Abstract
Yb2O3-Gd2O3-Y2O3 co-doped ZrO2 (YGYZ) is considered to be a promising material in thermal barrier coating (TBC) applications. In this study, 2Yb2O3-2Gd2O3-6Y2O3-90ZrO2 (mol.%) (10YGYZ) feedstock candidates for air plasma spraying (APS) were prepared by calcination of agglomerated powders at 1100, 1200, 1300, 1400, and 1500 °C for 3 h, respectively. Incomplete solid solution was observed in calcined powders at 1100, 1200 and 1300 °C, and the 1500 °C calcined powder exhibited poor flowability due to intense sintering effect. The 1400 °C calcined powders were eventually determined to be the optimized feedstock for proper phase structure (cubic phase), great flowability, suitable apparent density and particle size distribution, etc. 10YGYZ TBCs with the optimized feedstock were prepared by APS, exhibiting pure c phase and good chemical uniformity. Controllable preparation of coatings with different porosity (i.e., 7%–9% and 12%–14%) was realized by stand-off distance adjustment.
1. Introduction
Thermal barrier coatings (TBCs) have become indispensable in the practice of thermal protection towards hot section components of aircraft engines and gas turbines. Thanks to the wide application of TBCs, the operation temperatures of engines have continuously improved, which contributes to a great enhancement of engine efficiency and performance [1,2].
A TBC is typically composed of a ceramic top coat and a metallic bond coat. Owing to its excellent comprehensive properties, such as low thermal conductivity, good compatibility with the substrate originating from appropriate thermal expansion coefficient, and great mechanical properties, 7–8 wt.% Y2O3 stabilized ZrO2 (YSZ) with an initial metastable tetragonal prime (t’) has been widely accepted as a classic material for TBC topcoat [1,2,3,4,5]. However, the working temperature of YSZ TBCs is strictly limited to below 1200 °C, which seriously hinders the development of advanced engines with higher temperature capability. At higher temperatures above 1200 °C, YSZ TBCs suffer from undesirable phase transformation (t’ decomposes into cubic (c) and tetragonal (t) phases, with the t phase transforming deleteriously to monoclinic (m) phase on cooling), deterioration of strain tolerance and thermal insulation capacity resulting from coating densification, as well as severe corrosion either from environmental deposits under specific flight routes or from corrosive salts in low-quality fuels [6,7,8,9]. Consequently, the search for new TBC materials that could work stably for long term at higher working temperatures has never stopped.
Efforts to achieve this goal can be roughly categorized into two approaches: One is to apply novel non-zirconia-based material, such as rare-earth phosphates, rare earth zirconates and perovskite-structured materials [10,11,12]. These new materials have been constantly emerging in recent years, but most of them are far away from practical application. The other approach tries to establish more stable and effective zirconia-based systems with different stabilizer portfolio, among which defect cluster TBCs (ZrO2 stabilized with two or more rare earth oxides) attract much attention, mainly due to their capacity to stabilize high-temperature t′ phase or c phase to room temperature [13,14,15,16,17].
Among various multi-stabilized zirconia systems, Yb2O3-Gd2O3-Y2O3 co-stabilized zirconia (YGYZ) has been investigated extensively, and most studies have consistently pointed out its promising applicability, especially in the field of ultra-high temperature applications. Bahamirian et al. characterized YGYZ powders prepared by a chemical co-precipitation method with different calcination temperatures, reporting great resistance to phase destabilization when subjected to heat treatment at 1300 °C [18,19]. Guo et al. systematically studied the Yb2O3-Gd2O3-Y2O3-ZrO2 system with different doping concentration, and the results suggested that YGYZ exhibited some outstanding properties, such as excellent phase stability, superior thermal insulating property and improved sintering resistance [20,21,22]. Additionally, recent studies aimed at its hot corrosion evaluation also indicated that YGYZ possessed better resistance against molten salts than conventional YSZ [23,24,25]. Furthermore, a relevant multicomponent stabilized zirconia-based powder called Metco 206A, with the nominal composition of 5.86 mol.% Y2O3-1.99 mol.% Gd2O3-1.98 mol.% Yb2O3-ZrO2, was intended for use in harsh environments with service temperatures well beyond 1200 °C [26].
Much work has been conducted on YGYZ; however, limited attention has been paid to its spray powder preparation, especially the novel and effective feedstock preparation method for air plasma spraying (APS), which is the most widely used technique for coating deposition. Bahamirian et al., Zhao et al. and Jung et al. have long been engaged in YGYZ coating preparation and evaluation. A chemical coprecipitation method was used by Bahamirian et al. to prepare YGYZ spray powder [24,27], Zhao et al. used a conventional fusion and crushing method [28,29], while Jung et al. directly purchased commercial powder [25,30]. A relatively long processing chain is inherent to the chemical coprecipitation method, while for fusion and crushing method, it is strenuous to crush the sintered bulk material with high hardness.
In this study, a simple and effective method, first by agglomeration followed by high-temperature calcination, is proposed to prepare YGYZ feedstock for APS. Several 2Yb2O3-2Gd2O3-6Y2O3-90ZrO2 (mol.%) (inspired by Metco 206A) candidate powders for APS application, hereinafter referred to as 10YGYZ, were synthesized by calcination of agglomerated powders at a temperature range of 1100–1500 °C for 3 h. The optimal feedstock was determined based on phase structure, apparent density, flowability, particle size distribution, etc. Eventually, air plasma sprayed 10YGYZ TBCs with different microstructures by using the optimized feedstock are successfully prepared.
2. Materials and Methods
2.1. Research Route of This Study
This study can be clearly divided into two parts: one is the preparation of 10YGYZ feedstock suitable for APS, and the other is the fabrication of 10YGYZ TBCs with the optimal powders developed from the first part (see Figure 1).
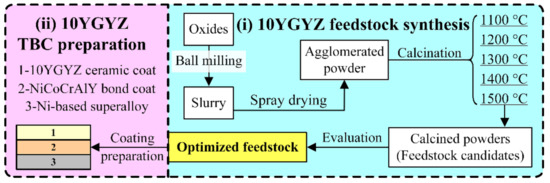
Figure 1.
Research route of this study.
2.1.1. Preparation of 10YGYZ Feedstocks for APS
Preparation of 10YGYZ powders for APS was based on a technologically simple solid-state reaction method, together with spray drying technique, with raw materials of high purity Yb2O3, Gd2O3, Y2O3 and ZrO2 (Yb2O3, Gd2O3, Y2O3: 99.99%, 1–3 μm, Beijing Deke Daojin Science and Technology Ltd., Beijing, China; ZrO2: m phase, 99.9%, 1–3 μm, Jiaozuo Weina Technology Ltd., Jiaozuo, China).
