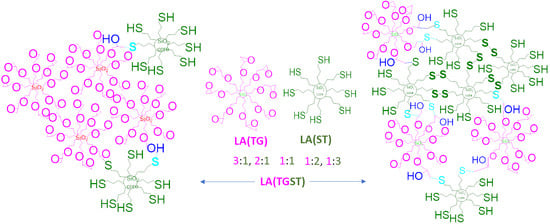
The low-alcohol epoxy silicate LA(TG) and thiol silicate LA(ST) colloids (
Scheme 1) interact with each other in two ways. First, formation of the siloxane (Si-O-Si) network by reaction of silanol groups on the surface of LA(TG) and LA(ST) colloids leads to an increase in particle size and aggregation. This directly results in an increase of the hydrophobic nature of the colloids. Second, there are the covalent interactions between LA(TG) and LA(ST) due to the simple nucleophilic epoxide ring opening by the thiol groups [
11], creating new C-S bonds and introducing new hydroxyl binding sites, thus increasing the crosslinking density. This reaction constitutes a polycondensation reaction and follows the kinetics of a step growth polymerization forming a network emerging from multifunctional epoxy and crosslinking thiol groups [
20]. The Si-O-Si network leads to the formation of the M-O-Si bonds with the metal substrate, facilitating strong interactions between the coating and the substrate, effectively reducing the availability of the metal surface to form metal oxides such as rust. Some of the non-polar thiol groups of LA(ST) act to provide a hydrophobic envelope which deters the diffusion of water and other polar species to the metal surface, while the other available thiol groups of LA(ST) shield the metal surface by creating a passive polymeric coating [
21,
22]. Finally, the formation of thiolates with the metal mitigates corrosion onset. Therefore, it is expected that a variation in the thiol concentration will (i) potentially affect the extent of epoxide ring opening by thiol groups and (ii) an increase in thiol concentration (as with LA(TGST) 1:2 and 1:3) will increase the accessibility of these groups to complement crosslinking mechanisms and further enhance the corrosion resistance properties of the LA(TGST) coatings. However, the elimination of the alcohols from the precursors before crosslinking is also expected to affect the rate of crosslinking reactions between the silica species and the thiol and epoxide groups directly impacting the microstructure, particle size, and aggregation and finally the corrosion resistance of these coatings. The LA(TGST) and the TGST coatings prepared with varying epoxy/thiol stoichiometry from 3:1 to 1:3 were fully characterized, and corrosion prevention performance was measured for both systems.
3.1. Structural Characterization
The structure of the various formulations was characterized by Raman and FTIR spectroscopy.
Figure 1a shows the Raman spectra for the low-alcohol LA(TG), LA(ST), and crosslinked LA(TGST) formulations contrasted with the TG, ST, and the crosslinked TGST versions in a 1:1 stoichiometry.
The characteristic absorbance values for the epoxy C-O-C ring symmetric stretch (1260 cm
−1), thiol S-H stretch at 2580 cm
−1, the S-H stretch for the thiol group attached to an alky group at 652 cm
−1, and the peaks associated with the silica stretches namely, Si-OH (880 cm
−1), Si-O-Si (1020 cm
−1), and Si-CH
2-R (1450 cm
−1) (
Figure 1a), confirm the formation of crosslinked colloidal silica networks from TG and ST [
23]. Furthermore, the reduced intensity of the epoxy and thiol peaks in LA(TGST) and TGST formulations relative to LA(TG), TG, and LA(ST), ST indicate that the epoxy–thiol curing is extensive in both systems. In addition, interfacial interactions between TG and ST to form TGST for the 1:1 hybrid have been shown to occur immediately [
3].
Figure 1b shows the FTIR spectra for the 1:3 LA(TGST) and TGST formulations that complement the structural identification by Raman spectroscopy. Characteristic peaks for the C-H of the three-membered epoxy ring (2978 cm
−1), C-O-C epoxy ring breathing (1248 cm
−1), S-H stretching vibration for the thiols groups (2615 cm
−1), silanol (Si-OH, 890 cm
−1), and Si-O-M (≈900 cm
−1), Si-alkoxy (Si-O-C) and siloxane (Si-O-Si) bonds at ≈1110 cm
−1, and the unbounded and intermolecularly bonded alcohol and silanol stretching vibrations above 3400 cm
−1 confirm the crosslinked nature of the hybrids [
3,
24].
