The Physical Properties of Submicron and Nano-Grained La0.7Sr0.3MnO3 and Nd0.7Sr0.3MnO3 Synthesised by Sol–Gel and Solid-State Reaction Methods
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, D.; Roul, B.; Singh, S.; Srinivasu, V. Possible observation of Griffith phase over large temperature range in plasma sintered La0.67Ca0.33MnO3. J. Magn. Magn. Mater. 2018, 448, 287–291. [Google Scholar] [CrossRef]
- Panchal, G.; Choudhary, R.J.; Phase, D.M. Magnetic properties of La0.7Sr0.3MnO3 film on ferroelectric BaTiO3 substrate. J. Magn. Magn. Mater. 2018, 448, 262–265. [Google Scholar] [CrossRef]
- Gong, J.; Zheng, D.; Li, D.; Jin, C.; Bai, H. Lattice distortion modified anisotropic magnetoresistance in epitaxial La0.67Sr0.33MnO3 thin films. J. Alloys Compd. 2018, 735, 1152–1157. [Google Scholar] [CrossRef]
- Jonker, G.; Van Santen, J. Ferromagnetic compounds of manganese with perovskite structure. Physica 1950, 16, 337–349. [Google Scholar] [CrossRef]
- Yang, H.; Hua, S.; Zhang, P.; Zhang, S.; Ge, H.; Pan, M. Effect of Fe substitution on charge order state and magnetocaloric effect in Pr0.5Sr0.5Mn1−xFexO3 (0.00 ≤ x ≤ 0.08) manganites. J. Alloys Compd. 2016, 680, 768–772. [Google Scholar] [CrossRef]
- Von Helmolt, R.; Wecker, J.; Holzapfel, B.; Schultz, L.; Samwer, K. Giant negative magnetoresistance in perovskitelike La2/3Ba1/3Mnx ferromagnetic films. Phys. Rev. Lett. 1993, 71, 2331. [Google Scholar] [CrossRef]
- Rozilah, R.; Ibrahim, N.; Yahya, A.K. Inducement of ferromagnetic–metallic phase and magnetoresistance behavior in charged ordered monovalent-doped Pr0.75Na0.25MnO3 manganite by Ni substitution. Solid State Sci. 2019, 87, 64–80. [Google Scholar] [CrossRef]
- Ahmed, A.; Mohamed, H.; Paixão, J.; Mohamed, S.A. Thermopower and magnetocaloric properties in NdSrMnO/CrO3 composites. J. Magn. Magn. Mater. 2018, 456, 217–222. [Google Scholar] [CrossRef]
- Zener, C. Interaction between the d-shells in the transition metals. II. Ferromagnetic compounds of manganese with perovskite structure. Phys. Rev. 1951, 82, 403. [Google Scholar] [CrossRef]
