Curing Behavior of Waterborne Paint Containing Catalyst Encapsulated in Micelle
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples of Surfactant
2.2. Paint Preparation
2.3. Paint Evaluation
2.3.1. Measurement of Paint Curing Behavior
2.3.2. Evaluation of Paint Storage Stability
2.4. Measurement of Change in Molecular Weight of Micelles
- n: refractive index of solvent
- MW: weight-average molecular weight
- C: concentration of solute
- dn/dC: amount of concentration increases in refractive index difference
- r: distance between light scattering substance and detection module
- NA: Avogadro’s number
- Rg2: inertial square radius
- q: scattering vector
2.5. Evaluation of Dynamic Properties of Catalyst
2.6. Measurement of Heat Flow of the Catalyst in the Surfactant Solution
3. Results and Discussion
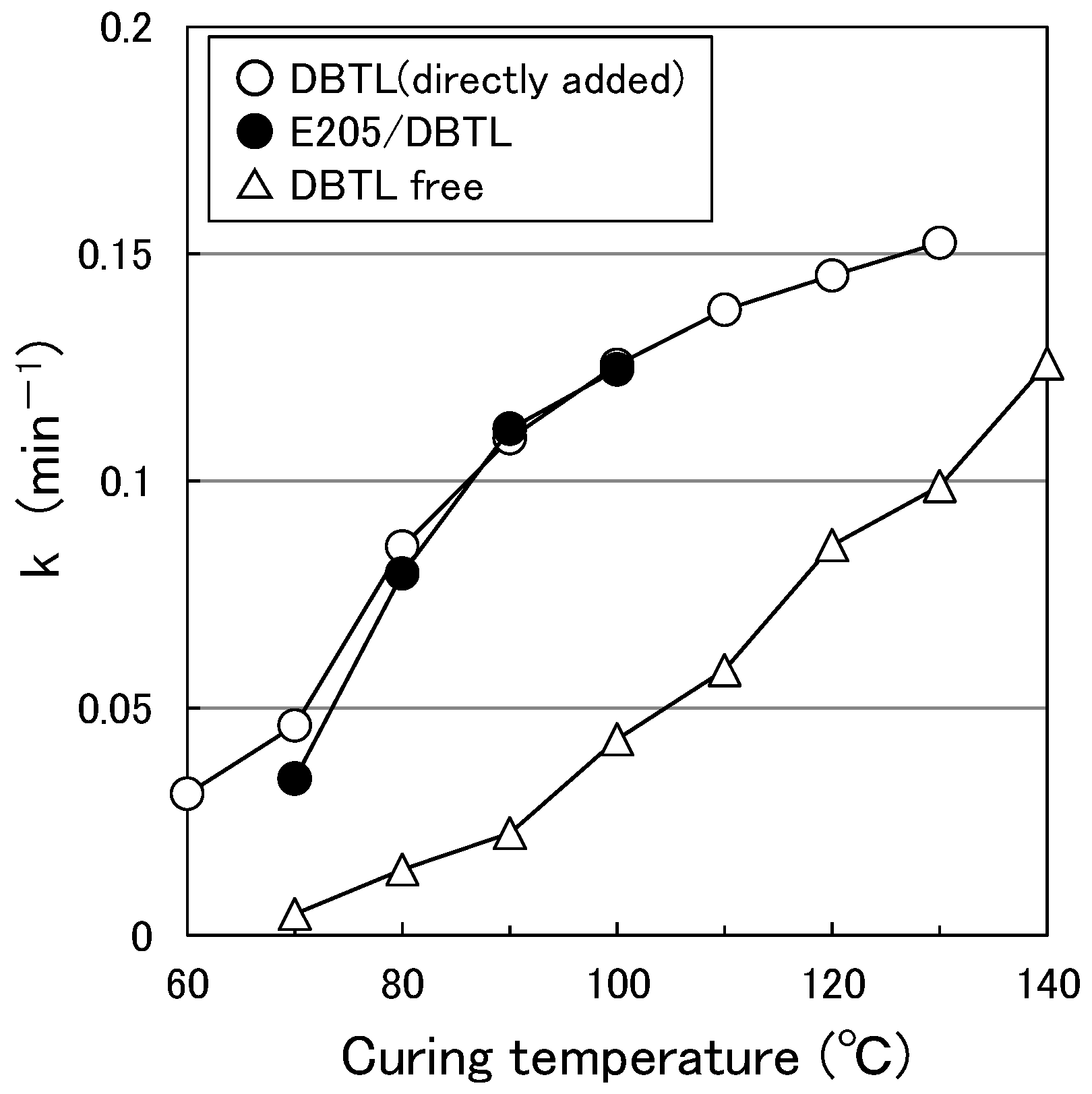
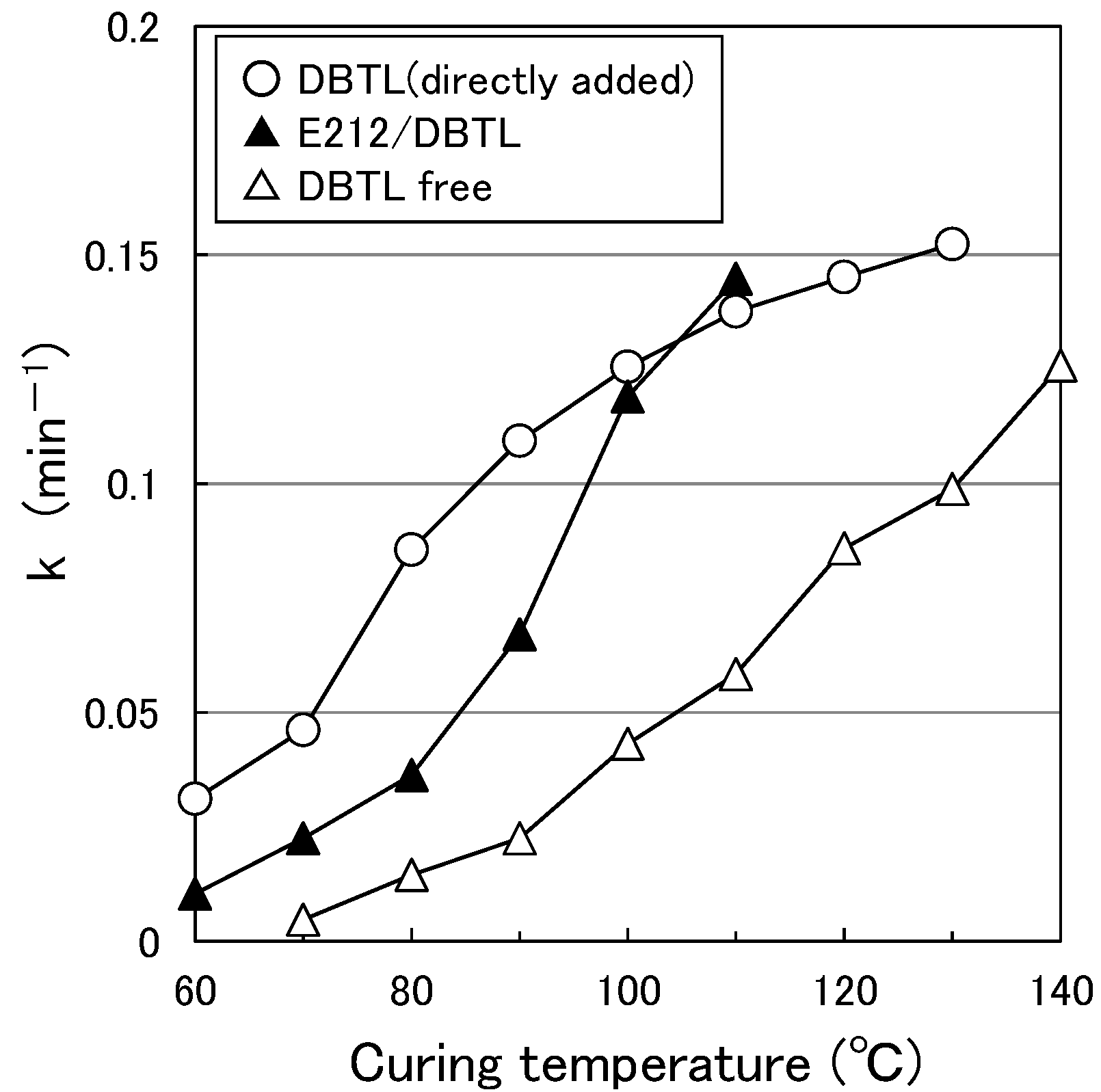
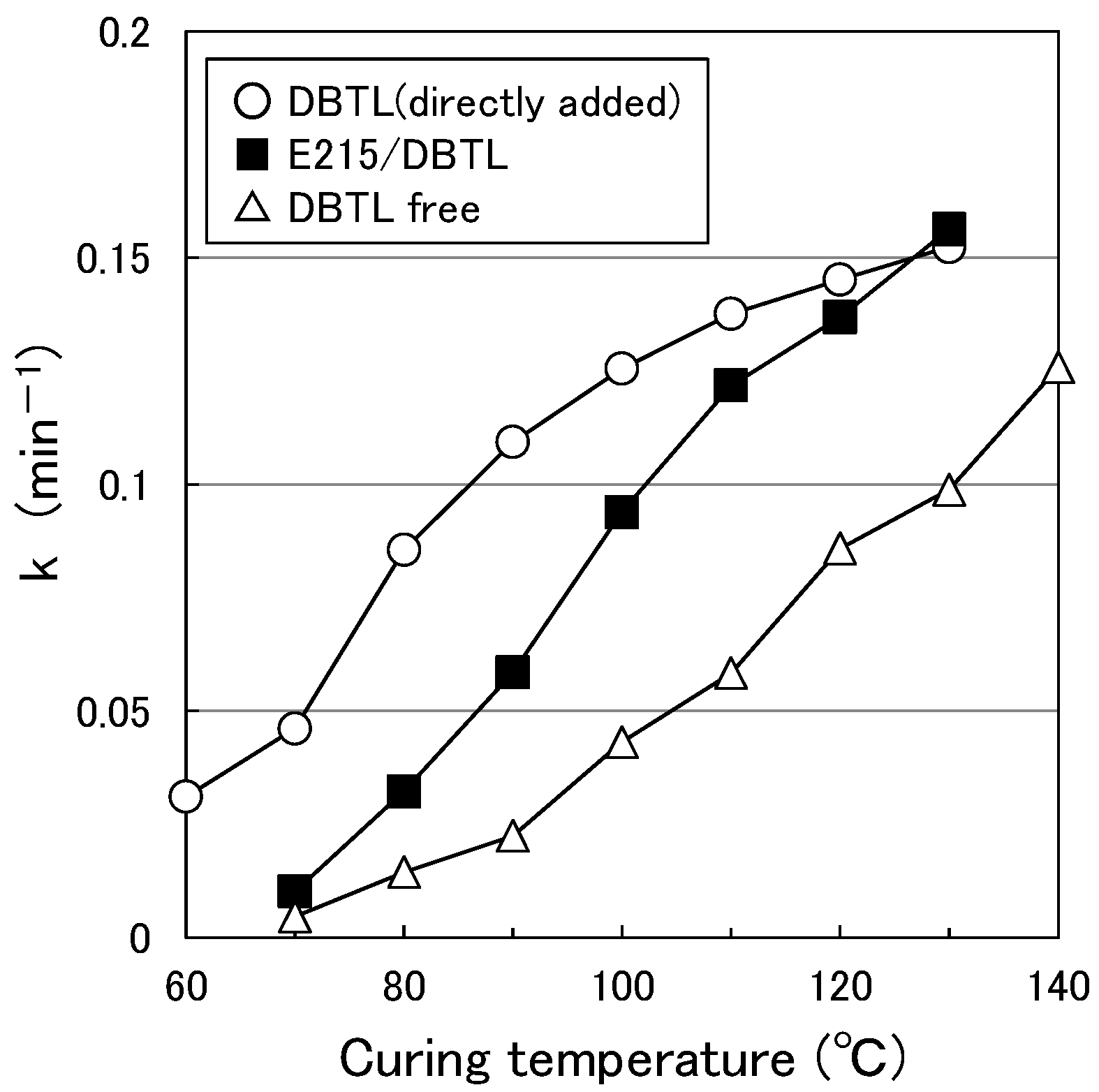
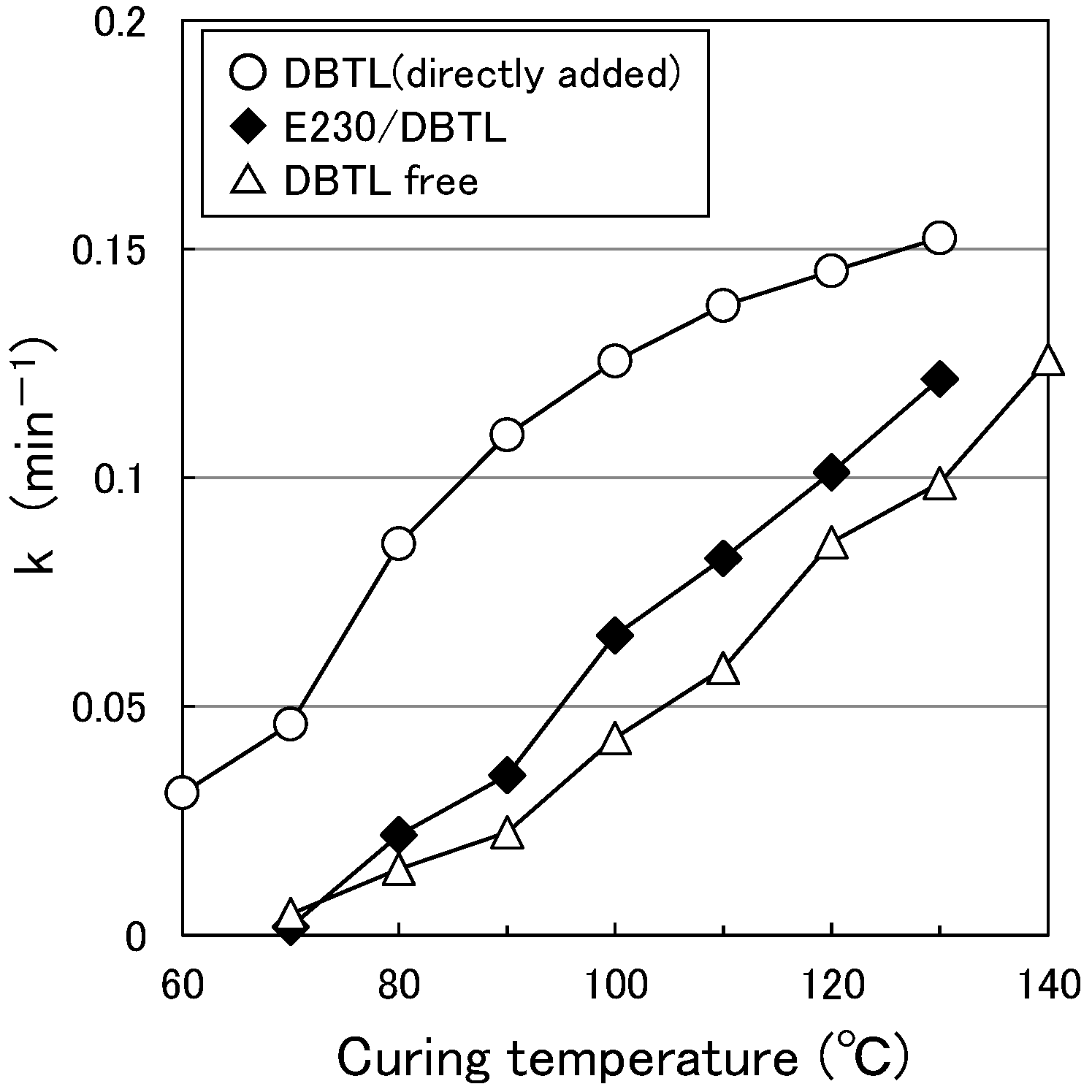
3.1. Paint Curing Behavior
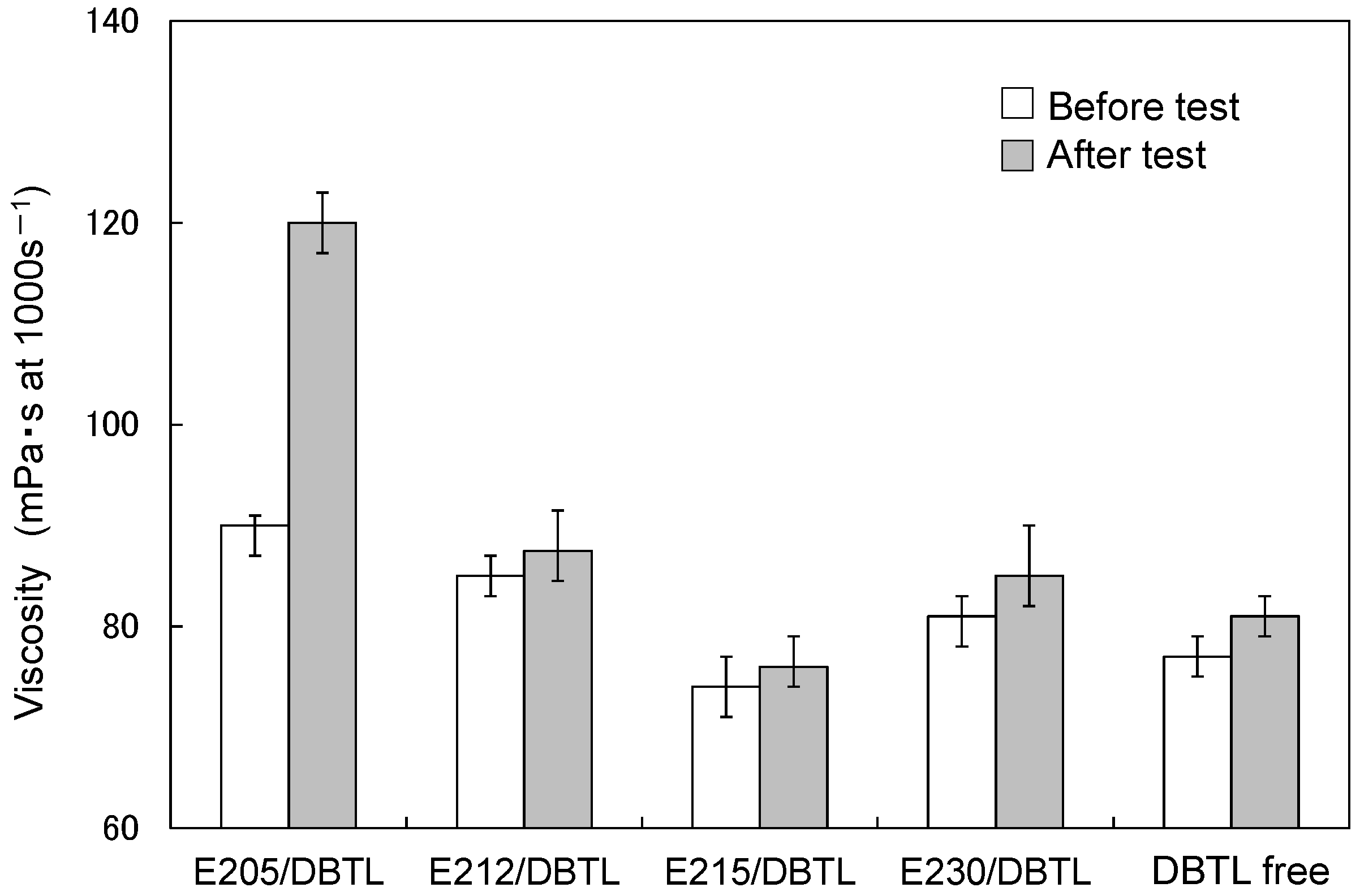
3.2. Paint Storage Stability
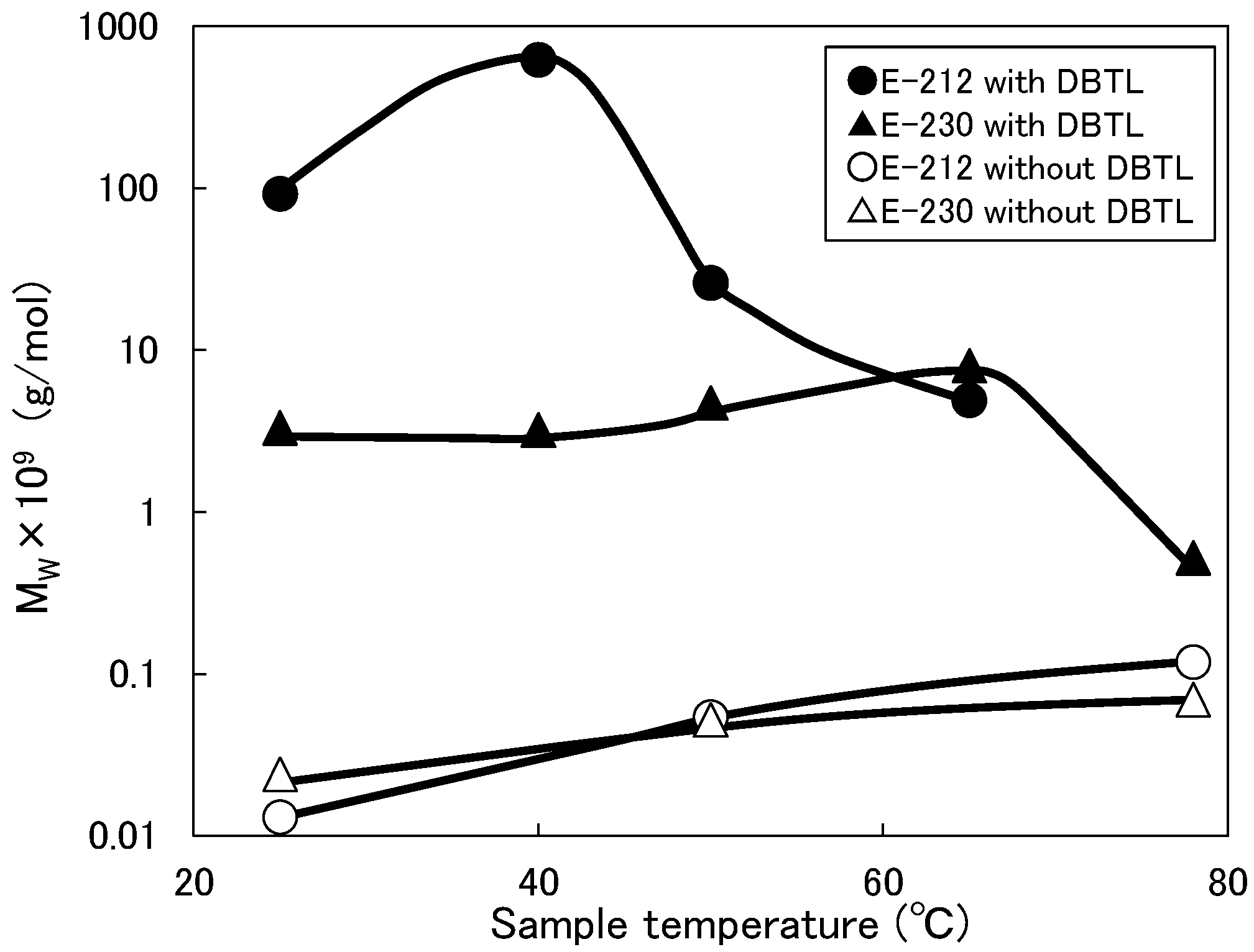
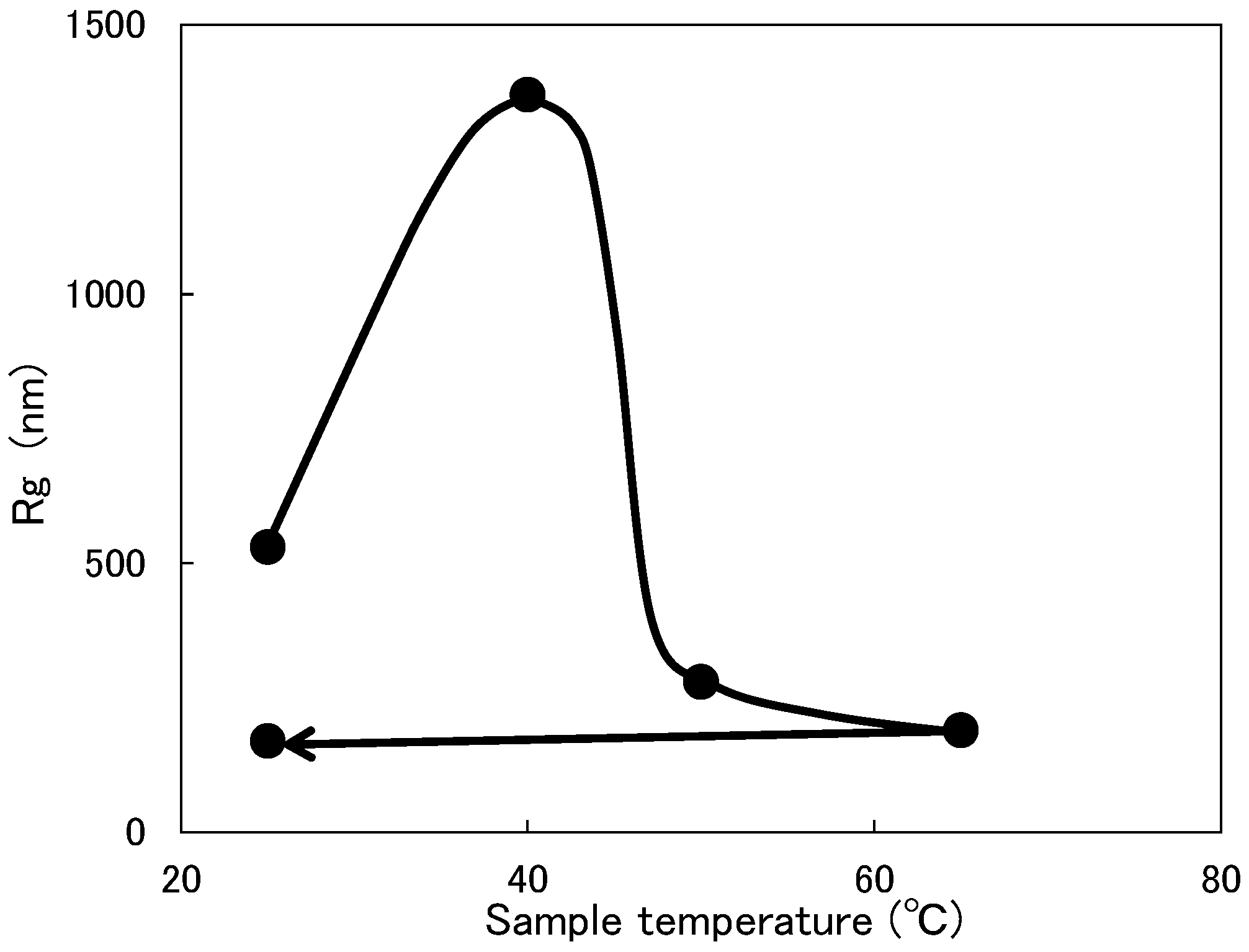
3.3. Change in Molecular Weight of Micelles
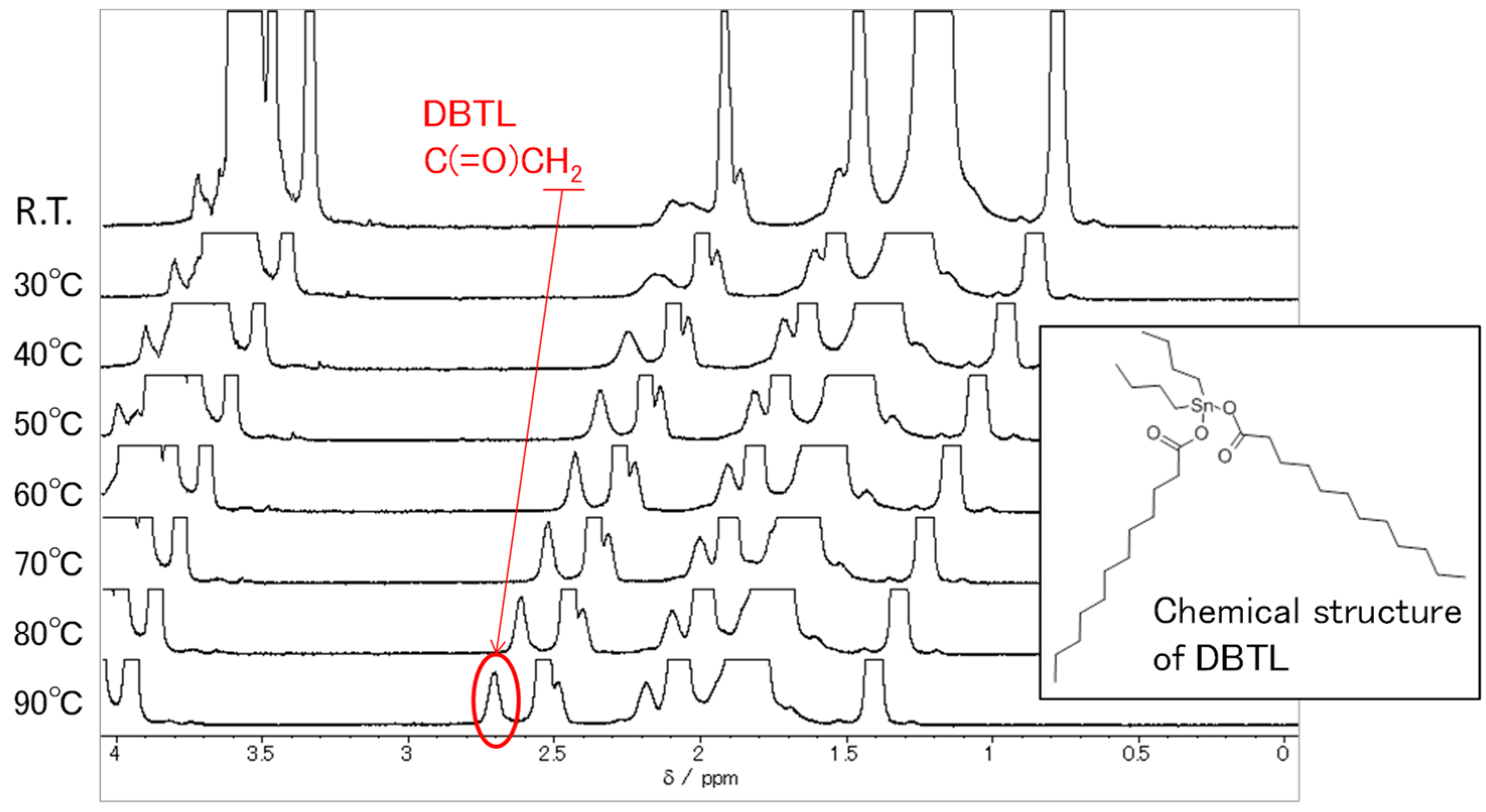