Figure 1c indicates the characteristic peak assignments for the thiol (C-S-H, 678 cm
−1), thioester (C-S-C, 632 cm
−1), the C-S (599 cm
−1), and S-S (580 cm
−1) bond vibrations associated with the disulfide (C-S-S-C) bonds in the fingerprint region [
25] for the 1:2 and 2:1 ratios, respectively. The presence of all the characteristic peaks in the crosslinked low-alcohol LA(TGST) formulations confirms that the removal of alcohols did not affect the structural integrity of the formulations (
Figure 1a,b). Spectra for all formulations in the fingerprint region are provided in
Figure S1 in the Supporting Information.
3.2. Effect of Stoichiometric Variation on Crosslinking Density in Low-Alcohol Formulations
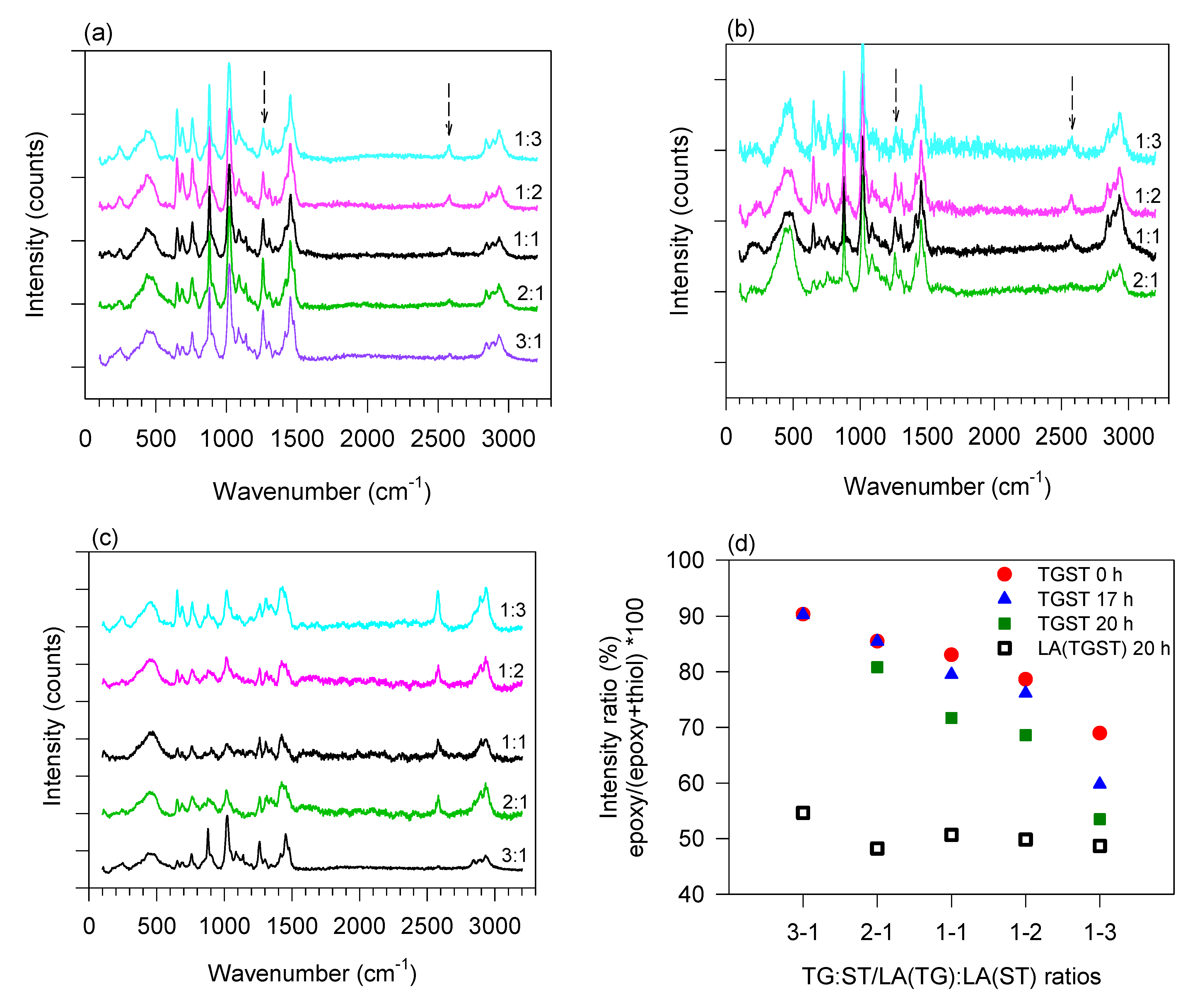
To investigate the impact of elimination of alcohols on the extent of crosslinking of the epoxy–thiol silicates and the sol–gel network formation for the varying ratios, Raman and IR spectroscopy of the TGST formulations was conducted to clearly elucidate the microstructural changes incurred by the LA(TGST) system when alcohols were absent. Raman spectrum for the TG:ST 1:1 formulation was measured again for this study and normalized to compare with the LA(TGST) 1:1 formulation.