- Tang, T.; Tien, C.; Hou, B. Low-field magnetoresistance of Ag-substituted perovskite-type manganites. Phys. B Condens. Matter 2008, 403, 2111–2115. [Google Scholar] [CrossRef]
- Nagaev, E.L. Colossal-magnetoresistance materials: Manganites and conventional ferromagnetic semiconductors. Phys. Rep. 2001, 346, 387–531. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Vasil’ev, A.N.; Maignan, A.; Szymczak, H. Critical behavior of La0.825Sr0.175MnO2.912 anion-deficient manganite in the magnetic phase transition region. JETP Lett. 2007, 85, 507–512. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Szymczak, H.; Botez, C.E.; Adair, A. Magnetotransport properties and mechanism of the a-site ordering in the Nd–Ba optimal-doped manganites. J. Low Temp. Phys. 2007, 149, 185–199. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Khomchenko, V.A.; Lobanovski, L.S.; Bushinsky, M.V.; Karpinsky, D.V.; Fedotova, V.V.; Troyanchuk, I.O.; Trukhanov, A.V.; Stepin, S.G.; Szymczak, R.; et al. Crystal structure and magnetic properties of Ba-ordered manganites Ln0.70Ba0.30MnO3−δ (Ln = Pr, Nd). J. Exp. Theor. Phys. 2006, 103, 398–410. [Google Scholar] [CrossRef]
- Ahsan, M.Z.; Ahsan, P.M.A.; Islam, M.A.; Asif, F.C. Structural and electrical properties of copper doped lanthanum manganite NPs. Results Phys. 2019, 15, 102600. [Google Scholar] [CrossRef]
- Mohamed, Z.; Ibrahim, N.; Ghani, M.A.; Safian, S.D.; Mohamed, S.N. Structural and electrical transport properties of (La0.7−xYx)Ca0.3MnO3 manganites. Results Phys. 2019, 12, 861–866. [Google Scholar] [CrossRef]
- Arun, B.; Suneesh, M.V.; Vasundhara, M. Comparative study of magnetic ordering and electrical transport in bulk and nano-grained Nd0.67Sr0.33MnO3 manganites. J. Magn. Magn. Mater. 2016, 418, 265–272. [Google Scholar] [CrossRef]
