotoxicity [185]. Moreover, compared to other commercial Ti-based alloys, cells on TiZr implants expressed a decrease in pro-inflammatory markers and an increase in anti-inflammatory markers, especially in the case of nanostructured alloy [126].
The formation of nanotubes, mechanical properties and inhibition of bacterial growth of S. aureus was studied on pure Ti and Zr alloys, as well as on several TixZr binary alloys (x = 10, 30, 50, 70 at.%), noting that nanotubular formations inhibit bacterial growth [89]. In the case of TiO2 nanotubes, it has been observed that annealing can increase the inhibitory effect on S. aureus and Pseudomonas aeruginosa and even that it increases depending on the temperature used [186].
The properties of nanotubes formed on Ti50Zr by two-step anodizing were also evaluated, in vitro, on E. coli culture. It was observed that the antibacterial efficacy depends on the diameter of the tubes formed, a size that can be controlled by the potential used for anodizing. Tubes with a diameter of less than 20–30 nm demonstrated a better bacteriostatic and bactericidal effect than those with a larger diameter of 50–70 nm [187].
Nanotubes can also be loaded with therapeutic agents, either as such or embedded in a polymer, by electrospray, lyophilization, immersion or vacuum impregnation techniques [188]. On a Ti50Zr alloy two types of nanostructures were formed, nanopores and nanotubes, that were loaded with gentamicin and coated with a layer of CS. It was observed that gentamicin was released from nanopores in 10 days, and from nanotubes in 21 days [68].
Another coating with a zwitterionic cysteine drug was investigated recently, reporting better performance in terms of stability, biocompatibility and antibacterial effect. The obtained structure evidenced large bands due to hydroxylic groups which formed a hydration layer and determined the increase in hydrophilic character. This layer was responsible for an antibacterial effect as well. The electrochemical tests performed in NaCl 0.9% as bioliquid confirmed the improved stability of the coated sample. The cell behavior in the presence of cysteine coating was determined with gingival fibroblasts, by measuring lactate dehydrogenase activity, concentrations of nitric oxide and the level of reactive oxygen species. The results obtained indicated that the coating is biocompatible, and no cytotoxicity was evidenced [94].
The cell morphology of MG63 cells (osteoblast-like cells) on Ti50Zr with a nanostructured surface also showed good biocompatibility [149], while the studies performed on RAW 264.7 macrophages showed a reduced reaction in inflammatory response in the case of unidimensional nanochannels [90].
The incorporation of Ag into Ti50Zr showed strong antibacterial activity on S. aureus [189] and coating a Ti50Zr alloy with poly (lactic acid), polycaprolactone and silver nanoparticles showed good inhibitory activity in E. coli and S. aureus [93].
Amorphous biomaterials such as Zr46Ti40Ag14 have been studied too as an alternative to conventional crystalline ones [189]. They do not have structural deficiencies characteristic of crystalline structures (dislocation, vacancy, twinning, grain boundary), which can lead to better mechanical properties and increased corrosion resistance, as well as antibacterial properties due to the Ag [190].
In the case of surfaces treated in alkaline solution, and subsequently, heat treated, better cell adhesion and proliferation were observed for SaOS2 [91]. Coating a Ti-based alloy with CaTiZr3(PO4)6 also showed that the coating promoted cell proliferation and bone formation [191].
3.7.2. The Influence of Surfaces In Vivo
TixZr implants (x = 13–17%) prepared by sandblasting and acid etching evaluated for 12 weeks in a study in rabbits show biocompatibility as good as in the case of cpTi [192]. In another study on titanium−zirconium (SLActive surface treatment) implants in rabbits, an increased quality was shown regarding bone formation in the case of TiZr compared with Ti. The evaluation was carried out through the removal of torque values [193].
Additonally, a better response in the case of SLActive® (nanostructured surface) highlighted in a study on human bone mesenchymal stem cells (hBMSCs) [194] is confirmed by a retrospective evaluation in another study of 154 Roxolid implants with SLActive® in 107 patients that showed high survival rates [122]. TiZr implant alloys, in general, proved positive outcomes in numerous studies [120,121,123,195,196,197]. Despite the success rate of dental TiZr implants, avoiding the acute surgical site infections in some patients is still challenging [198] due to several unsuccessful events [199] and researchers suggested coating therapeutic drugs on the biomaterials surface to locally release antibiotics in a controlled manner [200].
By using a cathodic polarization setup, a doxycycline loading of a TiZr alloy with a Zr content of 13–17% was performed. The method of preparation of the sample was that used for the preparation of Roxolid SLActive®, namely grit blasted and etching in sulfuric and hydrochloric acid. Samples were handled in a nitrogen atmosphere and stored in 0.9% NaCl. The coating was made by connecting the samples to the cathode, the anode being Pt. A constant current of 0.65 mA was used for each sample. The electrolyte was a 2 M acetate buffer solution in which doxycycline was dissolved to obtain 1 mg/mL. The process took 75 min and was performed at room temperature. The samples were further dried and stored in a nitrogen atmosphere. The coated surface evaluated in a rabbit tibial model showed increased markers related to bone formation [201].
Following the idea that coating implantable metal alloys with active substances could be a solution for controlling inflammation and post-interventional infections, the use of a doxycycline-coated TixZr alloy compared to the same doxycycline-free alloy was studied as well in dogs and rabbits. The coating did not produce different histological and histomorphometrical results compared to the control group [200].
Three binary TiZr alloys (Ti5Zr, Ti25Zr and Ti45Zr) coated with a layer of HAp-ZrO2-Ag were tested after implantation in pig tibia. It was observed that osseointegration was influenced both by the interaction between the tissue and the coating and by the chemical composition of the bulk alloy. The Ti45Zr alloy showed a better organizational structure of newly formed bone tissue, as well as increased proliferation and differentiation of osteoblasts [88].
Another in vivo study in mice shows that Ti materials with a nanostructured coating of TiO2 or ZrO2 have a positive influence on cell differentiation [202], while another study performed on sheep shows that the anodizing of both Ti and TixZr (x = 12–15 wt.%) improves osseointegration [203].
4. Conclusions
The critical approach decided upon in this review has led to the clear conclusion that TixZr alloys have all the characteristics of a good biomaterial. Not only do they exhibit great biocompatibility, but the mechanical properties are improved up to 2.5 times in Ti50Zr compared to Ti or Zr. Moreover, the surface modification methods described in the reviewed studies suggest that with the right combination of chemical and instrumental techniques, many characteristics and properties can be molded.
The alloys are naturally protected against corrosion through a layer of oxides, that can be easily further improved through thermal oxidation. More complex methods such as anodizing or sol-gel coatings can be used to obtain different nanostructures that improve cellular interaction, being similar in size. Of course, the risk of bacterial biofilm formation increases too, but besides the antibacterial effect of some of the nanostructures, antibiotic substances or other nanoparticles that inhibit bacterial growth may be used in the coating as well. Furthermore, coatings with hydroxyapatite can improve cell differentiation and proliferation. Additionally, all the studies performed in vivo, that we know of, showed promising results.
Taking into consideration all the above-mentioned aspects, we believe that TiZr may become one of the most used metallic biomaterials in the future.
Author Contributions
Conceptualization, D.I. and I.D.; investigation, R.N., D.I., and I.D.; data curation, R.N., D.I., and I.D.; writing—original draft preparation, R.N., D.I., and I.D.; writing—review and editing, D.I., and I.D.; visualization, R.N.; supervision, I.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not available.