The synthesis route is described as below (Figure 1): a slurry for spray drying was directly prepared by ball milling of individual oxides and binder (PVA). After the granulation process, the powders were calcined to complete composition homogenization and the formation of a certain phase structure. Finally, the optimized feedstock was determined by a comprehensive consideration of the calcined powders at different temperatures (feedstock candidates). Based on the above description, this route could be simply understood as “granulation first, and then calcination”. The core issue of this work lies in the optimization of calcination temperature. Thus, a series of calcination temperatures was designed: the agglomerated powders were heat-treated at 1100, 1200, 1300, 1400 and 1500 °C for 3 h in a box electric furnace (SX-G12163, Tianjin Zhonghuan Furnace Ltd., Tianjin, China), respectively, with the heating rate of 5 °C/min and furnace cooling. For detailed information about powders calcination, corundum crucibles with the size of ~φ15 cm × 15 cm were adopted, in which ~1.5 kg powders were loosely packed.
Details of ball milling and spray drying need to be further clarified, and most parameters involved were pre-optimized. Individual oxides, together with PVA, which accounts for 10% of the powders weight, were mixed by ball milling using a zirconia media for 12 h at 350 rpm, with the mass ratio of ball, powders and deionized water of 15:5:3. The obtained suspension was immediately granulated by a spray-dryer (LGZ-3, Wuxi Dongsheng Spray-granulating and Drying Equipment Plant, Wuxi, China) to avoid sedimentation. During the spray drying process, the slurry, with the feed rate of ~100 g/min, was transformed into spherical powders under the centrifugal effect of the high-speed rotating atomizer (10,000 r/min). The inlet and outlet temperatures were set at 300 and 140 °C, respectively.
2.1.2. Fabrication of 10YGYZ TBCs
The optimal feedstock was then used to prepare 10YGYZ TBCs, with the coating configuration composed of Ni-based superalloy, NiCoCrAlY bond coat and 10YGYZ top coat (Figure 1). Disk-shaped Ni-based alloy Inconel 718, with the dimension of φ25 × 5 mm2, was adopted as the metallic substrate. Prior to coating deposition, specimens were sand-blasted by Al2O3 particles with the size of ~500 μm to establish a fresh and rough surface. Then, APS method, by an APS system (GTV-MF-P-HVOF-K-ARC-200) equipped with a Metco F6 gun, was used to prepare the bond coat and ceramic top coat on the substrate, with the target thickness of 100 and 250 μm, respectively. Powders for bond coat and top coat were NiCoCrAlY (Amdry 365-2, 53–109 μm) and optimized 10YGYZ feedstock obtained in this study (45–125 μm). Two different coating microstructures, the relatively dense one and the relatively porous one, termed as 10YGYZ-1 and 10YGYZ-2, respectively, were designed via varied stand-off distances. Specifically, the coating porosity could be significantly increased by extending the stand-off distance, as was demonstrated by Bakan et al [31]. The spraying parameters for NiCoCrAlY and 10YGYZ are listed in Table 1.

Table 1.
APS parameters for 10YGYZ TBCs.
2.2. Characterizations
For the following tests, 10YGYZ calcined powders, in the form of loose bulks as a whole, were first set free using a 80# sieve. The particle size distributions of the calcined powders were measured by a laser scattering method (Mastersizer 3000, Malvern Instruments Ltd., Malvern, UK). For comparison, the agglomerated powders were also tested after 80# sieving. The apparent density and flowability of the calcined powders, with a particle size distribution of 45–125 μm after selected sieving, were tested by means of a Hall flowmeter funnel according to ASTM B0212-99 [32] and ASTM B213-03 [33], respectively. Additionally, the precise contents of rare earth oxides and impurities in calcined powders were determined by Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) method (725-ES, Agilent Technologies Inc., Santa Clara, CA, USA).
Both 10YGYZ powders and coatings need phase structure and microstructure analysis. Phase structure was identified by an X-ray diffraction (XRD, Bruker D8 Advanced, Karlsruhe, Germany) with CuKα radiation. Data were digitally collected in 2θ range of 20°–80° under a continuous scan mode, with a scanning rate of 0.2°/s. For the given 10YGYZ powder, in order to distinguish the c phase from t phase, a more extensive scan was conducted in the specific range 2θ = 72.5°–75.5°, with a step increment of 0.01° and a counting time of 1 s per step. Surface and cross-sectional morphologies were captured using a scanning electron microscope (SEM, SU5000, Hitachi Ltd., Tokyo, Japan), and composition analysis was carried out by an energy dispersive X-ray spectrometer (EDX, Bruker X flash 6130, Karlsruhe, Germany) attached on SEM. For the preparation of cross-section samples, a small amount of powder and a small piece of coating were first mounted by resin and then carefully polished. Microstructure image processing (MIP) software was employed to determine the coating porosity by using the cross-section samples.
3. Results and Discussion
3.1. Characterization of 10YGYZ Agglomerated Powders
As-obtained 10YGYZ agglomerated powders were first detected by XRD, and the results are shown in Figure 2a. Distinct diffraction peaks ascribed to four individual raw materials (m-ZrO2, Y2O3, Gd2O3 and Yb2O3) can be clearly observed, indicating that the oxides are well mixed during ball milling. Particle size distribution of the agglomerated powders was then measured, as is shown in Figure 2b. 10 vol.% of the powders have a diameter of less than DV (10) = 31.1 μm and 90 vol.% of the powders have a diameter of less than DV (90) = 101 μm, which is basically consistent with the results in our previous studies under the same spray drying parameters.
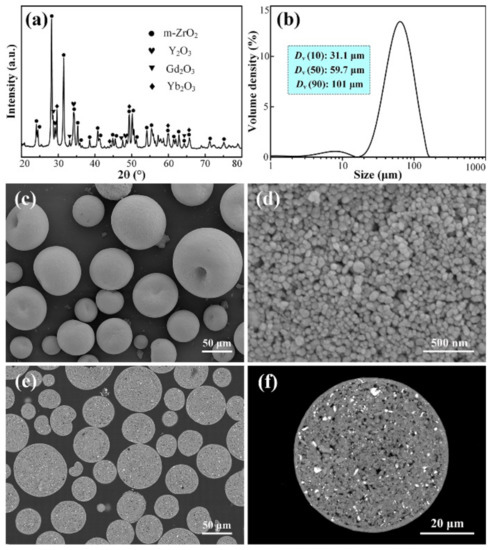
Figure 2.
XRD pattern (a), particle size distribution (b), surface morphologies (c,d) and cross-sectional microstructures (e,f) of 10YGYZ agglomerated powders. (d,f) Show enlarged SEM images of the surface and cross section of a single powder, respectively.
