RETRACTED: Effects of Cr2O3 Content on Microstructure and Mechanical Properties of Al2O3 Matrix Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
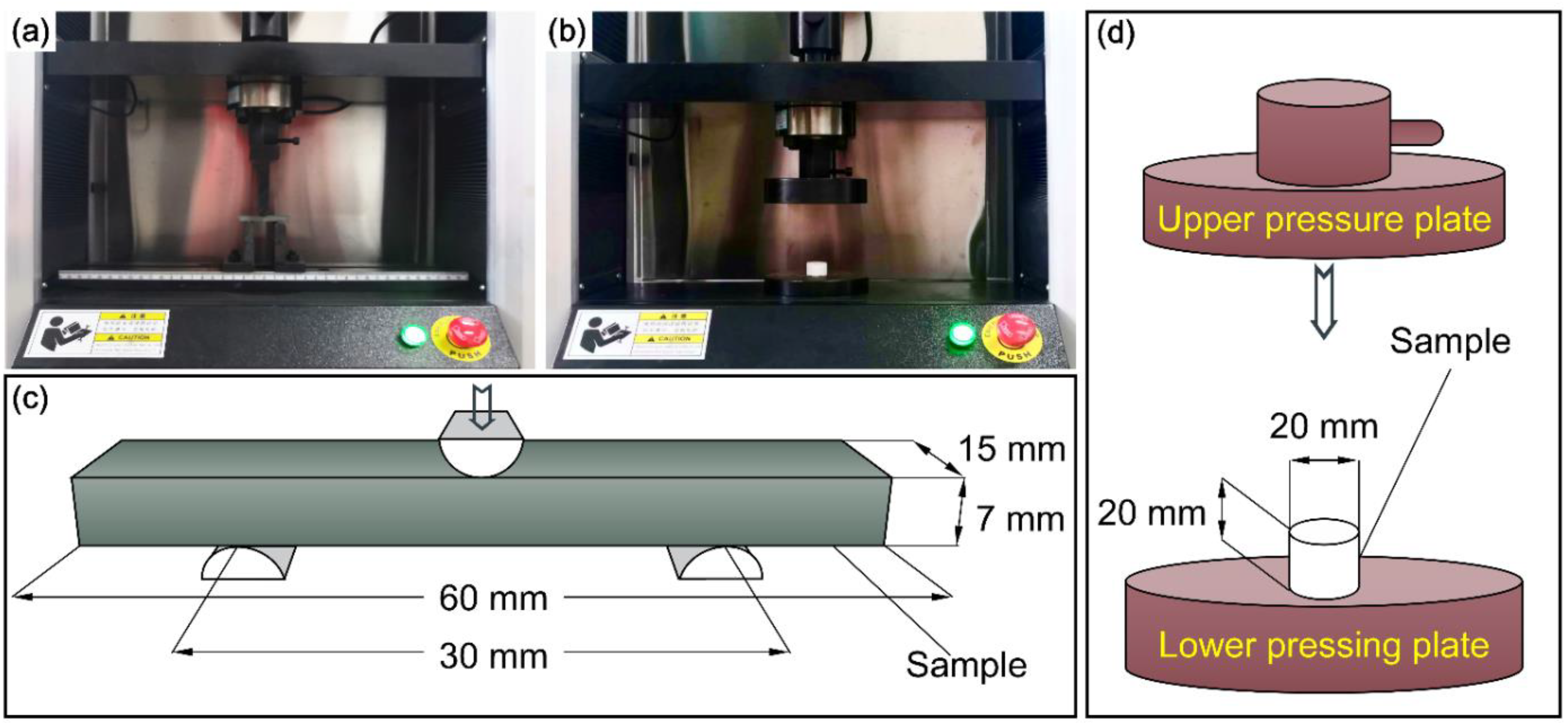
2.2. Characterization
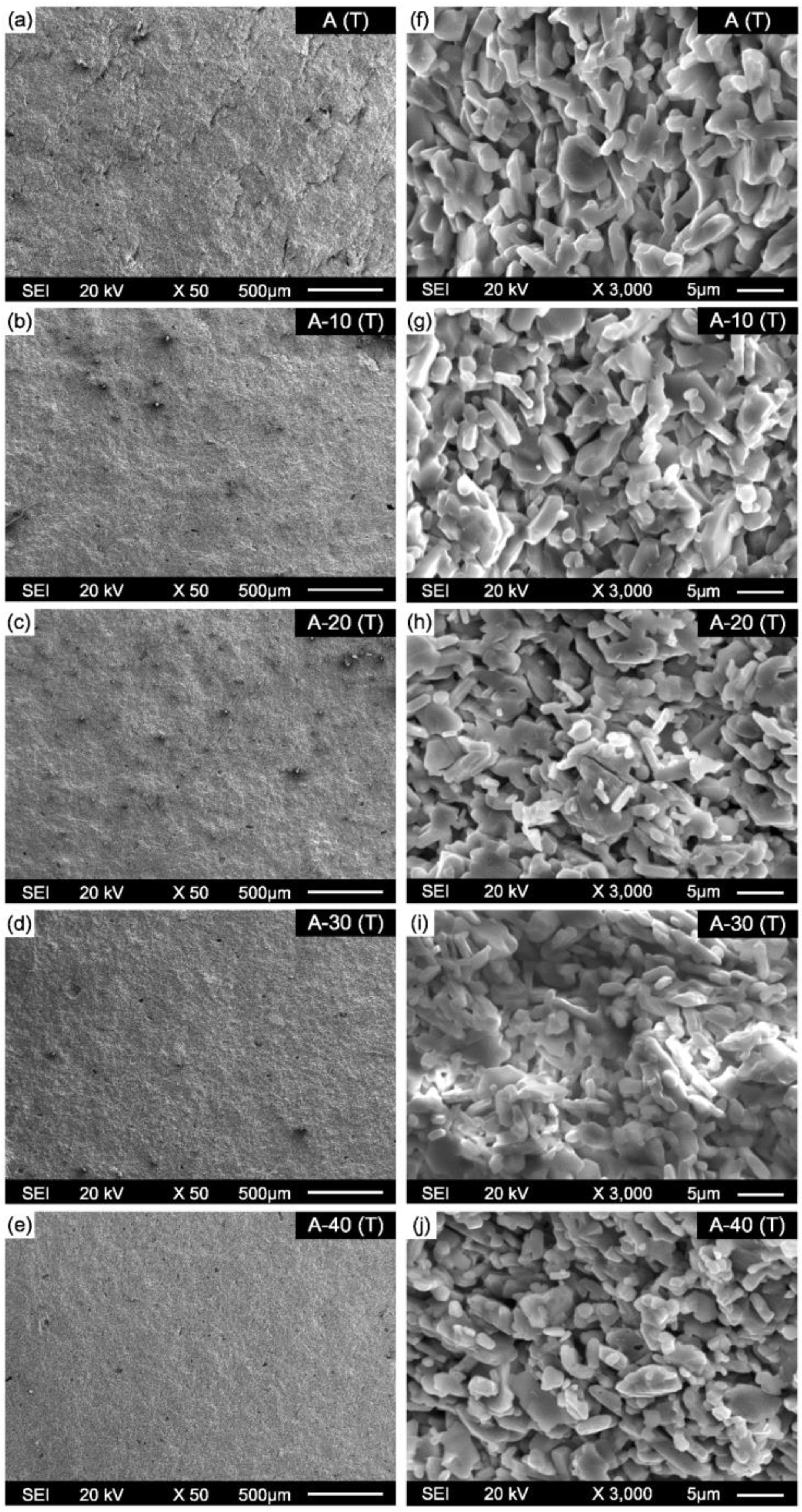
