Tribocorrosion Performance of Cr/CrN Hybrid Layer as a Coating for Machine Components Used in a Chloride Ions Environment
Abstract
1. Introduction
- A comparative assessment of resistance to tribocorrosion in 3.5% NaCl of the analyzed material solutions;
- The identification and quantitative evaluation of the synergy of friction and corrosion in the total material loss;
- The identification of the probable mechanism of the friction–corrosion synergy effect under tribocorrosion conditions.
2. Materials and Methods
2.1. Coatings and Identification Methods
2.2. Tribocorrosion Tests and Wear
3. Results
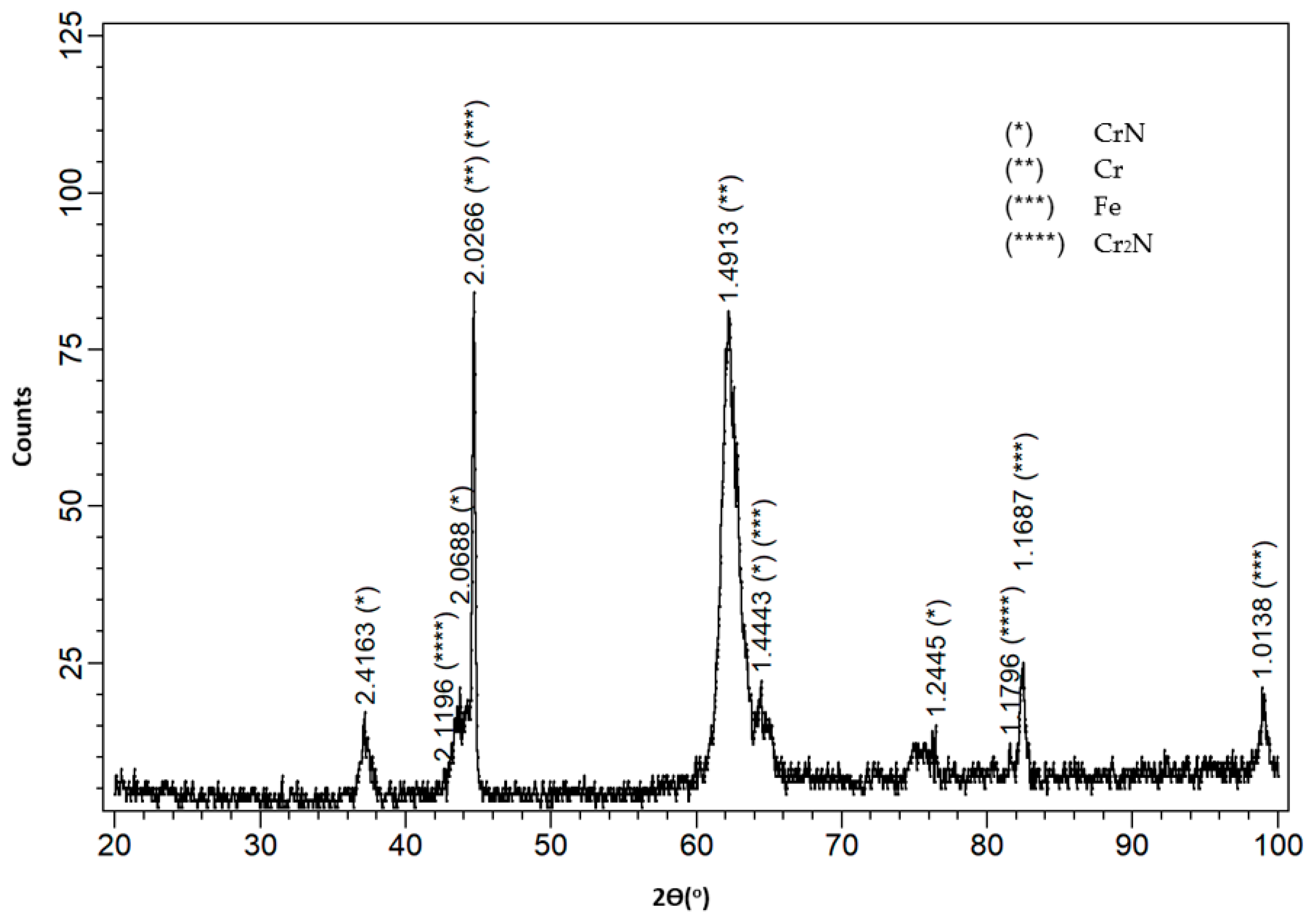
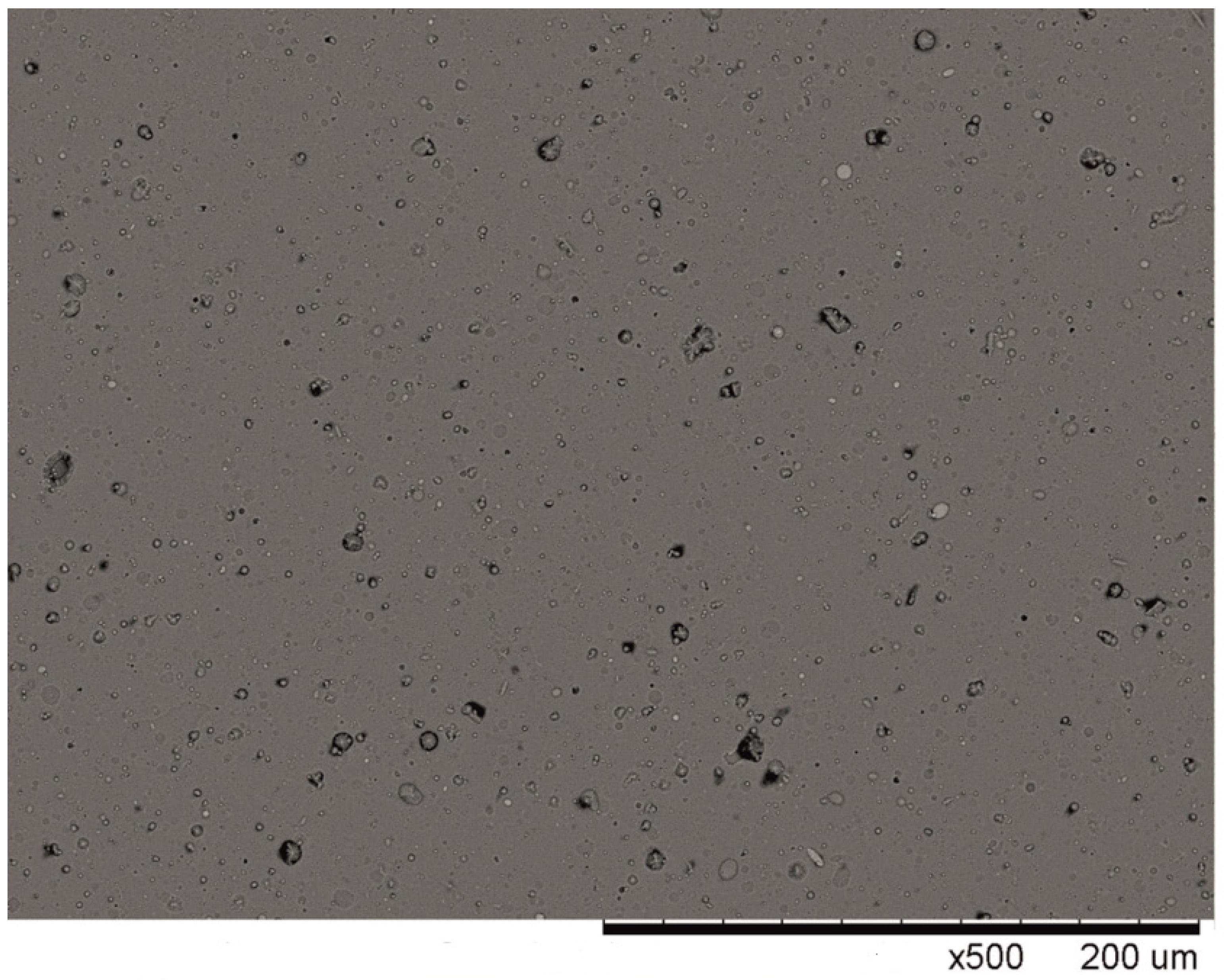
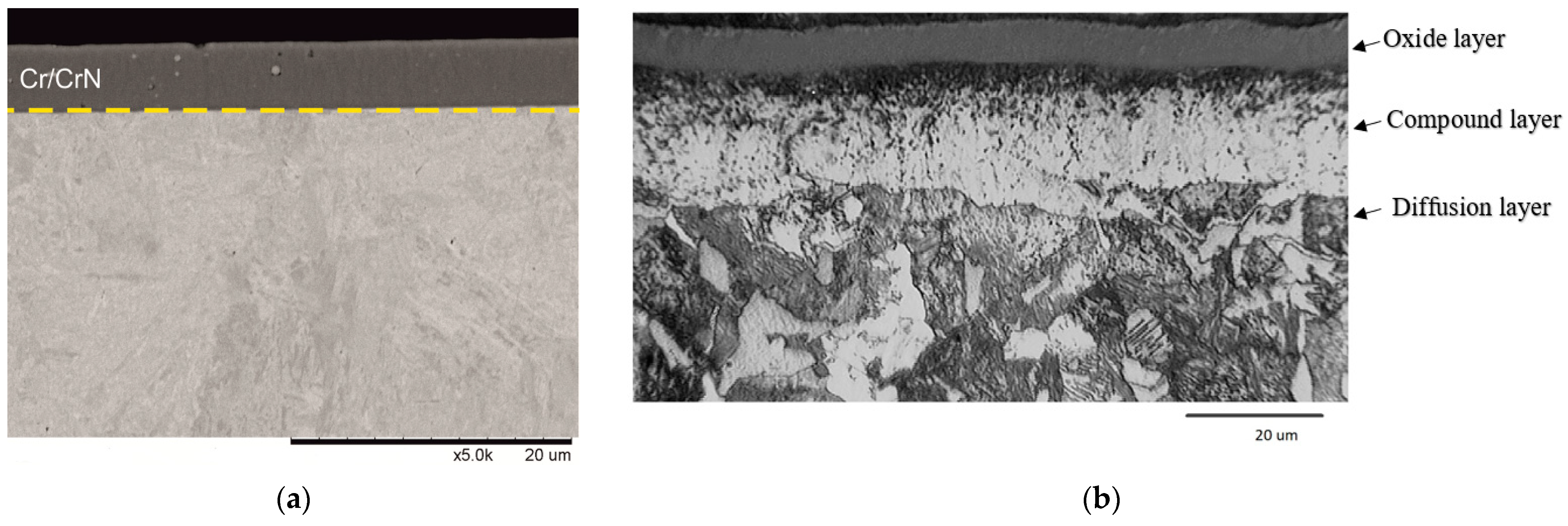
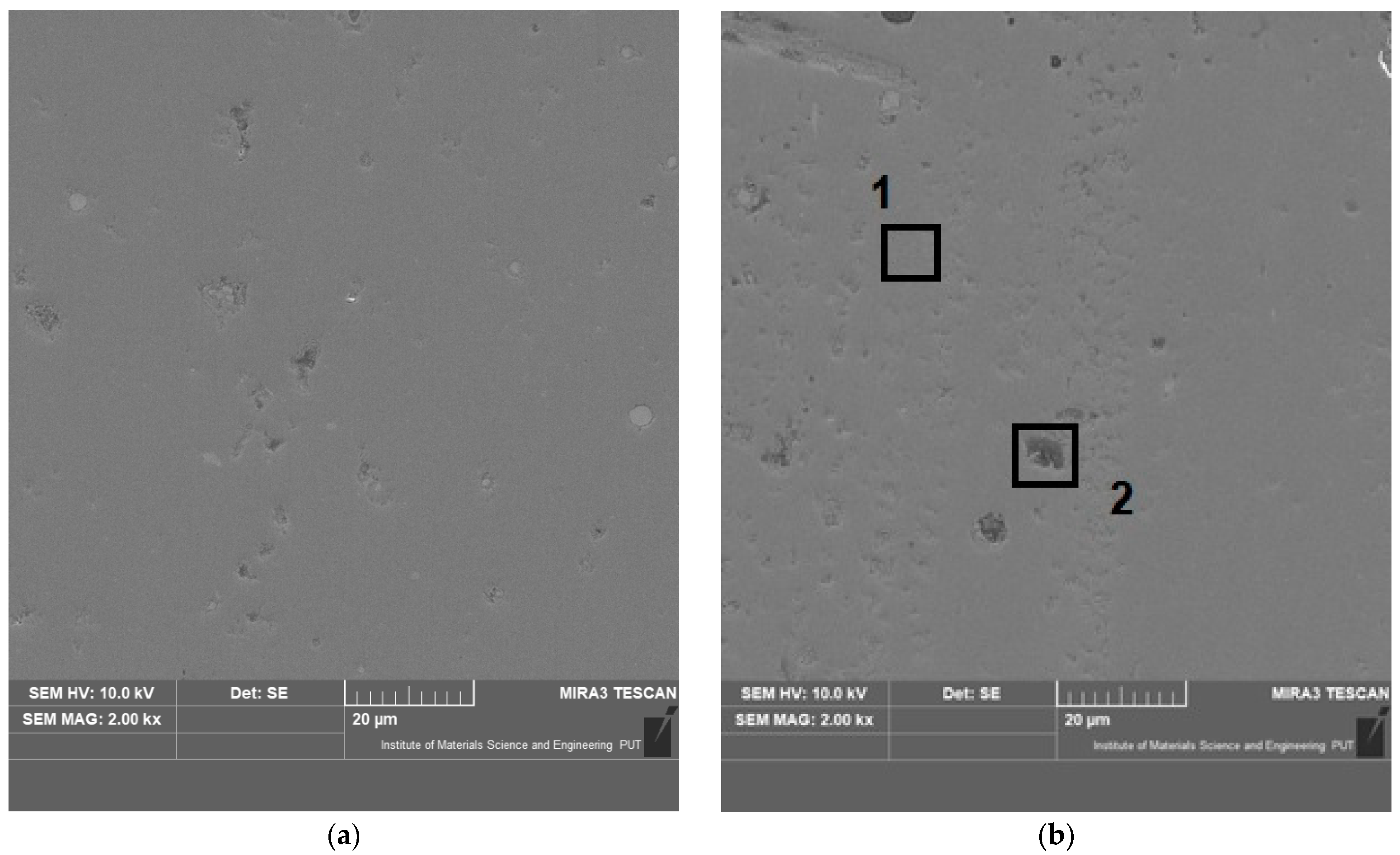
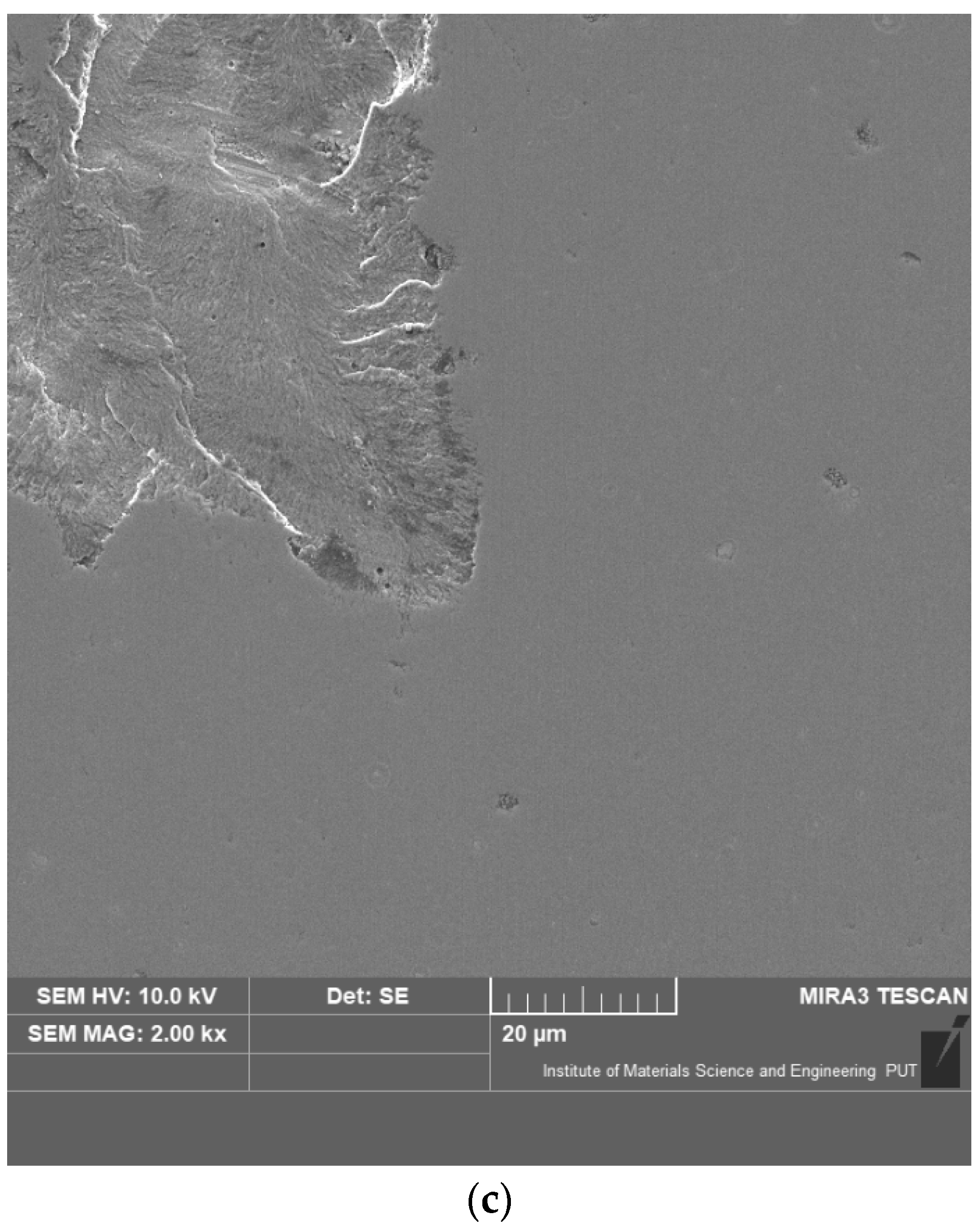
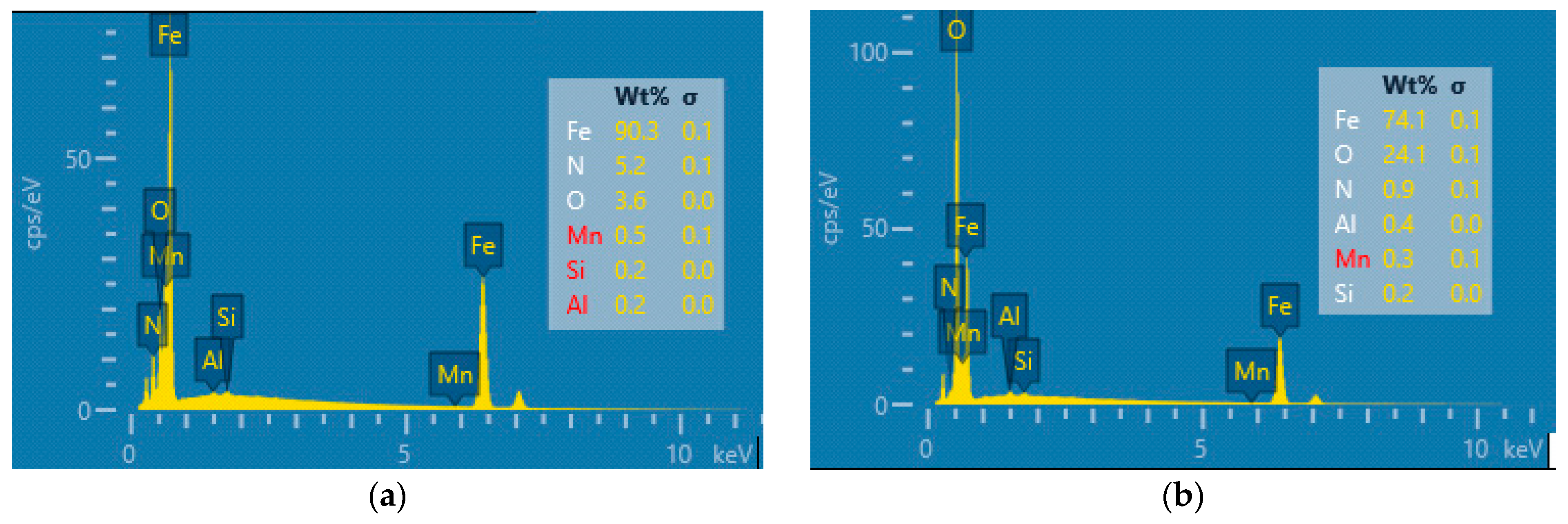
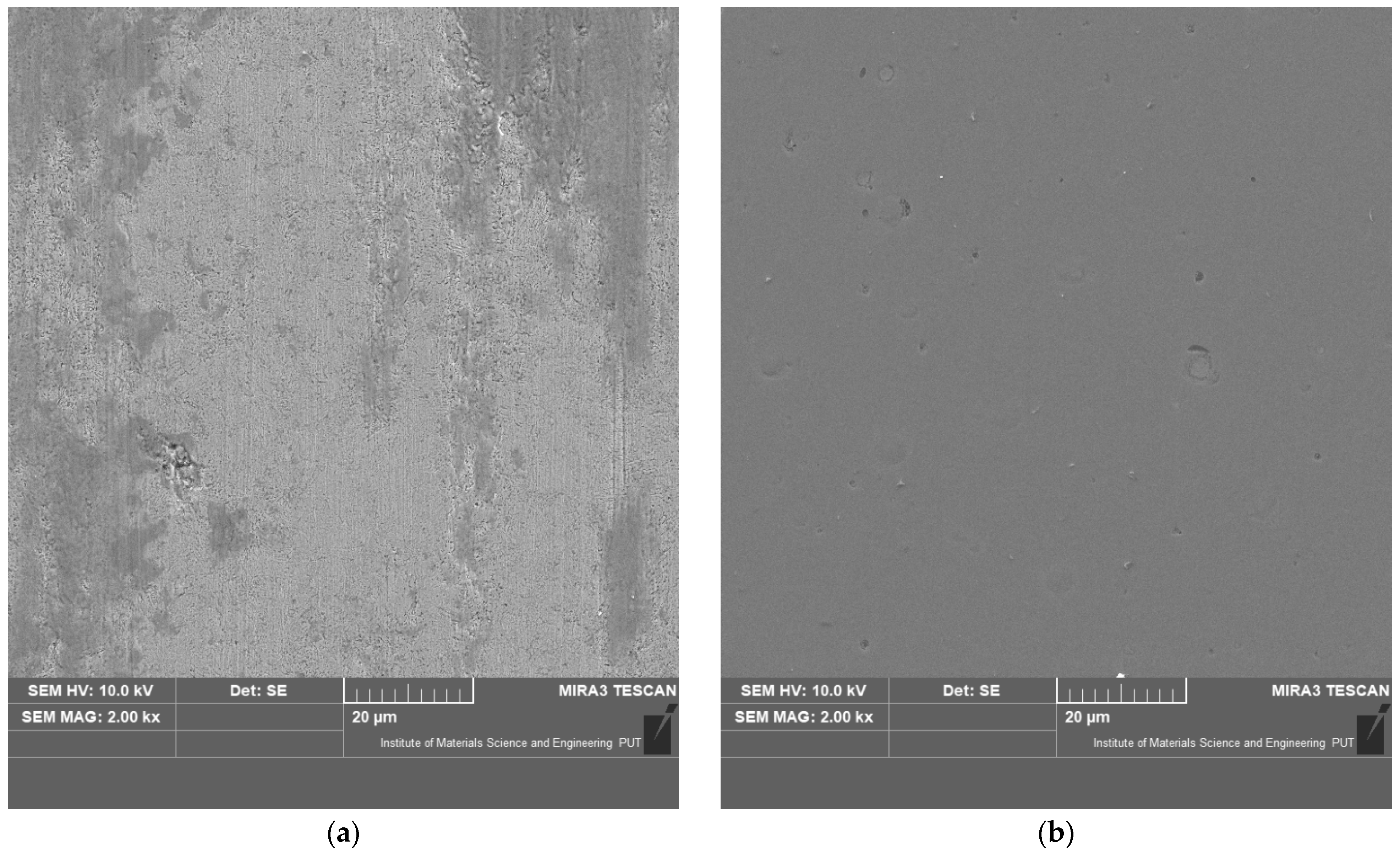
3.1. Characterisation of the Coatings
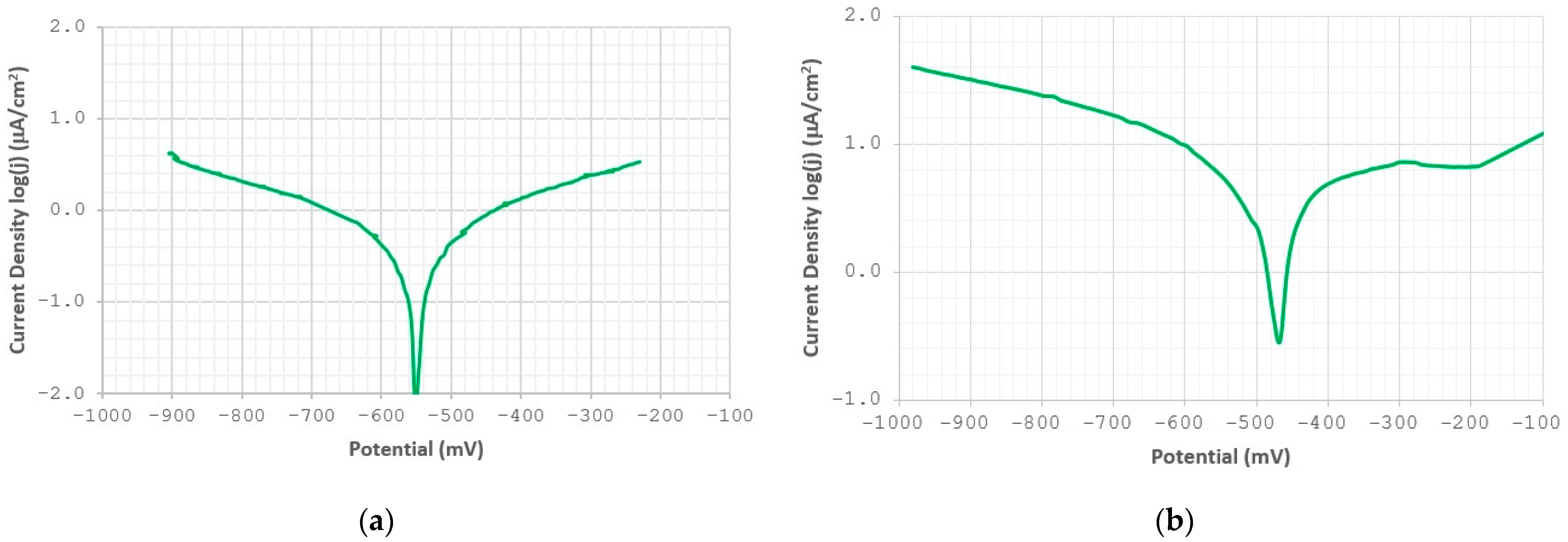
3.2. Corrosion Resistance
3.3. Tribocorrosion Performance
4. Discussion
5. Conclusions