Figure 2c shows the SEM micrograph of 10YGYZ agglomerated powders. A typical spherical shape of these particles could be clearly observed, and the particle size of these powders falls in the range of 20–120 μm, which is in good agreement with the results obtained from the particle size analysis. A closer observation of individual particles was made at a higher magnification, revealing a rough surface and a porous microstructure composed of many fine particles with sizes less than 100 nm (Figure 2d). To better understand the inner structure of the 10YGYZ granulated powders, their cross-sections were also studied. As shown in Figure 2e, all the powders possess a solid structure, with few traces of hollow architecture. Low PVA addition often leads to the formation of non-spherical particles, while excessive PVA addition often leads to the formation of large number of undesired oversized powders with the size larger than 100 μm [34]. In addition, the internal structure of agglomerated powders depends mainly on the amount of water added (or solid content), that is, the appearance of most powders with a hollow structure is a sign of excessive water addition (or low solid content) [35]. Combined with the surface morphology, size distribution and the inner structure of the powder, it is concluded that the amount of PVA and water added during suspension preparation and the parameters for spray drying are highly suitable. Detailed observation of an individual particle (Figure 2f) shows that the particle is composed of phases with different contrasts, that is, a small amount of bright and dark phases is randomly distributed on the gray contrast matrix. These phases are further identified by EDS composition analysis and element mapping, and the results are presented in Figure 3.
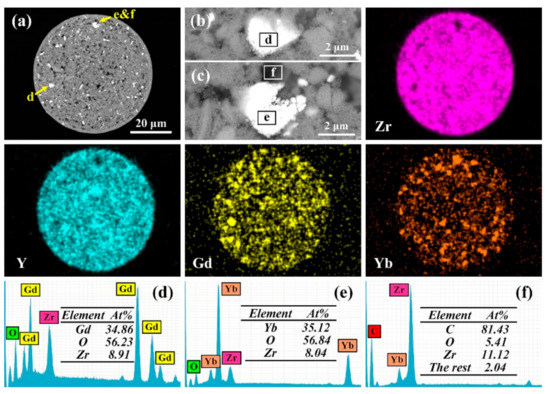
Figure 3.
Cross-sectional microstructure (a), enlarged SEM images of the Gd-rich region (b) and the Yb-rich region (c), and corresponding EDS mapping results of Zr, Y, Gd and Yb elements of a single 10YGYZ agglomerated powder. (d–f) are the EDS results of the marked regions in (b,c).
On the basis of the EDS mapping results of Zr, Y, Gd and Yb elements, it is obvious that Zr and Y are relatively evenly distributed in the whole powder, while the distributions of Gd and Yb are rather discrete. The content of each element (actually referring to the corresponding oxides) shown in the mapping results also agrees well with the peak intensity of XRD pattern in Figure 2a. Comparing the Gd-rich and Yb-rich micro regions with the powder cross-sectional image, it is found that both the Gd-rich and Yb-rich regions correspond to the bright phases in Figure 3a. EDS analysis of the white phases in different regions (region A and region B) showed that A has Gd, Zr and O, while B mainly consists of Yb and O, indicating that they are recognized as Gd2O3 and Yb2O3, respectively. Both Gd2O3 and Yb2O3 exhibit bright phase in backscattered electron image (BSE) because they have similar atomic mass. Please note that a certain amount of Zr could also be detected in both regions, which can be explained by the assumption that the electron beam can easily penetrate the small white phases into the zirconia matrix. In addition, the dark contrast phase in Figure 3a was also analyzed by EDS, and the result shows that C is the main component, which corresponds to the PVA added during ball milling. From the aforementioned analysis, it is implied that a chemically homogenous agglomerated powder can hardly be realized by ball milling and spray drying, and subsequent high temperature calcination is necessary to achieve composition homogenization and the formation of a certain phase structure.
3.2. Characterization of 10YGYZ Calcined Powders at Various Temperatures
3.2.1. Characterization of 10YGYZ Calcined Powders at 1100 °C
The agglomerated powders were first calcined at 1100 °C for 3 h and its XRD pattern is shown in Figure 4a. Diffraction peaks ascribed to the newly formed cubic phase can be clearly observed (identification of c phase will be explained later), which indicates that dissolution of rare earth oxides into zirconia occurs at 1100 °C. Further identifications of different rare earth oxides peaks show that the peaks of Y2O3 almost disappear, together with the greatly weakened Gd2O3 peaks, while the peaks of Yb2O3 almost remain unchanged, implying that Y2O3 is the first or the easiest to be dissolved into zirconia among these three rare earth oxides. After calcination at 1100 °C, the macro morphology of calcined powders has no obvious change compared with that of agglomerated powders (Figure 4b versus Figure 2c). However, the enlarged image of an individual powder shows that interconnection between fine particles and slight grain growth occurs, as shown in Figure 4c. Fine white phases could still be observed distributing dispersedly in the cross section of the powder (Figure 4d), but the amount of these phases decreases slightly (Figure 4e), implying partial dissolution of Gd2O3 occurs at 1100 °C. It should be noted that after heat treatment at 1100 °C for 3 h, the dark phase corresponding to PVA should be disappearing in the cross section of the powder (PVA will be removed at high-temperature heat treatment). However, the dark phase can still be observed, which is the result of the infiltration of liquid resin into the powders via the porous surface during the sample preparation, as was mentioned in Section 2.2. Gd and Yb maps are also provided. The presence of evident Gd-rich and Yb-rich regions with bright contrast clearly indicate that a large amount of Gd2O3 and Yb2O3 remains undissolved at 1100 °C.
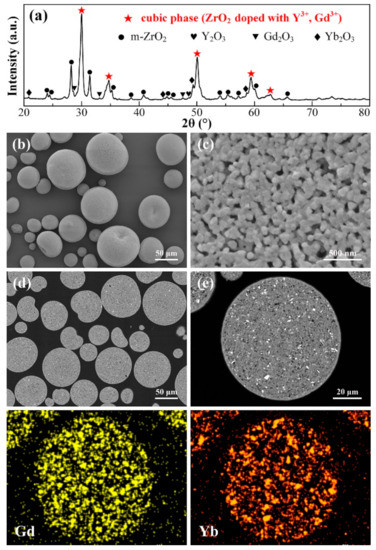
Figure 4.
XRD pattern (a), surface morphologies (b,c) and cross-sectional microstructures (d,e) of 10YGYZ agglomerated powders calcined at 1100 °C for 3 h. (c,e) are enlarged SEM images of the surface and cross section of a single powder, respectively. Corresponding EDS Gd and Yb maps of (e) are also provided.