3. Results and Discussion
3.1. Phase Identification
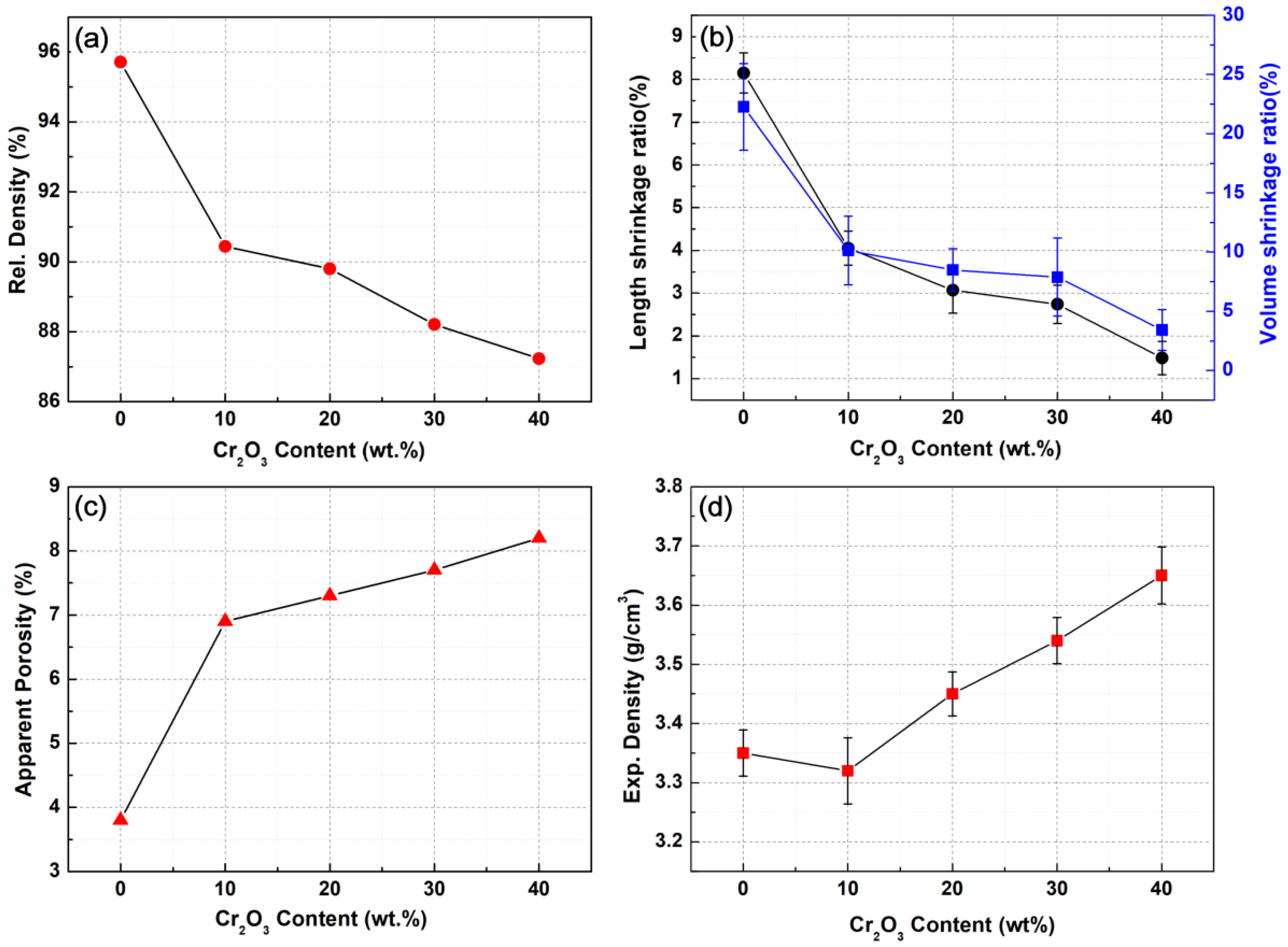
3.2. Consolidation Behaviour
3.3. Effect of Cr2O3 on Mechanical Properties and Thermal Shock Properties of Al2O3-Cr2O3 Composites
