Sensitivity of Corrosion Behavior for Fe-Based Amorphous Coating to Temperature and Chloride Concentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating Preparation
2.2. Electrochemical Tests
2.3. Microstructure Characterization
2.4. XPS Characterization
3. Results
3.1. Characterization of the AMCs
3.2. Anodic Polarization Behavior
3.3. Potentiostatic Polarization Tests
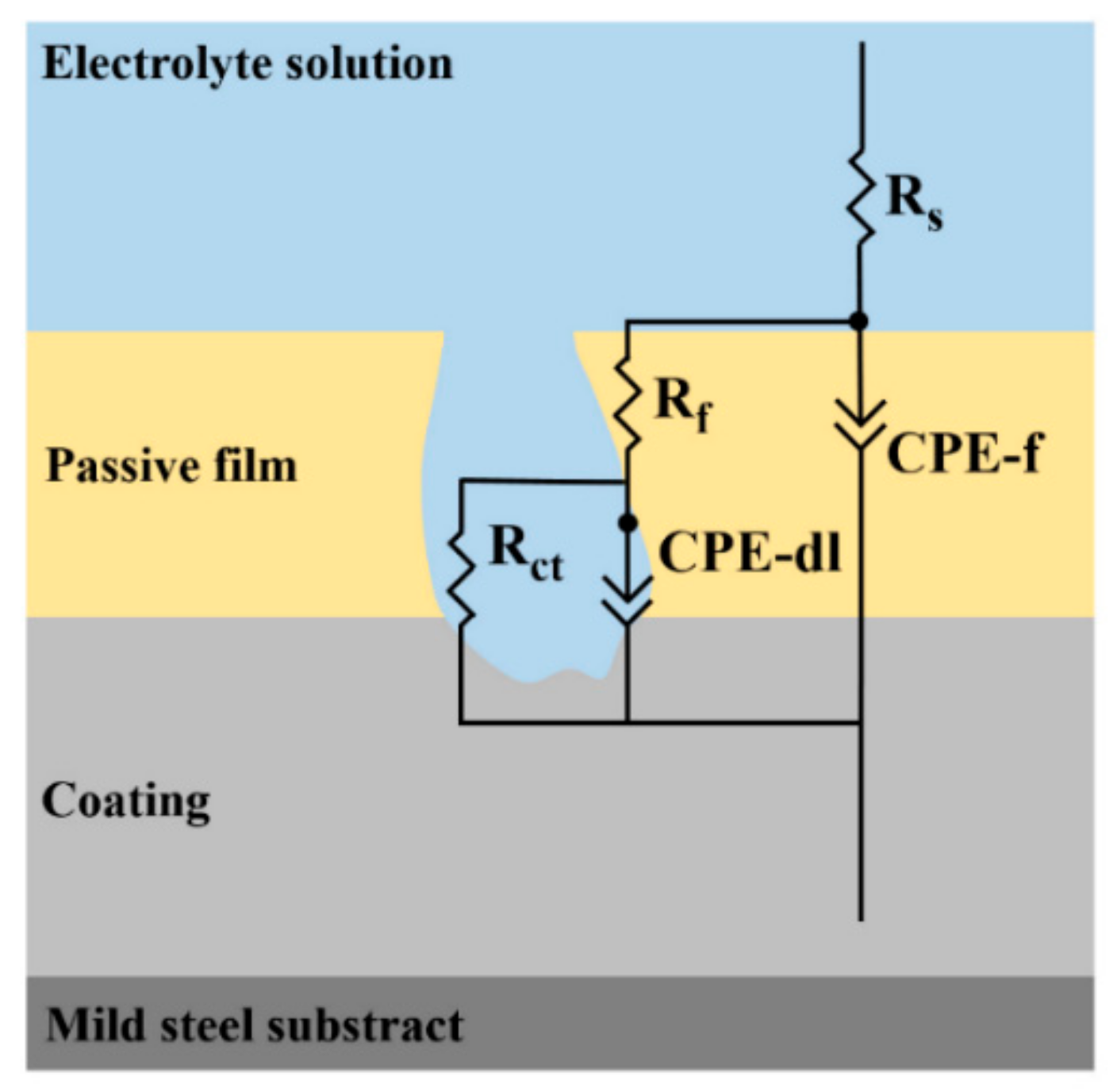
3.4. Electrochemical Impedance Analysis