- Abdel-Khalek, E.K.; Salem, A.F.; Mohamed, E.A. Study on the influence of magnetic phase transitions on the magnetocaloric effect in Sm0.7Sr0.3Mn0.95Fe0.05O3 manganite. J. Alloys Compd. 2014, 608, 180–184. [Google Scholar] [CrossRef]
- Xia, W.; Li, L.; Wu, H.; Xue, P.; Zhu, X. Structural, morphological, and magnetic properties of sol-gel derived La0.7Ca0.3MnO3 manganite nanoparticles. Ceram. Int. 2017, 43, 3274–3283. [Google Scholar] [CrossRef]
- Ng, S.; Lim, K.; Halim, S.; Jumiah, H. Grain size effect on the electrical and magneto-transport properties of nanosized Pr0.67Sr0.33MnO3. Results Phys. 2018, 9, 1192–1200. [Google Scholar] [CrossRef]
- Shokr, F.S.; Hussein, M. Physical properties of La0.67Ca0.13Sr0.2Mn1−xFexO3 compounds doped Fe. Results Phys. 2018, 8, 186–189. [Google Scholar] [CrossRef]
- Hintze, C.E.; Fuchs, D.; Merz, M.; Amari, H.; Kübel, C.; Huang, M.-J.; Powell, A.; v. Löhneysen, H. Size-induced changes of structural and ferromagnetic properties in La1−xSrxMnO3 nanoparticles. J. Appl. Phys. 2017, 121, 214303. [Google Scholar] [CrossRef]
- Paul, S.; Nath, T. Microstructural, electrical-, magneto-transport properties of grain size modulated nanocrystalline Nd0.67Sr0.33MnO3 CMR manganites. MRS Online Proc. Libr. 2008, 1118, 3–8. [Google Scholar] [CrossRef]
- Paul, S.; Nath, T.K. Magneto-transport and magnetic properties of Fe doped nanometric polycrystalline La0.7Sr0.3MnO3 CMR manganites. MRS Online Proc. Libr. (OPL) 2009, 1183. [Google Scholar] [CrossRef]
- Dey, P.; Nath, T. Tunable room temperature low-field spin polarized tunneling magnetoresistance of La0.7Sr0.3MnO3 nanoparticles. Appl. Phys. Lett. 2006, 89, 163102. [Google Scholar] [CrossRef]
- Ngida, R.E.A.; Zawrah, M.F.; Khattab, R.M.; Heikal, E. Hydrothermal synthesis, sintering and characterization of nano La-manganite perovskite doped with Ca or Sr. Ceram. Int. 2019, 45, 4894–4901. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, A.K. Investigation of structural and magnetic properties of Nd0.7Ba0.3Mn1−xTixO3 (x = 0.05, 0.15 and 0.25) manganites synthesized through a single-step process. J. Magn. Magn. Mater. 2019, 469, 264–273. [Google Scholar] [CrossRef]