Raman spectra showing the variation of the normalized intensities of epoxy and thiol peaks with change in epoxy–thiol silicate stoichiometry after completion of the crosslinking reaction (crosslinking time
t = 0 h) and then at
t = 20 h of curing time are shown in
Figure 2a,b.
Figure 2c shows the spectra for LA(TGST) ratios at 20 h.
Figure 2d quantifies the extent of curing as a percentage with changing TG:ST and LA(TG):LA(ST) ratios at the different crosslinking times using the ratio of the intensity of the epoxide and thiol peaks.
Table 1 shows the relative curing with the example of TGST ratios with progressing crosslinking time. The spectral trends seen at
t = 17 h crosslinking time are provided in the Supporting Information (
Figure S2).
As expected, the peak for the epoxy (C-O-C stretch, 1260 cm
−1,
Figure 2a–c) reduced in relative intensity with decreasing TG content in the formulations from TGST 3:1 to 1:3 at all crosslinking times. Concurrently, a marked increase in the intensity of the thiol peak (S-H stretch, 2580 cm
−1,
Figure 2a,b) was observed with increasing ST content. Interestingly, the relative intensities of the peaks for the TGST 3:1 and 2:1 ratio remain unchanged from
t = 0 h to
t = 17 h (
Figure 2d and
Table 1), but a rapid densification and gelation was observed at
t = 20 h. At 20 h, TGST 3:1 was the only formulation that had gelled completely (
Table 1). Therefore, the unchanged relative intensity at
t = 0 h and
t = 17 h for TGST 2:1 (
Table 1) indicated that the high epoxy concentration in these formulations remained in excess after completely reacting with the thiol functional groups. In TGST 2:1, which has a lower epoxy concentration, curing was evidenced by the reduced relative intensity (
Figure 2d and
Table 1) at
t = 20 h. Therefore, it would be expected that for a similarly high concentration of thiol groups in TGST 1:3 and 1:2 formulations, a similar trend would be observed. However, a decreasing trend of relative intensities at all crosslinking times was seen, indicating higher curing (
Table 1). Such a trend is attributed to two factors: (i) higher relative thiol to epoxy concentration leading to extended curing and (ii) the development of disulfide dimers. The excess concentration of sulfur containing ST in TGST 1:2 and 1:3 most likely promoted dimerization to form disulfide (S-S) bonds, which decreased the availability of functionally reactive free thiol S-H bonds in the formulation (
Scheme 1). S-S dimerization in ST is consistent with the observed increase in average particle size of ST (2.4 ± 0.5) compared to TG (1.1 ± 0.6) [
3]. Furthermore, TGST 1:1 showed the highest difference of relative intensity at 2.362 (
Table 1), implying the presence of fewer thiol groups available to crosslink with, which can be explained by the transformation of available thiol groups to disulfide dimers in ST.
In contrast to the trends observed in the TGST system, the LA(TGST) formulations achieved a consistent (circa 50%) extent of crosslinking at
t = 20 h across all ratios (
Figure 2d). This indicates that irrespective of the epoxy/thiol stoichiometry, the same number of functional groups are available for crosslinking. In addition, LA(TGST) 2:1 and 1:1 have a lower thiol content than LA(TGST) 1:3, but their relative epoxy–thiol intensities are similar and are also close to that of TGST 1:3 (
Figure 2d), indicating that there is considerable aggregation and dimerization in these ratios because of the removal of alcohols.
An increase in crosslinking density and disulfide formation associated with the increase in thiol content was confirmed by FTIR for all formulations.