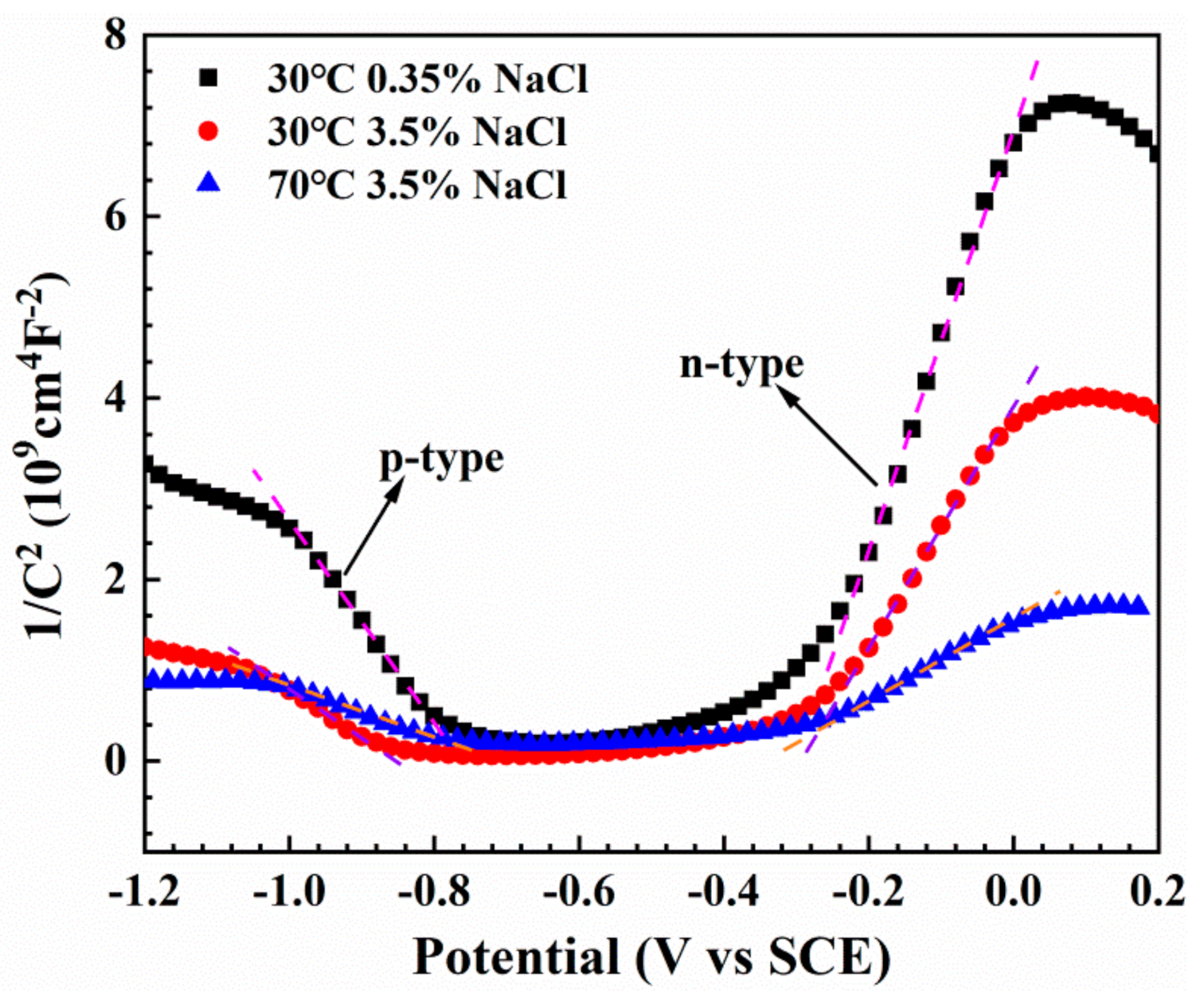
3.5. Mott–Schottky Results
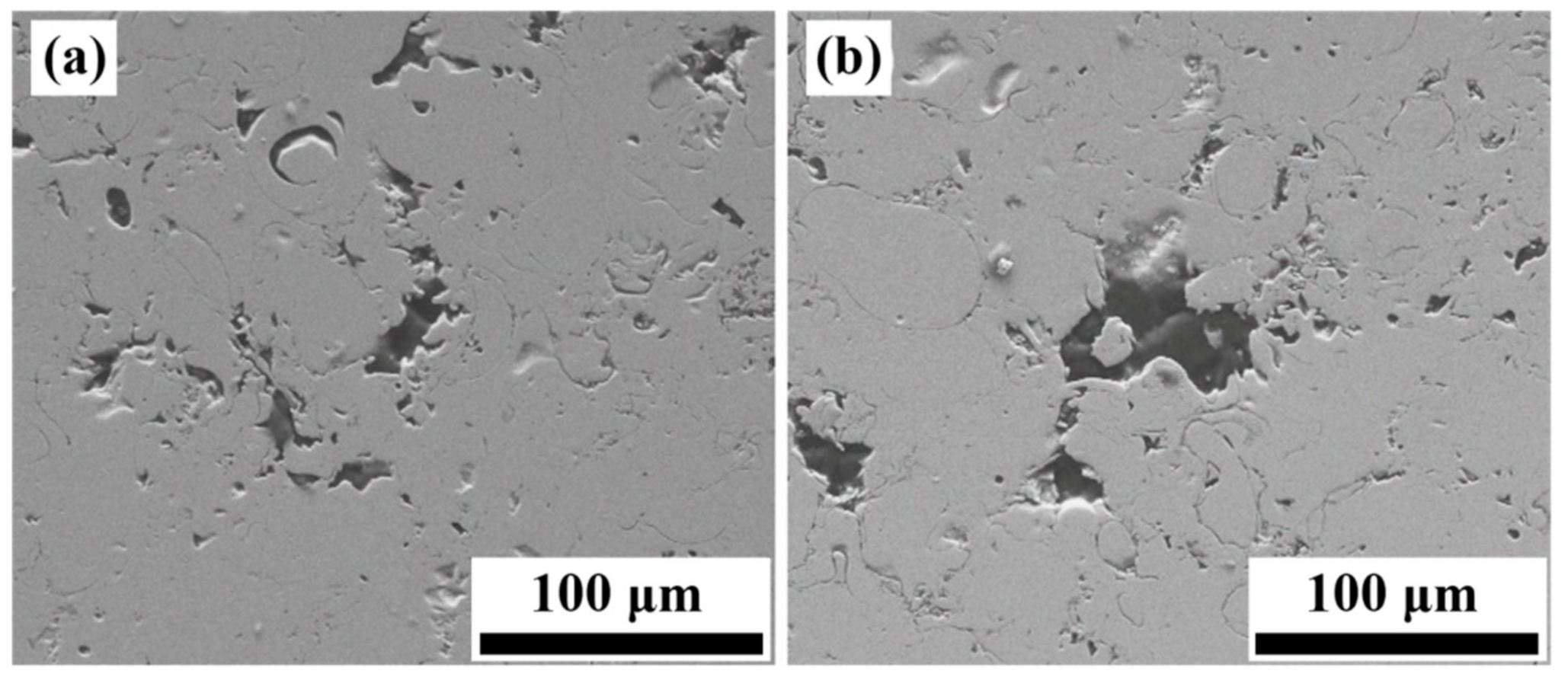
3.6. Corrosion Morphologies of Fe-Based AMCs
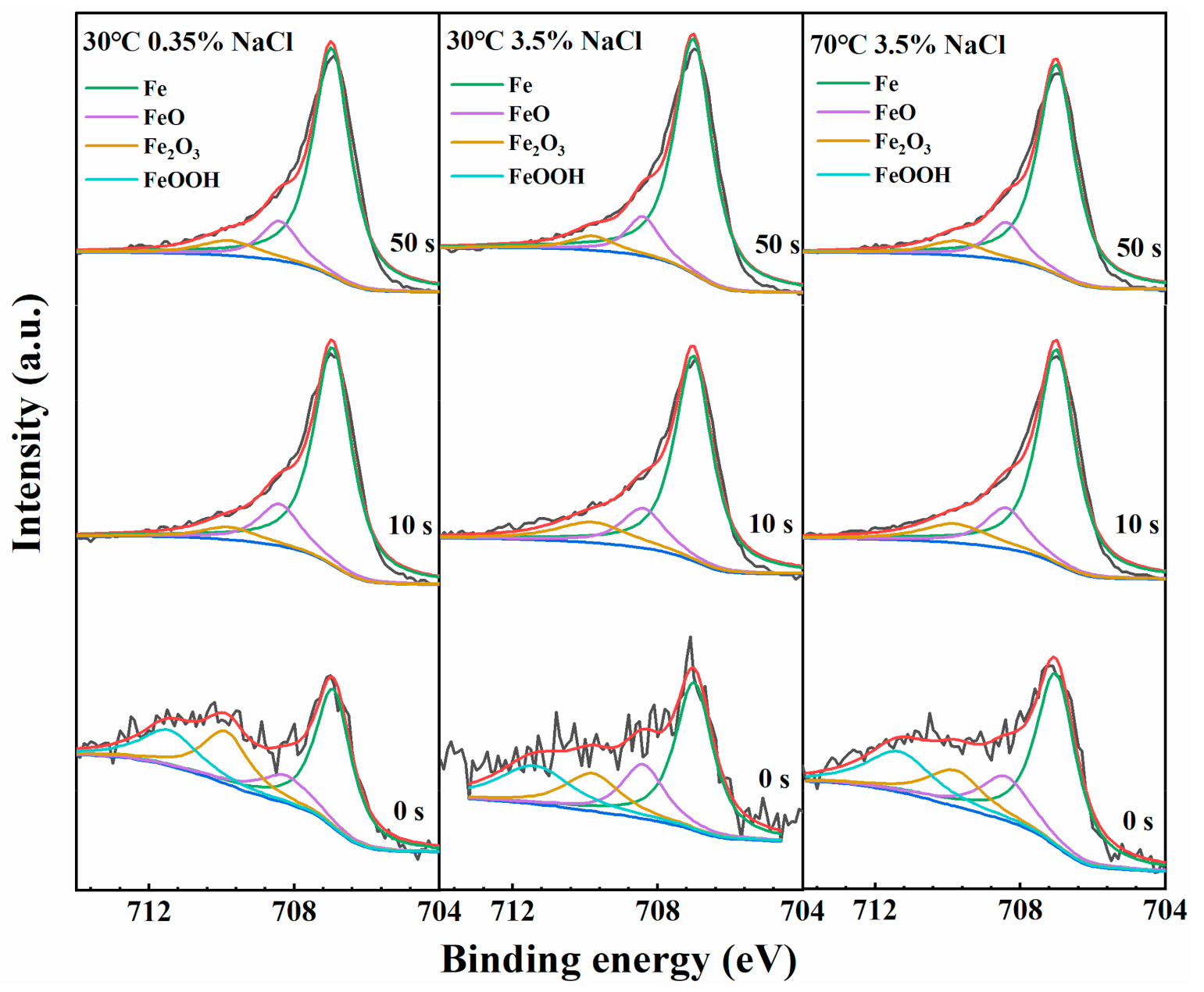
3.7. XPS Analysis
4. Discussion
4.1. Effects of Concentration of Chloride Ion and Solution Temperature on the Passive Behavior
4.2. The Evolution of Band Structure of the Passive Film
5. Conclusions
- The fabricated coating was basically amorphous state and had a homogeneous microstructure. As temperature and solution concentration increased, the passive current for the Fe-based AMCs increased and the pitting potential decreased obviously. The increase of temperature and solution concentration decreased the passivation index and the polarization resistance and deteriorated the protective performance of the passive film, which contributed to the decrease of induction time (tm) for the growth of corrosion pit.