Conflicts of Interest
The authors declare no conflict of interest
References
- Iftikhar, S.; Jahanzeb, N.; Saleem, M.; Rehman, S.; Matinlinna, J.P.; Khan, A.S. The trends of dental biomaterials research and future directions: A mapping review. Saudi Dent. J. 2021. [Google Scholar] [CrossRef]
- Ho, W.-F.; Chen, W.-K.; Wu, S.-C.; Hsu, H.-C. Structure, mechanical properties, and grindability of dental Ti–Zr alloys. J. Mater. Sci. Mater. Med. 2008, 19, 3179–3186. [Google Scholar] [CrossRef]
- He, X.; Reichl, F.X.; Milz, S.; Michalke, B.; Wu, X.; Sprecher, C.M.; Yang, Y.; Gahlert, M.; Röhling, S.; Kniha, H.; et al. Titanium and zirconium release from titanium-and zirconia implants in mini pig maxillae and their toxicity in vitro. Dent. Mater. 2020, 36, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jansen, J.A.; Walboomers, X.F.; van den Beucken, J.J. Mechanical aspects of dental implants and osseointegration: A narrative review. J. Mech. Behav. Biomed. Mater. 2020, 103, 103574. [Google Scholar] [CrossRef]
- Osman, R.B.; Swain, M.V. A critical review of dental implant materials with an emphasis on titanium versus zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef]
- Shekhawat, D.; Singh, A.; Banerjee, M.K.; Singh, T.; Patnaik, A. Bioceramic composites for orthopaedic applications: A comprehensive review of mechanical, biological, and microstructural properties. Ceram. Int. 2021, 47, 3013–3030. [Google Scholar] [CrossRef]
- Ionita, D.; Bajenaru-Georgescu, D.; Totea, G.; Mazare, A.; Schmuki, P.; Demetrescu, I. Activity of vancomycin release from bioinspired coatings of hydroxyapatite or TiO2 nanotubes. Int. J. Pharm. 2017, 517, 296–302. [Google Scholar] [CrossRef]
- Stoian, A.B.; Surdu-Bob, C.; Anghel, A.; Ionita, D.; Demetrescu, I. Investigation of High Voltage Anodic Plasma (HVAP) Ag-DLC Coatings on Ti50Zr with Different Ag Amounts. Coatings 2019, 9, 792. [Google Scholar] [CrossRef]
- Mani, G.; Feldman, M.D.; Patel, D.; Agrawal, C.M. Coronary stents: A materials perspective. Biomaterials 2007, 28, 1689–1710. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhou, C.; Zhao, Y.; He, F.; Wang, X. Development and properties of dental Ti–Zr binary alloys. J. Mech. Behav. Biomed. Mater. 2020, 112, 104048. [Google Scholar] [CrossRef]
- Altuna, P.; Lucas-Taulé, E.; Gargallo-Albiol, J.; Figueras-Álvarez, O.; Hernández-Alfaro, F.; Nart, J. Clinical evidence on titanium-zirconium dental implants: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2016, 45, 842–850. [Google Scholar] [CrossRef]
- Ionita, D.; Pirvu, C.; Stoian, A.B.; Demetrescu, I. The Trends of TiZr Alloy Research as a Viable Alternative for Ti and Ti16 Zr Roxolid Dental Implants. Coatings 2020, 10, 422. [Google Scholar] [CrossRef]
- Sharma, A.; Waddell, J.N.; Li, K.C.; Sharma, L.A.; Prior, D.J.; Duncan, W.J. Is titanium–zirconium alloy a better alternative to pure titanium for oral implant? Composition, mechanical properties, and microstructure analysis. Saudi Dent. J. 2020, 4–11. [Google Scholar] [CrossRef]
- Michelle Grandin, H.; Berner, S.; Dard, M. A review of Titanium Zirconium (TiZr) alloys for use in endosseous dental implants. Materials 2012, 5, 1348–1360. [Google Scholar] [CrossRef]
- Kondoh, K.; Fukuo, M.; Kariya, S.; Shitara, K.; Li, S.; Alhazaa, A.; Umeda, J. Quantitative strengthening evaluation of powder metallurgy Ti–Zr binary alloys with high strength and ductility. J. Alloys Compd. 2021, 852, 156954. [Google Scholar] [CrossRef]
- Möller, B.; Terheyden, H.; Ail, Y.; Purcz, N.M.; Hertrampf, K.; Tabakov, A.; Behrens, E.; Wiltfang, J. A comparison of biocompatibility and osseointegration of ceramic and titanium implants: An in vivo and in vitro study. Int. J. Oral Maxillofac. Surg. 2012, 41, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Tan, J.; Wang, D.; Qiu, J.; Liu, X. Surface alloyed Ti–Zr layer constructed on titanium by Zr ion implantation for improving physicochemical and osteogenic properties. Prog. Nat. Sci. Mater. Int. 2020, 30, 635–641. [Google Scholar] [CrossRef]
- Cui, W.F.; Shao, C.J. The improved corrosion resistance and anti-wear performance of Zr-xTi alloys by thermal oxidation treatment. Surf. Coat. Technol. 2015, 283, 101–107. [Google Scholar] [CrossRef]
- Vardaki, M.; Pantazi, A.; Demetrescu, I.; Enachescu, M. Assessing the Functional Properties of TiZr Nanotubular Structures for Biomedical Applications, through Nano-Scratch Tests and Adhesion Force Maps. Molecules 2021, 26, 900. [Google Scholar] [CrossRef]
- Ionita, D.; Vardaki, M.; Stan, M.S.; Dinischiotu, A.; Demetrescu, I. Enhance Stability and in vitro Cell Response to a Bioinspired Coating on Zr Alloy with Increasing Chitosan Content. J. Bionic Eng. 2017, 14, 459–467. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Wen, J.; Chu, X.; Cao, Y.; Li, N. Effects of Al on Precipitation Behavior of Ti-Nb-Ta-Zr Refractory High Entropy Alloys. Metals 2021, 11, 514. [Google Scholar] [CrossRef]
- Soni, V.; Gwalani, B.; Alam, T.; Dasari, S.; Zheng, Y.; Senkov, O.N.; Miracle, D.; Banerjee, R. Phase inversion in a two-phase, BCC+B2, refractory high entropy alloy. Acta Mater. 2020, 185, 89–97. [Google Scholar] [CrossRef]
- Todai, M.; Nagase, T.; Hori, T.; Matsugaki, A.; Sekita, A.; Nakano, T. Novel TiNbTaZrMo high-entropy alloys for metallic biomaterials. Scr. Mater. 2017, 129, 65–68. [Google Scholar] [CrossRef]
- Ji, P.F.; Li, B.; Chen, B.H.; Wang, F.; Ma, W.; Zhang, X.Y.; Ma, M.Z.; Liu, R.P. Effect of Nb addition on the stability and biological corrosion resistance of Ti-Zr alloy passivation films. Corros. Sci. 2020, 170. [Google Scholar] [CrossRef]
- Pitchi, C.S.; Priyadarshini, A.; Sana, G.; Narala, S.K.R. A review on alloy composition and synthesis of β-Titanium alloys for biomedical applications. Mater. Today Proc. 2020, 26, 3297–3304. [Google Scholar] [CrossRef]
- Pintaõ, C.A.F.; Correa, D.R.N.; Grandini, C.R. Torsion modulus as a tool to evaluate the role of thermo-mechanical treatment and composition of dental Ti-Zr alloys. J. Mater. Res. Technol. 2019, 8, 4631–4641. [Google Scholar] [CrossRef]
- Ji, P.; Chen, B.; Li, B.; Tang, Y.; Zhang, G.; Zhang, X.; Ma, M.; Liu, R. Influence of Nb addition on microstructural evolution and compression mechanical properties of Ti-Zr alloys. J. Mater. Sci. Technol. 2021, 69, 7–14. [Google Scholar] [CrossRef]
- Wen, C.E.; Yamada, Y.; Hodgson, P.D. Fabrication of novel TiZr alloy foams for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1439–1444. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, H.; Huang, Q.; Zhao, D.; He, J.; Cao, Y.; Song, M.; Liu, B.; Ouyang, S. Strong-yet-ductile Ti−Zr alloys through high concentration of oxygen strengthening. Trans. Nonferrous Met. Soc. China 2020, 30, 2449–2458. [Google Scholar] [CrossRef]