- The Cr/CrN PVD coating produced on AISI 1045 medium carbon steel provides better tribocorrosion resistance than the gas nitrided with subsequent oxidation and impregnation coating in a 3.5% NaCl environment. This relation applies to the entire range of loads in the contact zone which were applied during the tribocorrosion tests.
- In tribocorrosion tests, a clear synergy effect of friction and corrosion was identified for both tested material solutions. It was over 30% in both cases. A similar percentage of the synergy effect, despite the clear difference in the total tribocorrosive loss, may indicate that similar elementary destruction mechanisms occur in the case of both analyzed material solutions.
- According to the diagnosed fundamental tribocorrosion mechanisms, the synergy effect is caused by the fact that corrosion processes create local cavities which at successive time intervals are most likely to intensify mechanical wear. The area around the cavities facilitates plastic deformation, the initiation of cracking of cyclically deformed layers and the tearing off larger fragments of material (especially at higher unit pressures in the frictional contact zones).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, S.; Mischler, S. Modeling tribocorrosion of passive metals—A review. Curr. Opin. Solid State Mater. Sci. 2018, 22, 127–141. [Google Scholar] [CrossRef]
- Ghanbarzadeh, A.; Salehi, F.M.; Bryant, M.; Neville, A. A new asperity-scale mechanistic model of tribocorossive wear: Synergistic effect pf mechanical wear and corrosion. J. Tribol. 2019, 141, 1–12. [Google Scholar] [CrossRef]
- Jemmely, P.; Mischler, S.; Landolt, D. Electrochemical modeling of passivation phenomena in tribocorrosion. Wear 2000, 237, 63–76. [Google Scholar] [CrossRef]
- Ghanbarzadeh, A.; Salehi, F.M.; Bryant, M.; Neville, A. Modelling the evolution of electrochemical current in potentiostatic condition using and asperity-scale model of tribocorrosion. Biotribology 2019, 17, 19–29. [Google Scholar] [CrossRef]
- Landolt, D.; Mischler, S.; Stemp, M. Electrochemical methods in tribocorrosion: A critical appraisal. Electrochim. Acta 2001, 46, 3913–3929. [Google Scholar] [CrossRef]
