Chemical Solution Deposition of La-Substituted BiFe0.5Sc0.5O3 Perovskite Thin Films on Different Substrates
Abstract
1. Introduction
2. Materials and Methods
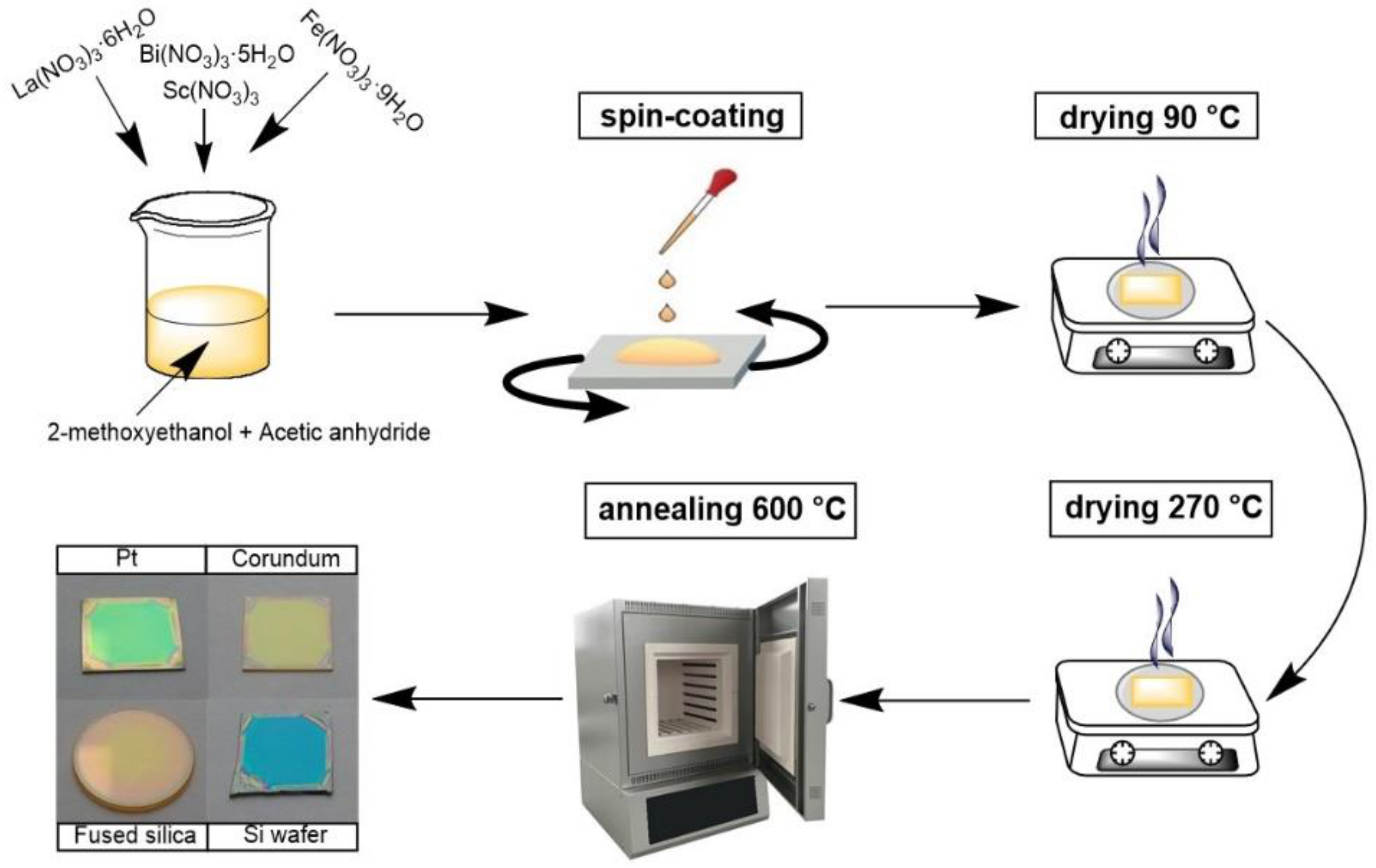
2.1. Preparation of Precursor Solution
2.2. Deposition of Thin Films
2.3. Characterization
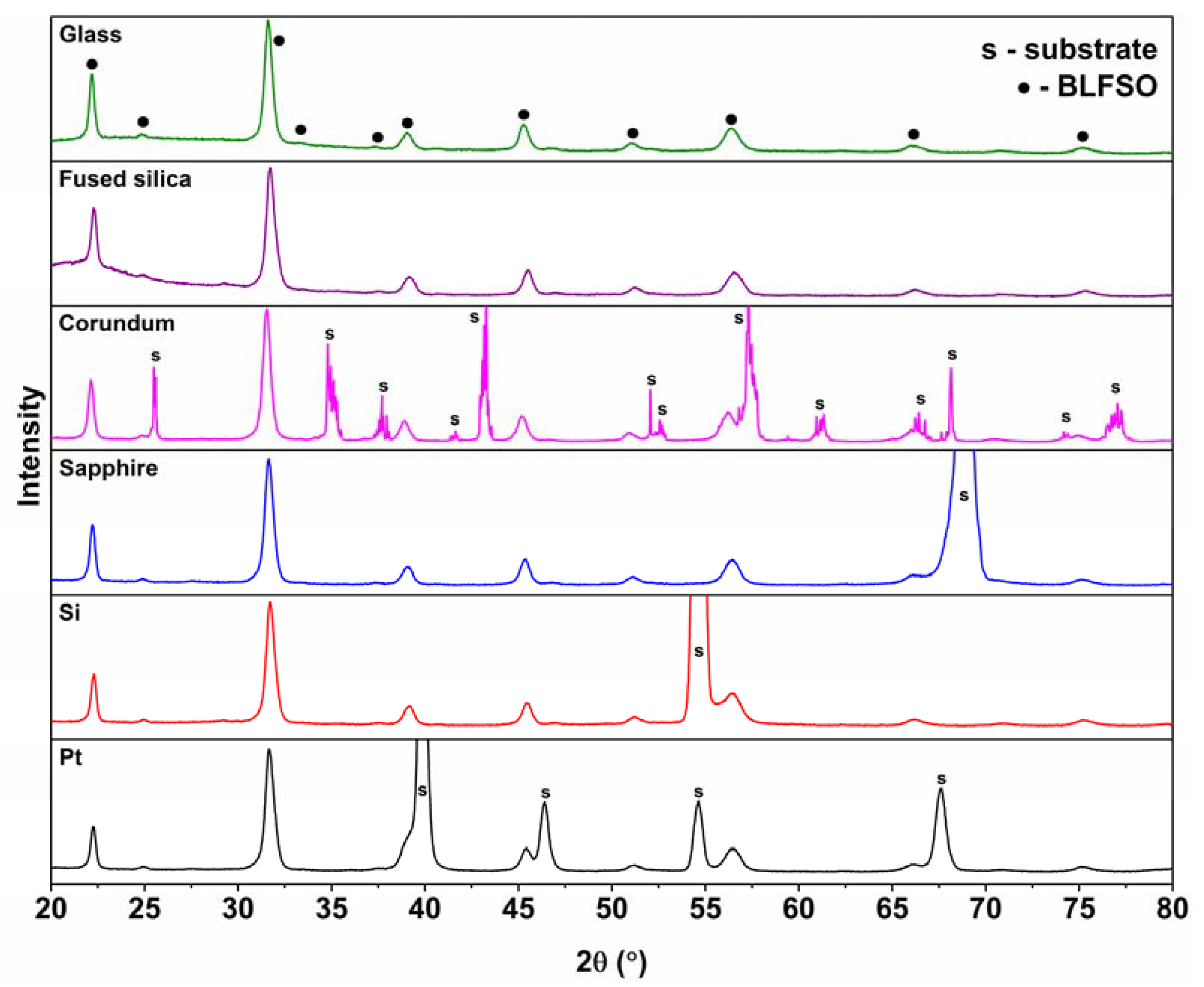
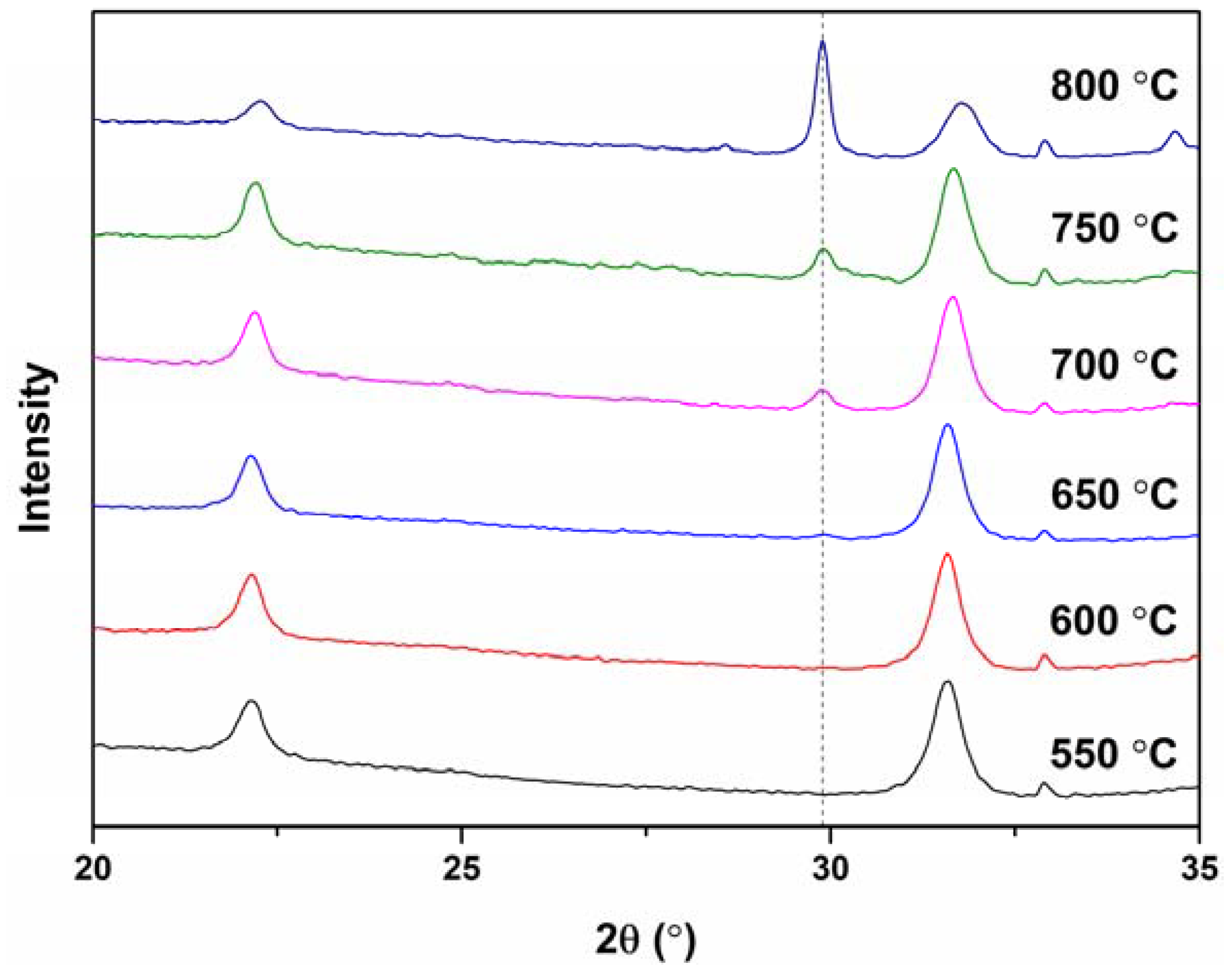
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vopson, M.M. Fundamentals of multiferroic materials and their possible applications. Crit. Rev. Solid State Mater. Sci. 2015, 40, 223–250. [Google Scholar] [CrossRef]
- Lu, C.; Wu, M.; Lin, L.; Liu, J.-M. Single-phase multiferroics: New materials, phenomena, and physics. Natl. Sci. Rev. 2019, 6, 653–668. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X. A brief review on perovskite multiferroics. Ferroelectrics 2017, 507, 69–85. [Google Scholar] [CrossRef]
- Catalan, G.; Scott, J.F. Physics and applications of bismuth ferrite. Adv. Mater. 2009, 21, 2463–2485. [Google Scholar] [CrossRef]
- Molak, A.; Mahato, D.K.; Szeremeta, A.Z. Synthesis and characterization of electrical features of bismuth manganite and bismuth ferrite: Effects of doping in cationic and anionic sublattice: Materials for applications. Prog. Cryst. Growth Charact. Mater. 2018, 64, 1–22. [Google Scholar] [CrossRef]