- Vicente, F.B.; Correa, D.R.N.; Donato, T.A.G.; Arana-Chavez, V.E.; Buzalaf, M.A.R.; Grandini, C.R. The influence of small quantities of oxygen in the structure, microstructure, hardness, elasticity modulus and cytocompatibility of Ti-Zr alloys for dental applications. Materials 2014, 7, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, A.; Wang, J.; Abdel-Hady Gepreel, M.; Wen, C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Correa, D.R.N.; Kuroda, P.A.B.; Lourenço, M.L.; Buzalaf, M.A.R.; Mendoza, M.E.; Archanjo, B.S.; Achete, C.A.; Rocha, L.A.; Grandini, C.R. Microstructure and selected mechanical properties of aged Ti-15Zr-based alloys for biomedical applications. Mater. Sci. Eng. C 2018, 91, 762–771. [Google Scholar] [CrossRef]
- Correa, D.R.N.; Vicente, F.B.; Donato, T.A.G.; Arana-Chavez, V.E.; Buzalaf, M.A.R.; Grandini, C.R. The effect of the solute on the structure, selected mechanical properties, and biocompatibility of Ti-Zr system alloys for dental applications. Mater. Sci. Eng. C 2014, 34, 354–359. [Google Scholar] [CrossRef]
- Sngfeng, E.; Shi, L.; Guo, Z.G.; Liu, W.M. The recent progress of tribological biomaterials. Biosurface Biotribol. 2015, 1, 81–97. [Google Scholar] [CrossRef]
- Sarswat, P.K.; Sarkar, S.; Murali, A.; Huang, W.; Tan, W.; Free, M.L. Additive manufactured new hybrid high entropy alloys derived from the AlCoFeNiSmTiVZr system. Appl. Surf. Sci. 2019, 476, 242–258. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Y.; Liu, B.; Zhang, W. Precipitation behavior during hot deformation of powder metallurgy Ti-Nb-Ta-Zr-Al high entropy alloys. Intermetallics 2018, 100, 95–103. [Google Scholar] [CrossRef]
- Hsu, H.C.; Wu, S.C.; Sung, Y.C.; Ho, W.F. The structure and mechanical properties of as-cast Zr-Ti alloys. J. Alloys Compd. 2009, 488, 279–283. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, M.G.; Jiménez, O.; Alvarado-Hernández, F.; Flores, M.; Andrade, E.; Canto, C.E.; Ávila, C.; Espinoza-Beltrán, F. The effect of C content on the mechanical properties of Ti-Zr coatings. J. Mech. Behav. Biomed. Mater. 2015, 49, 269–276. [Google Scholar] [CrossRef]
- Matuła, I.; Dercz, G.; Zubko, M.; Maszybrocka, J.; Jurek-Suliga, J.; Golba, S.; Jendrzejewska, I. Microstructure and porosity evolution of the ti–35zr biomedical alloy produced by elemental powder metallurgy. Materials 2020, 13, 4539. [Google Scholar] [CrossRef]
- Annur, D.; Kartika, I.; Supriadi, S.; Suharno, B. Titanium and titanium based alloy prepared by spark plasma sintering method for biomedical implant applications—A review. Mater. Res. Express 2021, 8. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Wong, X.P.C.; Song, S.M.; Tsai, P.H.; Jang, J.S.C.; Tsao, I.Y.; Lin, C.H.; Nguyen, V.C. Open-cell tizr-based bulk metallic glass scaffolds with excellent biocompatibility and suitable mechanical properties for biomedical application. J. Funct. Biomater. 2020, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.T.; Thampi, V.V.A.; Terada-Nakaishi, M.; Chen, P.; Hanawa, T.; Nandakumar, A.K.; Subramanian, B. Zirconium-based metallic glass and zirconia coatings to inhibit bone formation on titanium. Biomed. Mater. 2020, 15. [Google Scholar] [CrossRef]
- Hanawa, T. Zirconia versus titanium in dentistry: A review. Dent. Mater. J. 2020, 39, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Teffo, M.L.; Nyakane, N.E.; Seerane, M.; Shongwe, M.B.; Machaka, R. The effect of alloying elements on densification and mechanical behaviour of titanium based alloy. Mater. Today Proc. 2021, 38, 1203–1208. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, Y.; Wang, X. Effects of Zr addition on the deformation behavior, microstructure and mechanical properties of dental Ti alloys. Mater. Sci. Eng. A 2020, 794, 139808. [Google Scholar] [CrossRef]
- Ali, F.; Mehmood, M.; Qasim, A.M.; Ahmad, J.; Naeem-ur-Rehman; Iqbal, M.; Qureshi, A.H. Comparative study of the structure and corrosion behavior of Zr-20%Cr and Zr-20%Ti alloy films deposited by multi-arc ion plating technique. Thin Solid Films 2014, 564, 277–283. [Google Scholar] [CrossRef]
- Akimoto, T.; Ueno, T.; Tsutsumi, Y.; Doi, H.; Hanawa, T.; Wakabayashi, N. Evaluation of corrosion resistance of implant-use Ti-Zr binary alloys with a range of compositions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Atalay, B.; Aybar, B.; Ergüven, M.; Emes, Y.; Bultan, Ö.; Akça, K.; Yalçin, S.; Baysal, U.; Işsever, H.; Çehreli, M.C.; et al. The effects of pulsed electromagnetic field (PEMF) on osteoblast-like cells cultured on titanium and titanium-zirconium surfaces. J. Craniofac. Surg. 2013, 24, 2127–2134. [Google Scholar] [CrossRef]
- Pérez, R.A.; Gargallo, J.; Altuna, P.; Herrero-Climent, M.; Gil, F.J. Fatigue of narrow dental implants: Influence of the hardening method. Materials 2020, 13, 1429. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ruan, W.; Liu, J.; Zhang, T.; Yang, H.; Ruan, J. Microstructure, mechanical properties, and preliminary biocompatibility evaluation of binary Ti–Zr alloys for dental application. J. Biomater. Appl. 2019, 33, 766–775. [Google Scholar] [CrossRef]
- Hsu, H.C.; Wu, S.C.; Hsu, S.K.; Sung, Y.C.; Ho, W.F. Effects of heat treatments on the structure and mechanical properties of Zr-30Ti alloys. Mater. Charact. 2011, 62, 157–163. [Google Scholar] [CrossRef]
- Byeli, A.V.; Kukareko, V.A.; Kononov, A.G. Titanium and zirconium based alloys modified by intensive plastic deformation and nitrogen ion implantation for biocompatible implants. J. Mech. Behav. Biomed. Mater. 2012, 6, 89–94. [Google Scholar] [CrossRef]
- Jones, F.H. Teeth and bones: Applications of surface science to dental materials and related biomaterials. Surf. Sci. Rep. 2001, 42, 75–205. [Google Scholar] [CrossRef]
- Filipović, U.; Dahmane, R.G.; Ghannouchi, S.; Zore, A.; Bohinc, K. Bacterial adhesion on orthopedic implants. Adv. Colloid Interface Sci. 2020, 283, 1–12. [Google Scholar] [CrossRef]
- Shah, F.A.; Thomsen, P.; Palmquist, A. Osseointegration and current interpretations of the bone-implant interface. Acta Biomater. 2019, 84, 1–15. [Google Scholar] [CrossRef]
- Puleo, D.A.; Thomas, M.V. Implant Surfaces. Dent. Clin. N. Am. 2006, 50, 323–338. [Google Scholar] [CrossRef]
