Obtaining, Evaluation, and Optimization of Doxycycline-Loaded Microparticles Intended for the Local Treatment of Infectious Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Microcapsules
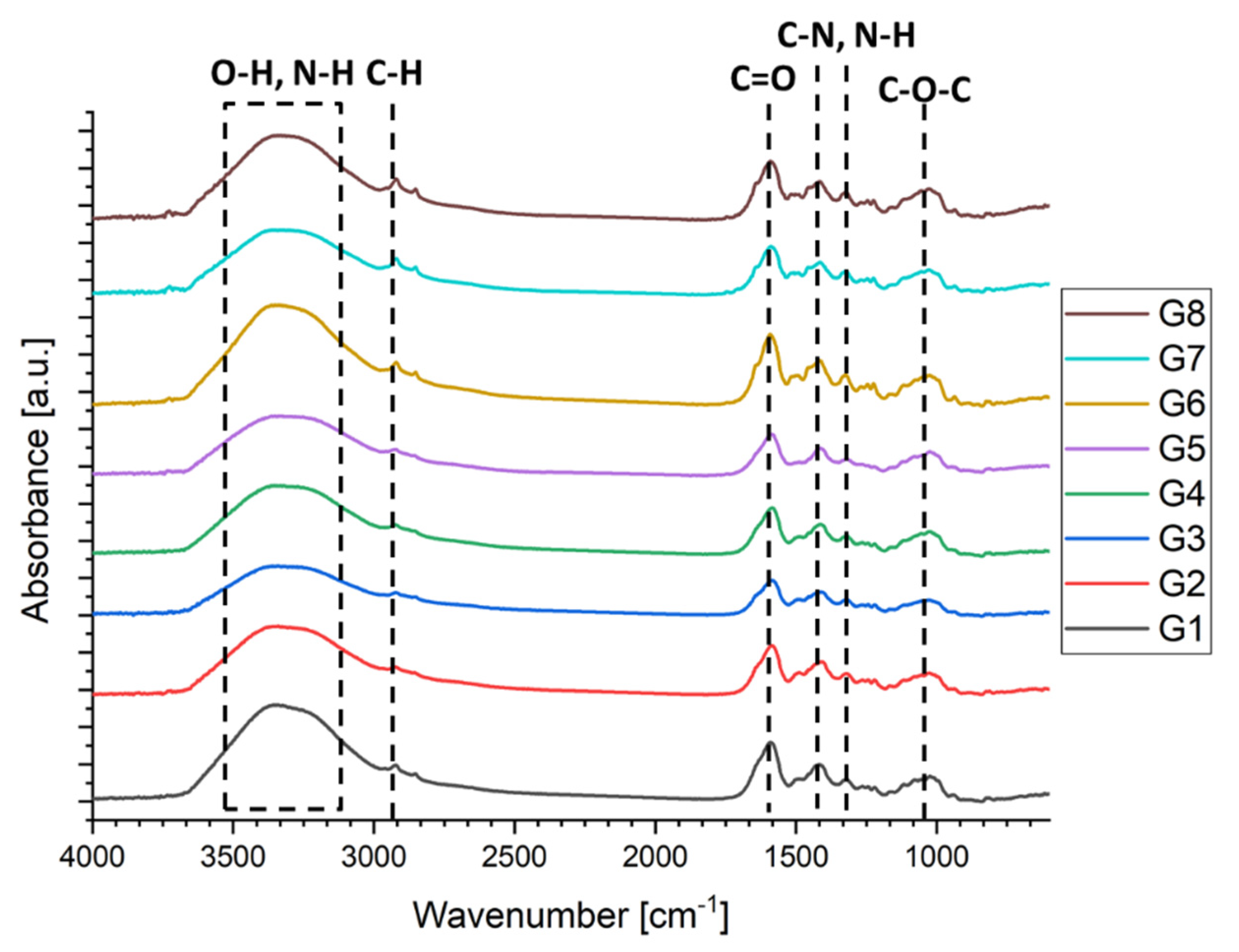
2.2.2. Fourier-Transform Infrared Spectroscopy (FTIR)
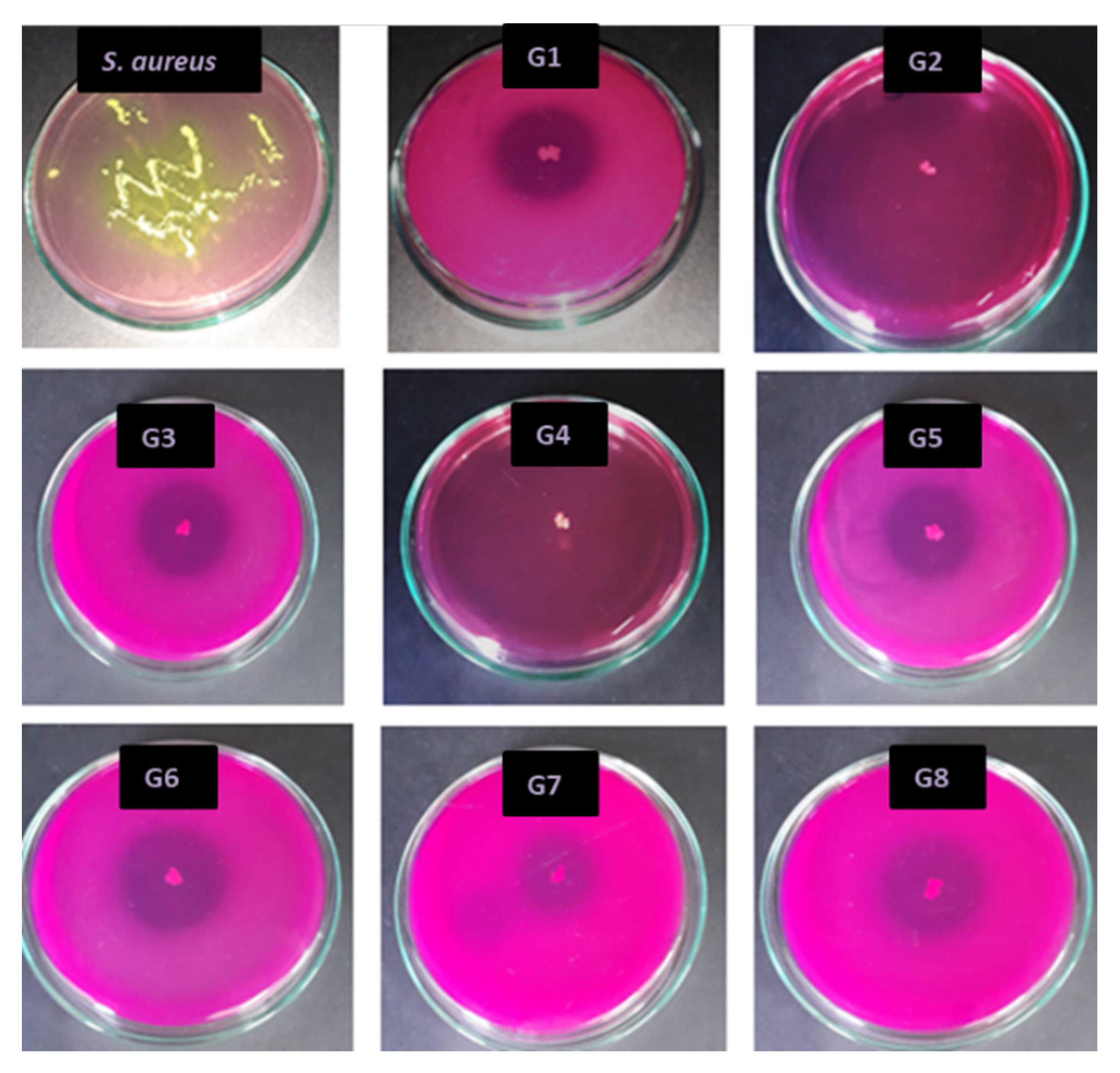
2.2.3. Antimicrobial Activity against Staphylococcus Aureus (S. aureus) Using “Agar Diffusion Method”
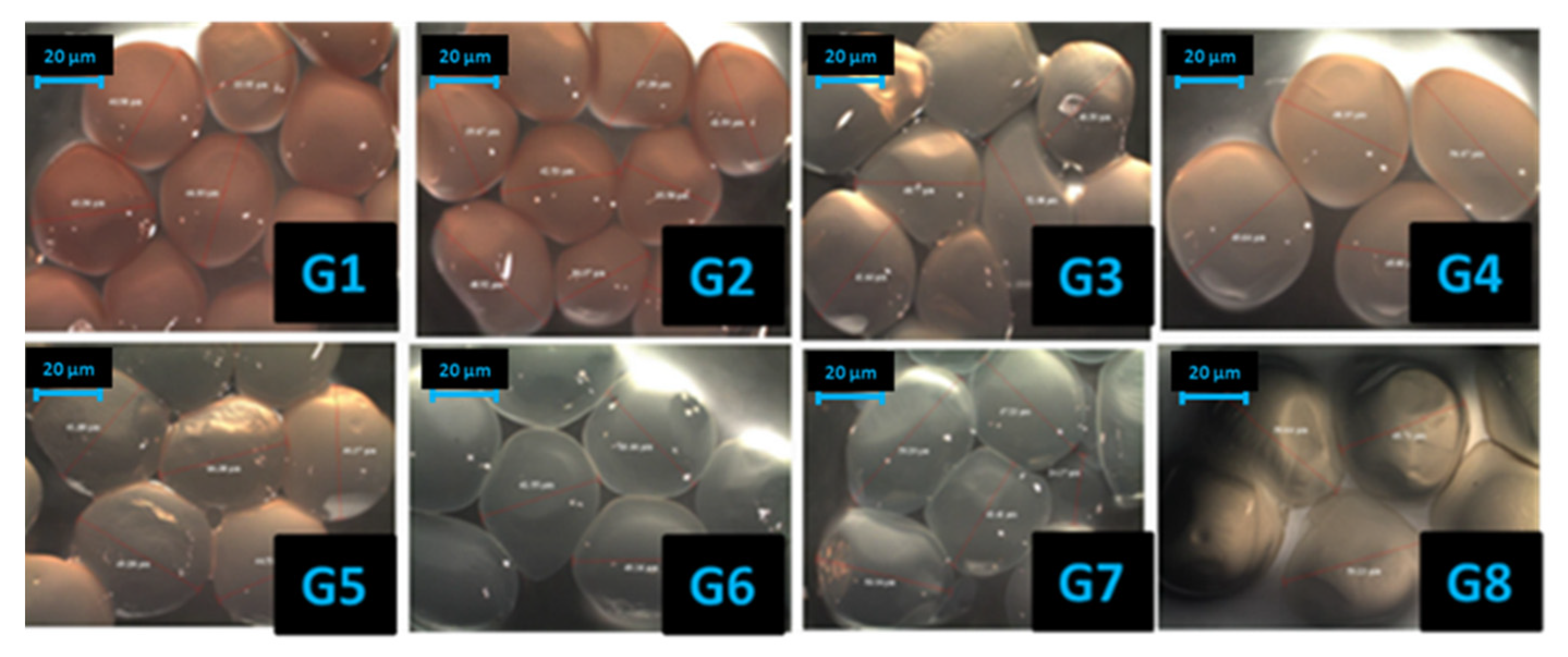
2.2.4. Optical Microscopy Images
2.2.5. Enzymatic Degradation
2.2.6. Drug Encapsulation Efficiency (EE)
2.2.7. Experimental Design and Optimization Technique
3. Results and Discussion
3.1. FTIR
3.2. Antimicrobial Activity Against S. aureus Using the “Agar Diffusion Method”
3.3. Optical Microscopy Images
3.4. Enzymatic Degradation
3.5. Drug Encapsulation Efficiency
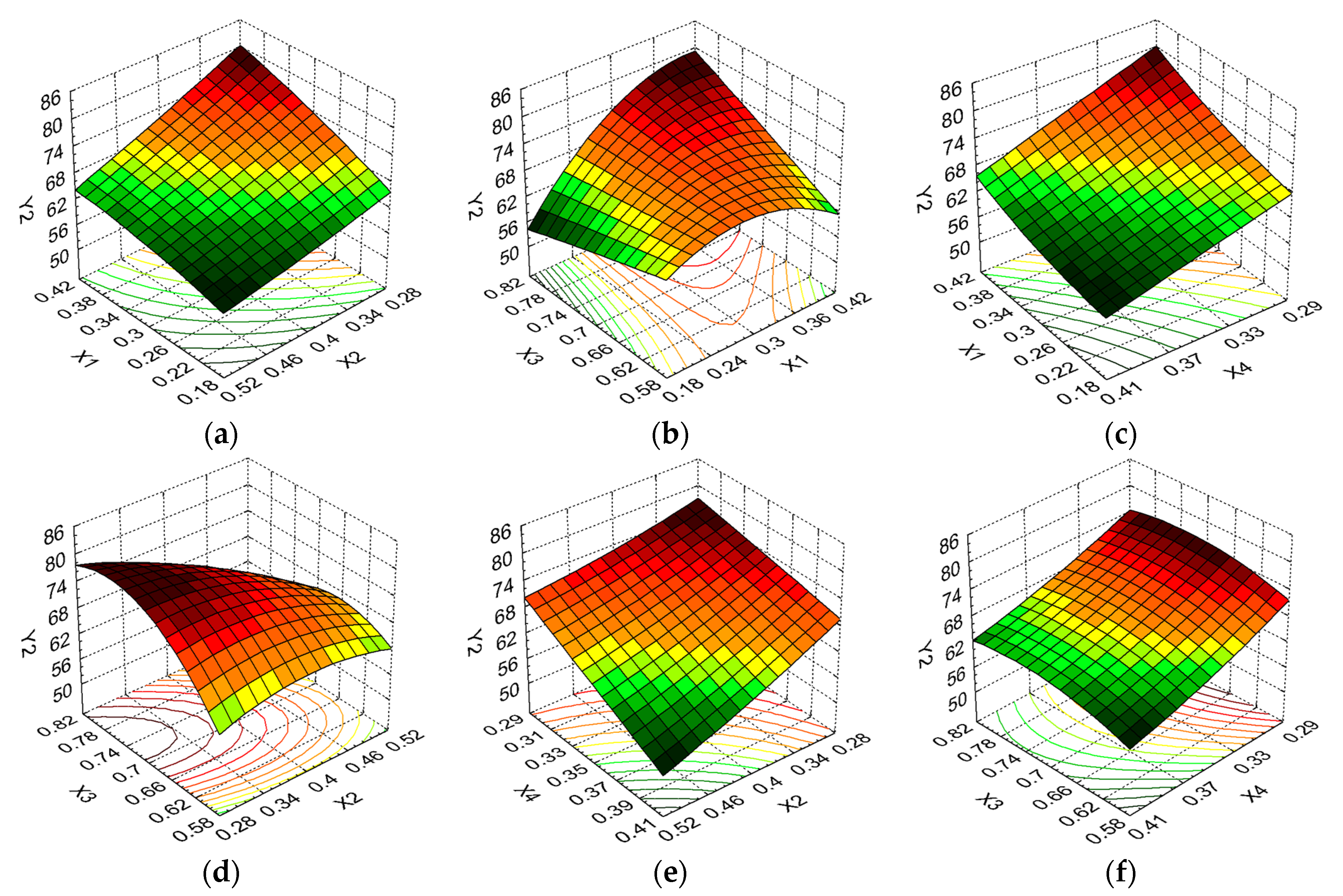
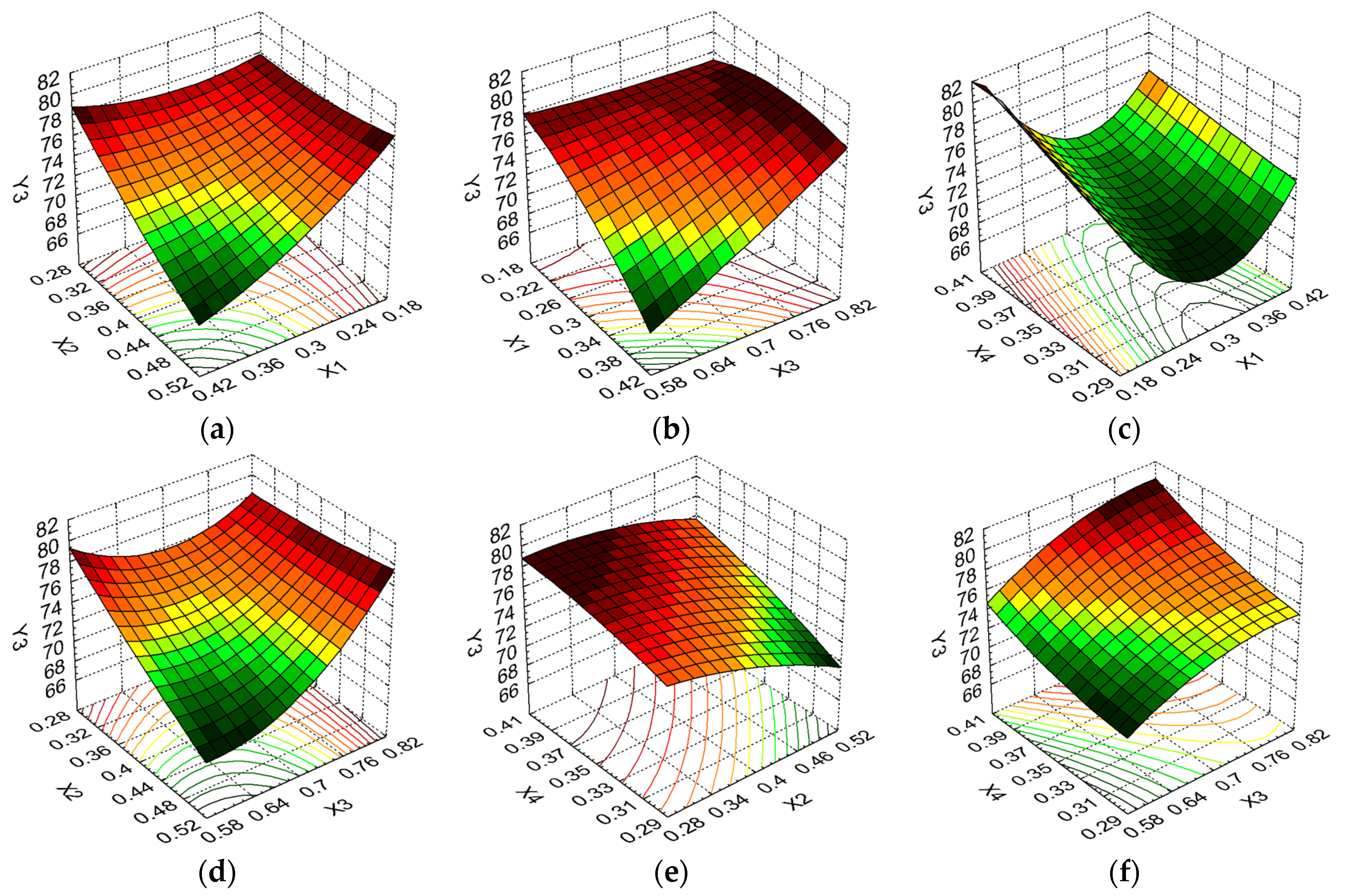
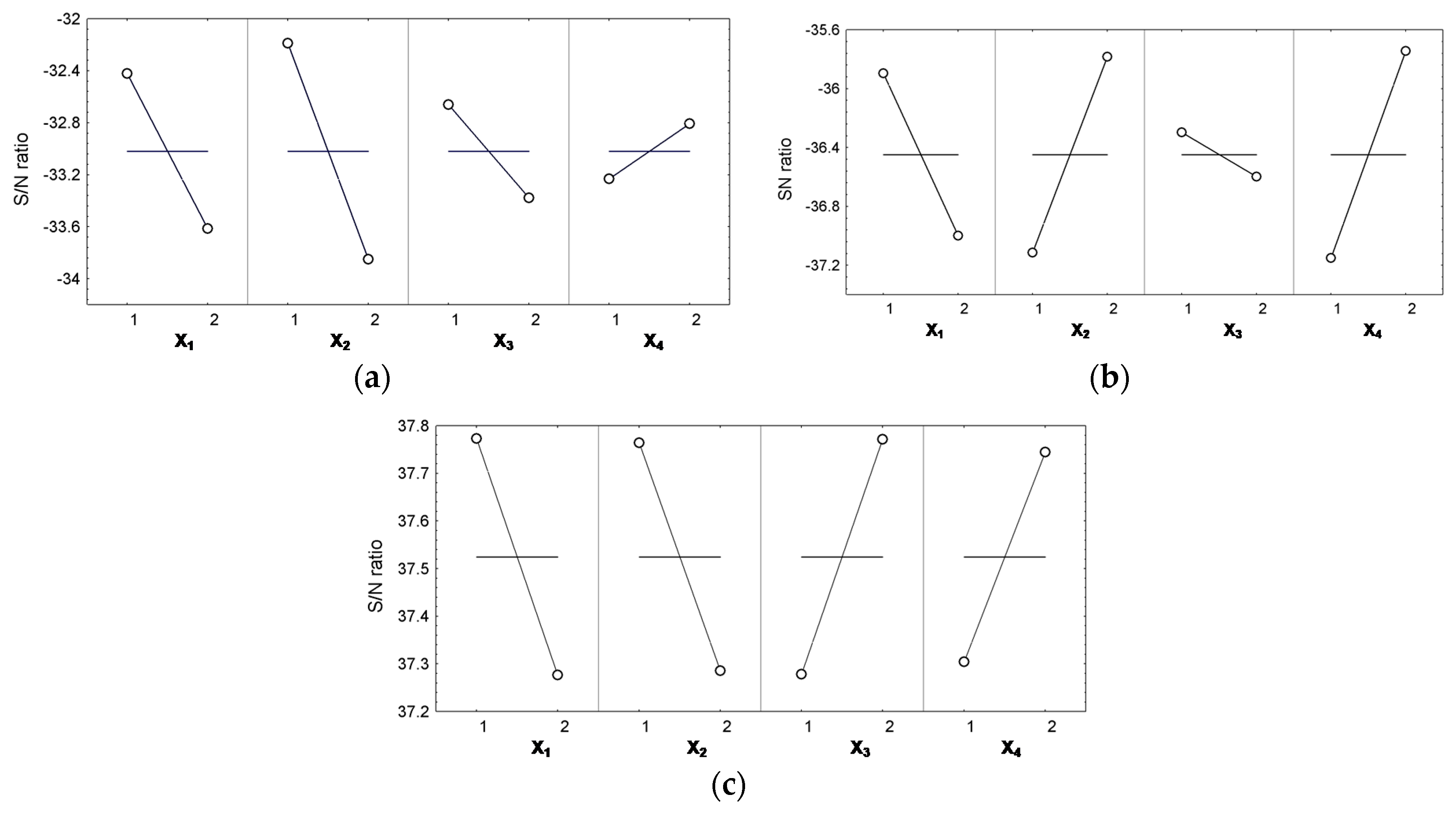
3.6. The Optimization Technique
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nade, S. Septic arthritis. Best Pract. Res. Clin. Rheumatol. 2003, 17, 183–200. [Google Scholar] [CrossRef]
- Kaandorp, C.J.; Van Schaardenburg, D.; Krijnen, P.; Habbema, J.D.; van de Laar, M.A. Risk factors for septic arthritis in patients with joint disease. A prospective study. Arthritis Rheum. 1995, 38, 1819–1825. [Google Scholar] [CrossRef]
- Edwards, A.M.; Massey, R.C. How does Staphylococcus aureus escape the bloodstream? Trends Microbiol. 2011, 19, 184–910. [Google Scholar] [CrossRef] [PubMed]
- Bremell, T.; Abdelnour, A.; Tarkowski, A. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect. Immun. 1992, 60, 2976–2985. [Google Scholar] [CrossRef] [PubMed]