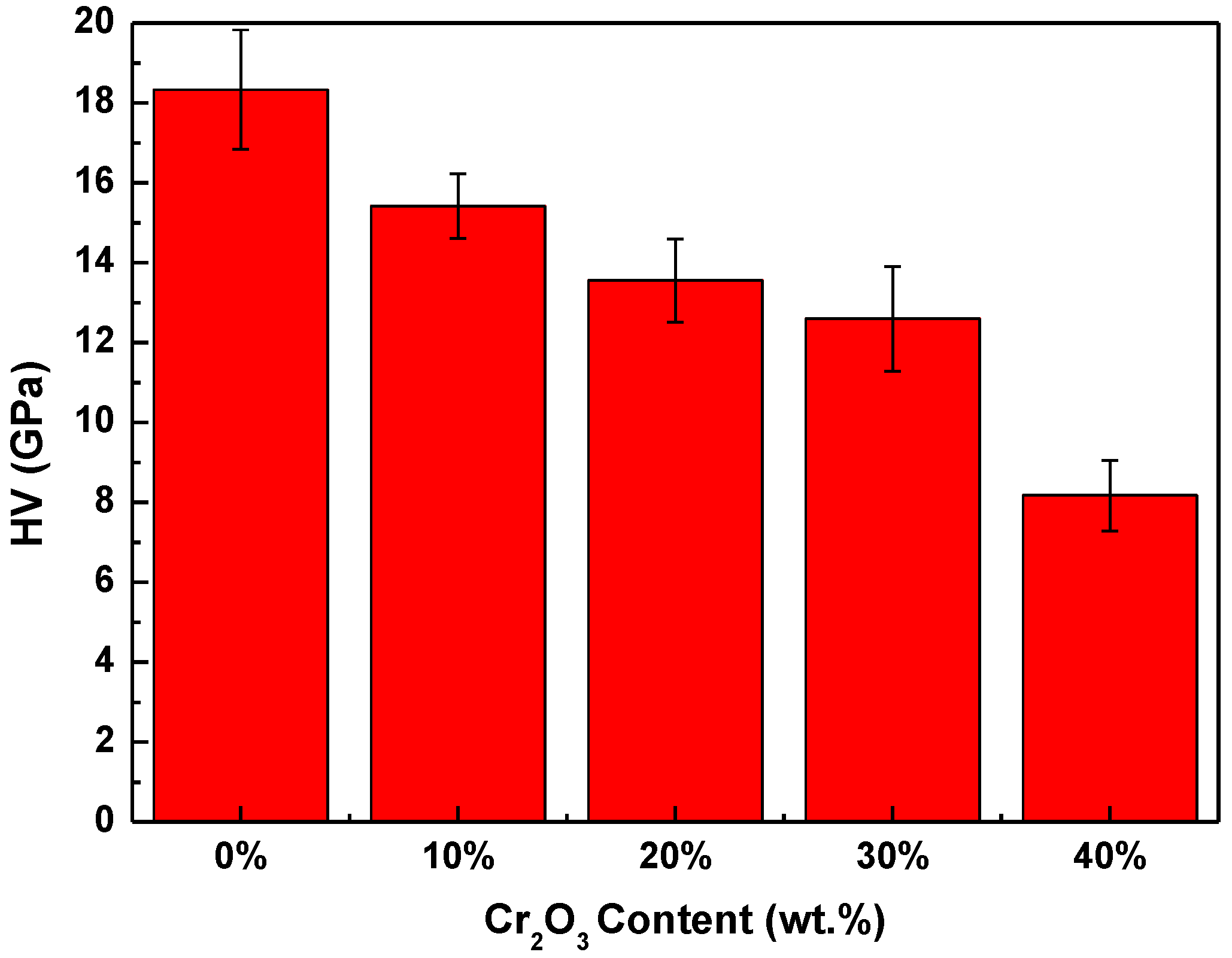
3.3.1. Hardness
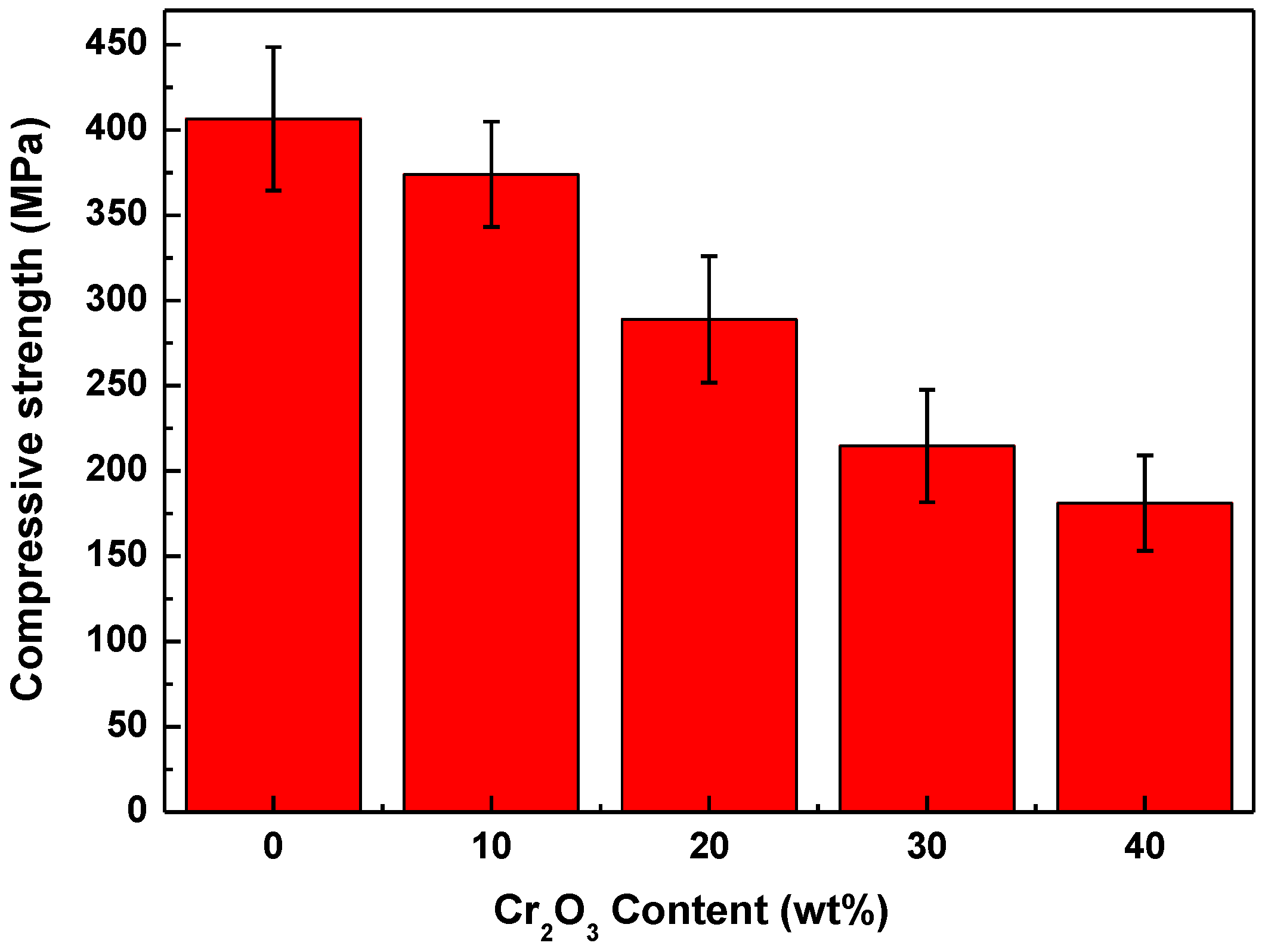
3.3.2. Compressive Strength
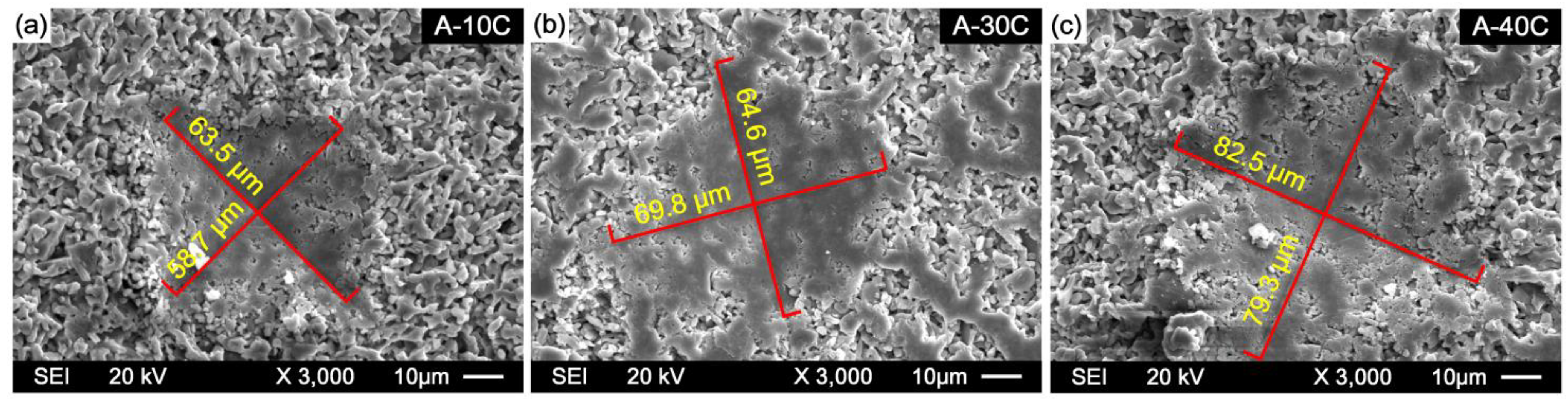
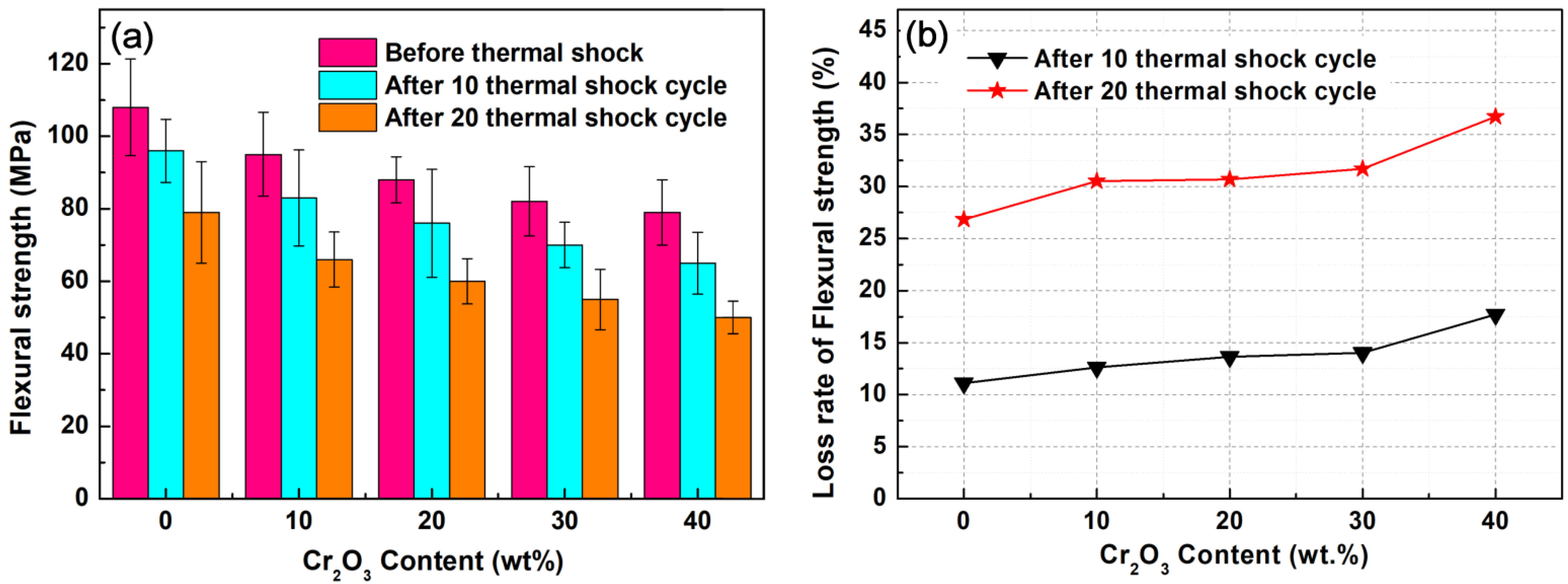
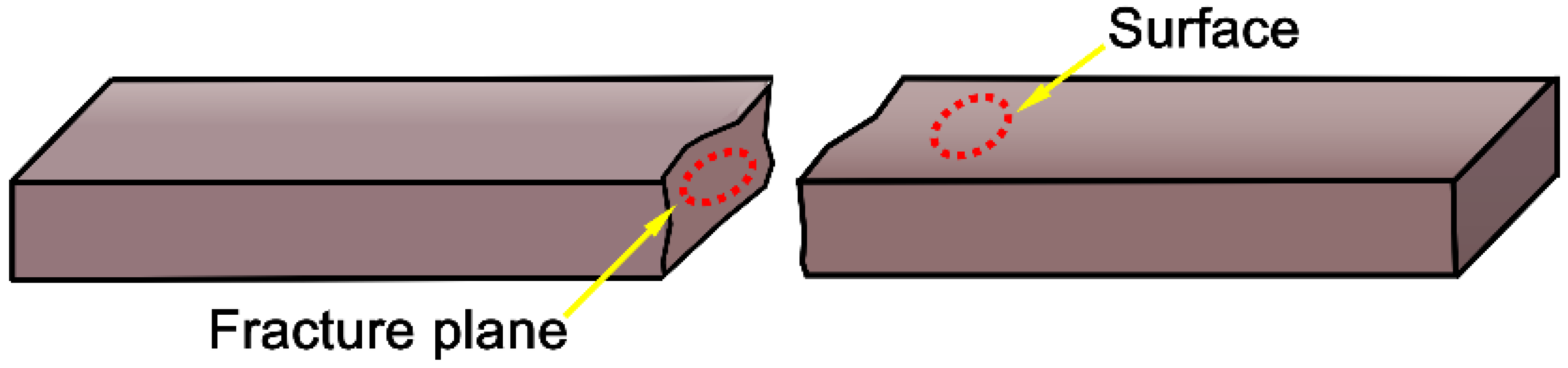
3.3.3. Thermal Shock Resistance and Flexural Strength
4. Conclusions
- The grain size and porosity increase gradually due to the formation of Al2O3-Cr2O3 solid solution phases; thus, the densification behavior of materials Al2O3-Cr2O3 gradually get worse along with the increase of content of Cr2O3. When the Cr2O3 content is 40 wt.%, the relative density and volume shrinkage rate of the Al2O3-Cr2O3 system achieve the minimum combined with the maximum porosity.