- Chen, L.; Cheng, Z.; Xu, W.; Meng, X.; Yuan, G.; Liu, J.; Liu, Z. Electrical and mechanical switching of ferroelectric polarization in the 70 nm BiFeO3 film. Sci. Rep. 2016, 6, 19092. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Sanchez, O.; Sanchez-Martinez, A.; Flores-Ruiz, F.J.; Huerta-Flores, A.M.; Torres-Martínez, L.M.; Ruelas, R.; García-Guaderrama, M. Study of BiFeO3 thin film obtained by a simple chemical method for the heterojunction-type solar cell design. J. Alloys Compd. 2020, 832, 154923. [Google Scholar] [CrossRef]
- Catalano, M.R.; Spedalotto, G.; Condorelli, G.G.; Malandrino, G. MOCVD growth of perovskite multiferroic BiFeO3 films: The effect of doping at the A and/or B sites on the structural, morphological and ferroelectric properties. Adv. Mater. Interfaces 2017, 4, 1601025. [Google Scholar] [CrossRef]
- McLeod, J.A.; Pchelkina, Z.V.; Finkelstein, L.D.; Kurmaev, E.Z.; Wilks, R.G.; Moewes, A.; Solovyev, I.V.; Belik, A.A.; Takayama-Muromachi, E. Electronic structure of BiMO3 multiferroics and related oxides. Phys. Rev. B 2010, 81, 144103. [Google Scholar] [CrossRef]
- Arnold, D.C. Composition-driven structural phase transitions in rare-earth-doped BiFeO3 ceramics: A review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 62–82. [Google Scholar] [CrossRef]
- Fertman, E.L.; Fedorchenko, A.V.; Khalyavin, D.D.; Salak, A.N.; Baran, A.; Desnenko, V.A.; Kotlyar, O.V.; Cizmar, E.; Feher, A.; Syrkin, E.S.; et al. Multiferroic Bi0.65La0.35Fe0.5Sc0.5O3 perovskite: Magnetic and thermodynamic properties. J. Magn. Magn. Mater. 2017, 429, 177–181. [Google Scholar] [CrossRef]