- Verdrengh, M.; Carlsten, H.; Ohlsson, C.; Tarkowski, A. Rapid systemic bone resorption during the course of Staphylococcus aureus-induced arthritis. J. Infect. Dis. 2006, 194, 1597–1600. [Google Scholar] [CrossRef]
- Kong, Y.Y.; Feige, U.; Sarosi, I.; Bolon, B.; Tafuri, A.; Morony, S.; Capparelli, C.; Li, J.; Elliott, R.; McCabe, S.; et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoproteger in ligand. Nature 1999, 402, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Campagnuolo, G.; Bolon, B.; Feige, U. Kinetics of bone protection by recombinant osteoproteger in therapy in Lewis rats with adjuvant arthritis. Arthritis Rheum. 2002, 46, 1926–1936. [Google Scholar] [CrossRef]
- Shirtliff, M.E.; Mader, J.T. Acute septic arthritis. Clin. Microbiol. Rev. 2002, 15, 527–544. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Vangsness, C.T., Jr.; Burke, W.S.; Narvy, S.J.; MacPhee, R.D.; Fedenko, A.N. Human knee synovial fluid cytokines correlated with grade of knee osteoarthritis—A pilot study. Bull. NYU Hosp. Jt. Dis. 2011, 69, 122–127. [Google Scholar] [PubMed]
- Colavite, P.M.; Sartori, A. Septic arthritis: Immunopathogenesis, experimental models and therapy. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 19–27. [Google Scholar] [CrossRef]
- Clerc, O.; Prod’hom, G.; Greub, G.; Zanetti, G.; Senn, L. Adult native septic arthritis: A review of 10 years of experience and lessons for empirical antibiotic therapy. J. Antimicrob. Chemother. 2011, 66, 1168–1173. [Google Scholar] [CrossRef]
- Howard-Jones, A.R.; Isaacs, D.; Gibbons, P.J. Twelve-month outcome following septic arthritis in children. J. Pediatr. Orthop. B 2013, 22, 486–490. [Google Scholar] [CrossRef]