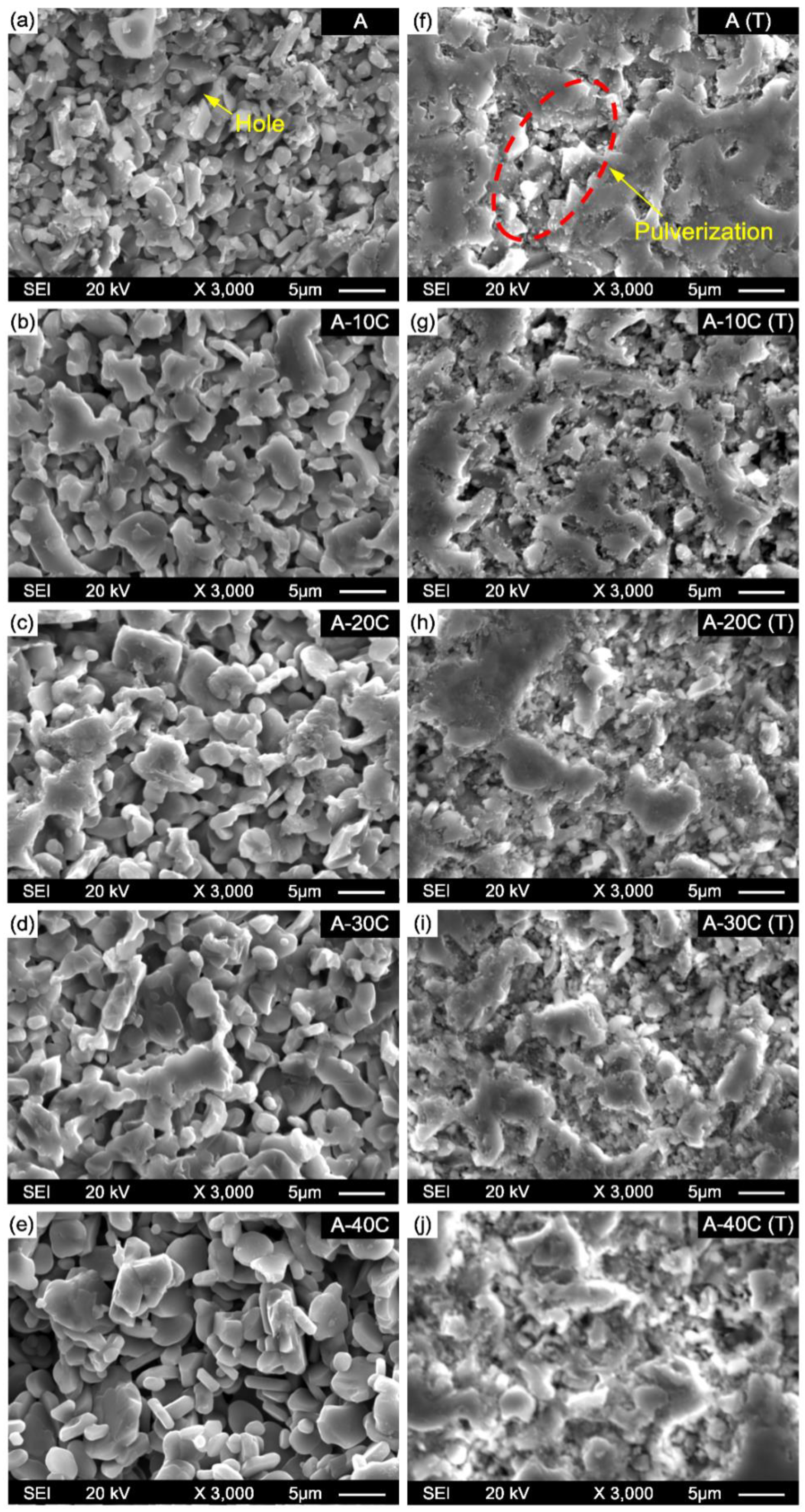
- Due to the reduction of densification degree, the material becomes progressively porous, and the fracture mode of the material changes from transgranular and intergranular mixed fracture to intergranular fracture mode. Meanwhile, the hardness, compressive strength, and flexural strength of Al2O3-Cr2O3 composites all decreased. When the content of Cr2O3 in the system exceeds 30 wt.%, the mechanical properties of the Al2O3-Cr2O3 material decrease significantly.
- After 10 and 20 cyclic thermal shocks, the flexural strength of the sample is reduced to varying degrees, and the fracture mode of composites is dominated by intercrystalline fracture. The flexural strength loss rate of samples gradually increases with the increase of Cr2O3 content. The maximum bending strength loss rate was observed when the Cr2O3 content is 40 wt.% after 10 and 20 thermal shock cycles; the flexural strength loss rates of sample A-40C were 17.72% and 36.71%, respectively. In addition, with the increase of Cr2O3 addition, the surface pulverization after thermal shock gradually becomes serious.