- Fedorchenko, A.V.; Fertman, E.L.; Desnenko, V.A.; Kotlyar, O.V.; Cizmar, E.; Shvartsman, V.V.; Lupascu, D.C.; Salamon, S.; Wende, H.; Salak, A.N.; et al. Magnetic properties of the Bi0.65La0.35Fe0.5Sc0.5O3 perovskite. Acta Phys. Pol. A 2017, 131, 1069–1071. [Google Scholar] [CrossRef]
- Khalyavin, D.D.; Salak, A.N.; Lopes, A.B.; Olekhnovich, N.M.; Pushkarev, A.V.; Radyush, Y.V.; Fertman, E.L.; Desnenko, V.A.; Fedorchenko, A.V.; Manuel, P.; et al. Magnetic structure of an incommensurate phase of La-doped BiFe0.5Sc0.5O3: Role of antisymmetric exchange interactions. Phys. Rev. B 2015, 92, 224428. [Google Scholar] [CrossRef]
- Khalyavin, D.D.; Salak, A.N.; Manuel, P.; Olekhnovich, N.M.; Pushkarev, A.V.; Radysh, Y.V.; Fedorchenko, A.V.; Fertman, E.L.; Desnenko, V.A.; Ferreira, M.G.S. Antisymmetric exchange in La-substituted BiFe0.5Sc0.5O3 system: Symmetry adapted distortion modes approach. Z. Krist. Cryst. Mater. 2015, 230, 767–774. [Google Scholar] [CrossRef]
- Salak, A.N.; Khalyavin, D.D.; Zamaraite, I.; Stanulis, A.; Kareiva, A.; Shilin, A.D.; Rubanik, V.V.; Radyush, Y.V.; Pushkarev, A.V.; Olekhnovich, N.M.; et al. Metastable perovskite Bi1−xLaxFe0.5Sc0.5O3 phases in the range of the compositional crossover. Phase Transit. 2017, 90, 831–839. [Google Scholar] [CrossRef]
- Lu, C.; Hu, W.; Tian, Y.; Wu, T. Multiferroic oxide thin films and heterostructures. Appl. Phys. Rev. 2015, 2, 021304. [Google Scholar] [CrossRef]