3.2.2. Characterization of 10YGYZ Calcined Powders at 1200 °C
The XRD pattern of 10YGYZ agglomerated powders calcined at 1200 °C for 3 h is shown in Figure 5a. Diffraction peaks of rare earth oxides can hardly be observed, with only peaks of c phase and t-ZrO2 left. It should be noted that the t-ZrO2 with weak diffraction intensity is the product resulting from unreacted raw material m-ZrO2 transformation (the transition temperature of m-ZrO2 → t-ZrO2 is close to 1200 °C), suggesting that the solid solution is not yet completed in this case. The surface SEM images are presented in Figure 5b,c. The macroscopical surface morphology of the calcined powders is almost the same as that of the agglomerated counterpart. In the enlarged image, strong interconnection between individual particles is established, and the grains have experienced slight growth, with the grain size of ~200–300 nm. Figure 5d shows the cross section of an individual powder after 1200 °C heat treatment (cross section image with low magnification is omitted here). Except for a few large white phases, most of the fine bright phases disappear, indicating that the solid solution process is accelerated at 1200 °C. This obvious dissolution of rare earth oxides could account for their very weak diffraction peaks in Figure 5a. In addition, the elongated aggregate in Figure 5d is an exception, and is rarely observed in other individual powders, the presence of which is possibly due to the exceptional large particles from raw materials or abnormal grain growth during calcination.
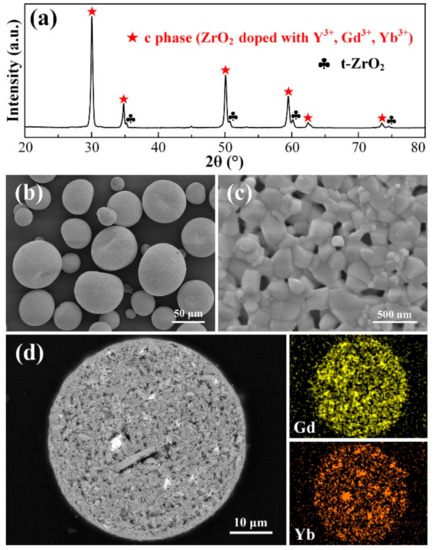
Figure 5.
XRD pattern (a), surface morphologies (b,c), cross-sectional microstructure (d) and corresponding EDS Gd and Yb mapping results of 10YGYZ agglomerated powders calcined at 1200 °C for 3 h. (c) is the enlarged SEM surface image of a single powder.
To better understand the order, or the difficulty, of different rare earth oxides when dissolving into the zirconia matrix, EDS mapping of Gd and Yb elements are implemented. It is apparent that the Gd map exhibits a more uniform distribution than that of Yb, and most undissolved bright phases in Figure 5d point clearly to the Yb-rich regions in the Yb map, implying that Gd2O3 gets dissolved more easily in zirconia than Yb2O3, which agrees well with the previous research [20]. Consequently, in combination with the findings observed in calcined powders at 1100 °C, it could be concluded that Y3+ is the easiest to substitutes for Zr4+ among Y3+, Gd3+ and Yb3+, and it is the most difficult for Yb3+ to replace Zr4+. The order or difficulty of dopants substitution for host cation mainly depends on the difference in the ionic radii and atomic mass between RE3+ and Zr4+. Table 2 shows that although the ionic radius of Yb3+ is the closest to that of Zr4+, Yb3+ is actually the most difficult to dissolve into zirconia, which can be attributed to the huge mass misfit between Yb3+ and Zr4+, implying that compared with the ionic radius difference, mass difference is the dominating factor governing the solid solution process in this case.

Table 2.
Ionic radius and atomic mass of Zr4+, Y3+, Gd3+ and Yb3+.
3.2.3. Characterization of 10YGYZ Calcined Powders at 1300 °C
When the calcination temperature is increased to 1300 °C, much more significant changes occur on the agglomerated powders, as compared to the calcined powders at 1100 and 1200 °C. The XRD spectrum (Figure 6a) reveals dominating peaks ascribed to c phase, with very weak t-ZrO2 peaks left, suggesting that solid solution is basically completed in this case. Distinguishing c phase from t phase could be realized by closer recognition at 2θ regions of 72.5°–75.5°. Within this range, the t phase presents two typical separated diffraction peaks, specifically (004) and (400), whereas c phase has no splitting characteristics at all these positions [37]. The absence of two splitted peaks at certain positions marked in the inset image in Figure 6a confirms the identity of c phase. Furthermore, as the doping concentration of stabilizers is increased to ~10 mol.%, zirconia is prone to be fully stabilized to form a cubic structure, as is also demonstrated in other studies [20,26].
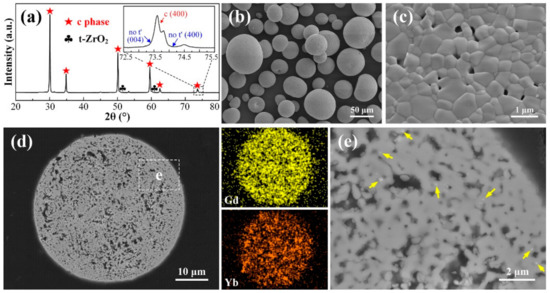
Figure 6.
XRD pattern (a), surface morphologies (b,c), cross-sectional microstructure (d,e), corresponding EDS Gd and Yb mapping results of 10YGYZ agglomerated powders calcined at 1300 °C for 3 h. (e) is the enlarged image of the marked region of (d).
Careful observation on the surface morphology of an individual powder (Figure 6b,c) reveals that a relatively dense microstructure is formed during 1300 °C calcination process: many pores between the particles are sealed by the obvious growth of the grains due to enhanced sintering effect. A typical cross-sectional morphology of an individual powder is provided in Figure 6d. The powder exhibits a consistent gray contrast as a whole, without striking phases with bright contrast, which is in accordance with the XRD result. It seems that rare earth components are fully dissolved into the matrix; however, EDS mapping results show that the distributions of Gd and Yb, especially Yb, although dissolved into matrix, are not uniform, implying that fully mixed constituents with composition homogenization have not yet been completed under this heat treatment condition. This could also be confirmed by the tiny dots with white contrast in a certain micro-region, as marked by arrows in Figure 6e.
3.2.4. Characterization of 10YGYZ Calcined Powders at 1400 °C
1400 °C was preset as another target calcination temperature. The XRD spectrum (Figure 7a) reveals well-defined diffraction peaks ascribed to c phase, without any peaks either from stabilizers (Y2O3, Gd2O3 and Yb2O3) or t-ZrO2, suggesting that the solid solution process is completed. Additionally, the enhanced peak intensity and narrowed width are indicators of enhanced crystallinity and increased crystallite size of cubic phase. The particle size distribution of the 1400 °C calcined powders was also measured, as is shown in Figure 7b (particle size distribution curve of the agglomerated powders is also given here as a baseline for comparison). Obviously, the particle size distribution curve of the 1400 °C calcined powders is significantly shifted to the left compared to the curve of the agglomerated powders, indicating intense shrinkage of powders happens during calcination. Specifically, DV (10), DV (50), and DV (90) of the agglomerated powders are reduced from 31.1, 59.7, and 101 μm to 26.7, 48.1 and 81.5 μm of the 1400 °C calcined powders, respectively.