- Le Dantec, L.; Maury, F.; Flipo, R.M.; Laskri, S.; Cortet, B.; Duquesnoy, B.; Delcambre, B. Peripheral pyogenic arthritis. A study of one hundred seventy-nine cases. Rev. Du Rhum. (Engl. Ed.) 1996, 63, 103–110. [Google Scholar]
- Morgan, D.S.; Fisher, D.; Merianos, A.; Currie, B.J. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol. Infect. 1996, 117, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.J.; Kavanagh, R.; Wall, P.G.; Hazleman, B.L. Bacterial joint infections in England and Wales: Analysis of bacterial isolates over a four year period. Br. J. Rheumatol. 1997, 36, 370–373. [Google Scholar] [CrossRef]
- Manjanna, K.M.; Shivakumar, B.; Kumar, T.M.P. Microencapsulation: An acclaimed novel drug-delivery system for NSAIDs in arthritis. Crit. Rev. Ther. Drug Carr. Syst. 2010, 27, 501–532. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, D.T. Microparticulate drug delivery systems. Drug Dev. Ind. Pharm. 2008, 12, 117–122. [Google Scholar]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef]
- De Vos, P.; Lazarjani, H.A.; Poncelet, D.; Faas, M.M. Polymers in cell encapsulation from an enveloped cell perspective. Adv. Drug Deliv. Rev. 2014, 67, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Hoad, C.L.; Rayment, P.; Cox, E.; Wright, P.; Butler, M.; Spiller, R.C.; Gowland, P.A. Investigation of alginate beads for gastro-intestinal functionality, Part 2: In vivo characterisation. Food Hydrocoll. 2009, 23, 833–839. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Chevallay, B.; Herbage, D. Collagen-based biomaterials as 3D scaffold for cell cultures: Applications for tissue engineering and gene therapy. Med Biol. Eng. Comput. 2000, 38, 211–218. [Google Scholar] [CrossRef]