- In the potential ranges below and above the EFB, the passive film formed on the Fe-based AMCs exhibited a p-type and n-type semiconductor properties, respectively. The declining corrosion resistance for the Fe-based AMCs in relatively higher temperature and higher concentration NaCl solution was derived from the reduced ratio of Cr2O3, the increase of carrier densities and the decrease of WF for the passive film formed in this condition.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farmer, J.C.; Choi, J.S.; Saw, C.; Haslam, J.; Day, D.; Hailey, P.; Lian, T.; Rebak, R.; Perepezko, J.; Payer, J.; et al. Iron-Based Amorphous-Metals: High-Performance Corrosion-Resistant Material (HPCRM) Development. Metall. Mater. Trans. A 2009, 40, 1289–1305. [Google Scholar] [CrossRef]
- Bolelli, G.; Bonferroni, B.; Laurila, J.; Lusvarghi, L.; Milanti, A.; Niemi, K.; Vuoristo, P. Micromechanical properties and sliding wear behaviour of HVOF-sprayed Fe-based alloy coatings. Wear 2012, 276–277, 29–47. [Google Scholar] [CrossRef]
- Otsubo, F.; Era, H.; Kishitake, K. Formation of amorphous Fe-Cr-Mo-8P-2C coatings by the high velocity oxy-fuel process. J. Therm. Spray Technol. 2000, 9, 494–498. [Google Scholar] [CrossRef]
- Kobayashi, A.; Yano, S.; Kimura, H.; Inoue, A. Mechanical property of Fe-base metallic glass coating formed by gas tunnel type plasma spraying. Surf. Coat. Technol. 2008, 202, 2513–2518. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Y.; Hou, G.; An, Y.; Liu, G. The effect of high-velocity oxy-fuel spraying parameters on microstructure, corrosion and wear resistance of Fe-based metallic glass coatings. J. Non·Cryst. Solids 2014, 406, 37–44. [Google Scholar] [CrossRef]
- Sadeghimeresht, E.E.; Markocsan, N.; Nylén, P. Microstructural characteristics and corrosion behavior of HVAF- and HVOF-sprayed Fe-based coatings. Surf. Coat. Technol. 2017, 318, 365–373. [Google Scholar] [CrossRef]
- Wu, N.C.; Chen, K.; Sun, W.H.; Wang, J.Q. Correlation between particle size and porosity of Fe-based amorphous coating. Surf. Eng. 2018, 1–9. [Google Scholar] [CrossRef]