- Thombare, B.; Dusane, P.; Kekade, S.; Salunkhe, A.; Choudhary, R.J.; Phase, D.M.; Devan, R.S.; Patil, S.I. Influence of nano-dimensionality on magnetotransport, magnetic and electrical properties of Nd1−xSrxMnO3−δ (0.3 ≤ x ≤ 0.7). J. Alloys Compd. 2019, 770, 257–266. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: London, UK, 2013. [Google Scholar]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Ezaami, A.; Sfifir, I.; Cheikhrouhou-Koubaa, W.; Koubaa, M.; Cheikhrouhou, A. Critical properties in La0.7Ca0.2Sr0.1MnO3 manganite: A comparison between sol-gel and solid state process. J. Alloys Compd. 2017, 693, 658–666. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, Y.; Chen, Y.; Wang, G.; Dong, X.; Tang, X. Structure and magnetic properties of La0.67Sr0.33MnO3 thin films prepared by sol–gel method. J. Sol-Gel Sci. Technol. 2013, 67, 170–174. [Google Scholar] [CrossRef]
- Souza, A.D.; Babu, P.; Rayaprol, S.; Murari, M.; Mendonca, L.D.; Daivajna, M. Size control on the magnetism of La0.7Sr0.3MnO3. J. Alloys Compd. 2019, 797, 874–882. [Google Scholar] [CrossRef]
- Feng, X.; Wen, H.; Shen, Y. Microwave absorption properties of Nd0.7Sr0.3MnO3 prepared using high-energy ball milling. J. Alloys Compd. 2013, 555, 145–149. [Google Scholar] [CrossRef]
- Lim, K.P.; Ng, S.W.; Lau, L.N.; Kechik, M.M.A.; Chen, S.K.; Halim, S.A. Unusual electrical behaviour in sol–gel-synthesised PKMO nano-sized manganite. Appl. Phys. A 2019, 125, 745. [Google Scholar] [CrossRef]
- Jeddi, M.; Gharsallah, H.; Bekri, M.; Dhahri, E.; Hlil, E. Structural characterization and ZFC/FC magnetization study of La0.6Ca0.4−xSrxMnO3 nanoparticle compounds. Appl. Phys. A 2020, 126, 6. [Google Scholar] [CrossRef]
- Bouzidi, S.; Gdaiem, M.A.; Rebaoui, S.; Dhahri, J.; Hlil, E. Large magnetocaloric effect in La0.75Ca0.25−xNaxMnO3 (0 ≤ x ≤ 0.10) manganites. Appl. Phys. A 2020, 126, 60. [Google Scholar] [CrossRef]