- Agarwal, R.; García, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Azens, A.; Ekdahl, K.N.; Granqvist, C.G.; Nilsson, B. Material-specific thrombin generation following contact between metal surfaces and whole blood. Biomaterials 2005, 26, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, B.; Carroll, W. The evolution of cardiovascular stent materials and surfaces in response to clinical drivers: A review. Acta Biomater. 2009, 5, 945–958. [Google Scholar] [CrossRef]
- Kurup, A.; Dhatrak, P.; Khasnis, N. Surface modification techniques of titanium and titanium alloys for biomedical dental applications: A review. Mater. Today Proc. 2020, 39, 84–90. [Google Scholar] [CrossRef]
- Chang, Y.R.; Xu, F.F.; Li, J.; You, Y.H.; Liu, C.; Yin, L.H. Surface morphology and surface properties of Ti and TiZr implant materials. Zhonghua Kou Qiang Yi Xue Za Zhi 2019, 54, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nissan, B.; Choi, A.H.; Bendavid, A. Mechanical properties of inorganic biomedical thin films and their corresponding testing methods. Surf. Coat. Technol. 2013, 233, 39–48. [Google Scholar] [CrossRef]
- Hanawa, T. Biofunctionalization of titanium for dental implant. Jpn. Dent. Sci. Rev. 2010, 46, 93–101. [Google Scholar] [CrossRef]
- Stoian, A.B.; Demetrescu, I.; Ionita, D. Nanotubes and nano pores with chitosan construct on TiZr serving as drug reservoir. Colloids Surfaces B Biointerfaces 2020, 185, 110535. [Google Scholar] [CrossRef]
- Ionita, D.; Grecu, M.; Ungureanu, C.; Demetrescu, I. Antimicrobial activity of the surface coatings on TiAlZr implant biomaterial. J. Biosci. Bioeng. 2011, 112, 630–634. [Google Scholar] [CrossRef]
- Micksch, T.; Herrmann, E.; Scharnweber, D.; Schwenzer, B. A modular peptide-based immobilization system for ZrO2, TiZr and TiO2 surfaces. Acta Biomater. 2015, 12, 290–297. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Chen, C.; Zhao, Z. Review of the biocompatibility of micro-arc oxidation coated titanium alloys. Mater. Des. 2015, 85, 640–652. [Google Scholar] [CrossRef]
- Mahapatro, A. Bio-functional nano-coatings on metallic biomaterials. Mater. Sci. Eng. C 2015, 55, 227–251. [Google Scholar] [CrossRef]
- Tan, D.; Zhou, S.; Qiu, J.; Khusro, N. Preparation of functional nanomaterials with femtosecond laser ablation in solution. J. Photochem. Photobiol. C Photochem. Rev. 2013, 17, 50–68. [Google Scholar] [CrossRef]
- Jaworek, A.; Sobczyk, A.T. Electrospraying route to nanotechnology: An overview. J. Electrostat. 2008, 66, 197–219. [Google Scholar] [CrossRef]
- Jaworek, A. Micro-and nanoparticle production by electrospraying. Powder Technol. 2007, 176, 18–35. [Google Scholar] [CrossRef]
- Izquierdo, J.; Bolat, G.; Cimpoesu, N.; Trinca, L.C.; Mareci, D.; Souto, R.M. Electrochemical characterization of pulsed layer deposited hydroxyapatite-zirconia layers on Ti-21Nb-15Ta-6Zr alloy for biomedical application. Appl. Surf. Sci. 2016, 385, 368–378. [Google Scholar] [CrossRef]
- Leite, D.M.C.; de Alencar, M.C.; Mucsi, C.S.; Araujo, J.V.S.; Tavares, L.A.; Berbel, L.O.; Aranha, L.C.; Rossi, J.L. Modifications of titanium and zirconium alloy surfaces for use as dental implants. Mater. Sci. Forum 2020, 1012, 477–482. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Zhang, Z.; Dong, S.; Liu, Y. Atomic oxygen treatment effects on magnetron sputtered Zr-Ti binary films. Appl. Surf. Sci. 2015, 324, 669–676. [Google Scholar] [CrossRef]
- Oliveira, N.T.C.; Biaggio, S.R.; Piazza, S.; Sunseri, C.; Di Quarto, F. Photo-electrochemical and impedance investigation of passive layers grown anodically on titanium alloys. Electrochim. Acta 2004, 49, 4563–4576. [Google Scholar] [CrossRef]
- Habazaki, H.; Uozumi, M.; Konno, H.; Shimizu, K.; Nagata, S.; Asami, K.; Matsumoto, K.; Takayama, K.; Oda, Y.; Skeldon, P.; et al. Influences of structure and composition on growth of anodic oxide films on Ti-Zr alloys. Electrochim. Acta 2003, 48, 3257–3266. [Google Scholar] [CrossRef]
- Bolat, G.; Izquierdo, J.; Mareci, D.; Sutiman, D.; Souto, R.M. Electrochemical characterization of ZrTi alloys for biomedical applications. Part 2: The effect of thermal oxidation. Electrochim. Acta 2013, 106, 432–439. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Faverani, L.P.; Grandini, C.R.; Rangel, E.C.; da Cruz, N.C.; Nociti Junior, F.H.; Almeida, A.B.; Vicente, F.B.; Morais, B.R.G.; Barão, V.A.R.; et al. Characterization of chemically treated Ti-Zr system alloys for dental implant application. Mater. Sci. Eng. C 2018, 92, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; McQuillan, A.J.; Sharma, L.A.; Waddell, J.N.; Shibata, Y.; Duncan, W.J. Spark anodization of titanium–zirconium alloy: Surface characterization and bioactivity assessment. J. Mater. Sci. Mater. Med. 2015, 26. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.J.; Walter, M.S.; Bucko, M.M.; Pamula, E.; Lyngstadaas, S.P.; Haugen, H.J. Polarization of modified titanium and titanium-zirconium creates nano-structures while hydride formation is modulated. Appl. Surf. Sci. 2013, 282, 7–16. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Pantaroto, H.N.; Paschoaleto, E.M.; Rangel, E.C.; da Cruz, N.C.; Sukotjo, C.; Barão, V.A.R. Synthesis of biofunctional coating for a TiZr alloy: Surface, electrochemical, and biological characterizations. Appl. Surf. Sci. 2018, 452, 268–278. [Google Scholar] [CrossRef]
- Calderon Moreno, J.M.; Osiceanu, P.; Vasilescu, C.; Anastasescu, M.; Drob, S.I.; Popa, M. Obtaining, structural and corrosion characterization of anodized nanolayers on Ti-20Zr alloy surface. Surf. Coat. Technol. 2013, 235, 792–802. [Google Scholar] [CrossRef]
- Kim, W.G.; Choe, H.C.; Ko, Y.M.; Brantley, W.A. Nanotube morphology changes for Ti-Zr alloys as Zr content increases. Thin Solid Films 2009, 517, 5033–5037. [Google Scholar] [CrossRef]
- Trincă, L.C.; Mareci, D.; Souto, R.M.; Lozano-Gorrín, A.D.; Izquierdo, J.; Burtan, L.; Motrescu, I.; Vulpe, V.; Pavel, G.; Strungaru, S.; et al. Osseointegration evaluation of ZrTi alloys with hydroxyapatite-zirconia-silver layer in pig’s tibiae. Appl. Surf. Sci. 2019, 487, 127–137. [Google Scholar] [CrossRef]
- Pantazi, A.; Vardaki, M.; Mihai, G.; Ionita, D.; Stoian, A.B.; Enachescu, M.; Demetrescu, I. Understanding surface and interface properties of modified Ti50Zr with nanotubes. Appl. Surf. Sci. 2020, 506, 144661. [Google Scholar] [CrossRef]
- Ion, R.; Stoian, A.B.; Dumitriu, C.; Grigorescu, S.; Mazare, A.; Cimpean, A.; Demetrescu, I.; Schmuki, P. Nanochannels formed on TiZr alloy improve biological response. Acta Biomater. 2015, 24, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Y.; Hodgson, P.D.; Wen, C. In vitro behavior of human osteoblast-like cells (SaOS2) cultured on surface modified titanium and titanium-zirconium alloy. Mater. Sci. Eng. C 2011, 31, 1545–1552. [Google Scholar] [CrossRef]