- Martin, L.W.; Chu, Y.H.; Ramesh, R. Advances in the growth and characterization of magnetic, ferroelectric, and multiferroic oxide thin films. Mater. Sci. Eng. R Rep. 2010, 68, 89–133. [Google Scholar] [CrossRef]
- Yan, F.; Zhu, T.J.; Lai, M.O.; Lu, L. Enhanced multiferroic properties and domain structure of La-doped BiFeO3 thin films. Scr. Mater. 2010, 63, 780–783. [Google Scholar] [CrossRef]
- Chen, X.; Hu, G.; Yan, J.; Wang, X.; Yang, C.; Wu, W. Enhanced multiferroic properties of (1 1 0)-oriented BiFeO3 film deposited on Bi3.5Nd0.5Ti3O12-buffered indium tin oxide/Si substrate. J. Phys. D Appl. Phys. 2008, 41, 225402. [Google Scholar] [CrossRef]
- Zhang, Q.; Sando, D.; Nagarajan, V. Chemical route derived bismuth ferrite thin films and nanomaterials. J. Mater. Chem. C 2016, 4, 4092–4124. [Google Scholar] [CrossRef]
- Deng, X.; Huang, J.; Sun, Y.; Liu, K.; Gao, R.; Cai, W.; Fu, C. Effect of processing parameters on the structural, electrical and magnetic properties of BFO thin film synthesized via RF magnetron sputtering. J. Alloys Compd. 2016, 684, 510–515. [Google Scholar] [CrossRef]
- Deng, X.; Zeng, Z.; Xu, R.; Qin, X.; Li, X.; Wang, Y.; Gao, R.; Wang, Z.; Chen, G.; Cai, W.; et al. Effect of annealing atmosphere on structural and multiferroic properties of BiFeO3 thin film prepared by RF magnetron sputtering. J. Mater. Sci. Mater. Electron. 2019, 30, 16502–16509. [Google Scholar] [CrossRef]