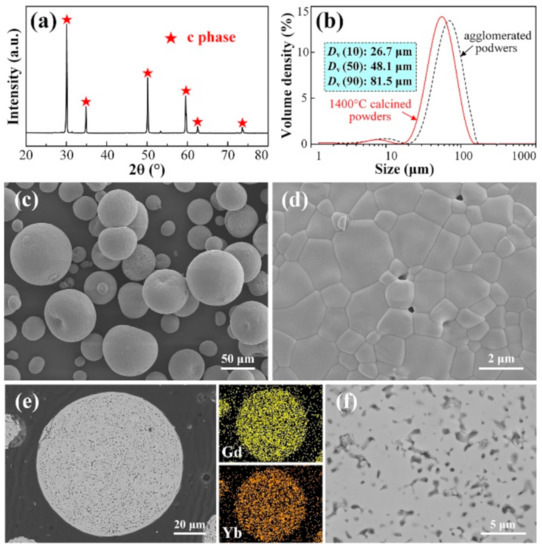
Figure 7.
XRD pattern (a), particle size distribution (b), surface morphologies (c,d), cross-sectional microstructure (e,f) and corresponding EDS Gd and Yb mapping results of 10YGYZ agglomerated powders calcined at 1400 °C for 3 h. (d) Shows the enlarged surface image of a single powder, while (f) is the enlarged image of certain region of (e).
After calcination at 1400 °C for 3 h, slight adhesion between adjacent powders occurs, as indicated in Figure 7c. This can be attributed to the intense sintering effect at high temperatures, which will be discussed in detail later. Fine microstructure of a single powder is then studied by SEM image with higher magnification and the result is given in Figure 7d. With the further growth of the grains, almost all the pores are healed, leaving a dense surface with almost no pores remaining. Figure 7e shows the SEM micrograph of the cross section of an individual powder, revealing a much denser microstructure with fewer pores. The mapping results show that Gd and Yb are uniformly distributed throughout the powder cross section and there are no tiny white spots that are not completely dissolved in the micro-region (Figure 7f), suggesting under the heat treatment of 1400 °C for 3 h, different stabilizers are fully dissolved into the zirconia matrix and chemical homogenization is achieved.
It is well accepted that feedstock for APS should have a particle size of 45–125 μm in order to obtain a high-quality coating, because coarse powders do not melt well, while fine powders have poor fluidity and often result in low coating adhesion due to lower inertia when colliding onto the substrate [24,38]. Based on this, the as-calcined powders were sieved to meet this particle size requirement. Then the flowability and the apparent density were measured, which turned out to be 25.58 s/50 g and 2.44 g/cm3. Previous study has pointed out that powders with apparent density >1.7 g/cm3 and mean particle size >20 μm can be directly used for plasma spraying without further densification [39]. Thereby, it is concluded that these powders with good flowability and proper apparent density are highly suitable for plasma spraying.
3.2.5. Characterization of 10YGYZ Calcined Powders at 1500 °C
Finally, 10YGYZ agglomerated powder was subjected to calcination at 1500 °C for 3 h, and its surface morphology was investigated by SEM, as is shown is Figure 8a (the XRD pattern is omitted because of its high similarity with that of 1400 °C calcined powders). When the temperature rises to 1500 °C, the sintering effect gets greatly enhanced, which is manifested by the significant adhesion and fusion between individual powders. Here, two different sintering phenomena are defined: Type A and Type B, as marked in Figure 8a. Type A refers to the slight sintering effect, which is specifically the adhesion between individual powders as a result of powders softening and deformation at high temperatures (as is also seen in Figure 7c). The attached particles introduced by this effect can be successfully detached by vibration or sieving. Figure 8b shows a typical surface morphology of an individual powder after most attached particles fall off. Many craters are evident on the powder surface, once serving as the adhesion sites for other particles. This kind of sintering effect is not fatal to the overall fluidity of the spray powders, for most of the individual spherical particles can be retained by certain physical means, as is confirmed by the good flowability of 1400 °C calcined powders.
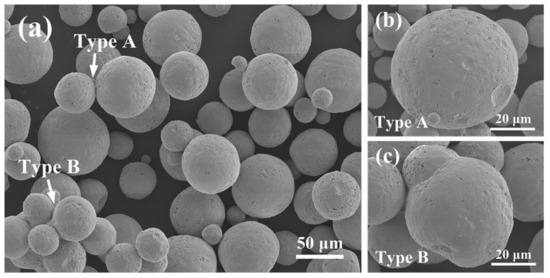
Figure 8.
Surface morphologies of 10YGYZ agglomerated powders calcined at 1500 °C for 3 h (a–c). Two types of sintering effects are illustrated by the enlarged images (b,c).
On the other hand, Type B is a severe sintering effect, specifically referring to the establishment of metallurgical bonding realized by mutual fusion between powders. A typical example of this Type B effect is given in Figure 8c. It can be found that an irregular powder is formed with a robust bonding between two particles, and it is obvious that each particle forming this “freak” cannot be separated by above physical methods. This kind of sintering undoubtedly poses a fatal effect on powders fluidity: when there is a large amount of the aforementioned irregular powders in spray powders, the overall fluidity of spray powders will be sharply weakened or even disappear completely, which is proven by the fact that 1500 °C calcined powders have no fluidity.
3.3. Determination of the Optimized YGYZ Calcined Powders for Plasma Spraying
A summary of properties of agglomerated powder and different calcined powders is provided in Table 3. Agglomerated powder with light density is composed of four individual oxides, and further calcination is necessary to promote densification and the dissolution of stabilizers into zirconia to form a single-phase structure. Then, several YGYZ calcined powders at various temperatures are listed as the candidates for plasma sprayable feedstock. In addition, the optimal calcined powder is determined by overall considerations in terms of phase structure, apparent density, flowability, particle size distribution, etc.

Table 3.
Summary of properties of agglomerated powder and different calcined powders.
It can be seen from Table 3 that with the increase of calcining temperature, stabilizers gradually dissolve into zirconia until the solid solution process is fully completed at 1400 °C. Therefore, calcined powders at 1100, 1200 and 1300 °C are easily excluded when determining optimal feedstock for the obvious presence of other phases besides c-phase. On the other hand, as calcination temperature increases, the powders apparent density increases, a better fluidity is achieved (except 1500 °C), and the powder size gets smaller (except 1500 °C). From previous analysis (Section 3.2.5.), it is evident that 1500 °C calcined powders suffer from serious sintering effect, leading to unfavorable adhesion and fusion between individual particles. This phenomenon, on one hand, causes the measured particle size to become larger (compared with that of 1400 °C calcined powder); while on the other hand, it greatly damages the overall fluidity for the presence of irregular powders. As far as we know, there is no absolute and uniform standard to define the flowability of feedstock suitable for plasma spraying. According to our previous experience, the flow time of powder is acceptable within 1 min and a shorter flow time is always more favorable, while the “inert” powder without any flowability is unacceptable, for it makes spraying process difficult or even impossible. Thus, 1500 °C calcined powder is also not suitable for plasma spraying because of its poor fluidity.