- Wen, C.E.; Xu, W.; Hu, W.Y.; Hodgson, P.D. Hydroxyapatite/titania sol-gel coatings on titanium-zirconium alloy for biomedical applications. Acta Biomater. 2007, 3, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Prodana, M.; Nistor, C.E.; Stoian, A.B.; Ionita, D.; Burnei, C. Dual nanofibrous bioactive coatings on TiZr implants. Coatings 2020, 10, 526. [Google Scholar] [CrossRef]
- Demetrescu, I.; Dumitriu, C.; Totea, G.; Nica, C.I.; Dinischiotu, A.; Ionita, D. Zwitterionic cysteine drug coating influence in functionalization of implantable Ti50Zr alloy for antibacterial, biocompatibility and stability properties. Pharmaceutics 2018, 10, 220. [Google Scholar] [CrossRef] [PubMed]
- Vardaki, M.; Ionita, D.; Stoian, A.B.; Demetrescu, I. Increasing corrosion resistance of a ZrTi alloy with a bioinspired coating with low porosity. Mater. Corros. 2017, 68, 988–994. [Google Scholar] [CrossRef]
- Matusiewicz, H. Potential release of in vivo trace metals from metallic medical implants in the human body: From ions to nanoparticles—A systematic analytical review. Acta Biomater. 2014, 10, 2379–2403. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Beline, T.; Ribeiro, A.L.R.; Rangel, E.C.; da Cruz, N.C.; Landers, R.; Faverani, L.P.; Vaz, L.G.; Fais, L.M.G.; Vicente, F.B.; et al. Development of binary and ternary titanium alloys for dental implants. Dent. Mater. 2017, 33, 1244–1257. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Vasilescu, C.; Drob, S.I.; Neacsu, E.I.; Mirza Rosca, J.C. Surface analysis and corrosion resistance of a new titanium base alloy in simulated body fluids. Corros. Sci. 2012, 65, 431–440. [Google Scholar] [CrossRef]
- Souto, R.M.; Burstein, G.T. A preliminary investigation into the microscopic depassivation of passive titanium implant materials in vitro. J. Mater. Sci. Mater. Med. 1996, 7, 337–343. [Google Scholar] [CrossRef]
- Burstein, G.T.; Liu, C.; Souto, R.M.; Vines, S.P. Origins of pitting corrosion. Corros. Eng. Sci. Technol. 2004, 39, 25–30. [Google Scholar] [CrossRef]
- Burstein, G.T.; Souto, R.M. Observations of localised instability of passive titanium in chloride solution. Electrochim. Acta 1995, 40, 1881–1888. [Google Scholar] [CrossRef]
- Burstein, G.T.; Liu, C.; Souto, R.M. The effect of temperature on the nucleation of corrosion pits on titanium in Ringer’s physiological solution. Biomaterials 2005, 26, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Revathi, A.; Borrás, A.D.; Muñoz, A.I.; Richard, C.; Manivasagam, G. Degradation mechanisms and future challenges of titanium and its alloys for dental implant applications in oral environment. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017, 76, 1354–1368. [Google Scholar] [CrossRef]
- Izquierdo, J.; Mareci, D.; Bolat, G.; Santana, J.J.; Rodríguez-Raposo, R.; Fernández-Mérida, L.C.; Burtan, L.; Trincă, L.C.; Souto, R.M. Improvement of the corrosion resistance of biomedical zr-ti alloys using a thermal oxidation treatment. Metals 2020, 10. [Google Scholar] [CrossRef]
- Mareci, D.; Bolat, G.; Cailean, A.; Santana, J.J.; Izquierdo, J.; Souto, R.M. Effect of acidic fluoride solution on the corrosion resistance of ZrTi alloys for dental implant application. Corros. Sci. 2014, 87, 334–343. [Google Scholar] [CrossRef]
- Mareci, D.; Sutiman, D.; Chelariu, R.; Leon, F.; Curteanu, S. Evaluation of the corrosion resistance of new ZrTi alloys by experiment and simulation with an adaptive instance-based regression model. Corros. Sci. 2013, 73, 106–122. [Google Scholar] [CrossRef]
- Bolat, G.; Izquierdo, J.; Santana, J.J.; Mareci, D.; Souto, R.M. Electrochemical characterization of ZrTi alloys for biomedical applications. Electrochim. Acta 2013, 88, 447–456. [Google Scholar] [CrossRef]
- Izquierdo, J.; Bolat, G.; Mareci, D.; Munteanu, C.; González, S.; Souto, R.M. Electrochemical behaviour of ZrTi alloys in artificial physiological solution simulating in vitro inflammatory conditions. Appl. Surf. Sci. 2014, 313, 259–266. [Google Scholar] [CrossRef]
- Calderon Moreno, J.M.; Vasilescu, E.; Drob, P.; Osiceanu, P.; Vasilescu, C.; Drob, S.I.; Popa, M. Surface analysis and electrochemical behavior of Ti-20Zr alloy in simulated physiological fluids. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2013, 178, 1195–1204. [Google Scholar] [CrossRef]
- Narendrakumar, K.; Kulkarni, M.; Addison, O.; Mazare, A.; Junkar, I.; Schmuki, P.; Sammons, R.; Iglič, A. Adherence of oral streptococci to nanostructured titanium surfaces. Dent. Mater. 2015, 31, 1460–1468. [Google Scholar] [CrossRef]
- Elias, C.N.; Oshida, Y.; Lima, J.H.C.; Muller, C.A. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J. Mech. Behav. Biomed. Mater. 2008, 1, 234–242. [Google Scholar] [CrossRef]
- Roehling, S.; Astasov-Frauenhoffer, M.; Hauser-Gerspach, I.; Braissant, O.; Woelfler, H.; Waltimo, T.; Kniha, H.; Gahlert, M. In Vitro Biofilm Formation on Titanium and Zirconia Implant Surfaces. J. Periodontol. 2017, 88, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, D.A.; Guida, L.; Sridhar, S.; Valderrama, P.; Wilson, T.G.; Rodrigues, D.C. Evaluation of oral microbial corrosion on the surface degradation of dental implant materials. J. Periodontol. 2019, 90, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Chopra, D.; Gulati, K.; Ivanovski, S. Understanding and Optimizing the Antibacterial Functions of Anodized Nano-Engineered Titanium Implants. Acta Biomater. 2021. [Google Scholar] [CrossRef] [PubMed]
- Antunes, R.A.; De Oliveira, M.C.L. Corrosion fatigue of biomedical metallic alloys: Mechanisms and mitigation. Acta Biomater. 2012, 8, 937–962. [Google Scholar] [CrossRef] [PubMed]
- Tejero, R.; Anitua, E.; Orive, G. Toward the biomimetic implant surface: Biopolymers on titanium-based implants for bone regeneration. Prog. Polym. Sci. 2014, 39, 1406–1447. [Google Scholar] [CrossRef]
- Kaluderović, M.R.; Krajnović, T.; Maksimović-Ivanić, D.; Graf, H.L.; Mijatović, S. Ti-SLActive and TiZr-SLActive dental implant surfaces promote fast osteoblast differentiation. Coatings 2017, 7, 102. [Google Scholar] [CrossRef]
- Marco, F.; Milena, F.; Gianluca, G.; Vittoria, O. Peri-implant osteogenesis in health and osteoporosis. Micron 2005, 36, 630–644. [Google Scholar] [CrossRef]
- Cabrera-Domínguez, J.J.; Castellanos-Cosano, L.; Torres-Lagares, D.; Pérez-Fierro, M.; Machuca-Portillo, G. Clinical performance of titanium-zirconium implants with a hydrophilic surface in patients with controlled type 2 diabetes mellitus: 2-year results from a prospective case-control clinical study. Clin. Oral Investig. 2020, 24, 2477–2486. [Google Scholar] [CrossRef]