- Lau, L.N.; Ibrahim, N.B.; Baqiah, H. Influence of precursor concentration on the structural, optical and electrical properties of indium oxide thin film prepared by a sol–gel method. Appl. Surf. Sci. 2015, 345, 355–359. [Google Scholar] [CrossRef]
- Kurchania, R.; Rathore, D.; Pandey, R. Size dependent strain and nanomagnetism in CoFe2O4 nanoparticles. J. Mater. Sci. Mater. Electron. 2015, 26, 9355–9365. [Google Scholar] [CrossRef]
- Arun, B.; Akshay, V.R.; Chandrasekhar, K.D.; Mutta, G.R.; Vasundhara, M. Comparison of structural, magnetic and electrical transport behavior in bulk and nanocrystalline Nd-lacunar Nd0.67Sr0.33MnO3 manganites. J. Magn. Magn. Mater. 2019, 472, 74–85. [Google Scholar] [CrossRef]
- Verma, M.K.; Sharma, N.D.; Sharma, S.; Choudhary, N.; Singh, D. High magnetoresistance in La0.5Nd0.15Ca0.25A0.1MnO3 (A = Ca, Li, Na, K) CMR manganites: Correlation between their magnetic and electrical properties. Mater. Res. Bull. 2020, 125, 110813. [Google Scholar] [CrossRef]
- Navin, K.; Kurchania, R. The effect of particle size on structural, magnetic and transport properties of La0.7Sr0.3MnO3 nanoparticles. Ceram. Int. 2018, 44, 4973–4980. [Google Scholar] [CrossRef]
- Baaziz, H.; Tozri, A.; Dhahri, E.; Hlil, E.K. Effect of particle size reduction on the structural, magnetic properties and the spin excitations in ferromagnetic insulator La0.9Sr0.1MnO3 nanoparticles. Ceram. Int. 2015, 41, 2955–2962. [Google Scholar] [CrossRef]
- Giri, S.K.; Yusuf, S.M.; Mukadam, M.D.; Nath, T.K. Emergence of Griffiths phase and glassy mixed phase in Sm0.5Ca0.5MnO3 nanomanganites. J. Alloys Compd. 2014, 591, 181–187. [Google Scholar] [CrossRef]
- Mandal, S.; Nath, T.; Rao, V. Effect of nanometric grain size on electronic-transport, magneto-transport and magnetic properties of La0.7Ba0.3MnO3 nanoparticles. J. Phys. Condens. Matter 2008, 20, 385203. [Google Scholar] [CrossRef] [PubMed]