- Zhang, C.; Chan, K.C.; Wu, Y.; Liu, L. Pitting initiation in Fe-based amorphous coatings. Acta Mater. 2012, 60, 4152–4159. [Google Scholar] [CrossRef]
- Zhang, S.D.; Wu, J.; Qi, W.B.; Wang, J.Q. Effect of porosity defects on the long-term corrosion behaviour of Fe-based amorphous alloy coated mild steel. Corros. Sci. 2016, 110, 57–70. [Google Scholar] [CrossRef]
- Zhang, S.D.; Zhang, W.L.; Wang, S.G.; Gu, X.J.; Wang, J.Q. Characterisation of three-dimensional porosity in an Fe-based amorphous coating and its correlation with corrosion behaviour. Corros. Sci. 2015, 93, 211–221. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; He, D.Y.; Wang, F.C.; Liu, Y.B. Microstructure and Wear Resistance of Fe-Based Amorphous Metallic Coatings Prepared by HVOF Thermal Spraying. J. Therm. Spray Technol. 2010, 19, 1287–1293. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Wang, F.C.; Zhang, H.F.; Liu, Y.B.; Xu, S.H. Formation and corrosion behavior of Fe-based amorphous metallic coatings by HVOF thermal spraying. Surf. Coat. Technol. 2009, 204, 563–570. [Google Scholar] [CrossRef]
- Koga, G.Y.; Nogueira, R.P.; Roche, V.; Yavari, A.R.; Melle, A.K.; Gallego, J.; Bolfarini, C.; Kiminami, C.S.; Botta, W.J. Corrosion properties of Fe–Cr–Nb–B amorphous alloys and coatings. Surf. Coat. Technol. 2014, 254, 238–243. [Google Scholar] [CrossRef]
- Zois, D.; Lekatou, A.; Vardavoulias, M. Preparation and characterization of highly amorphous HVOF stainless steel coatings. J. Alloys Compd. 2010, 504, S283–S287. [Google Scholar] [CrossRef]
- Li, D.; Chen, X.; Hui, X.; Wang, J.; Jin, P.; Li, H. Effect of amorphicity of HVOF sprayed Fe-based coatings on their corrosion performances and contacting osteoblast behavior. Surf. Coat. Technol. 2017, 310, 207–213. [Google Scholar] [CrossRef]
- Katakam, S.; Santhanakrishnan, S.; Dahotre, N. Fe-Based Amorphous Coatings on AISI 4130 Structural Steel for Corrosion Resistance. JOM 2012, 64, 709–715. [Google Scholar] [CrossRef]