- Although the increase of Cr2O3 content deteriorates the mechanical properties of Al2O3-Cr2O3 composites, the composites still have better mechanical properties when the Cr2O3 content is 20–30% and can meet the service requirements of molten reduction ironmaking.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Meijer, K.; Zeilstra, C.; Teerhuis, C.; Ouwehand, M.; Van Der Stel, J. Developments in Alternative Ironmaking. Trans. Indian Inst. Met. 2013, 66, 475–481. [Google Scholar] [CrossRef]
- Kurunov, I.F. The direct production of iron and alternatives to the blast furnace in iron metallurgy for the 21st century. Metallurgist 2010, 54, 335–342. [Google Scholar] [CrossRef]
- Pervaiz, M.; Ahmad, I.; Yousaf, M.; Kirn, S.; Munawar, A.; Saeed, Z.; Rashid, A. Synthesis, spectral and antimicrobial studies of amino acid derivative Schiff base metal (Co, Mn, Cu, and Cd) complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Hasanbeigi, A.; Arens, M.; Price, L. Alternative emerging ironmaking technologies for energy-efficiency and carbon dioxide emissions reduction: A technical review. Renew. Sustain. Energy Rev. 2014, 33, 645–658. [Google Scholar] [CrossRef]
- Kashif, M.; Ngaini, Z.; Harry, A.V.; Vekariya, R.L.; Ahmad, A.; Zuo, Z.; Alarifi, A. An experimental and DFT study on novel dyes incorporated with natural dyes on titanium dioxide (TiO2) towards solar cell application. Appl. Phys. A 2020, 126, 1–13. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, L.; Chen, X.D. Analysis of Improving COREX-3000 Competence. J. Iron Steel Res. Int. 2009, 16, 1225–1227. [Google Scholar]
- Hussain, S.; Khan, A.J.; Arshad, M.; Javed, M.S.; Ahmad, A.; Shah, S.S.A.; Khan, M.R.; Akram, S.; Zulfiqar, S.; Ali, S.; et al. Charge storage in binder-free 2D-hexagonal CoMoO4 nanosheets as a redox active material for pseudocapacitors. Ceram. Int. 2020. [Google Scholar] [CrossRef]
- Li, J.Q.; Wang, W.W.; Gan, F.F.; Wu, J.G. Study on Corrosion of magnesium chromium materials by iron bath smelting reduction slag. Refractory 2011, 45, 1–5. (In Chinese) [Google Scholar]
- Zhang, X.Z.; Xu, P.H.; Liu, G.W.; Ahmad, A.; Chen, X.H.; Zhu, Y.L.; Qiao, G.J. Synthesis, characterization and wettability of Cu-Sn alloy on the Si-implanted 6H-SiC. Coatings 2020, 10, 906. [Google Scholar] [CrossRef]
- Ahmad, A.; Jini, D.; Aravind, M.; Parvathiraja, C.; Ali, R.; Kiyani, M.Z.; Alothman, A. A novel study on synthesis of egg shell based activated carbon for degradation of methylene blue via photocatalysis. Arab. J. Chem. 2020, 13, 8717–8722. [Google Scholar] [CrossRef]
- Aravind, M.; Ahmad, A.; Ahmad, I.; Amalanathan, M.; Naseem, K.; Mary, S.M.M.; Parvathiraja, C.; Hussain, S.; Algarni, T.S.; Pervaiz, M.; et al. Critical green routing synthesis of silver NPs using jasmine flower extract for biological activities and photocatalytical degradation of methylene blue. J. Environ. Chem. Eng. 2021, 9, 104877. [Google Scholar] [CrossRef]
- Naseem, K.; Rehman, M.Z.U.; Ahmad, A.; Dubal, D.; Algarni, T.S. Plant Extract Induced Biogenic Preparation of Silver Nanoparticles and Their Potential as Catalyst for Degradation of Toxic Dyes. Coatings 2020, 10, 1235. [Google Scholar] [CrossRef]
- Ahmad, A.; Mubharak, N.; Naseem, K.; Tabassum, H.; Rizwan, M.; Najda, A.; Kashif, M.; Bin-Jumah, M.; Hussain, A.; Shaheen, A.; et al. Recent advancement and development of chitin and chitosan-based nanocomposite for drug delivery: Critical approach to clinical research. Arab. J. Chem. 2020, 13, 8935–8964. [Google Scholar] [CrossRef]
- Zhan, M.; Hussain, S.; Algarni, T.S.; Shah, S.; Liu, J.; Zhang, X.; Ahmad, A.; Javed, M.S.; Qiao, G.; Liu, G. Facet controlled polyhedral ZIF-8 MOF nanostructures for excellent NO2 gas-sensing applications. Mater. Res. Bull. 2021, 136, 111133. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, J.; Zhang, Y.; Wei, Y.; Han, B.; Li, Y.; Zhang, S.; Li, N. Corrosion mechanism of Cr2O3-Al2O3-ZrO2 refractories in a coal-water slurry gasifier: A post-mortem analysis. Corros. Sci. 2020, 163, 108250. [Google Scholar] [CrossRef]