- Manh, D.H.; Phong, P.T.; Thanh, T.D.; Hong, L.V.; Phuc, N.X. Low-field magnetoresistance of La0.7Ca0.3MnO3 perovskite synthesized by reactive milling method. J. Alloys Compd. 2010, 499, 131–134. [Google Scholar] [CrossRef]
- Anshul, A.; Amritphale, S.S.; Kaur, S.; Hada, R. Nanomaterials synthesis and effect of granular disorders on electrical behavior of lanthanum manganites. J. Mater. Sci. Technol. 2011, 27, 691–695. [Google Scholar] [CrossRef]
- Bhatt, R.C.; Singh, S.K.; Srivastava, P.C.; Agarwal, S.K.; Awana, V.P.S. Impact of sintering temperature on room temperature magneto-resistive and magneto-caloric properties of Pr2/3Sr1/3MnO3. J. Alloys Compd. 2013, 580, 377–381. [Google Scholar] [CrossRef]
- Sakthipandi, K.; Arunachalam, M.; Thamilmaran, P.; Sivabharathy, M.; Sankarrajan, S. Influence of ionic radius on magnetic phase transition in R1−xSrxMnO3 perovskites. AIP Conf. Proc. 2017, 1832, 030016. [Google Scholar]
- Sakthipandi, K.; Rajendran, V.; Jayakumar, T. Phase transitions of bulk and nanocrystalline La1−xSrxMnO3 (x = 0.35 and 0.37) perovskite manganite materials using in situ ultrasonic studies. Mater. Res. Bull. 2013, 48, 1651–1659. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Chen, Q.; Li, D.; Li, Z.; Zhang, Y. Effects of A-site cationic radius and cationic disorder on the electromagnetic properties of La0.7Ca0.3MnO3 ceramic with added Sr, Pb, and Ba. Ceram. Int. 2018, 44, 5378–5384. [Google Scholar] [CrossRef]
- Mohamed, H. Influence of sodium doping on the electrical and magnetic properties of La0.90Li0.10MnO3 manganites. J. Magn. Magn. Mater. 2017, 424, 44–52. [Google Scholar] [CrossRef]
- Venkataiah, G.; Venugopal Reddy, P. Electrical behavior of sol–gel prepared Nd0.67Sr0.33MnO3 manganite system. J. Magn. Magn. Mater. 2005, 285, 343–352. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, X.; Li, S. Structure, magnetic, electrical transport and magnetoresistance properties of La0.67Sr0.33Mn1−xFexO3 (x = 0–0.15) doped manganite coatings. Ceram. Int. 2017, 43, 3679–3687. [Google Scholar] [CrossRef]