- Wu, J.; Cui, J.P.; Zheng, Q.J.; Zhang, S.D.; Sun, W.H.; Yang, B.J.; Wang, J.Q. Insight into the corrosion evolution of Fe-based amorphous coatings under wet-dry cyclic conditions. Electrochim. Acta 2019, 319, 966–980. [Google Scholar] [CrossRef]
- Kim, J.-D.; Pyun, S.-I. Effects of electrolyte composition and applied potential on the repassivation kinetics of pure aluminium. Electrochim. Acta 1995, 40, 1863–1869. [Google Scholar] [CrossRef]
- Pyun, S.-I.; Lee, E.-J. Effect of halide ion and applied potential on repassivation behaviour of Al-1 wt.%Si-0.5 wt.%Cu alloy. Electrochim. Acta 1995, 40, 1963–1970. [Google Scholar] [CrossRef]
- Park, J.-J.; Pyun, S.-I.; Lee, W.-J.; Kim, H.-P. Effect of Bicarbonate Ion Additives on Pitting Corrosion of Type 316L Stainless Steel in Aqueous 0.5 M Sodium Chloride Solution. Corrosion 1999, 55, 380–387. [Google Scholar] [CrossRef]
- Qiao, Y.X.; Zheng, Y.G.; Ke, W.; Okafor, P.C. Electrochemical behaviour of high nitrogen stainless steel in acidic solutions. Corros. Sci. 2009, 51, 979–986. [Google Scholar] [CrossRef]
- Lee, J.-B. Effects of alloying elements, Cr, Mo and N on repassivation characteristics of stainless steels using the abrading electrode technique. Mater. Chem. Phys. 2006, 99, 224–234. [Google Scholar] [CrossRef]
- Galvele, J.R.; Torresi, R.M.; Carranza, R.M. Passivity breakdown, its relation to pitting and stress-corrosion-cracking processes. Corros. Sci. 1990, 31, 563–571. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Pitting corrosion of aluminum. Corros. Sci. 1999, 41, 1743–1767. [Google Scholar] [CrossRef]
- Zhao, X.; Zuo, Y.; Zhao, J.; Xiong, J.; Tang, Y. A study on the self-sealing process of anodic films on aluminum by EIS. Surf. Coat. Technol. 2006, 200, 6846–6853. [Google Scholar] [CrossRef]
- Hakiki, N.E.; Belo, M.D.; Simões, A.M.P.; Ferreira, M.G.S. Semiconducting Properties of Passive Films Formed on Stainless Steels: Influence of the Alloying Elements. J. Electrochem. Soc. 1998, 145, 3821–3829. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Fujimoto, S.; Chihara, O.; Shibata, T. Semiconductive behavior of passive films formed on pure Cr and Fe–Cr alloys in sulfuric acid solution. Electrochim. Acta 2002, 47, 4357–4366. [Google Scholar] [CrossRef]