- El-Sheikh, A.M.; Shihabuddin, O.F. Clinical and radiographic evaluation of narrow-diameter titanium-zirconium implants in unilateral atrophic mandibular distal extensions: A 1-year pilot study. J. Contemp. Dent. Pract. 2015, 15, 417–422. [Google Scholar] [CrossRef]
- Herrmann, J.; Hentschel, A.; Glauche, I.; Vollmer, A.; Schlegel, K.A.; Lutz, R. Implant survival and patient satisfaction of reduced diameter implants made from a titanium-zirconium alloy: A retrospective cohort study with 550 implants in 311 patients. J. Cranio Maxillofac. Surg. 2016, 44, 1940–1944. [Google Scholar] [CrossRef]
- Altinci, P.; Can, G.; Gunes, O.; Ozturk, C.; Eren, H. Stability and Marginal Bone Level Changes of SLActive Titanium-Zirconium Implants Placed with Flapless Surgery: A Prospective Pilot Study. Clin. Implant Dent. Relat. Res. 2016, 18, 1193–1199. [Google Scholar] [CrossRef]
- Vardaki, M.; Mohajernia, S.; Pantazi, A.; Nica, I.C.; Enachescu, M.; Mazare, A.; Demetrescu, I.; Schmuki, P. Post treatments effect on TiZr nanostructures fabricated via anodizing. J. Mater. Res. Technol. 2019, 8, 5802–5812. [Google Scholar] [CrossRef]
- Medvedev, A.E.; Molotnikov, A.; Lapovok, R.; Zeller, R.; Berner, S.; Habersetzer, P.; Dalla Torre, F. Microstructure and mechanical properties of Ti-15Zr alloy used as dental implant material. J. Mech. Behav. Biomed. Mater. 2016, 62, 384–398. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Sowers, K.T.; Olivares-Navarrete, R. Novel in vitro comparative model of osteogenic and inflammatory cell response to dental implants. Dent. Mater. 2019, 35, 176–184. [Google Scholar] [CrossRef]
- Murphy, M.; Walczak, M.S.; Thomas, A.G.; Silikas, N.; Berner, S.; Lindsay, R. Toward optimizing dental implant performance: Surface characterization of Ti and TiZr implant materials. Dent. Mater. 2017, 33, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.J.; Walter, M.S.; Lyngstadaas, S.P.; Wintermantel, E.; Haugen, H.J. Hydrogen content in titanium and a titanium-zirconium alloy after acid etching. Mater. Sci. Eng. C 2013, 33, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.A.; Surmeneva, M.A.; Shugurov, V.V.; Koval, N.N.; Shulepov, I.A.; Surmenev, R.A. Physico-mechanical properties of Ti-Zr coatings fabricated via ion-assisted arc-plasma deposition. Vacuum 2018, 149, 129–133. [Google Scholar] [CrossRef]
- Lin, M.T.; Wan, C.H.; Wu, W. Characterization and corrosion resistance of TiZr coating on SS304 stainless steel using cathodic arc evaporation techniques. Surf. Coat. Technol. 2017, 320, 217–225. [Google Scholar] [CrossRef]
- Romanov, D.A.; Sosnin, K.V.; Filyakov, A.D.; Nevskii, S.A.; Moskovskii, S. V The effect of bioinert electroexplosive coatings on stress distribution near the dental implant-bone interface. Mater. Res. Express 2021, 8. [Google Scholar] [CrossRef]
- Tsai, M.T.; Chang, Y.Y.; Huang, H.L.; Wu, Y.H.; Shieh, T.M. Micro-arc oxidation treatment enhanced the biological performance of human osteosarcoma cell line and human skin fibroblasts cultured on titanium–zirconium films. Surf. Coat. Technol. 2016, 303, 268–276. [Google Scholar] [CrossRef]
- Shin, K.R.; Ko, Y.G.; Shin, D.H. Surface characteristics of ZrO2-containing oxide layer in titanium by plasma electrolytic oxidation in K 4P 2O 7 electrolyte. J. Alloys Compd. 2012, 536, S226–S230. [Google Scholar] [CrossRef]
- Oleshko, O.; Deineka, V.; Husak, Y.; Korniienko, V.; Dryhval, B.; Dudko, J.; Solodovnyk, O.; Simka, W.; Michalska, J.; Mishchenko, O.; et al. Plasma Electrolytic Oxidation of TiZr Alloy in ZnONPs-Contained Solution: Structural and Biological Assessment. In Springer Proceedings in Physics; Springer: Berlin/Heidelberg, Germany, 2020; Volume 244, pp. 75–82. ISBN 9789811539954. [Google Scholar]
- Wang, X.; Fan, H.; Zhang, F.; Zhao, S.; Liu, Y.; Xu, Y.; Wu, R.; Li, D.; Yang, Y.; Liao, L.; et al. Antibacterial Properties of Bilayer Biomimetic Nano-ZnO for Dental Implants. ACS Biomater. Sci. Eng. 2020, 6, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Simonenko, E.P.; Simonenko, N.P.; Kopitsa, G.P.; Mokrushin, A.S.; Khamova, T.V.; Sizova, S.V.; Khaddazh, M.; Tsvigun, N.V.; Pipich, V.; Gorshkova, Y.E.; et al. A sol-gel synthesis and gas-sensing properties of finely dispersed ZrTiO4. Mater. Chem. Phys. 2019, 225, 347–357. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef]
- Viswanathan, V.; Laha, T.; Balani, K.; Agarwal, A.; Seal, S. Challenges and advances in nanocomposite processing techniques. Mater. Sci. Eng. R Reports 2006, 54, 121–285. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrothermal technology for nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef]
- Kumar, A.P.; Depan, D.; Singh Tomer, N.; Singh, R.P. Nanoscale particles for polymer degradation and stabilization-Trends and future perspectives. Prog. Polym. Sci. 2009, 34, 479–515. [Google Scholar] [CrossRef]
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering biocompatible implant surfaces: Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Manole, C.C.; Dinischiotu, A.; Nica, C.; Demetrescu, I.; Pirvu, C. Influence of electrospun TiO2 nanowires on corrosion resistance and cell response of Ti50Zr alloy. Mater. Corros. 2018, 69, 1609–1619. [Google Scholar] [CrossRef]
- Mendonça, G.; Mendonça, D.B.S.; Aragão, F.J.L.; Cooper, L.F. Advancing dental implant surface technology—From micron-to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Sarraf, M.; Nasiri-Tabrizi, B.; Yeong, C.H.; Madaah Hosseini, H.R.; Saber-Samandari, S.; Basirun, W.J.; Tsuzuki, T. Mixed oxide nanotubes in nanomedicine: A dead-end or a bridge to the future? Ceram. Int. 2021, 47, 2917–2948. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, C.J.; Brammer, K.S.; Noh, K.; Connelly, L.S.; Oh, S.; Chen, L.H.; Jin, S. Zirconium oxide nanotube surface prompts increased osteoblast functionality and mineralization. Mater. Sci. Eng. C 2011, 31, 1716–1722. [Google Scholar] [CrossRef]
- Neacsu, P.; Mazare, A.; Cimpean, A.; Park, J.; Costache, M.; Schmuki, P.; Demetrescu, I. Reduced inflammatory activity of RAW 264.7 macrophages on titania nanotube modified Ti surface. Int. J. Biochem. Cell Biol. 2014, 55, 187–195. [Google Scholar] [CrossRef]
- Minagar, S.; Berndt, C.C.; Wang, J.; Ivanova, E.; Wen, C. A review of the application of anodization for the fabrication of nanotubes on metal implant surfaces. Acta Biomater. 2012, 8, 2875–2888. [Google Scholar] [CrossRef]