- Wolf, K.; Alexander, S.; Schacht, V.; Coussens, L.M.; Von Andrian, U.H.; Van Rheenen, J.; Deryugina, E.; Friedl, P. Collagen-based cell migration models in vitro and in vivo. Semin. Cell Dev. Biol. 2009, 20, 931–941. [Google Scholar] [CrossRef]
- Wang, K.H.; Wan, R.; Chiu, L.H.; Tsai, Y.H.; Fang, C.L.; Bowley, J.F.; Chen, K.C.; Shih, H.N.; Lai, W.F.T. Effects of collagen matrix and bioreactor cultivation on cartilage regeneration of a full-thickness critical-size knee joint cartilage defects with subchondral bone damage in a rabbit model. PLoS ONE 2018, 13, e0199567. [Google Scholar] [CrossRef]
- Pulkkinen, H.; Tiitu, V.; Valonen, P.; Jurvelin, J.; Rieppo, L.; Töyräs, J.; Silvast, T.S.; Lammi, M.; Kiviranta, I. Repair of osteochondral defects with recombinant human type II collagen gel and autologous chondrocytes in rabbit. Osteoarthr. Cartil. 2013, 21, 481–490. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable cellulose-based hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Raval, J.P.; Naik, D.R.; Amin, K.A.; Patel, P.S. Controlled-release and antibacterial studies of doxycycline-loaded poly (e-caprolactone) microspheres. J. Saudi Chem. Soc. 2014, 18, 566–573. [Google Scholar] [CrossRef]
- Uyen, N.T.T.; Hamid, Z.A.A.; Thi, L.A.; Ahmad, N. Synthesis and characterization of curcumin loaded alginate microspheres for drug delivery. J. Drug Deliv. Sci. Technol. 2020, 58, 101796. [Google Scholar] [CrossRef]
- Yadav, S.K.; Khan, G.; Bonde, G.V.; Bansal, M.; Mishra, B. Design, optimization and characterizations of chitosan fortified calcium alginate microspheres for the controlled delivery of dual drugs. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1180–1193. [Google Scholar] [CrossRef]