- Bayon, R.; Igartua, A.; Fernandez, X.; Martinez, R.; Rodriguez, R.J.; Garcia, J.A.; de Frutos, A.; Arenas, M.A.; de Damborenea, J. Corrosion-wear behaviour of PVD Cr/CrN multilayer coatings for gear applications. Tribol. Int. 2009, 42, 591–599. [Google Scholar] [CrossRef]
- Ould, C.; Badiche, X.; Montmitonnet, P.; Gacho, Y. PVD coated mill rolls for cold rolling of stainless-steel strips—Tribological and mechanical laboratory tests. J. Manuf. Process. 2013, 15, 77–86. [Google Scholar] [CrossRef]
- Ginting, A.; Skein, R.; Cuaca, D.; Herdianto; Pieter, Z.; Masyithah, Z. The characteristics of CVD- and PVD-coated carbide tools in hard turning of AISI 4340. Measurement 2018, 129, 548–557. [Google Scholar] [CrossRef]
- Malvajerdi, S.S.; Malvajerdi, A.S.; Ghanaatshoar, M. Protection of CK45 carbon steel tillage tools using TiN coating deposited by an arc-PVD method. Ceram. Int. 2019, 45, 3816–3822. [Google Scholar] [CrossRef]
- Gupta, K.M.; Ramdev, K.; Dharmateja, S.; Sivarajan, S. Cutting characteristics of PVD coated cutting tools. Mater. Today Proc. 2018, 5, 11260–11267. [Google Scholar] [CrossRef]