- Grigorescu, S.; Pruna, V.; Titorencu, I.; Jinga, V.V.; Mazare, A.; Schmuki, P.; Demetrescu, I. The two step nanotube formation on TiZr as scaffolds for cell growth. Bioelectrochemistry 2014, 98, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Stoian, A.B.; Vardaki, M.; Ionita, D.; Enachescu, M.; Prodana, M.; Brancoveanu, O.; Demetrescu, I. Nanopores and nanotubes ceramic oxides elaborated on titanium alloy with zirconium by changing anodization potentials. Ceram. Int. 2018, 44, 7026–7033. [Google Scholar] [CrossRef]
- Ion, R.; Mazare, A.; Dumitriu, C.; Pirvu, C.; Schmuki, P.; Cimpean, A. Nanochannelar topography positively modulates osteoblast differentiation and inhibits osteoclastogenesis. Coatings 2018, 8, 294. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface nano-functionalization of biomaterials. Mater. Sci. Eng. R Rep. 2010, 70, 275–302. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol-gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Vardaki, M.; Romonti, D.C.; Ionita, D.; Demetrescu, I. Polymeric hybrid coatings on Ti50%Zr alloy. Mater. Plast. 2016, 53, 276–279. [Google Scholar]
- Mareci, D.; Trincă, L.C.; Căilean, D.; Souto, R.M. Corrosion resistance of ZrTi alloys with hydroxyapatite-zirconia-silver layer in simulated physiological solution containing proteins for biomaterial applications. Appl. Surf. Sci. 2016, 389, 1069–1075. [Google Scholar] [CrossRef]
- Padovani, G.C.; Feitosa, V.P.; Sauro, S.; Tay, F.R.; Durán, G.; Paula, A.J.; Durán, N. Advances in Dental Materials through Nanotechnology: Facts, Perspectives and Toxicological Aspects. Trends Biotechnol. 2015, 33, 621–636. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Ionita, D.; Grecu, M.; Ungureanu, C.; Demetrescu, I. Modifying the TiAlZr biomaterial surface with coating, for a better anticorrosive and antibacterial performance. Appl. Surf. Sci. 2011, 257, 9164–9168. [Google Scholar] [CrossRef]
- Micksch, T.; Liebelt, N.; Scharnweber, D.; Schwenzer, B. Investigation of the peptide adsorption on ZrO2, TiZr, and TiO2 surfaces as a method for surface modification. ACS Appl. Mater. Interfaces 2014, 6, 7408–7416. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Van Der Mei, H.C.; Subbiahdoss, G.; De Vries, J.; Rustema-Abbing, M.; Kuijer, R.; Busscher, H.J.; Ren, Y. Soft tissue integration versus early biofilm formation on different dental implant materials. Dent. Mater. 2014, 30, 716–727. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Monteiro, M.S.G.; Gupta, S.; Hubler, R.; de Oliveira, S.D. Influence of titanium and zirconia modified surfaces for rapid healing on adhesion and biofilm formation of Staphylococcus epidermidis. Arch. Oral Biol. 2020, 117. [Google Scholar] [CrossRef]
- Galli, S.; Jimbo, R.; Naito, Y.; Berner, S.; Dard, M.; Wennerberg, A. Chemically modified titanium–zirconium implants in comparison with commercially pure titanium controls stimulate the early molecular pathways of bone healing. Clin. Oral Implants Res. 2017, 28, 1234–1240. [Google Scholar] [CrossRef]
- Gallagher, W.M.; Lynch, I.; Allen, L.T.; Miller, I.; Penney, S.C.; O’Connor, D.P.; Pennington, S.; Keenan, A.K.; Dawson, K.A. Molecular basis of cell-biomaterial interaction: Insights gained from transcriptomic and proteomic studies. Biomaterials 2006, 27, 5871–5882. [Google Scholar] [CrossRef]
- Souza, J.G.S.; Bertolini, M.; Costa, R.C.; Lima, C.V.; Barão, V.A.R. Proteomic profile of the saliva and plasma protein layer adsorbed on Ti–Zr alloy: The effect of sandblasted and acid-etched surface treatment. Biofouling 2020, 36, 428–441. [Google Scholar] [CrossRef]
- Chen, S.; Tsoi, J.K.H.; Tsang, P.C.S.; Park, Y.-J.; Song, H.-J.; Matinlinna, J.P. Candida albicans aspects of binary titanium alloys for biomedical applications. Regen. Biomater. 2020, 7, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Florit, M.; Xing, R.; Ramis, J.M.; Taxt-Lamolle, S.; Haugen, H.J.; Lyngstadaas, S.P.; Monjo, M. Human gingival fibroblasts function is stimulated on machined hydrided titanium zirconium dental implants. J. Dent. 2014, 42, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Piattelli, A.; Farias Pontes, A.E.; Degidi, M.; Iezzi, G. Histologic studies on osseointegration: Soft tissues response to implant surfaces and components. A review. Dent. Mater. 2011, 27, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Sista, S.; Wen, C.; Hodgson, P.D.; Pande, G. Expression of cell adhesion and differentiation related genes in MC3T3 osteoblasts plated on titanium alloys: Role of surface properties. Mater. Sci. Eng. C 2013, 33, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Bordin, D.; Cavalcanti, I.M.G.; Jardim Pimentel, M.; Fortulan, C.A.; Sotto-Maior, B.S.; Del Bel Cury, A.A.; da Silva, W.J. Biofilm and saliva affect the biomechanical behavior of dental implants. J. Biomech. 2015, 48, 997–1002. [Google Scholar] [CrossRef]
- Pachaiappan, R.; Rajendran, S.; Show, P.L.; Manavalan, K.; Naushad, M. Metal/metal oxide nanocomposites for bactericidal effect: A review. Chemosphere 2020, 128607. [Google Scholar] [CrossRef]
- Huang, H.L.; Chang, Y.Y.; Liu, J.X.; Tsai, M.T.; Lai, C.H. Antibacterial activity and cell compatibility of TiZrN, TiZrCN, and TiZr-amorphous carbon coatings. Thin Solid Films 2015, 596, 111–117. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Llama-Palacios, A.; Fernández, E.; Figuero, E.; Marín, M.J.; León, R.; Blanc, V.; Herrera, D.; Sanz, M. An in vitro biofilm model associated to dental implants: Structural and quantitative analysis of in vitro biofilm formation on different dental implant surfaces. Dent. Mater. 2014, 30, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Tsoi, J.K.H.; Rodrigues, F.P.; Leprince, J.G.; Palin, W.M. Bacterial adhesion mechanisms on dental implant surfaces and the influencing factors. Int. J. Adhes. Adhes. 2016, 69, 58–71. [Google Scholar] [CrossRef]
- Myshin, H.L.; Wiens, J.P. Factors affecting soft tissue around dental implants: A review of the literature. J. Prosthet. Dent. 2005, 94, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, C.; Pirvu, C.; Mindroiu, M.; Demetrescu, I. Antibacterial polymeric coating based on polypyrrole and polyethylene glycol on a new alloy TiAlZr. Prog. Org. Coat. 2012, 75, 349–355. [Google Scholar] [CrossRef]