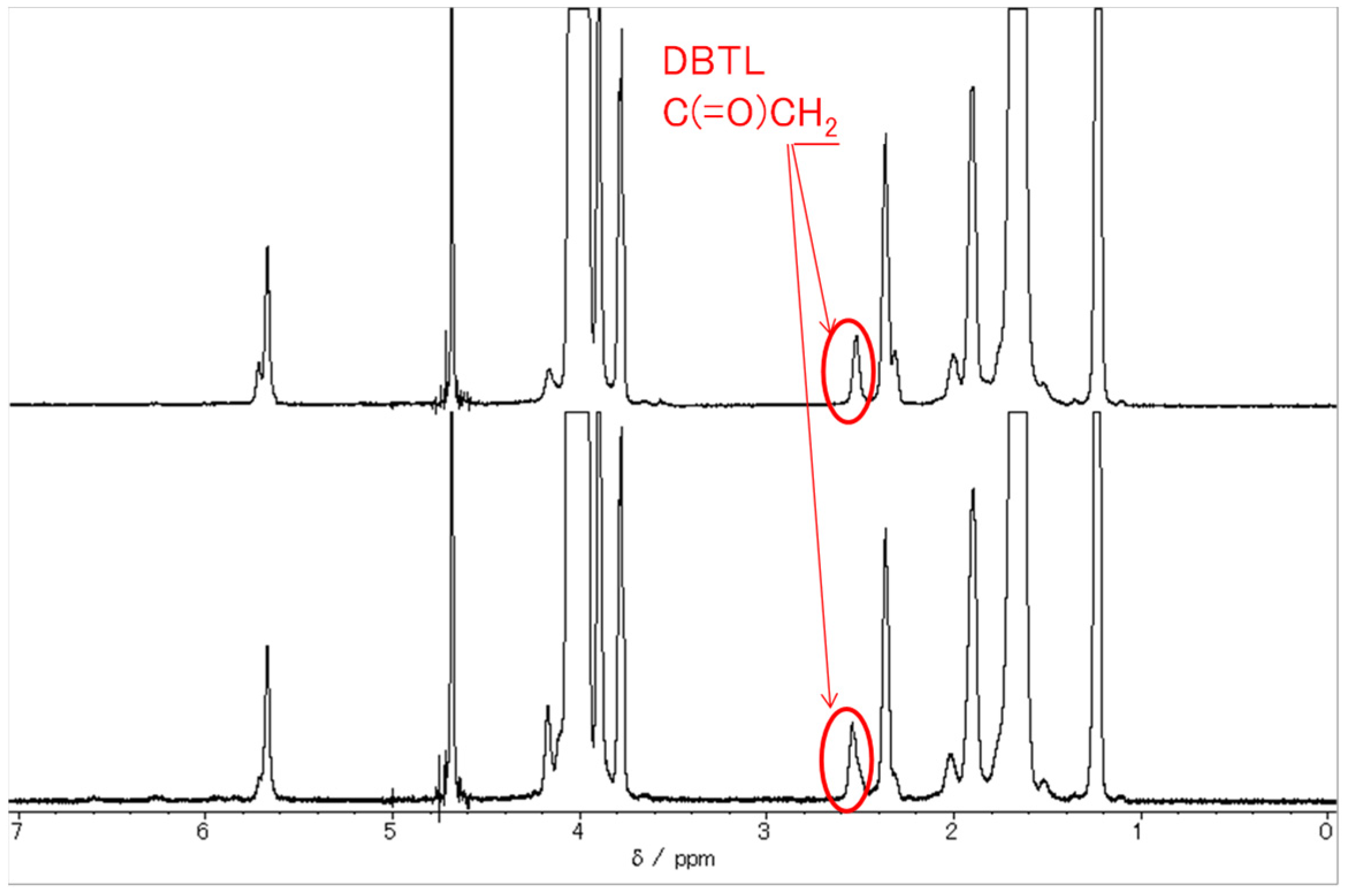
3.4. Dynamic Properties of Catalyst
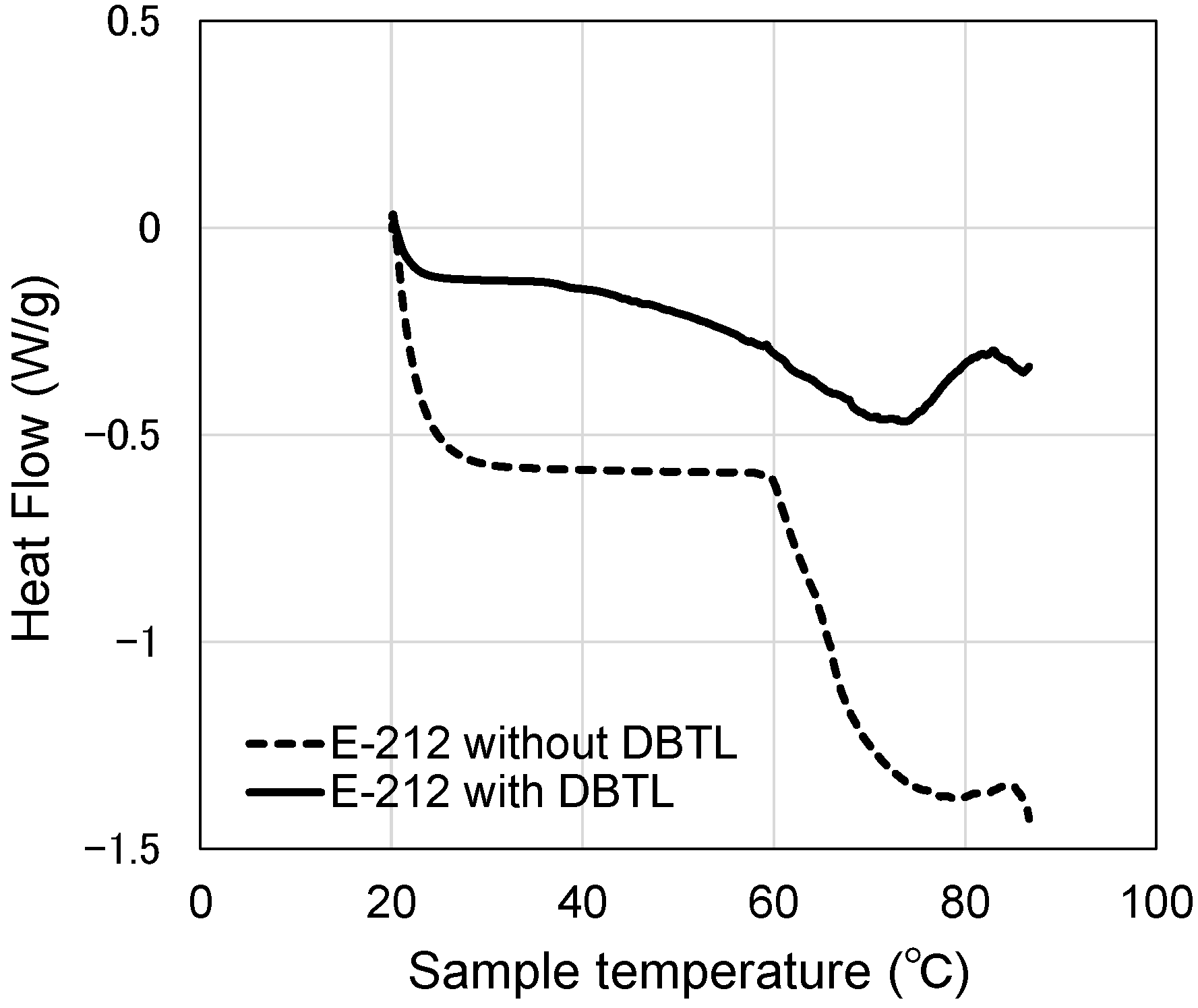
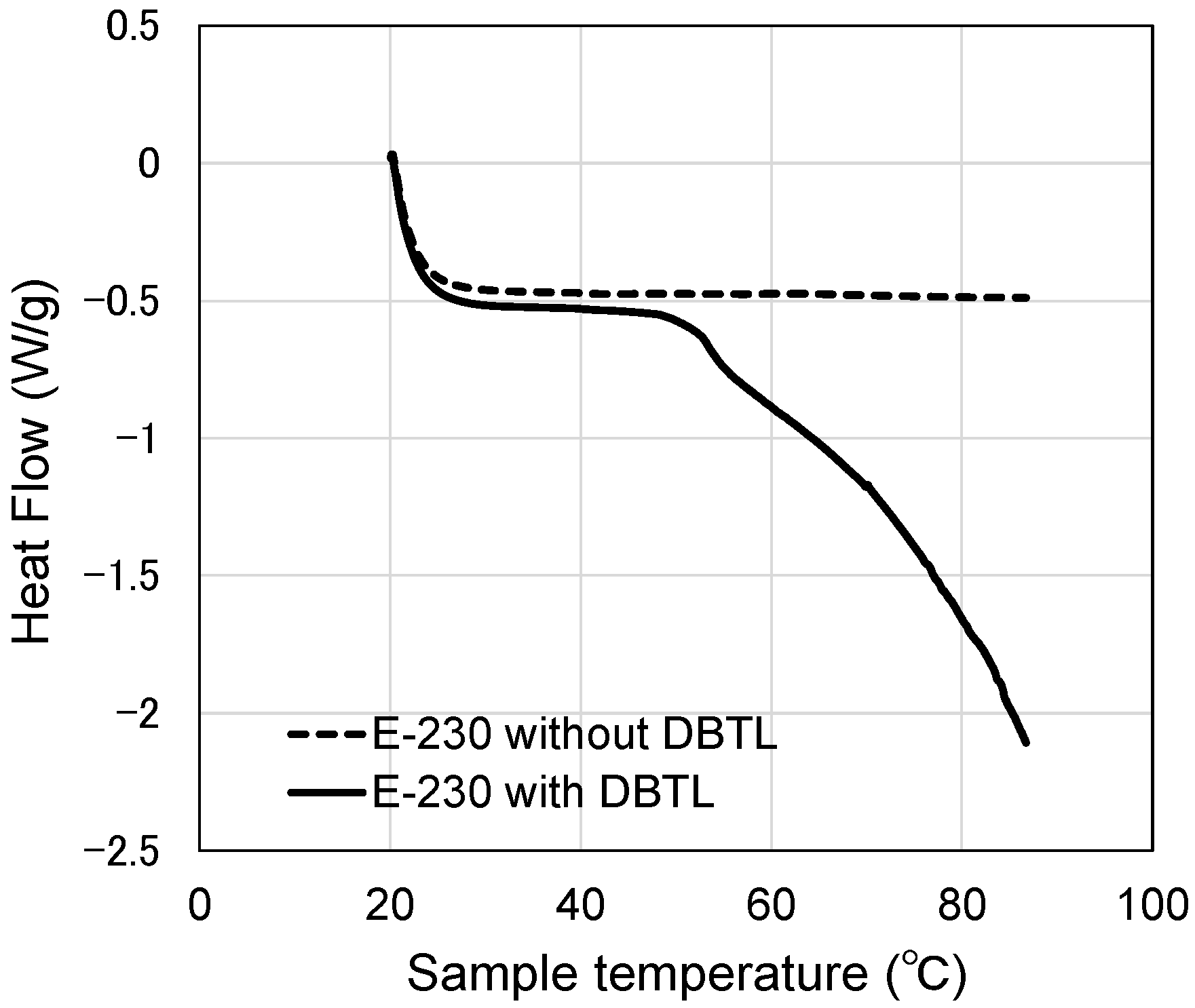
3.5. Heat Flow of Catalyst in the Surfactant Solution
3.6. Effect of HLB of the Surfactant on Curing Behavior
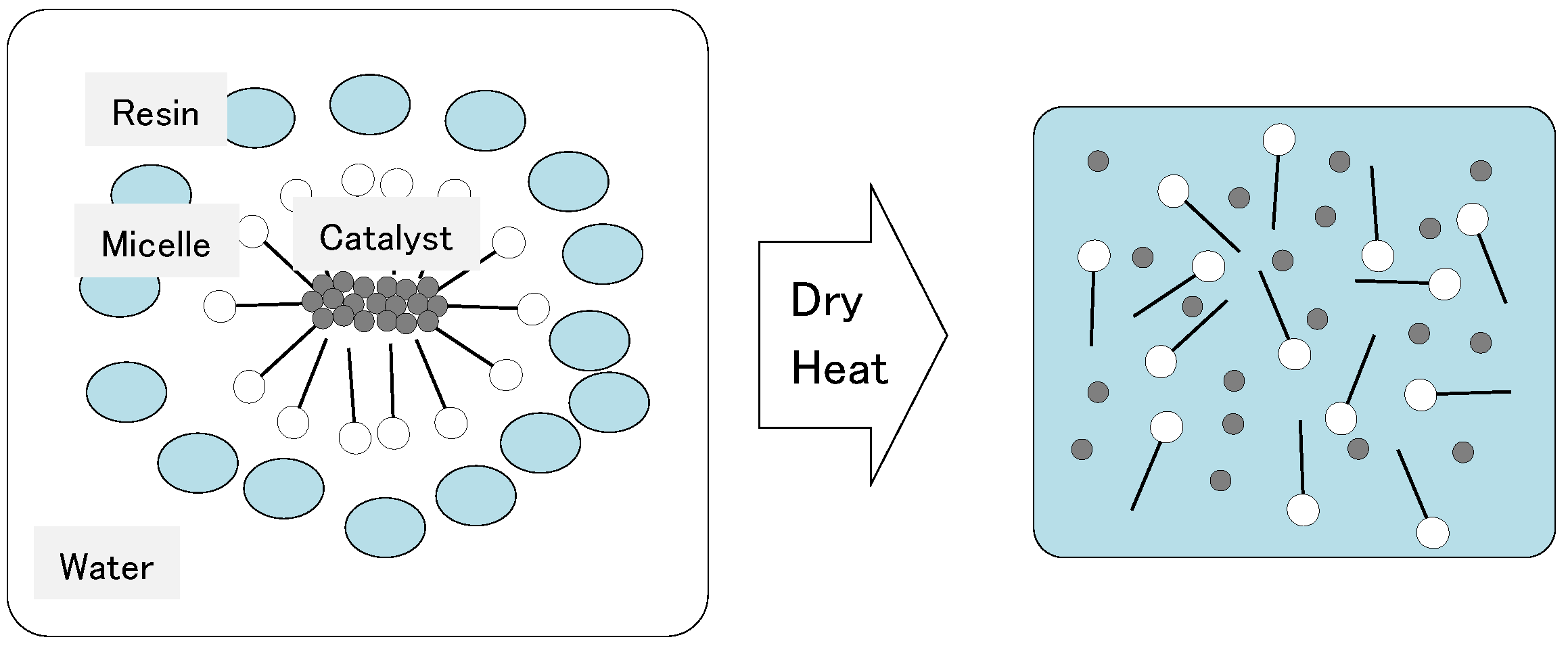
- Adding DBTL to a non-ionic surfactant aqueous solution increases MW and Rg values. One reason for this is because the DBTL molecules are constrained by the surfactant molecules (micelles). Since DBTL is highly hydrophobic, the hydrophobic portions of the surfactant molecules are more likely to be incorporated into the inwardly oriented micelles (Figure 1).
- Although the micelles grow as the temperature increases, the peak temperature for E-212 is 40 °C and the peak temperature for E-230 is 65 °C. At temperatures above these peaks, the MW and Rg values decrease. One reason for this is thought to be the gradual discharge of DBTL from the micelles, since the micelles start to break down at 40 °C or higher for E-212 and at 65 °C or higher for E-230.
- The switching function starting temperature based on the measured viscoelasticity results for the paint films (E-212: 80 °C, E-230: 120 °C or higher) is different in absolute terms from the results described in (2). As the former results were obtained from paint films with an extremely low water content, the motion of the DBTL molecules was also low. In contrast, as the latter results were obtained using aqueous solutions, the motion of the DBTL molecules was higher. This difference in motion is likely reflected in this absolute temperature difference.