Figure 3a,b show the increase in normalized intensity for the C-H vibration of the epoxide ring with a concurrent decrease in the thiol S-H peak intensity from 1:3 to 3:1 epoxy/thiol ratios in both the TGST (
Figure 3a) and LA(TGST) (
Figure 3b) systems. The gradual increase was attributed to effective crosslinking between the TG and ST components. A higher peak intensity of the Si-O-H (890 cm
−1) and Si-O-Si peaks (≈1110 cm
−1) for the 1:1, 1:2, and 1:3 formulations in contrast to the 3:1 and 2:1 formulations is potentially a result of enhanced hydrolysis and condensation of Si-alkoxy groups with the newly available OH groups emerging from the ring-opening of the epoxide by the higher thiol concentration (
Figure 2a,b). This explains the shift to lower frequencies for the peak centers for the Si-OH and Si-O-Si peaks (shown as the dashed red arrows in
Figure 3a) at 946 cm
−1 to 890 cm
−1 and 1110 cm
−1 to 1050 cm
−1 respectively from 3:1 to 1:3 ratios for TGST and LA(TGST) formulations, which is attributed to increased hydrogen bonding. The area under these peaks also corresponds to the degree of condensation between the hydrolyzed Si-O-H and the coated iron surface to form Si-O-Fe bonds. The coating–substrate covalent interactions augment the interfacial adhesion, which is an important mechanism for mitigating the onset of corrosion.
Figure 3c,d show the extent of crosslinking for the TGST and the LA(TGST) systems as a function of the changes in the thiol, epoxide ring, thioester, disulfide, and alcohol bond vibration frequencies. A linear correlation was observed for all the groups associated with ST, for example, C-S-H, C-S-C, C-S-S-C, in both systems exhibiting a direct increase in crosslinking density with increasing ST stoichiometry. The linear increase in the C-S-C and C-OH peak intensities from 3:1 to 1:3 TG:ST ratios also supports the growth in extent of crosslinking arising from the increased number of thiol groups available for nucleophilic attack at the strained epoxide ring, resulting in new thioester (C-S-C) and alcohol (C-O-H) moieties (
Scheme 1).
Interestingly, as the thiol content is increased in the formulations, disulfide content is also simultaneously elevated (C-S-S-C peak intensity in
Figure 3c,d) in both systems. This is explained by the self-association of the thiol silicate particles, as illustrated in
Scheme 1. An increased self-association of the more hydrophobic LA(ST) particles was also observed in the LA(TGST) system, where the alcohols were replaced with water in the formulations affecting formulation stability and increasing particle size, potentially recording increased absorbances for all peaks in
Figure 3b,d. It would be expected that the C-H peak intensity of the epoxide ring would be highest for the 3:1 TG:ST ratio and would linearly decrease from 3:1 to 1:3 TG:ST, notably; however, only a marginal increase in intensity was observed from 1:1 to 1:3 TG:ST (
Figure 3c). This increase in the intensity for 1:3 TGST despite improved crosslinking in high ST and LA(ST) ratios (
Figure 3c,d) is attributed to the competing disulfide formation, correspondingly mitigating extensive crosslinking with available epoxy groups. In addition, although the C-OH peak intensity may be attributed to both crosslinking interactions and the formation of diols via the acid-catalyzed epoxide ring opening by water, the C-OH peak intensity was observed to be lowest for 3:1 TGST and LA(TGST) (
♦ and
⟡ Figure 3c,d), indicating that the ring was not extensively opened by this mechanism. In LA(TGST), independent of stoichiometry, a consistent amount of LA(TG) was consumed by LA(ST) as is indicated by the almost constant intensity of C-H epoxy peak from 3:1 to 1:3 TG:ST (
Figure 3d). This implies that any expected increase in TG:ST crosslinking was mitigated by the dimerization/self-aggregation in LA(ST) and ST colloids. The self-aggregation of thiol silicates can be explained by the absence of the charge-stabilizing presence of alcohols that were removed from the LA(TGST) formulations.
Overall, three key results can be obtained from FTIR, which are important to enhancing corrosion prevention with thiol content in the LA(TGST) and TGST formulations: (i) the growing extent of crosslinking seen in the TGST systems from 3:1 to 1:3 TG:ST ratios, in contrast to the LA(TGST) systems, indicates consistent TG:ST crosslinking irrespective of stoichiometry; (ii) extensive silanol hydrolysis and condensation is achieved in formulations with higher ST ratios; and (iii) improved adhesion of the metal substrate and the coating is obtained.