- Modi, A.; Bhat, M.A.; Pandey, D.K.; Tarachand; Bhattacharya, S.; Gaur, N.K.; Okram, G.S. Structural, magnetotransport and thermal properties of Sm substituted La0.7−xSmxBa0.3MnO3 (0 ≤ x ≤ 0.2) manganites. J. Magn. Magn. Mater. 2017, 424, 459–466. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, X.; Li, S. Effect of particle size on magnetic and electric transport properties of La0.67Sr0.33MnO3 coatings. PCCP 2015, 17, 31161–31169. [Google Scholar] [CrossRef] [PubMed]
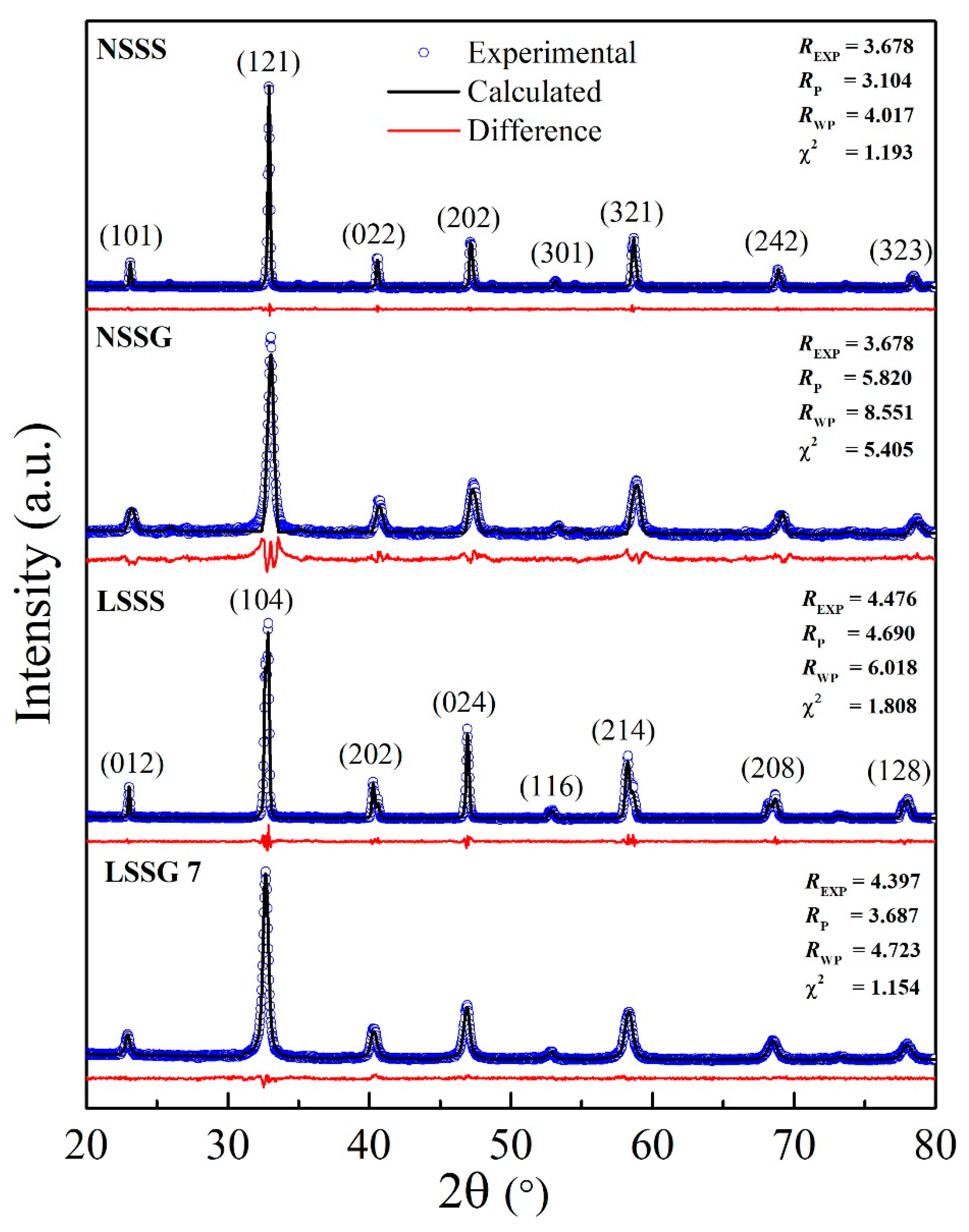
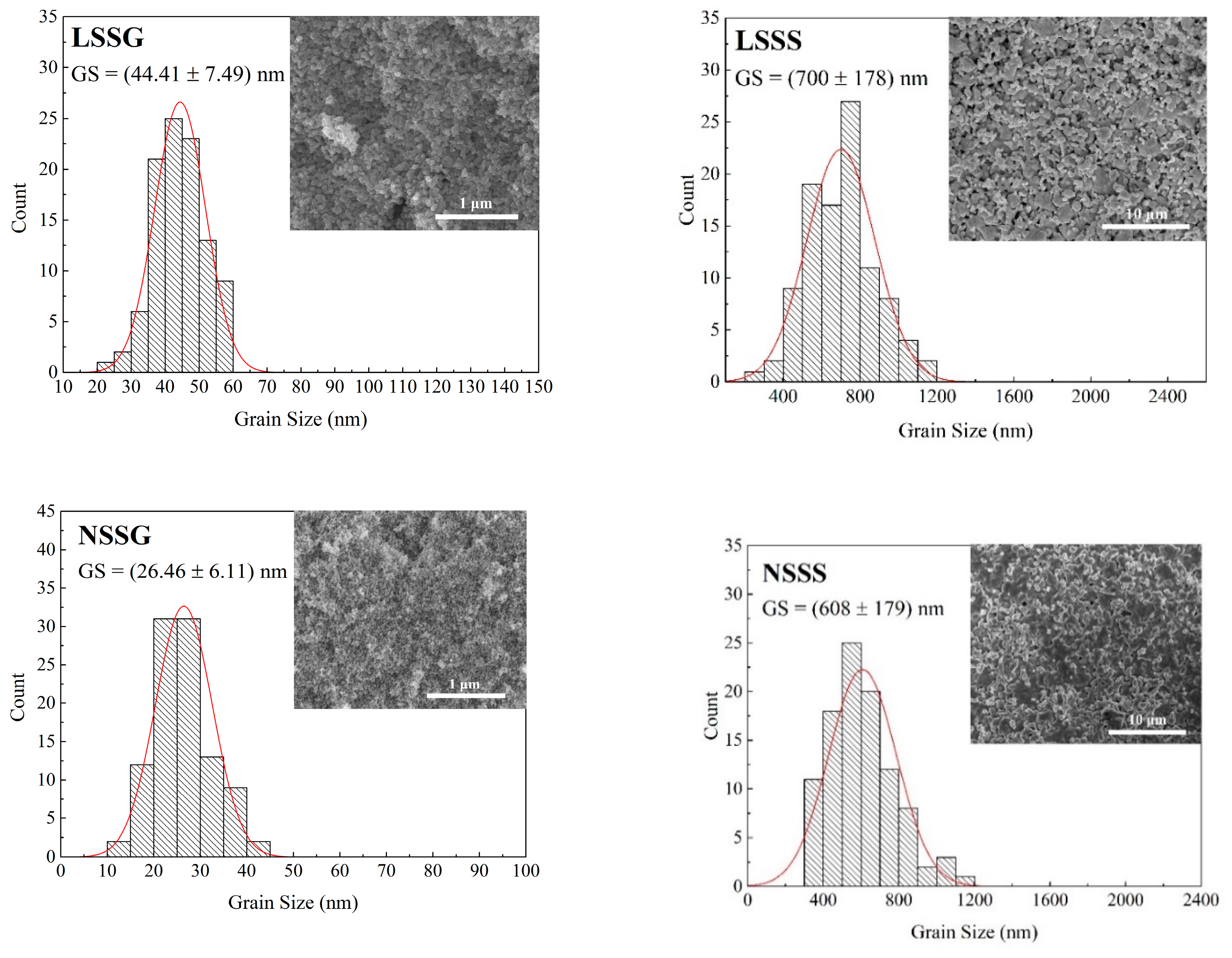
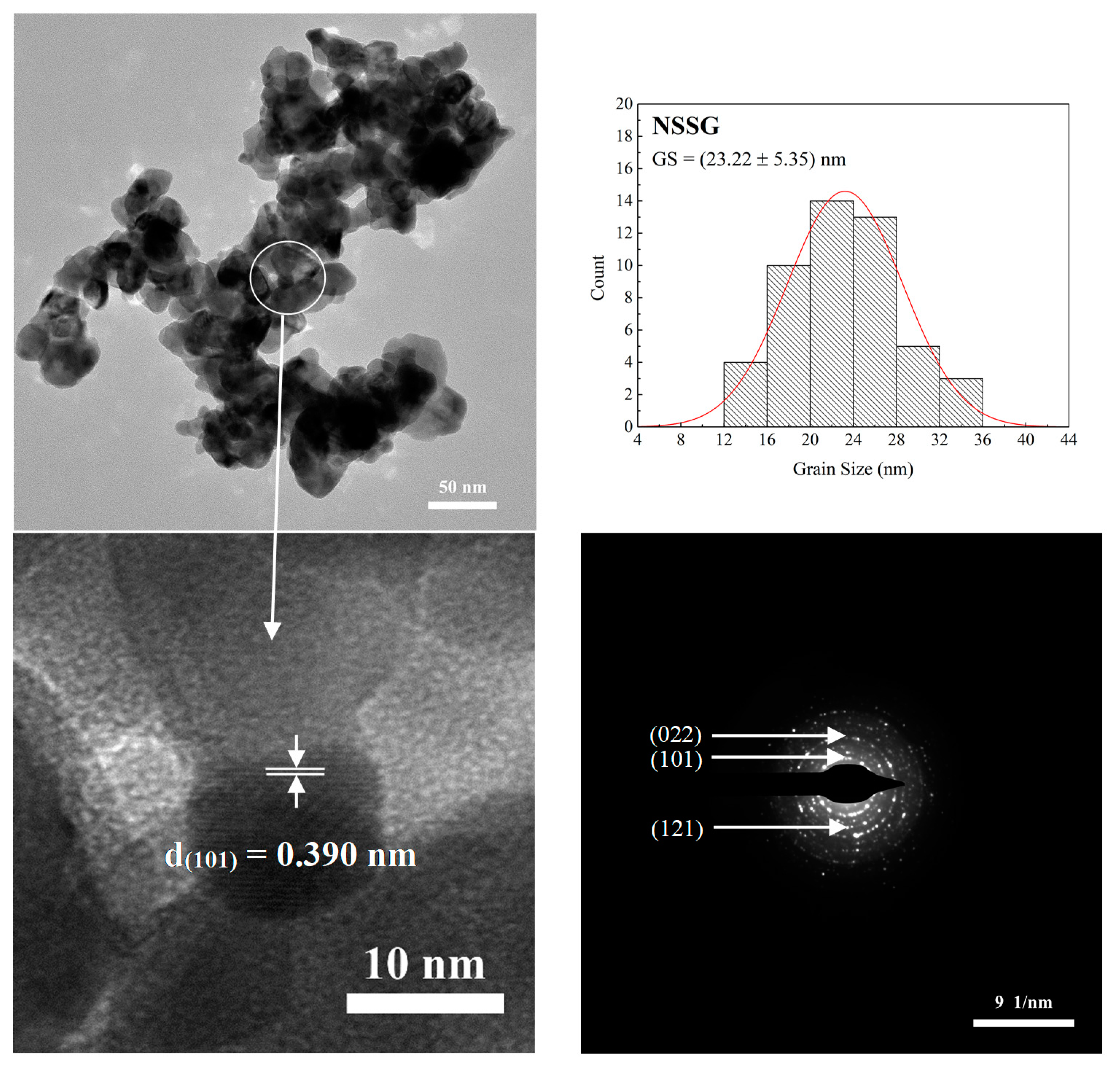
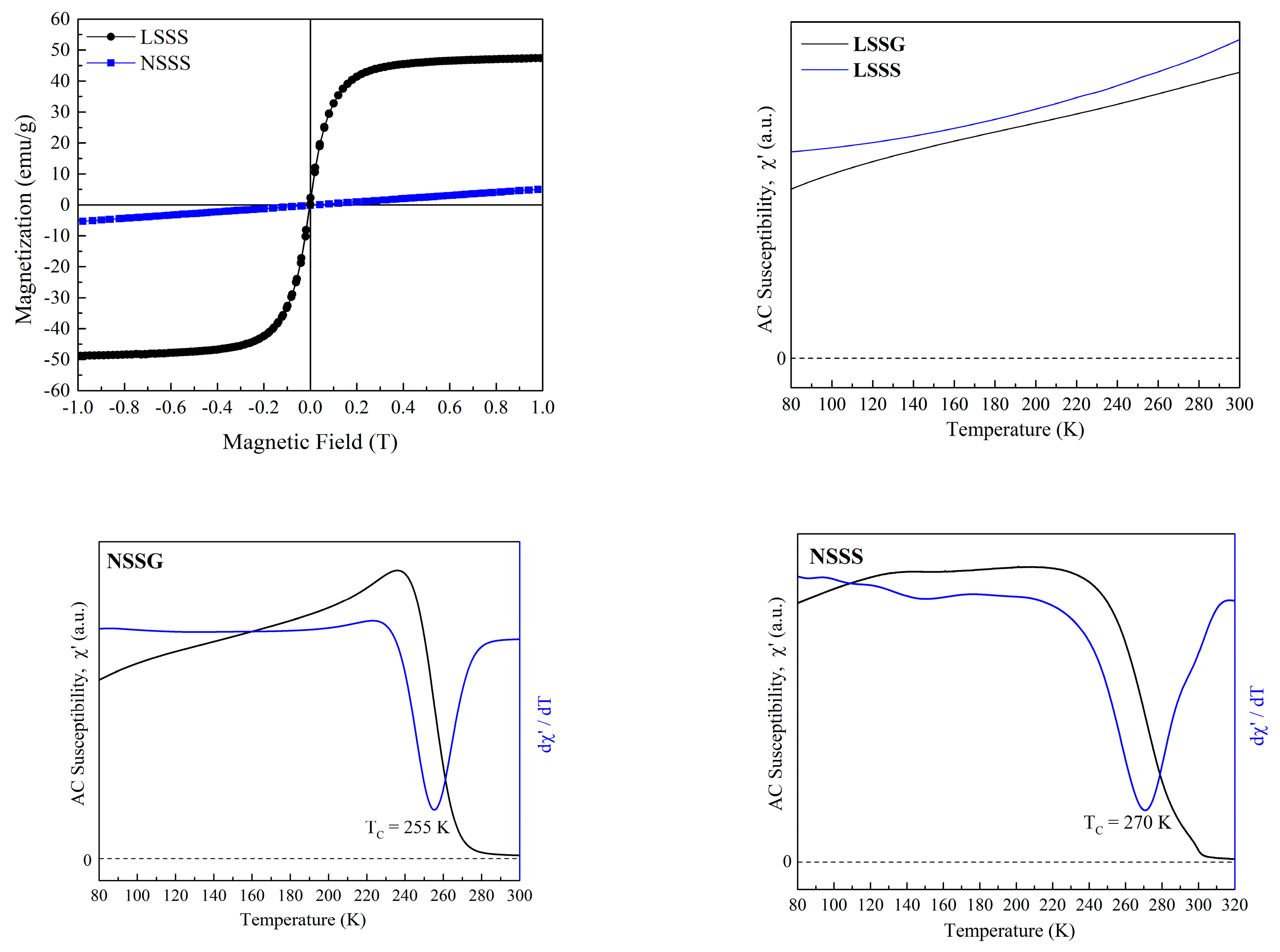


| Sample Code | LSSG | LSSS | NSSG | NSSS |
|---|---|---|---|---|
| Sample | La0.7Sr0.3MnO3 | Nd0.7Sr0.3MnO3 | ||
| ICSD Reference Code | 98-004-9739 | 98-003-7493 | ||
| Crystal Structure | Hexagonal | Orthorhombic | ||
| Space Group | R-3c (167) | Pnma (62) | ||
| Tolerance Factor, tG | 0.930 | 0.917 | ||
| Lattice Parameter | - | |||
| a (Å) | 5.486 | 5.505 | 5.437 | 5.450 |
| b (Å) | 5.486 | 5.505 | 7.670 | 7.700 |
| c (Å) | 13.381 | 13.366 | 5.480 | 5.466 |
| Volume (Å3) | 348.704 | 350.757 | 228.516 | 229.398 |
| Bond Angle and Length | - | - | - | - |
| ∠Mn-O1-Mn (°) | 166.496 | 161.681 | 159.880 | 165.804 |
| ∠Mn-O2-Mn (°) | - | - | 158.591 | 160.057 |
| Mn-O1 (Å) | 1.950 | 1.860 | 1.967 | 1.943 |
| Mn-O2 (Å) | - | - | 1.951 | 1.955 |
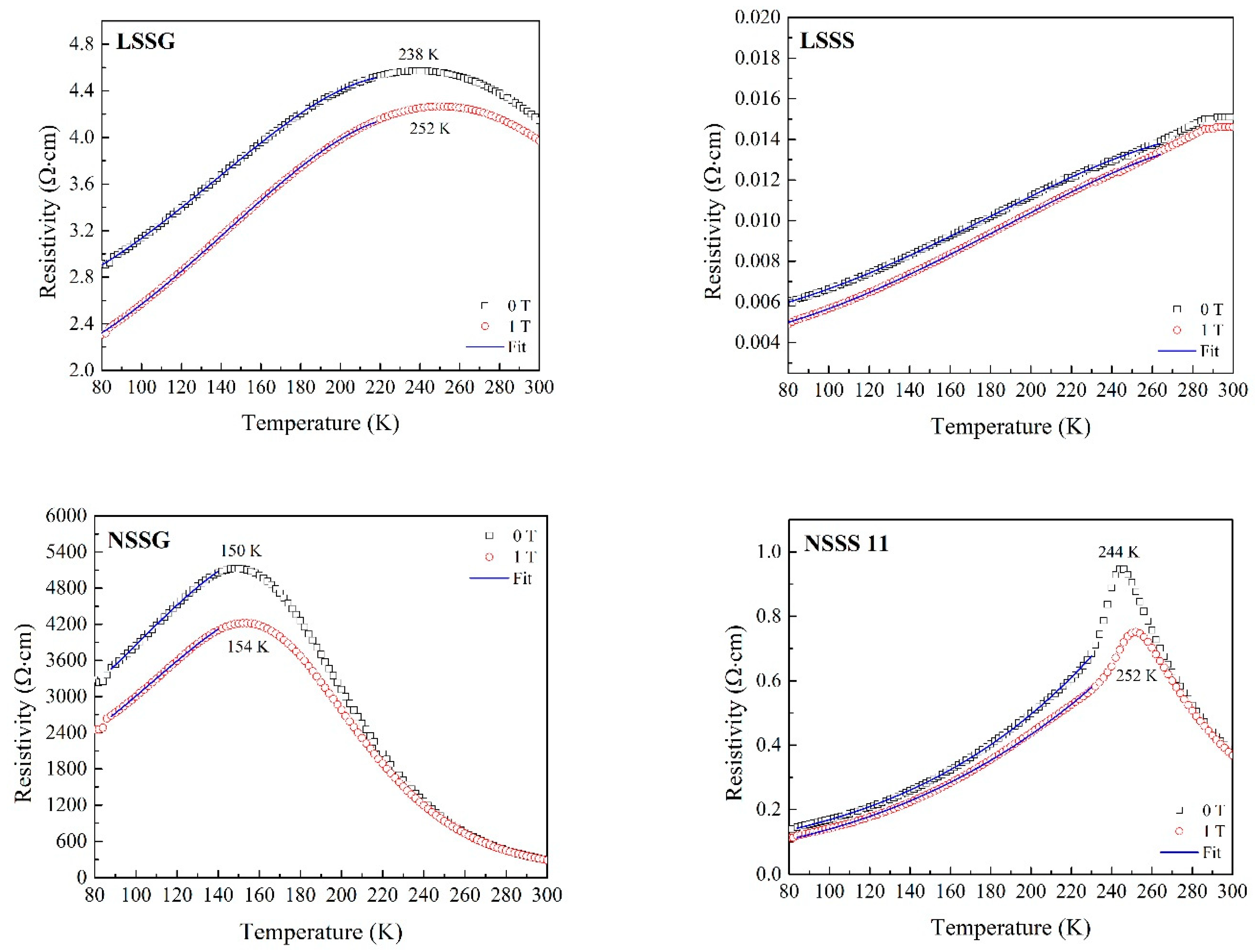
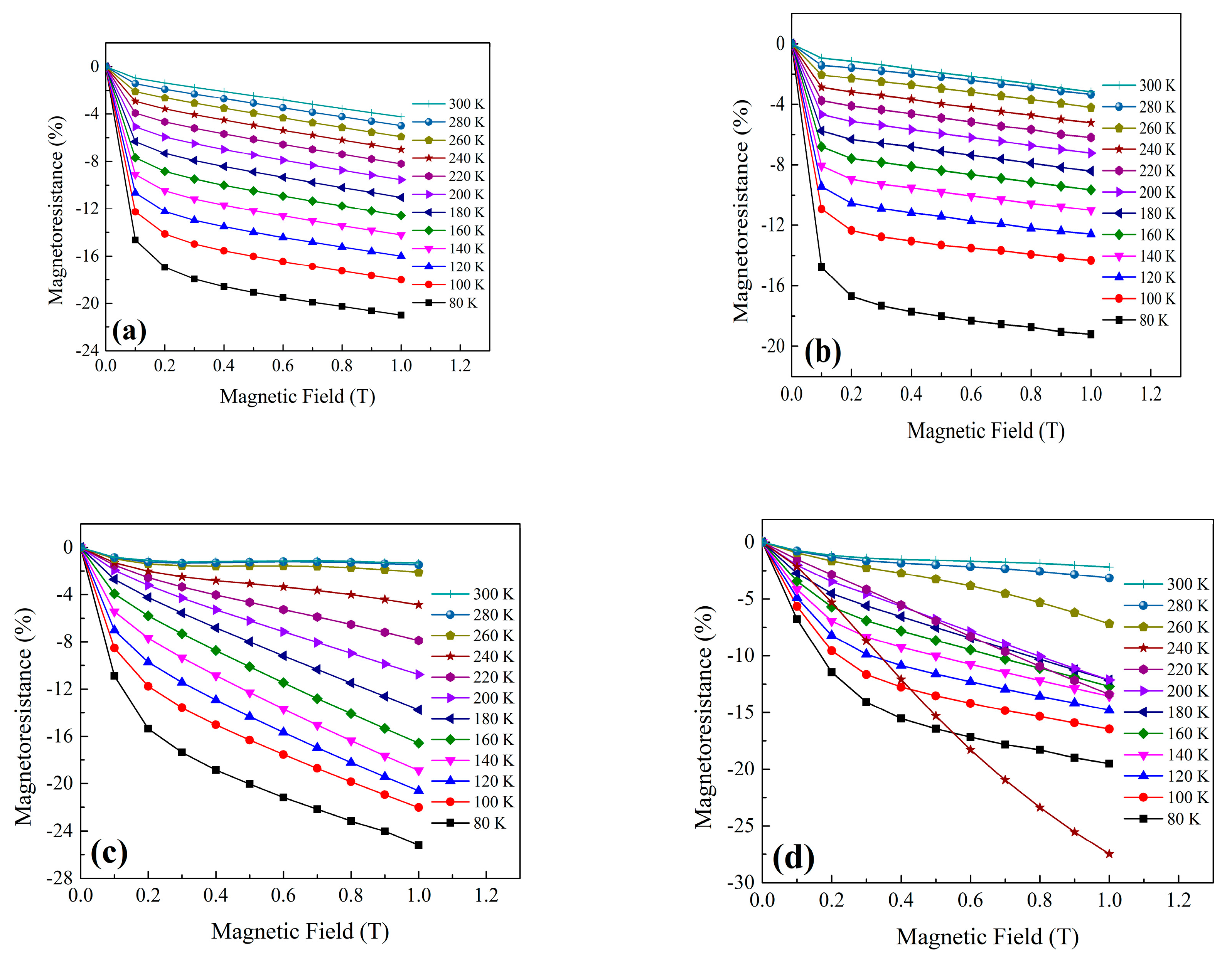
| Sample | FWHM (°) | Average Crystallite Size D (nm) | Average Grain Size GS (nm) | Curie Temperature, TC (K) | Metal–Insulator Temperature, TMI (K) | MR at 1 T (%) | |
|---|---|---|---|---|---|---|---|
| 80 K | TMI | ||||||
| LSSG | 0.417 | 22.6 | 44.4 | >300 | 238 | 21.0 | 7.1 |
| LSSS | 0.115 | 127.4 | 699.9 | >300 | >300 | 19.2 | − |
| NSSG | 0.434 | 21.6 | 26.5 | 255 | 150 | 25.2 | 17.8 |
| NSSS | 0.163 | 73.3 | 607.5 | 270 | 244 | 19.5 | 27.7 |