- Marin, M.M.; Albu Kaya, M.; Marin, S.; Danila, E.; Bumbeneci, G.; Aldea, C.; Coara, G.; Albu, L. Process for Obtaining Collagen Extracts From Bovine Cartilage For Medical Applications. CBI OSIM A 00840/26.10.2018. 26 October 2018. [Google Scholar]
- Ghica, M.V.; Albu, M.G.; Leca, M.; Popa, L.; Moisescu, S.T. Design and optimization of some collagen-minocycline based hydrogels potentially applicable for the treatment of cutaneous wound infections. Die Pharm. 2011, 66, 853–861. [Google Scholar]
- Ghica, M.V.; Popa, L.; Saramet, G.; Leca, M.; Lupuliasa, D.; Moisescu, S. Optimization of the pharmaceutical products and process design applying Taguchi quality engineering principles. Farmacia 2011, 59, 321–328. [Google Scholar]
- Ghica, M.V.; Albu, M.G.; Popa, L.; Moisescu, S. Response surface methodology and Taguchi approach to assess the combined effect of formulation factors on minocycline delivery from collagen sponges. Die Pharm. 2013, 68, 340–348. [Google Scholar]
- Ghica, M.V.; Kaya, M.G.A.; Dinu-Pîrvu, C.-E.; Lupuleasa, D.; Udeanu, D.I. Development, optimization and in vitro/in vivo characterization of collagen-dextran spongious wound dressings loaded with flufenamic acid. Molecules 2017, 22, 1552. [Google Scholar] [CrossRef] [PubMed]
- Kaya, D.A.; Ghica, M.V.; Dănilă, E.; Öztürk, Ş.; Türkmen, M.; Kaya, M.G.A.; Dinu-Pîrvu, C.-E. Selection of optimal operating conditions for extraction of Myrtus Communis L. essential oil by the steam distillation method. Molecules 2020, 25, 2399. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, microspheres, and microcapsules for advanced drug delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Del Prado Audelo, M.L.; Gómez Lizárraga, K.K.; Gómez, D.M.G.; Martínez Hernández, H.; Rodríguez Fuentes, N.; Castell Rodríguez, A.E.; Montufar, E.B.; Piña Barba, M.C. Development of collagen-EDC scaffolds for skin tissue engineering: Physicochemical and biological characterization. Int. J. Eng. Res. Sci. 2016, 2, 73–83. [Google Scholar]