- Baptista, A.; Silva, F.; Porteiro, J.; Miguez, J.; Pinto, G. Sputtering physical vapour deposition (PVD) coatings: A critical review on process improvement and market trend demands. Coatings 2018, 8, 402. [Google Scholar] [CrossRef]
- Sousa, V.F.C.; Silva, F.J.G. Recent advances on coated milling tool technology—A comprehensive review. Coatings 2020, 10, 235. [Google Scholar] [CrossRef]
- Liu, C.; Leyland, A.; Bi, Q.; Matthews, A. Corrosion resistance of multi-layered plasma-assisted physical vapor deposition TiN and CrN coatings. Surf. Coat. Technol. 2001, 141, 164–173. [Google Scholar] [CrossRef]
- Ibrahim, M.A.M.; Korablov, S.F.; Yoshimura, M. Corrosion of stainless steel coated with TiN, (TiAl)N and CrN in aqueous environments. Corros. Sci. 2002, 44, 815–828. [Google Scholar] [CrossRef]
- Su, Y.L.; Yao, S.H.; Wu, C.T. Comparison of characterizations and tribological performance of TiN and CrN deposited by cathodic arc plasma deposition process. Wear 1996, 199, 132–141. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, Y.; Xie, Z.; Chen, T.; Wan, Y.; Wang, H.; Gao, X.; Chen, Y.; Zhou, Y.; Guo, Y. Tribocorrosion behaviors of CrN coating in 3,5 wt% NaCl solution. Thin Solid Films 2017, 622, 41–47. [Google Scholar] [CrossRef]