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bailey, M.E.; Kirss, V.; Spaunburgh, R.G. Reactivity of Organic Isocyanates. Ind. Eng. Chem. 1956, 48, 794. [Google Scholar] [CrossRef]
- Wicks, D.A.; Wicks, Z.W., Jr. Blocked isocyanates III Part B: Uses and applications of blocked isocyanates. Prog. Org. Coat. 2001, 41, 1–83. [Google Scholar] [CrossRef]
- Modi, S.; Spubler, A.; Jin, J. Impact of Automated, Connected, Electric, and Shared (ACES) Vehicles on Design, Materials, Manufacturing and Business Models; Center for Automotive Research: Ann Arbor, MI, USA, 2018. [Google Scholar]
- Melchiors, M.; Sonntag, M.; Kobusch, C.; Juergens, E. Recent developments in aqueous two-component polyurethane (2K-PUR) coatings. Prog. Org. Coat. 2020, 40, 99–109. [Google Scholar] [CrossRef]
- Yomo, S.; Tachi, K.; Narita, T. Improving the appearance of 3-coat-l-bake multilayer films on automotive bodies. Prog. Org. Coat. 2018, 123, 299. [Google Scholar] [CrossRef]
- Wicks, D.A.; Wicks, Z.W., Jr. Blocked isocyanates III: Part A. Mechanisms and chemistry. Prog. Org. Coat. 1999, 36, 148–172. [Google Scholar] [CrossRef]
- Cram, D.J.; Cram, J.M. Monographs in Supramolecular Chemistry; The Royal Society of Chemistry: Cambridge, UK, 1994; Volume 4, p. 223. [Google Scholar]
- June, Y.G.; Jung, K.I.; Choi, M.; Lee, T.H.; Noh, S.M.; Jung, H.W. Effect of Urethane Crosslinking by Blocked Isocyanates with Pyrazole-Based Blocking Agents on Rheological and Mechanical Performance of Clearcoats. Coatings 2020, 10, 961. [Google Scholar] [CrossRef]
- Wang, L.; Zou, H.; Dong, Z.; Zhou, L.; Li, J.; Luo, Q.; Zhu, J.; Xu, J.; Liu, J. Temperature-Driven Switching of the Catalytic Activity of Artificial Glutathione Peroxidase by the Shape Transition between the Nanotubes and Vesicle-like Structures. Langmuir 2014, 30, 4013–4018. [Google Scholar] [CrossRef] [PubMed]
- Kremer, C.; Schnakenburg, G.; Lutzen, A. Towards allosteric receptors—Synthesis of beta-cyclodextrin-functionalised 2,2′-bipyridines and their metal complexes. Beilstein J. Org. Chem. 2014, 10, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xu, G.; Chamoreau, L.; Zhang, Y.; Mouries-Mansuy, V.; Fensterbank, L.; Bistri-Aslanoff, O.; Roland, S.; Sollogoub, M. Permethylated NHC-Capped alpha- and beta-Cyclodextrins (ICyDMe) Regioselective and Enantioselective Gold-Catalysis in Pure Water. Chem. Eur. J. 2020, 26, 15901–15909. [Google Scholar]
- Quach, Q.; Biehler, E.; Elzamzami, A.; Huff, C.; Long, J.L.M.; Abdei-Fattah, T.M. Catalytic Activity of Beta-Cyclodextrin-Gold Nanoparticles Network in Hydrogen Evolution Reaction. Catalysts 2021, 11, 118. [Google Scholar] [CrossRef]
- Ye, R.P.; Lin, L.; Liu, C.Q.; Chen, C.C.; Yao, Y.G. One-Pot Synthesis of Cyclodextrin-Doped Cu-SiO2 Catalysts for Efficient Hydrogenation of Dimethyl Oxalate to Ethylene Glycol. Chemcatchem 2017, 9, 4587–4597. [Google Scholar] [CrossRef]
- Ikeda, A.; Shinkai, S. Novel cavity design using calix[n]arene skeletons: Toward molecular recognition and metal binding. Chem. Rev. 1997, 97, 1713–1734. [Google Scholar] [CrossRef] [PubMed]
- Duchene, D. New Trends in Cyclodextrins and Derivatives; Edition de Sante: Paris, France, 1991; p. 449. [Google Scholar]
- Vaution, C. Cyclodextrins and Their Industrial Uses; Edition de Sante: Paris, France, 1987; p. 297. [Google Scholar]
- Kiasat, A.R.; Saghanezhad, S.J.; Noori, S. Beta-Cyclodextrin Based Nanosponges in Organic Synthesis. Curr. Org. Chem. 2019, 23, 2366–2377. [Google Scholar] [CrossRef]
- Mori, K.; Yamashita, H. Design of Colloidal and Supported Metal Nanoparticles: Their Synthesis, Characterization, and Catalytic Application. J. Jpn. Pet. Inst. 2011, 54, 1–14. [Google Scholar] [CrossRef]
- Szejtli, J. Cyclodextrins in food, cosmetics, and toiletries. Starch/Staerke 1982, 34, 379. [Google Scholar] [CrossRef]
- Hinze, W.L.; Pramauro, E. A critical-review of surfactant-mediated phase separations (cloud-point extractions)—Theory and applications. Crit. Rev. Anal. Chem. 1993, 24, 133–177. [Google Scholar] [CrossRef]
- Miller, C.A.; Raney, K.H. Solubilization emulsification mechanisms of detergency. Colloids and Surf. A-Physicochem. Eng. Asp. 1993, 74, 169. [Google Scholar] [CrossRef]
- Brown, W.; Schillen, K.; Almgren, M.; Hvidt, S.; Bahadur, P. Micelle and gel formation in a poly(ethylene oxide) poly(propylene oxide) poly(ethylene oxide) triblock copolymer in water solution—Dynamic and static light-scattering and oscillatory shear measurements. J. Phys. Chem. 1991, 95, 1850–1858. [Google Scholar] [CrossRef]
- NOF. Corporation Products Catalogue. Available online: https://www.nof.co.jp/business/oleo/pdf/comprehensive.pdf (accessed on 18 January 2021). (In Japanese).
- Mori, K. Method for Estimating Crosslink Density in Curing Process on Coating Films-Proportionality between Storage Modulus and Crosslink Density. J. Jpn. Soc. Color Mater. 2013, 86, 123. [Google Scholar] [CrossRef][Green Version]
- Zimm, B.H. The Scattering of Light and the Radial Distribution Function of High Polymer Solutions. J. Chem. Phys. 1948, 16, 1093–1099. [Google Scholar] [CrossRef]



| Product Name | Chemical Formula | HLB | Melting Point (°C) [23] |
|---|---|---|---|
| Nonion E-205 | C18H35O(C2H4O)nH | 9.0 | 4 |
| Nonion E-212 | 13.3 | 31 | |
| Nonion E-215 | 14.2 | 35 | |
| Nonion E-230 | 16.6 | 40 |
| Monomers | Blend Amount (wt.%) |
|---|---|
| Butyl acrylate | 22 |
| Butyl methacrylate | 19 |
| Methyl methacrylate | 10 |
| 2-hydroxyethyl methacrylate | 36 |
| Styrene | 10 |
| Acrylic acid | 3 |
| Total | 100 |
| Averaged molecular weight | 11,500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yomo, S. Curing Behavior of Waterborne Paint Containing Catalyst Encapsulated in Micelle. Coatings 2021, 11, 375. https://doi.org/10.3390/coatings11040375
Yomo S. Curing Behavior of Waterborne Paint Containing Catalyst Encapsulated in Micelle. Coatings. 2021; 11(4):375. https://doi.org/10.3390/coatings11040375
Chicago/Turabian StyleYomo, Shuji. 2021. "Curing Behavior of Waterborne Paint Containing Catalyst Encapsulated in Micelle" Coatings 11, no. 4: 375. https://doi.org/10.3390/coatings11040375
APA StyleYomo, S. (2021). Curing Behavior of Waterborne Paint Containing Catalyst Encapsulated in Micelle. Coatings, 11(4), 375. https://doi.org/10.3390/coatings11040375