- Al-Radha, A.S.D.; Dymock, D.; Younes, C.; O’Sullivan, D. Surface properties of titanium and zirconia dental implant materials and their effect on bacterial adhesion. J. Dent. 2012, 40, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Fackler, A.; Follo, M.; Hellwig, E.; Bächle, M.; Hannig, C.; Han, J.S.; Wolkewitz, M.; Kohal, R. In vivo study of the initial bacterial adhesion on different implant materials. Arch. Oral Biol. 2013, 58, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Hamlet, S.M.; Ivanovski, S. Tailoring the immuno-responsiveness of anodized nano-engineered titanium implants. J. Mater. Chem. B 2018, 6, 2677–2689. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Luo, H.; Zhang, D.; Lei, S.; Zhou, K. Dual-Purpose Magnesium-Incorporated Titanium Nanotubes for Combating Bacterial Infection and Ameliorating Osteolysis to Realize Better Osseointegration. ACS Biomater. Sci. Eng. 2019, 5, 5368–5383. [Google Scholar] [CrossRef]
- Huo, K.; Zhang, X.; Wang, H.; Zhao, L.; Liu, X.; Chu, P.K. Osteogenic activity and antibacterial effects on titanium surfaces modified with Zn-incorporated nanotube arrays. Biomaterials 2013, 34, 3467–3478. [Google Scholar] [CrossRef]
- Rahnamaee, S.Y.; Bagheri, R.; Heidarpour, H.; Vossoughi, M.; Golizadeh, M.; Samadikuchaksaraei, A. Nanofibrillated chitosan coated highly ordered titania nanotubes array/graphene nanocomposite with improved biological characters. Carbohydr. Polym. 2021, 254, 117465. [Google Scholar] [CrossRef]
- Prokopovich, P. Interactions between mammalian cells and nano-or micro-sized wear particles: Physico-chemical views against biological approaches. Adv. Colloid Interface Sci. 2014, 213, 36–47. [Google Scholar] [CrossRef]
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- Hanawa, T. Evaluation techniques of metallic biomaterials in vitro. Sci. Technol. Adv. Mater. 2002, 3, 289–295. [Google Scholar] [CrossRef]
- Park, Y.J.; Song, Y.H.; An, J.H.; Song, H.J.; Anusavice, K.J. Cytocompatibility of pure metals and experimental binary titanium alloys for implant materials. J. Dent. 2013, 41, 1251–1258. [Google Scholar] [CrossRef]
- Mazare, A.; Totea, G.; Burnei, C.; Schmuki, P.; Demetrescu, I.; Ionita, D. Corrosion, antibacterial activity and haemocompatibility of TiO2 nanotubes as a function of their annealing temperature. Corros. Sci. 2016, 103, 215–222. [Google Scholar] [CrossRef]
- Grigorescu, S.; Ungureanu, C.; Kirchgeorg, R.; Schmuki, P.; Demetrescu, I. Various sized nanotubes on TiZr for antibacterial surfaces. Appl. Surf. Sci. 2013, 270, 190–196. [Google Scholar] [CrossRef]
- Lyndon, J.A.; Boyd, B.J.; Birbilis, N. Metallic implant drug/device combinations for controlled drug release in orthopaedic applications. J. Control. Release 2014, 179, 63–75. [Google Scholar] [CrossRef]
- Jabed, A.; Khan, M.M.; Camiller, J.; Greenlee-Wacker, M.; Haider, W.; Shabib, I. Property optimization of Zr-Ti-X (X = Ag, Al) metallic glass via combinatorial development aimed at prospective biomedical application. Surf. Coat. Technol. 2019, 372, 278–287. [Google Scholar] [CrossRef]
- Huang, C.H.; Huang, Y.S.; Lin, Y.S.; Lin, C.H.; Huang, J.C.; Chen, C.H.; Li, J.B.; Chen, Y.H.; Jang, J.S.C. Electrochemical and biocompatibility response of newly developed TiZr-based metallic glasses. Mater. Sci. Eng. C 2014, 43, 343–349. [Google Scholar] [CrossRef]
- Heimann, R.B. Thermal spraying of biomaterials. Surf. Coat. Technol. 2006, 201, 2012–2019. [Google Scholar] [CrossRef]
- Jimbo, R.; Naito, Y.; Galli, S.; Berner, S.; Dard, M.; Wennerberg, A. Biomechanical and Histomorphometrical Evaluation of TiZr Alloy Implants: An in vivo Study in the Rabbit. Clin. Implant Dent. Relat. Res. 2015, 17, e670–e678. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Zhu, F.; Li, Z.; Zhang, P.; Lin, X.; Dard, M. The osseointegration behavior of titanium-zirconium implants in ovariectomized rabbits. Clin. Oral Implants Res. 2014, 25, 819–825. [Google Scholar] [CrossRef]
- Yin, L.; Chang, Y.; You, Y.; Liu, C.; Li, J.; Lai, H. Biological responses of human bone mesenchymal stem cells to Ti and TiZr implant materials. Clin. Implant Dent. Relat. Res. 2019, 21, 550–564. [Google Scholar] [CrossRef]
- Ghazal, S.; Huynh-Ba, G.; Aghaloo, T.; Dibart, S.; Froum, S.; O’Neal, R.; Cochran, D. Randomized Controlled Multicenter Clinical Study Evaluating Crestal Bone Level Change of Narrow-Diameter Versus Standard-Diameter Ti-Zr Implants for Single Tooth Replacement in Anterior and Premolar Region. Int. J. Oral Maxillofac. Implants 2019, 34, 708–718. [Google Scholar] [CrossRef]
- Quirynen, M.; Al-Nawas, B.; Meijer, H.J.A.; Razavi, A.; Reichert, T.E.; Schimmel, M.; Storelli, S.; Romeo, E.; Bräuer, I.; Castro-Laza, J.; et al. Small-diameter titanium Grade IV and titanium-zirconium implants in edentulous mandibles: Three-year results from a double-blind, randomized controlled trial. Clin. Oral Implants Res. 2015, 26, 831–840. [Google Scholar] [CrossRef]
- Müller, F.; Al-Nawas, B.; Storelli, S.; Quirynen, M.; Hicklin, S.; Castro-Laza, J.; Bassetti, R.; Schimmel, M. Small-diameter titanium grade IV and titanium-zirconium implants in edentulous mandibles: Five-year results from a double-blind, randomized controlled trial. BMC Oral Health 2015, 15, 1–10. [Google Scholar] [CrossRef]
- Zheng, X.; Mo, A. Immune System-Related Diseases and Dental Implant Treatment. In Dental Implant Treatment in Medically Compromised Patients; Springer International Publishing: Cham, Switzerland, 2020; pp. 129–147. ISBN 9783030285562. [Google Scholar]
- Karl, M.; Krafft, T.; Kelly, J. Fracture of a Narrow-Diameter Roxolid Implant: Clinical and Fractographic Considerations. Int. J. Oral Maxillofac. Implants 2014, 29, 1193–1196. [Google Scholar] [CrossRef]
- Rahmati, M.; Lyngstadaas, S.P.; Reseland, J.E.; Andersbakken, I.; Haugland, H.S.; López-Peña, M.; Cantalapiedra, A.G.; Guzon Muñoz, F.M.; Haugen, H.J. Coating doxycycline on titanium-based implants: Two in vivo studies. Bioact. Mater. 2020, 5, 787–797. [Google Scholar] [CrossRef]
- Walter, M.S.; Frank, M.J.; Satué, M.; Monjo, M.; Rønold, H.J.; Lyngstadaas, S.P.; Haugen, H.J. Bioactive implant surface with electrochemically bound doxycycline promotes bone formation markers in vitro and in vivo. Dent. Mater. 2014, 30, 200–214. [Google Scholar] [CrossRef]
- Mendonça, G.; Mendonça, D.B.S.; Simões, L.G.P.; Araújo, A.L.; Leite, E.R.; Golin, A.L.; Aragão, F.J.L.; Cooper, L.F. Nanostructured implant surface effect on osteoblast gene expression and bone-to-implant contact in vivo. Mater. Sci. Eng. C 2011, 31, 1809–1818. [Google Scholar] [CrossRef]
- Sharma, A.; McQuillan, A.J.; Shibata, Y.; Sharma, L.A.; Waddell, J.N.; Duncan, W.J. Histomorphometric and histologic evaluation of titanium–zirconium (aTiZr) implants with anodized surfaces. J. Mater. Sci. Mater. Med. 2016, 27. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).