- Shan, L.; Wang, Y.; Zhang, Y.; Zhang, Q.; Xue, Q. Tribocorrosion behaviors of PVD CrN coated stainless steel in seawater. Wear 2016, 362–363, 97–104. [Google Scholar] [CrossRef]
- Shan, L.; Zhang, Y.-R.; Wang, Y.-X.; Li, J.-L.; Jiang, X.; Chen, J.-M. Corrosion and wear behaviors of PVD CrN and CrSiN coatings in seawater. Trans. Nonferr. Met. Soc. China 2016, 26, 175–184. [Google Scholar] [CrossRef]
- Shan, L.; Wang, Y.; Li, J.; Jiang, X.; Chen, J. Improving tribological performance of CrN coatings in seawater by structure design. Tribol. Int. 2015, 82, 78–88. [Google Scholar] [CrossRef]
- Marin, E.; Offoiach, R.; Regis, M.; Fusi, S.; Lanzutti, A.; Fedrizzi, L. Diffusive thermal treatments combined with PVD coatings for tribological protection of titanium alloys. Mater. Des. 2016, 89, 314–322. [Google Scholar] [CrossRef]
- Bayon, R.; Nevshupa, R.; Zubizarreta, C.; Ruiz de Gopegui, U.; Barriga, J.; Igartua, A. Characterisation of tribocorrosion behaviour of multilayer PVD coatings. Anal. Bioanal. Chem. 2010, 396, 2855–2862. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Y.; Chen, H.; Li, J.; Yao, Y.; Wang, C. Doping carbon to improve the tribological performance of CrN coatings in seawater. Tribol. Int. 2015, 90, 362–371. [Google Scholar] [CrossRef]
- Song, G.-H.; Yang, X.-P.; Xiong, G.-L.; Lou, Z.; Chen, L.-J. The corrosive behawior of Cr/CrN multilayer coatings with different modulation periods. Vacuum 2013, 89, 136–141. [Google Scholar] [CrossRef]
- Kowalski, M. The Improvement of Tribocorrosion Performance of Structural Steel Components in Marine Environment. Ph.D. Thesis, Warsaw University of Technology, Warsaw, Poland, 2018. [Google Scholar]
- Michalski, J.; Iwanow, J.; Tacikowski, J.; Tarfa, T.N.; Tymowski, J.; Sułkowski, I.; Wach, P. Anticorrosion nitriding with post-oxidation and inhibitor impregnation, and its industrial applications. Heat Treat. Met. 2004, 2, 31–35. [Google Scholar]
- EN ISO 6507-1 Metallic Materials—Vickers Hardness Test—Part 1: Test Method; European Committee for Standarization: Brussels, Belgium, 2005.