| Independent Variables * | Coded Symbol | Coded and Uncoded Variation Levels | |
|---|---|---|---|
| Inferior (1) | Superior (2) | ||
| Collagen (%) | X1 | 0.2 | 0.4 |
| NaCMC (%) | X2 | 0.3 | 0.5 |
| Alg (%) | X3 | 0.6 | 0.8 |
| Doxycycline hyclate (%) | X4 | 0.3 | 0.4 |
| Dependent Variables | Coded Symbol | Constraints | |
| Diameter (μm) | Y1 | Minimize | |
| Enzymatic degradation—2 h (%) | Y2 | Minimize | |
| Drug encapsulation efficiency (%) | Y3 | Maximize | |
| Trials. No. | Exp. Name | Independent Variables (Coded Level) | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | ||
| 1 | G1 | 1 | 1 | 1 | 1 |
| 2 | G2 | 1 | 1 | 1 | 2 |
| 3 | G3 | 1 | 2 | 2 | 1 |
| 4 | G4 | 1 | 2 | 2 | 2 |
| 5 | G5 | 2 | 1 | 2 | 1 |
| 6 | G6 | 2 | 1 | 2 | 2 |
| 7 | G7 | 2 | 2 | 1 | 1 |
| 8 | G8 | 2 | 2 | 1 | 2 |
| Sample | Inhibition Area Diameter (mm) | Bacterial Strain | Evaluation | SD (Standard Deviation) for Three Determinations |
|---|---|---|---|---|
| G1 | 16 | Staphylococcus aureus | Satisfactory effect | 0.1 |
| G2 | Total inhibition | Staphylococcus aureus | Satisfactory effect | 0 |
| G3 | 17 | Staphylococcus aureus | Satisfactory effect | 0.4 |
| G4 | Total inhibition | Staphylococcus aureus | Satisfactory effect | 0 |
| G5 | 15 | Staphylococcus aureus | Satisfactory effect | 0.3 |
| G6 | 20 | Staphylococcus aureus | Satisfactory effect | 0.4 |
| G7 | 13 | Staphylococcus aureus | Satisfactory effect | 0.3 |
| G8 | 18 | Staphylococcus aureus | Satisfactory effect | 0.3 |