Based on proper phase structure, good fluidity, suitable apparent density and particle size (Table 3), together with the proper microstructure and great chemical uniformity (Figure 7), it is concluded that 1400 °C calcined powder is determined as the optimal feedstock. There is a concern regarding the production efficiency of this feedstock. Feedstock with desired particle distribution is obtained by selective screening, that is, coarse powders larger than 125 μm and fine powders smaller than 45 μm are eliminated. In this study, the yield rate of this feedstock is about 75% (mass ratio) and most eliminated powders are fine powders, which is the result of the relatively small particle size of agglomerated powders. To improve the yield rate, it is effective to increase the particle size of agglomerated powders by adjusting spray drying parameters, for example, slowing down the rotation rate of atomizer.
Additionally, it should be noted that calcined powder at 1400 °C for 3 h is not likely to be the “chosen one” that meets all the above requirements. Although there is a concern about chemical inhomogeneity, powders calcined at 1300 °C for 3 h have good fluidity (26.75 s/50 g) and suitable apparent density (2.18 g/cm3). Prolonging the holding time at 1300 °C may be a solution to this problem. Similarly, shortening the holding time of the powders at 1500 °C may also yield suitable feedstock through reduced sintering effect. These assumptions would be verified in our future work. Furthermore, it is observed that with the increase of calcining temperature, the grains of the powder undergo significant coarsening. It is convinced that the grain size of the powder would affect the powder melting behavior in the spraying process. However, the relationship between grain size and the melting behavior is not established in this study, and will also be our next work.
In addition, the chemical composition of the optimized feedstock needs further verification. Two aspects should be seriously considered. Firstly, does the chemical composition of the 1400 °C calcined powders meet the nominal composition? The precise chemical compositions of 1400 °C calcined powders was determined by using the ICP-OES method, and the results are listed in Table 4. The calculated Yb2O3:Gd2O3:Y2O3:ZrO2 (mol.%) is 1:1.02:3.06:46.22, which is very close to the nominal ratio (1:1:3:45). Secondly, what is the impurity level of the calcined powder? Impurity in feedstock poses a negative impact on coating performance. Park et al. suggested that plasma-sprayed YSZ coating using high purity feedstock exhibited greater phase stability and sintering resistance than the one using feedstock of regular purity [40]. Similarly, Lyu et al. reported that double-layered TBC consisting of YGYZ top layer and high-purity YSZ buffer layer has better thermal and mechanical durability than its counterpart with regular-purity YSZ buffer layer in thermally graded mechanical fatigue test [30]. It can be seen from Table 4 that the impurity level of the 1400 °C calcined powder is very low, with the contents of most impurities not higher than 0.01%. Therefore, by using this feedstock, a high-quality coating with good phase stability, sintering resistance and durability can be expected.

Table 4.
Chemical compositions of YGYZ calcined powders at 1400 °C for 3 h (in wt.%).
3.4. Characterization of 10YGYZ TBCs Prepared Using Optimal Feedstock
The optimized feedstock was then used for 10YGYZ coating deposition and two different coatings with varied porosity level (10YGYZ-1 and 10YGYZ-2) were prepared. The characterization results of these two coatings are shown in Figure 9. Figure 9a shows XRD patterns of the optimized feedstock (1400, 3 h calcined powder) and the 10YGYZ-1 top coat prepared by this feedstock. For brevity, the XRD spectrum of 10YGYZ-2 is omitted, as it is almost the same as that of 10YGYZ-1. The results suggest that the feedstock and the coating all have a single homogeneous c phase. No other phases could be detected in the coating after spraying, indicating the inherent excellent phase stability of 10YGYZ.

Figure 9.
XRD patterns of optimized 10YGYZ feedstock and as-sprayed 10YGYZ coating (a), surface morphology of 10YGYZ-1 coating (b), cross-sectional microstructures of 10YGYZ-1 coating (c), with corresponding EDS mapping results of Zr, Y, Gd and Yb, and 10YGYZ-2 coating (d). An enlarged image of the marked region in (d) is also provided (e).
Figure 9b,c depicts the surface and cross-sectional microstructure of 10YGYZ-1 coating. A bimodal microstructure consisting of rough region and smooth region is exhibited in Figure 9b. Further observation of the rough region shows the presence of numerous nanoparticles which refer to unmelted particles, while the smooth region evolves from the fully molten particles. Figure 9c presents a classic coating architecture composed of a bond coat and a 10YGYZ top coat, with the thickness of ~60 and 250 μm, respectively. The top coat has a relatively dense structure, with the estimated porosity of 7%–9%, indicating that powder melts well under this parameter. Further investigation shows that there are many micro defects embedded in the coating, such as pores, micro-cracks and splat boundaries in the lamellar structure, which are intrinsic characteristics of coatings prepared by APS. The EDS maps show that Zr, Y, Gd and Y are evenly distributed throughout the entire ceramic layer (the counting rates of Gd and Y are weak because of their low contents), indicating that almost no component burning and composition segregation occur during the spray process.
When the stand-off distance is increased to 195 mm (10YGYZ-2), significant changes occurred on the coating microstructure. Similar bimodal morphology could still be observed on coating surface, albeit with much more clusters of unmolten nanoparticles, implying a strikingly incomplete melting behavior of the feedstock (the surface SEM image is omitted here for brevity). The cross-sectional SEM image (Figure 9d) clearly shows a porous and loose microstructure (the porosity was measured to be 12%–14% for the top coat), with numerous rough regions embedded in it. An enlarged image of one of these regions is provided in Figure 9e. These regions are also termed nano zones for the typical appearance of agglomeration of nano-sized unmolten particles, and it is the presence of these nano zones that contributes to the great porosity increase.
In summary, the optimized 10YGYZ feedstock is proven to be feasible for preparation of high-quality coatings. The focus of our future work will be 10YGYZ TBCs evaluation (thermal shock resistance, corrosion resistance, etc.) and the effect of coating microstructure on these performances.