- Vidakis, N.; Antoniadis, A.; Bilalis, N. The VDI 3198 indentation test evaluation of a reliable qualitative control for layered compounds. J. Mater. Process. Technol. 2003, 143–144, 481–485. [Google Scholar] [CrossRef]
- Stachowiak, A.; Zwierzycki, W. Tribocorrosion modeling of stainless steel in a sliding pair of pin-on-plate type. Tribol. Int. 2011, 44, 1216–1224. [Google Scholar] [CrossRef]
- Kowalski, M.; Stachowiak, A. Tribocorrosion performance of Zn, Zn–Ni with magnesium electrodeposited on medium carbon steel in a chloride environment. Surf. Coat. Technol. 2019, 366, 75–85. [Google Scholar] [CrossRef]
- Mischler, S.; Debaud, S.; Landolt, D. Wear accelerated corrosion of passive metal in tribocorrosion systems. J. Electrochem. Soc. 1998, 145, 750–758. [Google Scholar] [CrossRef]
- Jemmely, P.; Mischler, S.; Landolt, D. Tribocorrosion behavior of Fe-17Cr stainless steel in acid and alkaline solutions. Tribol. Int. 1999, 32, 295–303. [Google Scholar] [CrossRef]
- ASTM G119-09. Standard Guide for Determining Synergism between Wear and Corrosion; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Sun, Y. Tribocorrosive behavior of low temperature plasma-nitrided PH stainless steel sliding against under linear reciprocation with and without trensvere oscillations. Wear 2016, 362–363, 105–113. [Google Scholar] [CrossRef]
- Baranowska, J.; Franklin, S.E.; Kochmanska, A. Wear behaviour of low-temperature gas nitrided austenitic stainless steel in a corrosive liquid environment. Wear 2007, 263, 669–673. [Google Scholar] [CrossRef]
- Hacisalihogu, I.; Yildiz, F.; Celik, A. Tribocorrosion behavior of plasma nitrided Hardox steels in NaCl solution. Tribol. Int. 2018, 120, 434–445. [Google Scholar] [CrossRef]
- Naghibi, S.A.; Ksolution, R.; Fathi, M.H. Corrosion and tribocorrosion behawior of T/TiN PVD coating on 316L stainless steel substrate in Ringer’s. Mater. Chem. Phys. 2014, 148, 614–623. [Google Scholar] [CrossRef]
- Kok, Y.N.; Akid, R.; Hovsepian, P.E. Tribocorrosion testing of stainless steel (SS) and PVD coated SS using a modified scanning reference electrode technique. Wear 2005, 295, 1472–1481. [Google Scholar] [CrossRef]







| Stage Number | Stage Name | Stage Parameters |
|---|---|---|
| 1 | submerged cleaning | time: 8 min, temperature 85 °C |
| 2 | submerged cleaning | time: 8 min, temperature 50 °C, ultrasounds |
| 3 | submerged cleaning | time: 8 min, temperature 85 °C |
| 4 | vapor cleaning | time: 8 min |
| Type of Process | Pressure (mbar) | Temperature (°C) | Polarization VoltageUbias (V) | Process Time (min) | Atmosphere |
|---|---|---|---|---|---|
| heating | <1 × 10−5 | 300 | – | 30 | – |
| etching by Ar ions | 5 × 10−3 | 300 | −300 | 25 | 100% Ar |
| etching by Cr ions | 5 × 10−3 | 400 | −300 | 15 | 100% Ar |
| Cr deposition | 5 × 10−3 | 400 | −50 | 5 | 100% Ar |
| CrN deposition | 3.5 × 10−3 | 380 | −150 | 120 | 100% N2 |
| Coating | Hardness HV |
|---|---|
| gas nitrided | 778 ± 72 |
| Cr/CrN | 2627 ± 260 |
| Material Type | Acting Force (N) | ||
|---|---|---|---|
| 9 | 13 | 19 | |
| Depth of the Tribocorrosion Wear Trace (µm) | |||
| gas nitrided coating | 6.44 ± 0.18 | 8.46 ± 0.46 | 11.72 ± 0.19 |
| Cr/CrN PVD | 0.975 ± 0.035 | 1.307 ± 0.095 | 2.09 ± 0.12 |
| Material Type | Acting Force (N) | ||
|---|---|---|---|
| 9 | 13 | 19 | |
| Volume Tribocorrosion Wear (mm3) | |||
| gas nitrided coating | 0.01098 ± 0.00054 | 0.01635 ± 0.00098 | 0.02679 ± 0.00056 |
| Cr/CrN PVD | 0.000642 ± 0.000035 | 0.000996 ± 0.00011 | 0.00203 ± 0.00017 |
| Material Type | Total Material Loss T (mm3) | Pure Wear W0 (mm3) | Pure Corrosion C0 (mm3) | Synergistic Effect S = T − (W0 − C0) (mm3) | S/T (%) |
|---|---|---|---|---|---|
| gas nitrided | 16.35 × 10−3 | 11.06 × 10−3 | 1.55 × 10−5 | 5.28 × 10−3 | 32 |
| Cr/CrN PVD | 0.99 × 10−3 | 0.65 × 10−3 | 7.59 × 10−7 | 0.34 × 10−3 | 34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalski, M.; Stachowiak, A. Tribocorrosion Performance of Cr/CrN Hybrid Layer as a Coating for Machine Components Used in a Chloride Ions Environment. Coatings 2021, 11, 242. https://doi.org/10.3390/coatings11020242
Kowalski M, Stachowiak A. Tribocorrosion Performance of Cr/CrN Hybrid Layer as a Coating for Machine Components Used in a Chloride Ions Environment. Coatings. 2021; 11(2):242. https://doi.org/10.3390/coatings11020242
Chicago/Turabian StyleKowalski, Marcin, and Arkadiusz Stachowiak. 2021. "Tribocorrosion Performance of Cr/CrN Hybrid Layer as a Coating for Machine Components Used in a Chloride Ions Environment" Coatings 11, no. 2: 242. https://doi.org/10.3390/coatings11020242
APA StyleKowalski, M., & Stachowiak, A. (2021). Tribocorrosion Performance of Cr/CrN Hybrid Layer as a Coating for Machine Components Used in a Chloride Ions Environment. Coatings, 11(2), 242. https://doi.org/10.3390/coatings11020242