| Sample | H (T) | ρο (Ω cm) | ρ2 (Ω cm K−2) | ρ4.5 (Ω cm K−4.5) | R2 |
|---|---|---|---|---|---|
| LSSG | 0 | 2.463 | 7.12 × 10−5 | −4.00 × 10−1 | 0.999 |
| 1 | 1.854 | 7.53× 10−5 | −3.89 × 10−1 | 0.999 | |
| LSSS | 0 | 4.79 × 10−3 | 1.91 × 10−7 | −5.47 × 10−1 | 0.999 |
| 1 | 3.77 × 10−3 | 1.95 × 10−7 | −5.21 × 10−1 | 0.999 | |
| NSSG | 0 | 1819 | 0.232 | −2.85 × 10−7 | 0.999 |
| 1 | 1293 | 0.192 | −2.08 × 10−7 | 0.999 | |
| NSSS | 0 | 8.30 × 10−2 | 8.18 × 10−6 | 3.79 × 10−12 | 0.999 |
| 1 | 5.33 × 10−2 | 8.43 × 10−6 | 1.89 × 10−12 | 0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, L.N.; Lim, K.P.; Ishak, A.N.; Awang Kechik, M.M.; Chen, S.K.; Ibrahim, N.B.; Miryala, M.; Murakami, M.; Shaari, A.H. The Physical Properties of Submicron and Nano-Grained La0.7Sr0.3MnO3 and Nd0.7Sr0.3MnO3 Synthesised by Sol–Gel and Solid-State Reaction Methods. Coatings 2021, 11, 361. https://doi.org/10.3390/coatings11030361
Lau LN, Lim KP, Ishak AN, Awang Kechik MM, Chen SK, Ibrahim NB, Miryala M, Murakami M, Shaari AH. The Physical Properties of Submicron and Nano-Grained La0.7Sr0.3MnO3 and Nd0.7Sr0.3MnO3 Synthesised by Sol–Gel and Solid-State Reaction Methods. Coatings. 2021; 11(3):361. https://doi.org/10.3390/coatings11030361
Chicago/Turabian StyleLau, Lik Nguong, Kean Pah Lim, Amirah Natasha Ishak, Mohd Mustafa Awang Kechik, Soo Kien Chen, Noor Baa’yah Ibrahim, Muralidhar Miryala, Masato Murakami, and Abdul Halim Shaari. 2021. "The Physical Properties of Submicron and Nano-Grained La0.7Sr0.3MnO3 and Nd0.7Sr0.3MnO3 Synthesised by Sol–Gel and Solid-State Reaction Methods" Coatings 11, no. 3: 361. https://doi.org/10.3390/coatings11030361
APA StyleLau, L. N., Lim, K. P., Ishak, A. N., Awang Kechik, M. M., Chen, S. K., Ibrahim, N. B., Miryala, M., Murakami, M., & Shaari, A. H. (2021). The Physical Properties of Submicron and Nano-Grained La0.7Sr0.3MnO3 and Nd0.7Sr0.3MnO3 Synthesised by Sol–Gel and Solid-State Reaction Methods. Coatings, 11(3), 361. https://doi.org/10.3390/coatings11030361