- Fujimoto, S.; Tsuchiya, H. Semiconductor properties and protective role of passive films of iron base alloys. Corros. Sci. 2007, 49, 195–202. [Google Scholar] [CrossRef]
- de Gryse, R.; Gomes, W.P.; Cardon, F.; Vennik, J. On the Interpretation of Mott-Schottky Plots Determined at Semiconductor/Electrolyte Systems. J. Electrochem. Soc. 1975, 122, 711–712. [Google Scholar] [CrossRef]
- Simões, A.M.P.; Ferreira, M.G.S.; Rondot, B.; Belo, M.D. Study of Passive Films Formed on AISI 304 Stainless Steel by Impedance Measurements and Photoelectrochemistry. J. Electrochem. Soc. 1990, 137, 82–87. [Google Scholar] [CrossRef]
- Marcus, P.; Maurice, V.; Strehblow, H.H. Localized corrosion (pitting): A model of passivity breakdown including the role of the oxide layer nanostructure. Corros. Sci. 2008, 50, 2698–2704. [Google Scholar] [CrossRef]
- Fernández-Domene, R.M.; Blasco-Tamarit, E.; García-García, D.M.; Antón, J.G. Passivity Breakdown of Titanium in LiBr Solutions. J. Electrochem. Soc. 2013, 161, C25–C35. [Google Scholar] [CrossRef]
- Wang, Z.M.; Ma, Y.T.; Zhang, J.; Hou, W.L.; Chang, X.C.; Wang, J.Q. Influence of yttrium as a minority alloying element on the corrosion behavior in Fe-based bulk metallic glasses. Electrochim. Acta 2008, 54, 261–269. [Google Scholar] [CrossRef]
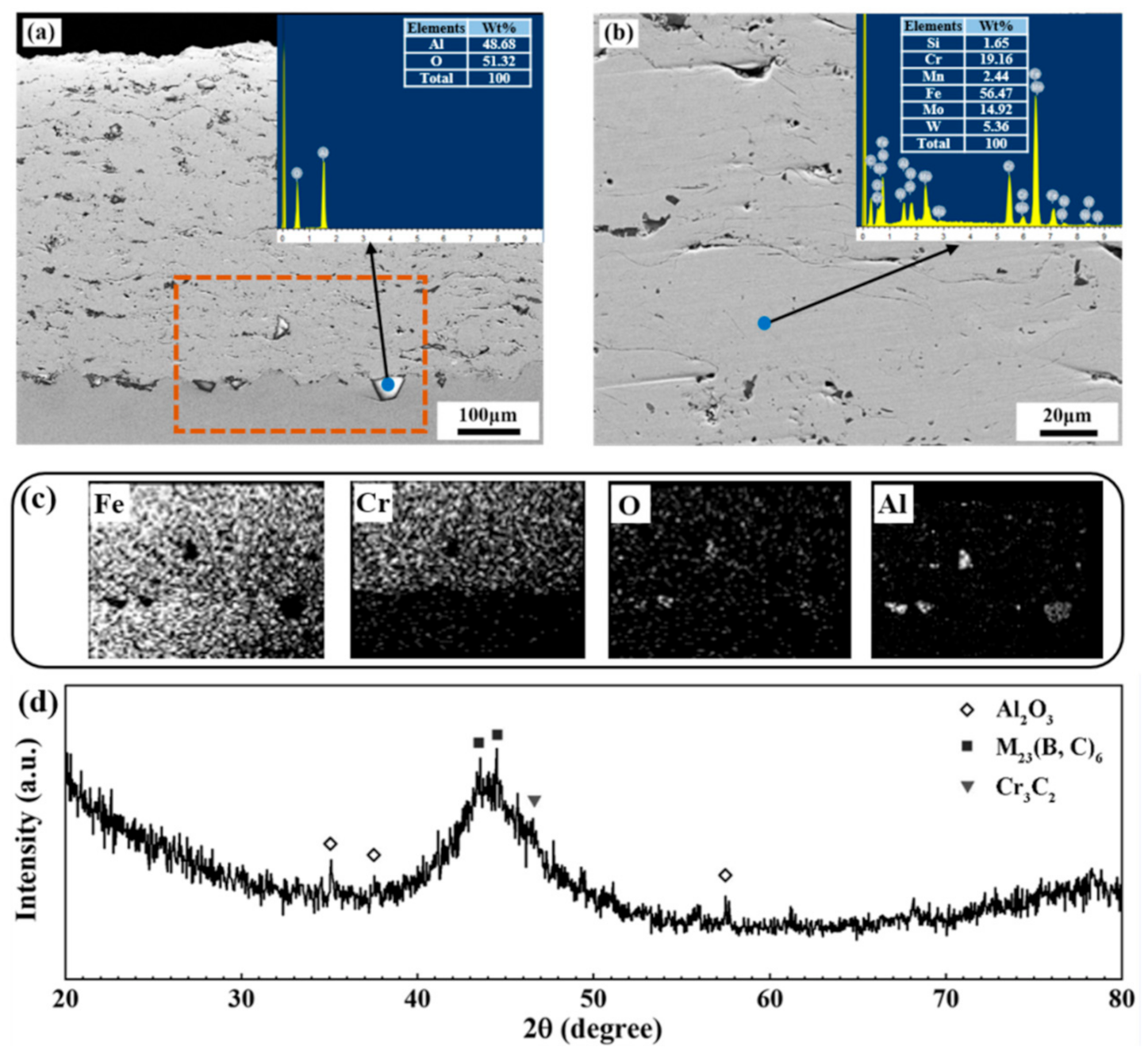
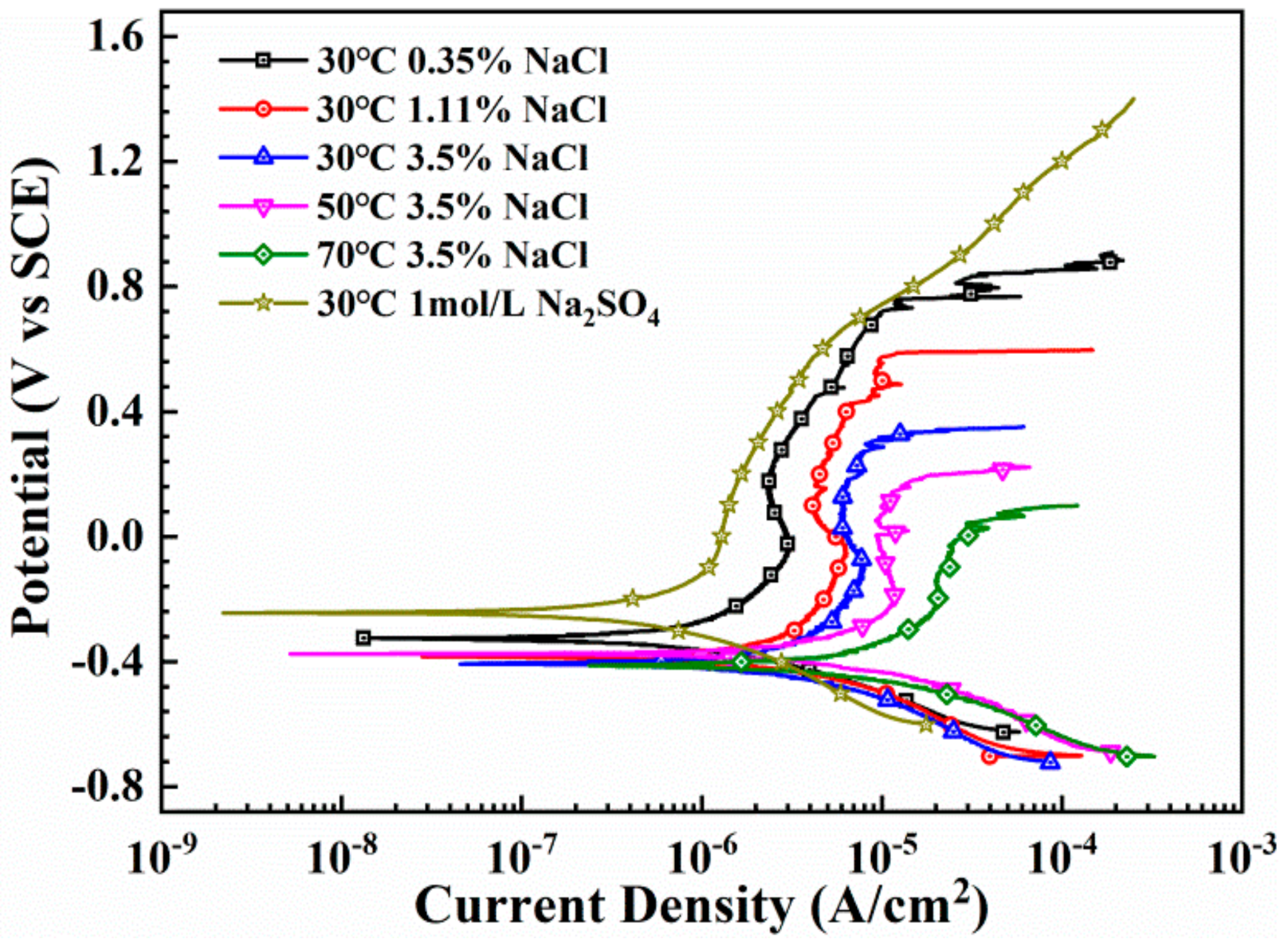
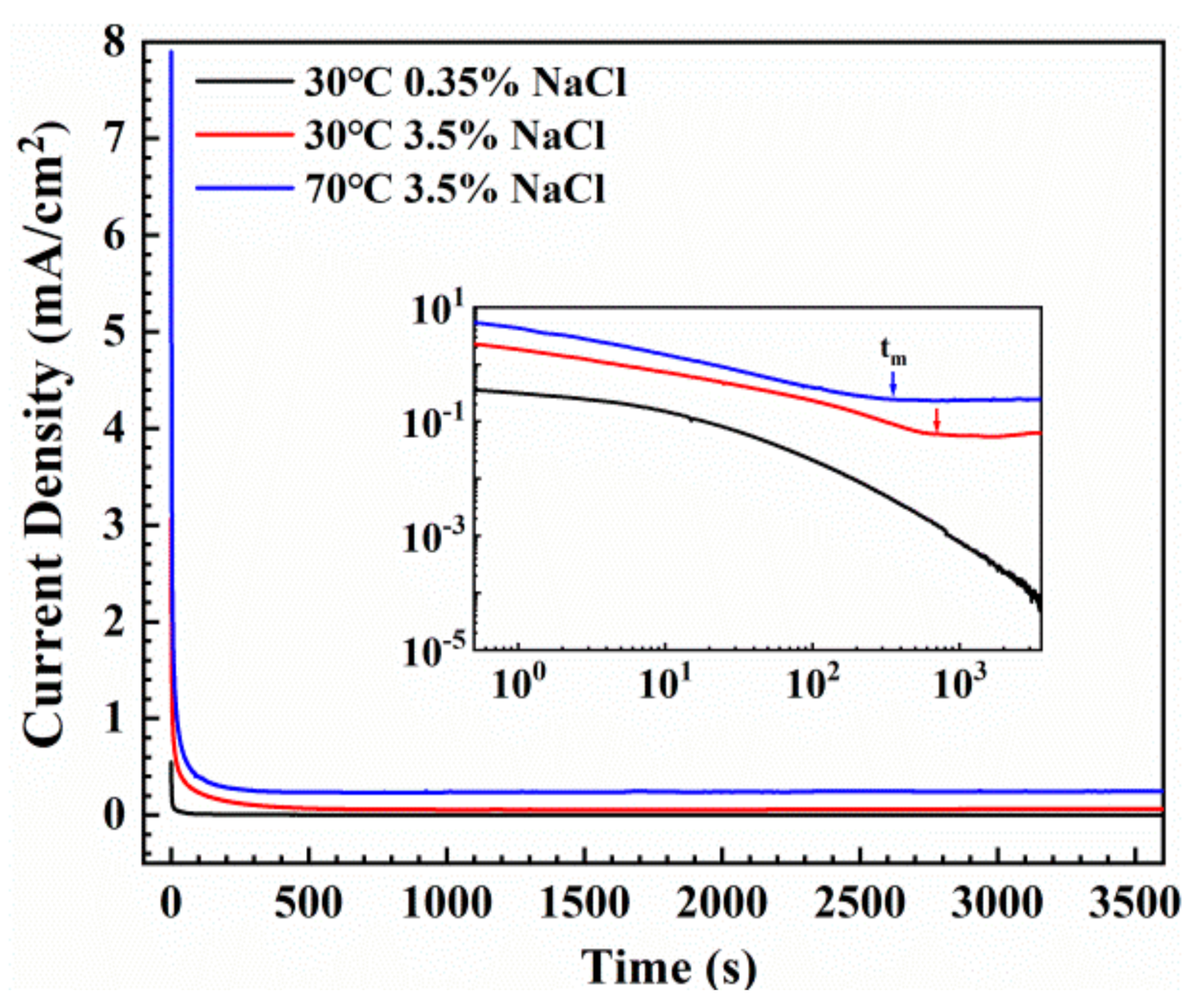
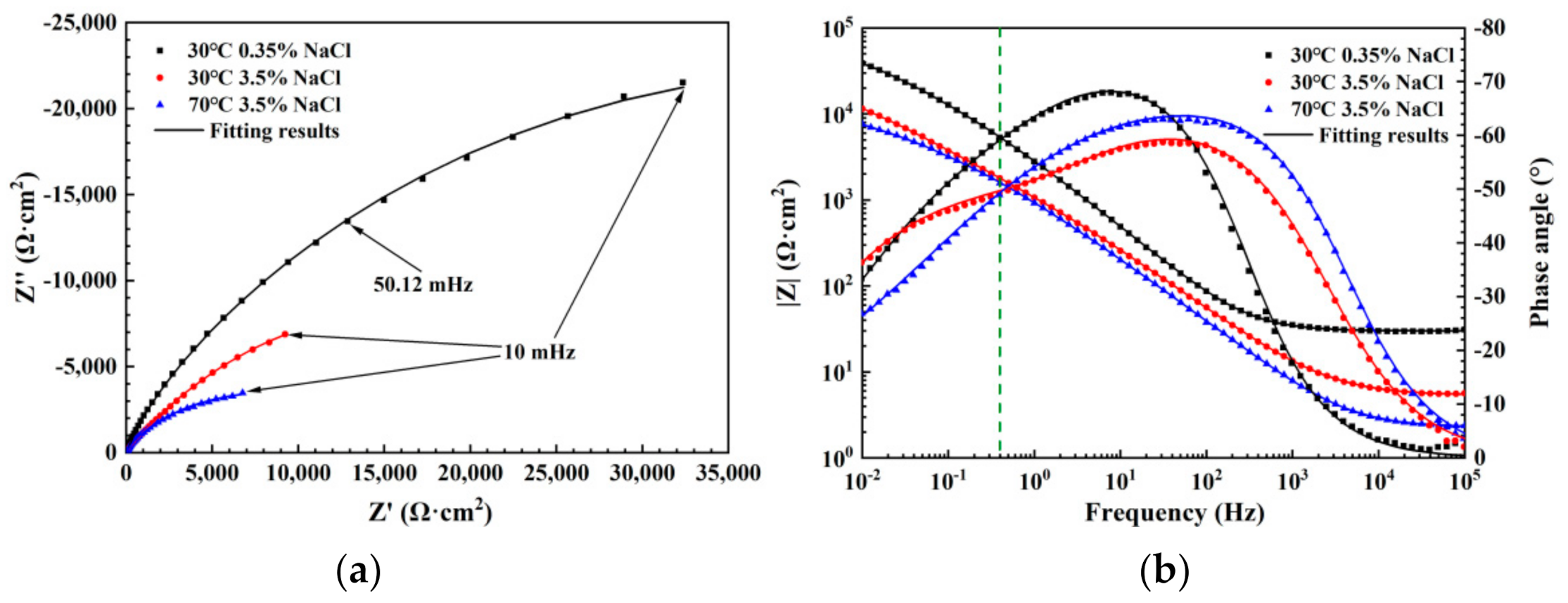



| Condition | iss (μA·cm−2) | n |
|---|---|---|
| 30 °C 0.35% NaCl | 1.23 ± 0.32 | 1.28 ± 0.07 |
| 30 °C 3.5% NaCl | 63.19 ± 7.46 | 0.83 ± 0.04 |
| 70 °C 3.5% NaCl | 247.63 ± 19.86 | 0.55 ± 0.02 |
| Solution Condition | Rs (Ω cm2) | CPE-f | Rf (Ω cm2) | CPE-dl | Rct (Ω cm2) | χ2 | ||
|---|---|---|---|---|---|---|---|---|
| Q (F/s(1−α) cm2) | α | Q (F/s(1−α) cm2) | α | |||||
| 30 °C 0.35% | 29.83 | 6.79 × 10−5 | 0.82 | 7866 | 5.86 × 10−5 | 0.50 | 82897 | 6.66 × 10−4 |
| 30 °C 3.5% | 5.30 | 1.84 × 10−4 | 0.72 | 1640 | 2.03 × 10−5 | 0.52 | 33557 | 1.48 × 10−4 |