- Hu, C.-W.; Yen, C.-M.; Feng, Y.-C.; Chen, L.-H.; Liao, B.-Z.; Chen, S.-C.; Liao, M.-H. Multi-ferroic properties on BiFeO3/BaTiO3 multi-layer thin-film structures with the strong magneto-electric effect for the application of magneto-electric devices. Coatings 2021, 11, 66. [Google Scholar] [CrossRef]
- Abramov, A.; Alikin, D.; Sobol, A.; Myakishev, D.; Slabov, V.; Trusov, L.; Safina, V.; Turygin, A.; Vasiliev, A.; Shur, V.; et al. Chemical solution deposition of BiFeO3 films with layer-by-layer control of the coverage and composition. Coatings 2020, 10, 438. [Google Scholar] [CrossRef]
- Dubourdieu, C.; Kang, S.B.; Li, Y.Q.; Kulesha, G.; Gallois, B. Solid single-source metal organic chemical vapor deposition of yttria-stabilized zirconia. Thin Solid Film. 1999, 339, 165–173. [Google Scholar] [CrossRef]
- Murauskas, T.; Kubilius, V.; Saltyte, Z.; Plausinaitiene, V. Metalorganic chemical vapor deposition and investigation of nonstoichiometry of undoped BaSnO3 and La-doped BaSnO3 thin films. Thin Solid Film. 2019, 692, 137575. [Google Scholar] [CrossRef]
- Jiang, J.; Shen, W.; Hertz, J.L. Fabrication of epitaxial zirconia and ceria thin films with arbitrary dopant and host atom composition. Thin Solid Film. 2012, 522, 66–70. [Google Scholar] [CrossRef]
- Zarkov, A.; Stanulis, A.; Mikoliunaite, L.; Katelnikovas, A.; Jasulaitiene, V.; Ramanauskas, R.; Tautkus, S.; Kareiva, A. Chemical solution deposition of pure and Gd-doped ceria thin films: Structural, morphological and optical properties. Ceram. Int. 2017, 43, 4280–4287. [Google Scholar] [CrossRef]