3.3. XPS Analyses
The survey scan of the coated surfaces (
Figure 4a) shows the atomic percentage of key elements (C 1s, S 2p, Si 2p, O 1s) and their respective binding energies. The nitrogen and fluorine seen in the survey scan are components of the fluorosurfactant used in the coatings. The binding energies at 399.8 eV (N 1s) and 688.8 eV (F 1s) are indicative of nitrogen in amine [
26] and the -CF
2-CF
2- bond [
27] respectively, which are both expected from the surfactant.
Figure 4b shows the deconvolution spectra of sulfur 2p for TGST 2:1 typical of spectra for all formulations.
Figure 4c is plotted to reveal the correlations and extent of crosslinking between the various chemical species as a function of the LA(TG):LA(ST) and TG:ST stoichiometry and the impact of alcohol removal in the low VOC formulations.
The S 2p spectrum shown in
Figure 4b was fitted by six peaks corresponding to three major species each with their S 2p 3/2 and 1/2 peak splitting at binding energies 162.2, 163.38, 163.5, 164.5, and 167.5 and 168.9 eV. The binding energy at 162.2 and 163.38 eV is associated with the 1/2 and 3/2 spin peaks of the bound thiols forming metal thiolates with iron, including a surface state contribution of the monosulfide (S
2−) [
28,
29,
30], whereas the predominant envelope at 163.5 eV encompasses the S 2p
3/2 overlap of the unbound C-S-H (thiols), C-S-C (thioester), and C-S-S-C (disulfide) groups [
21,
28,
31,
32]. The lower intensity peak at 164.5 eV is attributed to the S 2p
½ binding energies for the C-S-C and C-S-H thiols groups commensurate with the spin-orbital degeneracy. Binding energies for metal sulfites and sulfates (SO
32− and SO
42−) for S 2p are observed at 167.5 eV and 168.9 eV, respectively [
33,
34]. The Si 2p at 102.91 eV can be fitted by a single broad peak attributed to Si-O representative of the predominant Si-O-Si network in all formulations (data not shown).
XPS analyses corroborate the results from Raman and FTIR spectroscopy. The growing extent of crosslinking between the low-alcohol and the TG and ST precursors and the increase in disulfide formation with corresponding increase in thiol content demonstrated in the infrared results is also seen in the XPS results (
Figure 4c). The atomic percent of sulfur species in C-S-H, C-S-S-C, and C-S-C (
●) as a function of the total atomic percent consistently increases from 2.9 atomic percent for the 3:1 to 5.7 atomic percent for the 1:3 TG:ST ratios. The C-S-H concentration in 3:1, 2:1, and 1:1 is identical and would be expected to remain unchanged at 2.9%; however, the significant increase in the C-S atomic percentage between 3.3% for 2:1 and 4.6% for 1:1 demonstrates the conversion of thiol to thioester and alkyl disulfide. This is consistent with the formation of crosslinked species and oxidation of thiols to disulfide in the presence of molecular oxygen in aqueous solutions [
35]. It is expected that the enhanced epoxy–thiol crosslinking and growth of hydrophobic moieties associated with increased disulfide formation improves corrosion resistance in combination with strong substrate-coating adhesion.
The XPS information for the TGST hybrids mirrors the data for the LA(TGST) coatings (
Figure 4c). Interestingly, metal sulfate formation (
■,
Figure 4c) is almost constant across the formulations independent of the starting concentration of thiols. This can be ascribed to the zero-order kinetics followed for the self-oxidation of thiols in the presence of atmospheric molecular oxygen. The oxidation is catalyzed by the presence of transition metal ions such as iron, although the rate of oxidation is dependent on both the pH and the nature of the transition metal [
35]. Therefore, development of the green color within 1 to 5 min of air-drying coatings in all coated samples across all ratios can be attributed to the formation of iron sulfates, which is also known as green vitriol. Moreover, adhesion between the metal substrate and coating remains consistent (
▲,
Figure 4c) independent of stoichiometric increase in the thiol functionality. Overall, these XPS results clearly demonstrate the moieties involved in crosslinking and identify the bonds responsible for the green coloration of the coatings in the presence of atmospheric oxygen.