| 70 °C 3.5% | 2.27 | 1.79 × 10−4 | 0.77 | 59.87 | 1.99 × 10−4 | 0.35 | 20663 | 1.09 × 10−4 |
| Conditions | NA (1020 cm−3) | ND (1020 cm−3) | EFB (V) |
|---|---|---|---|
| 30 °C 0.35% NaCl | 7.64 ± 0.09 | 3.56 ± 0.03 | −0.311 ± 0.001 |
| 30 °C 3.5% NaCl | 17.47 ± 0.67 | 7.01 ± 0.13 | −0.326 ± 0.002 |
| 70 °C 3.5% NaCl | 28.62 ± 0.53 | 19.45 ± 0.17 | −0.358 ± 0.004 |
| Solutions | Sputting Time | Cr2O3 | Cr(OH)3 | FeO | Fe2O3 | FeOOH |
|---|---|---|---|---|---|---|
| T = 30 °C 0.35% NaCl | 0 s | 0.32 | 0.27 | 0.10 | 0.17 | 0.14 |
| 10 s | 0.23 | 0.05 | 0.52 | 0.20 | 0 | |
| 20 s | 0.19 | 0 | 0.52 | 0.29 | 0 | |
| 50 s | 0.17 | 0 | 0.53 | 0.30 | 0 | |
| T = 30 °C 3.5% NaCl | 0 s | 0.41 | 0.32 | 0.09 | 0.08 | 0.10 |
| 10 s | 0.31 | 0.22 | 0.25 | 0.22 | 0 | |
| 20 s | 0.19 | 0.06 | 0.47 | 0.28 | 0 | |
| 50 s | 0.22 | 0 | 0.52 | 0.26 | 0 | |
| T = 70 °C 3.5% NaCl | 0 s | 0.34 | 0.38 | 0.09 | 0.08 | 0.11 |
| 10 s | 0.19 | 0.12 | 0.39 | 0.30 | 0 | |
| 20 s | 0.15 | 0.07 | 0.54 | 0.24 | 0 | |
| 50 s | 0.18 | 0 | 0.52 | 0.30 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, W.; Wang, D.; Wang, Q.; Yang, F.; Li, T.; Shi, Y.; Zhang, S.; Yang, B. Sensitivity of Corrosion Behavior for Fe-Based Amorphous Coating to Temperature and Chloride Concentration. Coatings 2021, 11, 331. https://doi.org/10.3390/coatings11030331
Lu W, Wang D, Wang Q, Yang F, Li T, Shi Y, Zhang S, Yang B. Sensitivity of Corrosion Behavior for Fe-Based Amorphous Coating to Temperature and Chloride Concentration. Coatings. 2021; 11(3):331. https://doi.org/10.3390/coatings11030331
Chicago/Turabian StyleLu, Weiyan, Debin Wang, Qi Wang, Fan Yang, Tianrun Li, Yutong Shi, Suode Zhang, and Baijun Yang. 2021. "Sensitivity of Corrosion Behavior for Fe-Based Amorphous Coating to Temperature and Chloride Concentration" Coatings 11, no. 3: 331. https://doi.org/10.3390/coatings11030331
APA StyleLu, W., Wang, D., Wang, Q., Yang, F., Li, T., Shi, Y., Zhang, S., & Yang, B. (2021). Sensitivity of Corrosion Behavior for Fe-Based Amorphous Coating to Temperature and Chloride Concentration. Coatings, 11(3), 331. https://doi.org/10.3390/coatings11030331