4. Conclusions
A simple and efficient synthesis route, referred as “first agglomeration followed by calcination”, for preparation of 10YGYZ feedstock for APS was explored in this study. Feedstock candidates were prepared by calcination of spray-dried agglomerated powders at 1100, 1200, 1300, 1400, and 1500 °C for 3 h, respectively. Among these, obvious dissolution of Y2O3 and Gd2O3 into zirconia occurred at 1100 °C. Solid solution process was accelerated as temperature increased, and almost a single c phase was achieved at 1300 °C, however, with chemical inhomogeneity. Calcined powder at 1500 °C presented poor flowability due to intense sintering effect. Eventually, 1400 °C calcined powder was determined as the optimized feedstock for proper phase structure, great flowability, suitable apparent density and particle size, good chemical homogeneity and composition accuracy. Additionally, prolonging the holding time at 1300 °C or shortening the holding time at 1500 °C may also be a solution to obtain optimal feedstock. In addition, the sintering effect was intensified with the increase of calcination temperature, which was specifically manifested in powders shrinkage, densification, grain growth and mutual adhesion and fusion. By using the optimized feedstock, high-quality air plasma sprayed 10YGYZ TBCs were prepared, exhibiting pure c phase and good chemical uniformity. Coatings with different porosity (i.e., 7%–9% and 12%–14%) were realized by adjusting stand-off distance. Our future work will focus on 10YGYZ TBCs evaluation and the effect of coating microstructure on their performance.
Author Contributions
Conceptualization, H.P.; methodology, Z.Y. and H.P.; validation, Z.Y. and K.Y.; formal analysis, Z.Y. and K.Y.; investigation, Z.Y. and K.Y.; data curation, Z.Y.; writing—original draft preparation, Z.Y.; writing—review and editing, H.P.; supervision, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Major Science and Technology Projects of China (No. 2017-VⅡ-0012-0109).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank for the technical supports provided by Shanjie Li, Zhihuai Liu and Xia Wu.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Clarke, D.R.; Oechsner, M.; Padture, N.P. Thermal-barrier coatings for more efficient gas-turbine engines. MRS Bull. 2012, 37, 891–898. [Google Scholar] [CrossRef]
- Padture, N.P. Advanced structural ceramics in aerospace propulsion. Nat. Mater. 2016, 15, 804–809. [Google Scholar] [CrossRef]
- Vassen, R.; Jarligo, M.O.; Steinke, T.; Mack, D.E.; Stöver, D. Overview on advanced thermal barrier coatings. Surf. Coat. Technol. 2010, 205, 938–942. [Google Scholar] [CrossRef]
- Bakan, E.; Vaßen, R. Ceramic top coats of plasma-sprayed thermal barrier coatings: Materials, processes, and properties. J. Therm. Spray. Technol. 2017, 26, 992–1010. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, Y.T.; Li, W.G.; Liu, W.L.; Wu, Y.P.; Liu, F.K. Research progresses on ceramic materials of thermal barrier coatings on gas turbine. Coatings 2021, 11, 79. [Google Scholar] [CrossRef]
- Ren, X.R.; Pan, W. Mechanical properties of high-temperature-degraded yttria-stabilized zirconia. Acta Mater. 2014, 69, 397–406. [Google Scholar] [CrossRef]
- Poerschke, D.L.; Jackson, R.W.; Levi, C.G. Silicate deposit degradation of engineered coatings in gas turbines: Progress toward models and materials solutions. Annu. Rev. Mater. Res. 2017, 47, 297–330. [Google Scholar] [CrossRef]
- Kabir, M.R.; Sirigiri, A.K.; Naraparaju, R.; Schulz, U. Flow kinetics of molten silicates through thermal barrier coating: A numerical study. Coatings 2019, 9, 332. [Google Scholar] [CrossRef]
- Liu, H.; Cai, J.; Zhu, J.H. Hot corrosion behavior of BaLa2Ti3O10 thermal barrier ceramics in V2O5 and Na2SO4 + V2O5 molten salts. Coatings 2019, 9, 351. [Google Scholar] [CrossRef]
- Cao, X.Q.; Vassen, R.; Stöver, D. Ceramic materials for thermal barrier coatings. J. Eur. Ceram. Soc. 2004, 24, 1–10. [Google Scholar] [CrossRef]
- Feng, J.; Xiao, B.; Zhou, R.; Pan, W. Anisotropy in elasticity and thermal conductivity of monazite-type REPO4 (RE = La, Ce, Nd, Sm, Eu and Gd) from first-principles calculations. Acta Mater. 2013, 61, 7364–7383. [Google Scholar] [CrossRef]
- Feng, J.; Xiao, B.; Wan, C.L.; Qu, Z.X.; Huang, Z.C.; Chen, J.C.; Zhou, R.; Pan, W. Electronic structure, mechanical properties and thermal conductivity of Ln2Zr2O7 (Ln = La, Pr, Nd, Sm, Eu and Gd) pyrochlore. Acta Mater. 2011, 59, 1742–1760. [Google Scholar] [CrossRef]
- Zhu, D.M.; Miller, R.A. Development of advanced low conductivity thermal barrier coatings. Int. J. Appl. Ceram. Technol. 2004, 1, 86–94. [Google Scholar] [CrossRef]
- Sun, L.L.; Guo, H.B.; Peng, H.; Gong, S.K.; Xu, H.B. Influence of partial substitution of Sc2O3 with Gd2O3 on the phase stability and thermal conductivity of Sc2O3-doped ZrO2. Ceram. Int. 2013, 39, 3447–3451. [Google Scholar] [CrossRef]
- Song, X.W.; Xie, M.; Mu, R.D.; Zhou, F.; Jia, G.X.; An, S.L. Influence of the partial substitution of Y2O3 with Ln2O3 (Ln = Nd, Sm, Gd) on the phase structure and thermophysical properties of ZrO2-Nb2O5-Y2O3 ceramics. Acta Mater. 2011, 59, 3895–3902. [Google Scholar] [CrossRef]
- Raghavan, S.; Wang, H.; Porter, W.D. Thermal properties of zirconia co-doped with trivalent and pentanvalent oxides. Acta Mater. 2001, 49, 169–179. [Google Scholar] [CrossRef]