- Jonauske, V.; Stanionyte, S.; Chen, S.-W.; Zarkov, A.; Juskenas, R.; Selskis, A.; Matijosius, T.; Yang, T.C.K.; Ishikawa, K.; Ramanauskas, R.; et al. Characterization of sol-gel derived calcium hydroxyapatite coatings fabricated on patterned rough stainless steel surface. Coatings 2019, 9, 334. [Google Scholar] [CrossRef]
- Gul, E.; Stanulis, A.; Barushka, Y.; Garskaite, E.; Ramanauskas, R.; Morkan, A.U.; Kareiva, A. The influence of thermal processing on microstructure of sol–gel-derived SrSnO3 thin films. J. Mater. Sci. 2017, 52, 12624–12634. [Google Scholar] [CrossRef]
- Karoblis, D.; Griesiute, D.; Mazeika, K.; Baltrunas, D.; Karpinsky, D.V.; Lukowiak, A.; Gluchowski, P.; Raudonis, R.; Katelnikovas, A.; Zarkov, A.; et al. A facile synthesis and characterization of highly crystalline submicro-sized BiFeO3. Materials 2020, 13, 3035. [Google Scholar] [CrossRef] [PubMed]
- Carranza-Celis, D.; Cardona-Rodríguez, A.; Narváez, J.; Moscoso-Londono, O.; Muraca, D.; Knobel, M.; Ornelas-Soto, N.; Reiber, A.; Ramírez, J.G. Control of multiferroic properties in BiFeO3 nanoparticles. Sci. Rep. 2019, 9, 3182. [Google Scholar] [CrossRef] [PubMed]
- Selbach, S.M.; Einarsrud, M.-A.; Grande, T. On the thermodynamic stability of BiFeO3. Chem. Mater. 2009, 21, 169–173. [Google Scholar] [CrossRef]




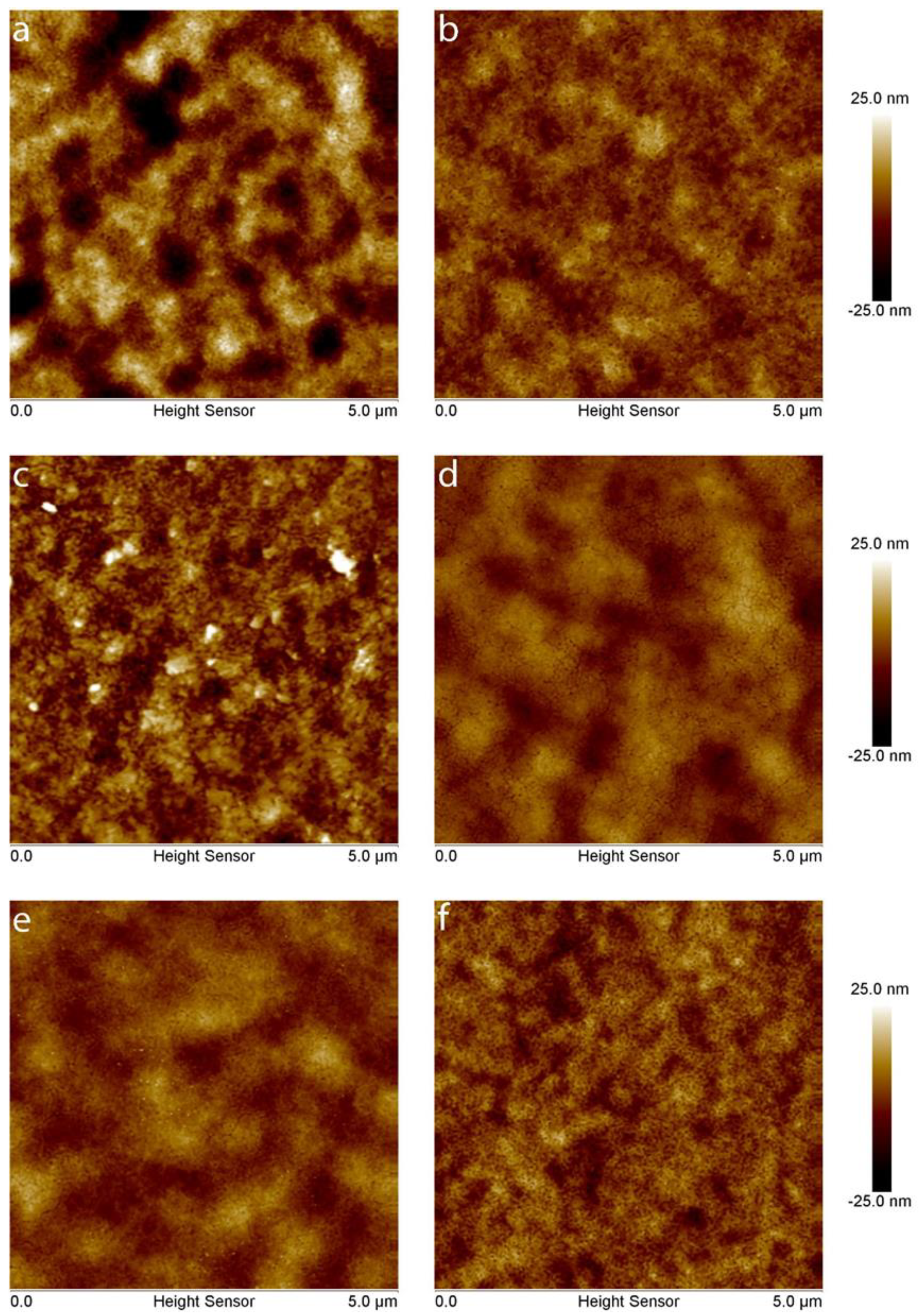