| Trials. No. | Exp. Name | Independent Variables (Coded Level/Physical Level) | Dependent Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 (g%) | X2 (g%) | X3 (g%) | X4 (g%) | Y1 (μm) | Y2 (%) | Y3 (%) | |||||
| Obs. | Pred. | Obs. | Pred. | Obs. | Pred. | ||||||
| 1 | G1 | 1 (0.2) | 1 (0.3) | 1 (0.6) | 1 (0.3) | 37.29 | 37.58 | 69.23 | 70.03 | 75.44 | 75.55 |
| 2 | G2 | 1 (0.2) | 1 (0.3) | 1 (0.6) | 2 (0.4) | 35.62 | 35.63 | 63.21 | 61.97 | 79.25 | 78.49 |
| 3 | G3 | 1 (0.2) | 2 (0.5) | 2 (0.8) | 1 (0.3) | 48.77 | 49.56 | 65.16 | 66.30 | 74.02 | 76.21 |
| 4 | G4 | 1 (0.2) | 2 (0.5) | 2(0.8) | 2 (0.4) | 47.09 | 46.31 | 52.96 | 52.87 | 81.04 | 80.14 |
| 5 | G5 | 2 (0.4) | 1 (0.3) | 2 (0.8) | 1 (0.3) | 45.75 | 46.09 | 81.88 | 82.10 | 76.48 | 75.07 |
| 6 | G6 | 2 (0.4) | 1 (0.3) | 2 (0.8) | 2 (0.4) | 45.06 | 44.15 | 73.84 | 74.04 | 78.10 | 79.01 |
| 7 | G7 | 2 (0.4) | 2 (0.5) | 1 (0.6) | 1 (0.3) | 53.19 | 52.42 | 72.89 | 71.12 | 67.68 | 67.57 |
| 8 | G8 | 2 (0.4) | 2 (0.5) | 1 (0.6) | 2 (0.4) | 48.18 | 49.17 | 56.99 | 57.69 | 70.57 | 70.52 |
| Responses | Sources of Variation | Sum of Squares | df | Mean Squares | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Y1 | Regression | 239.808 | 3 | 79.936 0.960 | 83.197 | 0.000464 < 0.01 |
| Residual | 3.843 | 4 | ||||
| Total | 243.651 | 7 | ||||
| Y2 | Regression | 615.758 | 3 | 205.252 1.793 | 114.422 | 0.000248 < 0.01 |
| Residual | 7.175 | 4 | ||||
| Total | 622.933 | 7 | ||||
| Y3 | Regression | 130.864 | 2 | 65.432 5.180 | 36.31 | 0.001053 < 0.01 |
| Residual | 9.009 | 5 | ||||
| Total | 139.873 | 7 |
| Control Factors (Input Variables) | Y1 | Y2 | Y3 | |||
|---|---|---|---|---|---|---|
| “Smaller-the-Better” | Effect Size | “Smaller-the-Better” | Effect Size | “Larger-the-Better” | Effect Size | |