- Shi, Q.Y.; Yuan, W.H.; Chao, X.Y.; Zhu, Z.F. Phase stability, thermal conductivity and crystal growth behavior of RE2O3 (RE = La, Yb, Ce, Gd) co-doped Y2O3 stabilized ZrO2 powder. J. Sol-Gel Sci. Techn. 2017, 84, 341–348. [Google Scholar] [CrossRef]
- Bahamirian, M.; Hadavi, S.M.M.; Rahimipour, M.R.; Farvizi, M.; Keyvani, A. Synthesis and characterization of yttria-stabilized zirconia nanoparticles doped with ytterbium and gadolinium: ZrO2 9.5 Y2O3 5.6 Yb2O3 5.2 Gd2O3. Metall. Mater. Trans. A 2018, 49, 2523–2532. [Google Scholar] [CrossRef]
- Bahamirian, M.; Hadavi, S.M.M.; Farvizi, M.; Rahimipour, M.R.; Keyvani, A. Phase stability of ZrO2 9.5 Y2O3 5.6 Yb2O3 5.2 Gd2O3 compound at 1100 °C and 1300 °C for advanced TBC applications. Ceram Int. 2019, 45, 7344–7350. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Guo, L.; Yang, Y.P.; Guo, H.B.; Zhang, H.J.; Gong, S.K. Influence of Gd2O3 and Yb2O3 co-doping on phase stability, thermo-physical properties and sintering of 8YSZ. Chin. J. Aeronaut. 2012, 25, 948–953. [Google Scholar] [CrossRef]
- Guo, L.; Guo, H.B.; Gong, S.K.; Xu, H.B. Improvement on the phase stability, mechanical properties and thermal insulation of Y2O3-stabilized ZrO2 by Gd2O3 and Yb2O3 co-doping. Ceram. Int. 2013, 39, 9009–9015. [Google Scholar] [CrossRef]
- Guo, L.; Li, M.Z.; Ye, F.X. Phase stability and thermal conductivity of RE2O3 (RE = La, Nd, Gd, Yb) and Yb2O3 co-doped Y2O3 stabilized ZrO2 ceramics. Ceram. Int. 2016, 42, 7360–7365. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, C.L.; Li, M.Z.; Sun, W.; Zhang, Z.Y.; Ye, F.X. Hot corrosion evaluation of Gd2O3-Yb2O3 co-doped Y2O3 stabilized ZrO2 thermal barrier oxides exposed to Na2SO4 + V2O5 molten salt. Ceram. Int. 2017, 43, 2780–2785. [Google Scholar] [CrossRef]
- Bahamirian, M.; Hadavi, S.M.M.; Farvizi, M.; Keyvanic, A.; Rahimipour, M.R. ZrO2 9.5 Y2O3 5.6 Yb2O3 5.2 Gd2O3; a promising TBC material with high resistance to hot corrosion. J. Asian Ceram. Soc. 2020, 8, 898–908. [Google Scholar] [CrossRef]
- Song, D.; Song, T.; Paik, U.; Lyu, G.; Kim, J.; Yang, S.C.; Jung, Y.G. Hot-corrosion resistance and phase stability of Yb2O3-Gd2O3-Y2O3 costabilized zirconia-based thermal barrier coatings against Na2SO4 + V2O5 molten salts. Surf. Coat. Tech. 2020, 400, 126197–126203. [Google Scholar] [CrossRef]
- Material Product Data Sheet: Zirconia Gadolinia Ytterbia Yttria Agglomerated and Sintered Thermal Spray Powder; Oerlikon Metco: Freienbach, Switzerland, 2017; DSMTS-0099.6.
- Bahamirian, M.; Hadavi, S.M.M.; Farvizi, M.; Keyvani, A.; Rahimipour, M.R. Microstructure and cyclic oxidation of yttria-stabilized zirconia/nanostructured ZrO2 9.5 Y2O3 5.6 Yb2O3 5.2 Gd2O3 thermal barrier coating at 1373 K. J. Mater. Eng. Perform. 2020, 29, 7080–7093. [Google Scholar] [CrossRef]
- Bobzin, K.; Zhao, L.D.; Öte, M.; Linke, T.F. Deposition and characterization of thermal barrier coatings of ZrO2–4 mol.% Y2O3–1 mol.% Gd2O3–1 mol.% Yb2O3. Surf. Coat. Technol. 2015, 268, 205–208. [Google Scholar] [CrossRef]
- Bobzin, K.; Zhao, L.D.; Öte, M.; Königstein, T. A highly porous thermal barrier coating based on Gd2O3–Yb2O3 co-doped YSZ. Surf. Coat. Technol. 2019, 366, 349–354. [Google Scholar] [CrossRef]
- Lyu, G.; Choi, B.G.; Lu, Z.; Park, H.M.; Jung, Y.G.; Zhang, J. Effect of thermal cycling frequency on the durability of Yb-Gd-Y-based thermal barrier coatings. Surf. Coat. Technol. 2019, 364, 187–195. [Google Scholar] [CrossRef]
- Bakan, E.; Mack, D.E.; Mauer, G.; Mucke, R.; Vaßen, R. Porosity–property relationships of plasma-sprayed Gd2Zr2O7/YSZ thermal barrier coatings. J. Am. Ceram. Soc. 2015, 98, 2647–2654. [Google Scholar] [CrossRef]
- ASTM B0212-99. Test Method for Apparent Density of Free Flowing Metal Powders Using the Hall Flowmeter Funnel; ASTM: West Conshohocken, PA, USA, 2003. [Google Scholar]
- ASTM B 213-03. Standard Test Method for Flow Rate of Metal Powders; ASTM: West Conshohocken, PA, USA, 2003. [Google Scholar]
- Loghman-Estarki, M.R.; Pourbafrany, M.; Razavi, R.S. Preparation of nanostructured YSZ granules by the spray drying method. Ceram. Int. 2014, 40, 3721–3729. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, D.R.; Dong, Y.C.; Chen, X.G.; Wang, L.; Chu, Z.H.; Zhang, J.X.; He, J.N. Preparing of nanostructured Al2O3-TiO2-ZrO2 composite powders and plasma spraying nanostructured composite coating. Vacuum 2013, 96, 39–45. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Gross, J.R.; Dutton, R.E. Phase stability, sintering, and thermal conductivity of plasma-sprayed ZrO2-Gd2O3 compositions for potential thermal barrier coating applications. Acta Mater. 2006, 54, 1615–1621. [Google Scholar] [CrossRef]
- Srinivasan, R.; Angelis, R.J.D.; Ice, G.; Davis, B.H. Identification of tetragonal and cubic structures of zirconia using synchrotron X-radiation source. J. Mater. Res. 1991, 6, 1287–1292. [Google Scholar] [CrossRef]
- Mauer, G.; Jarligo, M.O.; Mack, D.E. Plasma-sprayed thermal barrier coatings: New materials, processing issues, and solutions. J. Therm. Spray. Technol. 2013, 22, 646–658. [Google Scholar] [CrossRef]
- Cao, X.Q.; Vassen, R.; Schwartz, S.; Jungen, W.; Tietz, F.; Stöever, D. Spray-drying of ceramics for plasma-spray coating. J. Eur. Ceram. Soc. 2000, 20, 2433–2439. [Google Scholar] [CrossRef]
- Park, K.Y.; Jung, Y.G.; Kim, I.S.; Yang, B.I. Effects of purity and phase content of feedstock powder on thermal durability of zirconia-based thermal barrier coatings. J. Therm. Spray. Technol. 2017, 26, 1161–1167. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).