3.4. Formulation Stability: Viscosity and Pot-Life
Figure 5 shows the variation in viscosity for the TGST formulations at
t = 0 h and
t = 17 h to explore the formulation pot-life. Viscosity remained relatively unchanged with increasing TG:ST ratio immediately after reaction at
t = 0 h (
Figure 5). For all formulations, viscosity measurements were restricted to a maximum of
t = 17 h to avoid the risk of gelation in the viscometer. The
t = 17 h data point for TGST 3:1 was not measured due to signs of gelation. A significant decrease in viscosity between formulations was observed as the thiol content increased. In addition, a trend of decreasing difference in viscosity between
t = 0 h and
t = 17 h was observed with increasing TG:ST ratio (
Figure 5). This is attributed to two main reasons: (i) the greater crosslinking between epoxy and thiol groups in formulations where the thiol content was lower but more available for reaction instead of dimerization (TGST 3:1 and 2:1) and (ii) decreasing TGST particle agglomeration. The increase in viscosity with increase in
t has previously been shown to be associated with molecular weight gain and the gradually growing aggregate size of TGST particles [
3]. However, when the thiol content increases from TG:ST 1:1 to 1:3 in the formulation, ST dimerization competes with TG:ST crosslinking density, which mitigates an increase in viscosity.
Pot-life was tested for the TGST system to compare with the low-alcohol LA(TGST) system to determine if the removal of alcohols from the TG and ST precursor solutions impacted the LA(TGST) formulation stability with increasing crosslinking time
t. All solutions of TGST and LA(TGST) were clear solutions at
t = 0 h but became progressively cloudy on standing overnight at
t = 17 h and showed full gelation/sedimentation at
t = 20 h (
Figures S3 and S4 in the Supporting Information).
Figure 6a,b shows the formulation stability of the TGST and the LA(TGST) systems at
t = 17 h respectively. The solutions with higher TG content (3:1 and 2:1) showed signs of gel formation at
t = 17 h and were fully gelled within 20 h. Solutions with higher ST content showed increased precipitation of TGST/ST particles in the formulation, leading to the formation of suspensions. The rate of gelation and suspension were relatively slow until
t = 17 h but rapidly increased between
t = 17 and 20 h (
Figure S2 in the Supporting Information). The stability of formulations with respect to LA(TG): LA(ST) and TG:ST stoichiometry was observed to be 2:1, 1:1, and 1:2 greater than 3:1 and 1:3.
Figure 7a–d show the formulation stability of the precursor formulations TG, LA(TG), ST, and LA(ST). A significant difference in formulation stability between the TG and the ST dispersion and their corresponding low-alcohol precursors was observed. The TG and the low-alcohol LA(TG) formulations displayed excellent stability, maintaining their viscosity for over 30 days after preparation (
Figure 7a,b). ST (where no alcohols were removed) remained stable for over 30 h under ambient conditions (
Figure 7c); however, the LA(ST) formulation (
Figure 7d) turned cloudy within 17 h when the alcohols were replaced with water. Although the alcohols were replaced with water to prepare a low VOC system, the thiol group that is relatively less polar than the epoxide is also less stable in a predominantly aqueous environment, thus reducing the pot-life of LA(ST) substantially. This explains the reduced stability of the LA(TGST) formulations (
Figure 6b and
Figure S3 in the Supporting Information) for the corresponding crosslinking times as compared to the TGST system (
Figure 6a). Visibly, the LA(TGST) 1:2 and 1:3 formulations (
Figure 6b) look less cloudy than their TGST counterparts at
t = 17 h (
Figure 6a) and
t = 20 h; however, this is due to the increased sedimentation caused by the lower stability of the TGST/ST particles in the low-alcohol formulation. The bilayer in the formulation formed due to sedimentation is visible for TGST 1:2 at
t = 20 h in
Figure S3 in the Supporting Information. Expectedly, all formulations with a higher epoxy ratio (LA(TGST) and TGST 3:1, 2:1) showed gelation/sedimentation of LA(T)/ST later than the LA(TGST) and TGST 1:1, 1:2, or 1:3 formulations, which was attributed to the lower LA(ST)/ST concentration precipitating out of the dispersions. It is of note that all formulations of the LA(TGST) and TGST systems exhibited a pot-life >15 h and were readily coated on steel panels at that time, indicating that the formulations were adequately stable over a period sufficient for commercial application of the surface treatment.