| X1 | 1 | 0.596 | 1 | 0.552 | 1 | 0.248 |
| X2 | 1 | 0.831 | 2 | 0.665 | 1 | 0.239 |
| X3 | 1 | 0.358 | 1 | 0.149 | 2 | 0.246 |
| X4 | 2 | 0.212 | 2 | 0.703 | 2 | 0.220 |
| S/N ratio expected (dB) | −31.021 | – | −34.377 | – | 38.479 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, M.M.; Albu Kaya, M.G.; Iovu, H.; Stavarache, C.E.; Chelaru, C.; Constantinescu, R.R.; Dinu-Pîrvu, C.-E.; Ghica, M.V. Obtaining, Evaluation, and Optimization of Doxycycline-Loaded Microparticles Intended for the Local Treatment of Infectious Arthritis. Coatings 2020, 10, 990. https://doi.org/10.3390/coatings10100990
Marin MM, Albu Kaya MG, Iovu H, Stavarache CE, Chelaru C, Constantinescu RR, Dinu-Pîrvu C-E, Ghica MV. Obtaining, Evaluation, and Optimization of Doxycycline-Loaded Microparticles Intended for the Local Treatment of Infectious Arthritis. Coatings. 2020; 10(10):990. https://doi.org/10.3390/coatings10100990
Chicago/Turabian StyleMarin, Maria Minodora, Mădălina Georgiana Albu Kaya, Horia Iovu, Cristina Elena Stavarache, Ciprian Chelaru, Rodica Roxana Constantinescu, Cristina-Elena Dinu-Pîrvu, and Mihaela Violeta Ghica. 2020. "Obtaining, Evaluation, and Optimization of Doxycycline-Loaded Microparticles Intended for the Local Treatment of Infectious Arthritis" Coatings 10, no. 10: 990. https://doi.org/10.3390/coatings10100990
APA StyleMarin, M. M., Albu Kaya, M. G., Iovu, H., Stavarache, C. E., Chelaru, C., Constantinescu, R. R., Dinu-Pîrvu, C.-E., & Ghica, M. V. (2020). Obtaining, Evaluation, and Optimization of Doxycycline-Loaded Microparticles Intended for the Local Treatment of Infectious Arthritis. Coatings, 10(10), 990. https://doi.org/10.3390/coatings10100990