- Ahmad, A.; Mubarak, N.; Jannat, F.T.; Ashfaq, T.; Santulli, C.; Rizwan, M.; Najda, A.; Bin-Jumah, M.; Abdel-Daim, M.M.; Hussain, S.; et al. A Critical Review on the Synthesis of Natural Sodium Alginate Based Composite Materials: An Innovative Biological Polymer for Biomedical Delivery Applications. Processes 2021, 9, 137. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Ahmad, A.; Karri, R.R.; Pakalapati, H. Functionalized multi-walled carbon nanotubes and hydroxyapatite nanorods reinforced with polypropylene for biomedical application. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- KKashif, M.; Jafaar, E.; Sahari, S.K.; Low, F.W.; Hoa, N.D.; Ahmad, A.; Abbas, A.; Ngaini, Z.; Shafa, M.; Qurashi, A. Organic sensitization of graphene oxide and reduced graphene oxide thin films for photovoltaic applications. Int. J. Energy Res. 2021. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, P.; Chen, J.; Zhao, H.; He, K. Corrosion resistance of Al–Cr-slag containing chromium–corundum refractories to slags with different basicity. Ceram. Int. 2018, 44, 12162–12168. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Bai, C. Microstructure and oxidation behaviour of Si–MoSi2 functionally graded coating on Mo substrate. Ceram. Int. 2017, 43, 6250–6256. [Google Scholar] [CrossRef]
- Pan, D.; Zhao, H.; Zhang, H.; Zhao, P.; Li, Y.; Zou, Q. Corrosion mechanism of spray refractory in COREX slag with varying basicity. Ceram. Int. 2019, 45, 24398–24404. [Google Scholar] [CrossRef]
- Li, P.T.; Sun, H.G.; Li, J.Q.; Yan, S.Z.; Du, Y.H. Different compositions of Al2O3-Cr2O3 Study on the resistance to smelting reduction slag of No.3 brick. Refractory 2016, 50, 352–358. (In Chinese) [Google Scholar]
- Yıldız, B.K.; Yılmaz, H.; Tür, Y.K. Evaluation of mechanical properties of Al2O3–Cr2O3 ceramic system prepared in different Cr2O3 ratios for ceramic armour components. Ceram. Int. 2019, 45, 20575–20582. [Google Scholar] [CrossRef]
- Xu, X.; Li, J.; Wu, J.; Tang, Z.; Chen, L.; Li, Y.; Lu, C. Preparation and thermal shock resistance of corundum-mullite composite ceramics from andalusite. Ceram. Int. 2017, 43, 1762–1767. [Google Scholar] [CrossRef]
- Kirkaldy, J.S. Flux-independent theory of nonlinear diffusion for Vegard’s law solutions. Mater. Sci. Eng. A 2007, 444, 104–111. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, H.; Yu, J.; Zhang, H.; Gao, H.; Chen, Q. Crystal structure and properties of Al2O3-Cr2O3 solid solutions with different Cr2O3 contents. Ceram. Int. 2018, 44, 1356–1361. [Google Scholar] [CrossRef]
- Bondioli, F.; Ferrari, A.M.; Leonelli, C.; Manfredini, T.; Linati, L.; Mustarelli, P. Reaction Mechanism in Alumina/Chromia (Al2O3-Cr2O3) Solid Solutions Obtained by Coprecipitation. J. Am. Ceram. Soc. 2000, 83, 2036–2040. [Google Scholar] [CrossRef]
- Nath, M.; Kumar, P.; Maldhure, A.V.; Sinhamahapatra, S.; Dana, K.; Ghosh, A.; Tripathi, H.S. Anomalous densification behaviour of Al2O3–Cr2O3 system. Mater. Charact. 2016, 111, 8–13. [Google Scholar] [CrossRef]
- Shu, X.M.; Sang, S.B.; Wu, S.J.; Xia, C.Y.; Wu, L.S. Preparation of lightweight mullite aggregate from kaolin and its effect on properties of mullite SiC refractories. Refractory 2020, 54, 19–23. (In Chinese) [Google Scholar]
- Xiao, C.J.; Li, J.; Li, Z.X. Effect of carbon content on Al2O3 Effect of MgO-C refractories on properties. Foshan Ceram. 2015, 25, 9–11. (In Chinese) [Google Scholar]
- Yi, J.G.; Zhu, B.Q.; Li, X.C. Effect of carbon embedded heat treatment temperature on phase composition, microstructure and mechanical properties of low carbon MgO-C materials. Refractory 2014, 48, 170–173. (In Chinese) [Google Scholar]
- Tong, Y.; Zhu, W.; Bai, S.; Hu, Y.; Xie, X.; Li, Y. Thermal shock resistance of continuous carbon fiber reinforced ZrC based ultra-high temperature ceramic composites prepared via Zr-Si alloyed melt infiltration. Mater. Sci. Eng. A 2018, 735, 166–172. [Google Scholar] [CrossRef]



| Material | Particle Diameter (μm) | Purity (wt.%) | Resource |
|---|---|---|---|
| Al2O3 | 1–8 | >99.5% | Henan, China/Ou shang |
| Cr2O3 | 0.1–1 | >99.5% | Hebei, China/Qing guang |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, K.; Zhang, Y.; Fu, T.; Hussain, S.; Saad Algarni, T.; Wang, J.; Zhang, X.; Ali, S. RETRACTED: Effects of Cr2O3 Content on Microstructure and Mechanical Properties of Al2O3 Matrix Composites. Coatings 2021, 11, 234. https://doi.org/10.3390/coatings11020234
Cui K, Zhang Y, Fu T, Hussain S, Saad Algarni T, Wang J, Zhang X, Ali S. RETRACTED: Effects of Cr2O3 Content on Microstructure and Mechanical Properties of Al2O3 Matrix Composites. Coatings. 2021; 11(2):234. https://doi.org/10.3390/coatings11020234
Chicago/Turabian StyleCui, Kunkun, Yingyi Zhang, Tao Fu, Shahid Hussain, Tahani Saad Algarni, Jie Wang, Xu Zhang, and Shafaqat Ali. 2021. "RETRACTED: Effects of Cr2O3 Content on Microstructure and Mechanical Properties of Al2O3 Matrix Composites" Coatings 11, no. 2: 234. https://doi.org/10.3390/coatings11020234
APA StyleCui, K., Zhang, Y., Fu, T., Hussain, S., Saad Algarni, T., Wang, J., Zhang, X., & Ali, S. (2021). RETRACTED: Effects of Cr2O3 Content on Microstructure and Mechanical Properties of Al2O3 Matrix Composites. Coatings, 11(2), 234. https://doi.org/10.3390/coatings11020234