| Substrate | Roughness of Substrate, nm | Roughness of Film on the Respective Substrate, nm |
|---|---|---|
| Pt/TiO2/SiO2/Si, Si/SiO2 | 4.2 ± 0.1 | 7.3 ± 0.8 |
| Silicon | 0.6 ± 0.3 | 3.8 ± 0.1 |
| Sapphire | 1.2 ± 0.2 | 5.8 ± 0.9 |
| Corundum | 9.2 ± 2 | 4.8 ± 0.2 |
| Fused silica | 0.8 ± 0.1 | 3.1 ± 0.5 |
| Glass | 1.1 ± 0.1 | 4.2 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griesiute, D.; Karoblis, D.; Mikoliunaite, L.; Zarkov, A.; Salak, A.N.; Kareiva, A. Chemical Solution Deposition of La-Substituted BiFe0.5Sc0.5O3 Perovskite Thin Films on Different Substrates. Coatings 2021, 11, 307. https://doi.org/10.3390/coatings11030307
Griesiute D, Karoblis D, Mikoliunaite L, Zarkov A, Salak AN, Kareiva A. Chemical Solution Deposition of La-Substituted BiFe0.5Sc0.5O3 Perovskite Thin Films on Different Substrates. Coatings. 2021; 11(3):307. https://doi.org/10.3390/coatings11030307
Chicago/Turabian StyleGriesiute, Diana, Dovydas Karoblis, Lina Mikoliunaite, Aleksej Zarkov, Andrei N. Salak, and Aivaras Kareiva. 2021. "Chemical Solution Deposition of La-Substituted BiFe0.5Sc0.5O3 Perovskite Thin Films on Different Substrates" Coatings 11, no. 3: 307. https://doi.org/10.3390/coatings11030307
APA StyleGriesiute, D., Karoblis, D., Mikoliunaite, L., Zarkov, A., Salak, A. N., & Kareiva, A. (2021). Chemical Solution Deposition of La-Substituted BiFe0.5Sc0.5O3 Perovskite Thin Films on Different Substrates. Coatings, 11(3), 307. https://doi.org/10.3390/coatings11030307









