Hypothesis on the Influence of the Magnetic Behaviour of Hydrogen Doped Zinc Oxide during Its Plasma Sputtering Process
Abstract
1. Introduction
2. Experimental
2.1. Thin Film Deposition
2.2. Spectral Data Collection from the Plasma Emissions
2.3. Sample Characterization
3. Results and Discussion
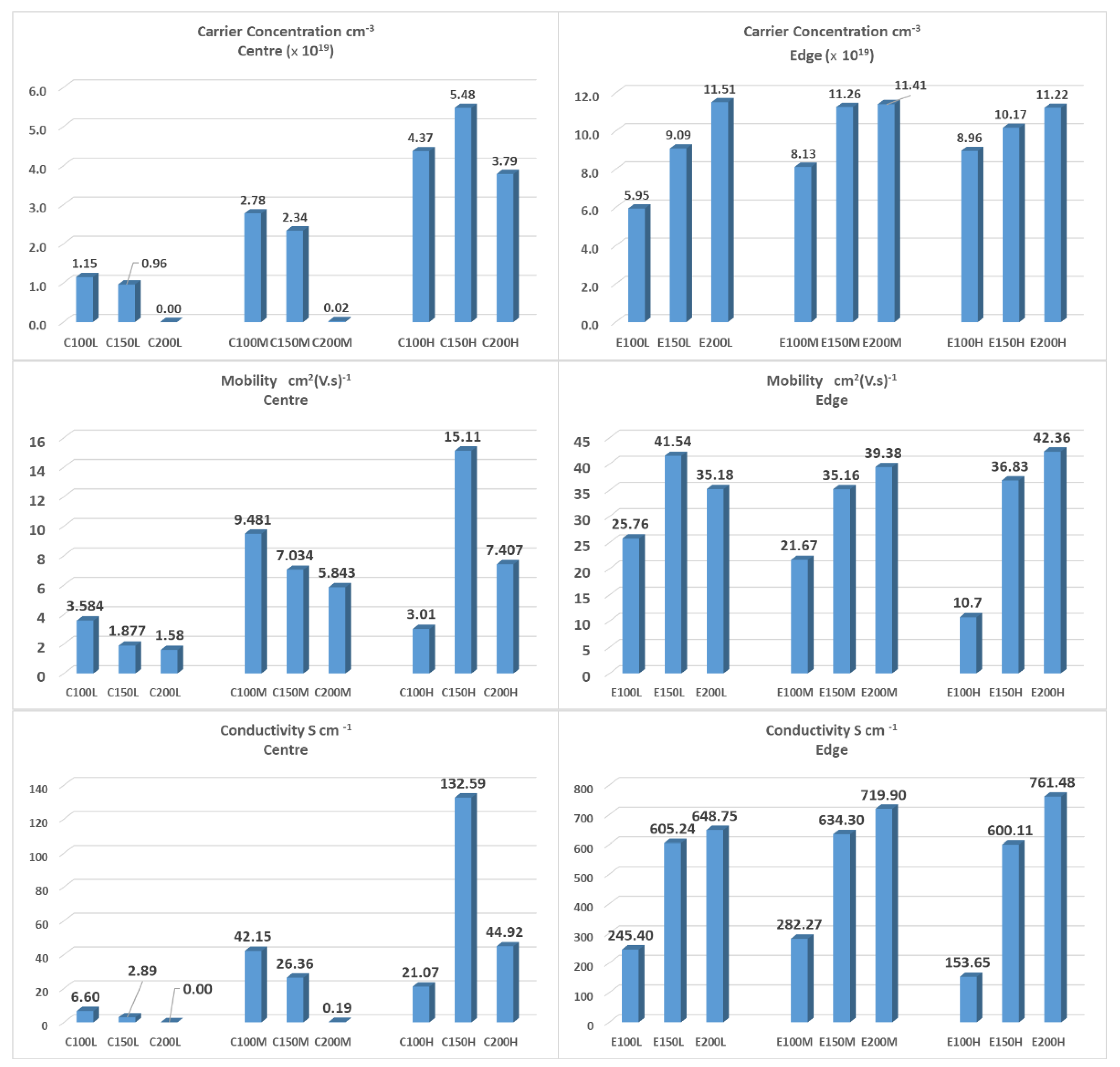
3.1. Electrical Properties
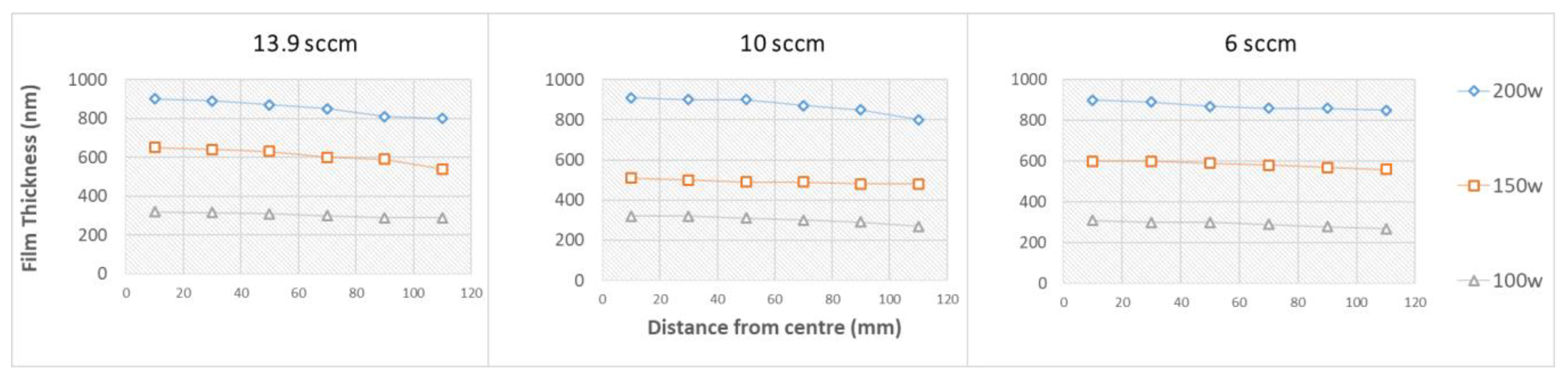
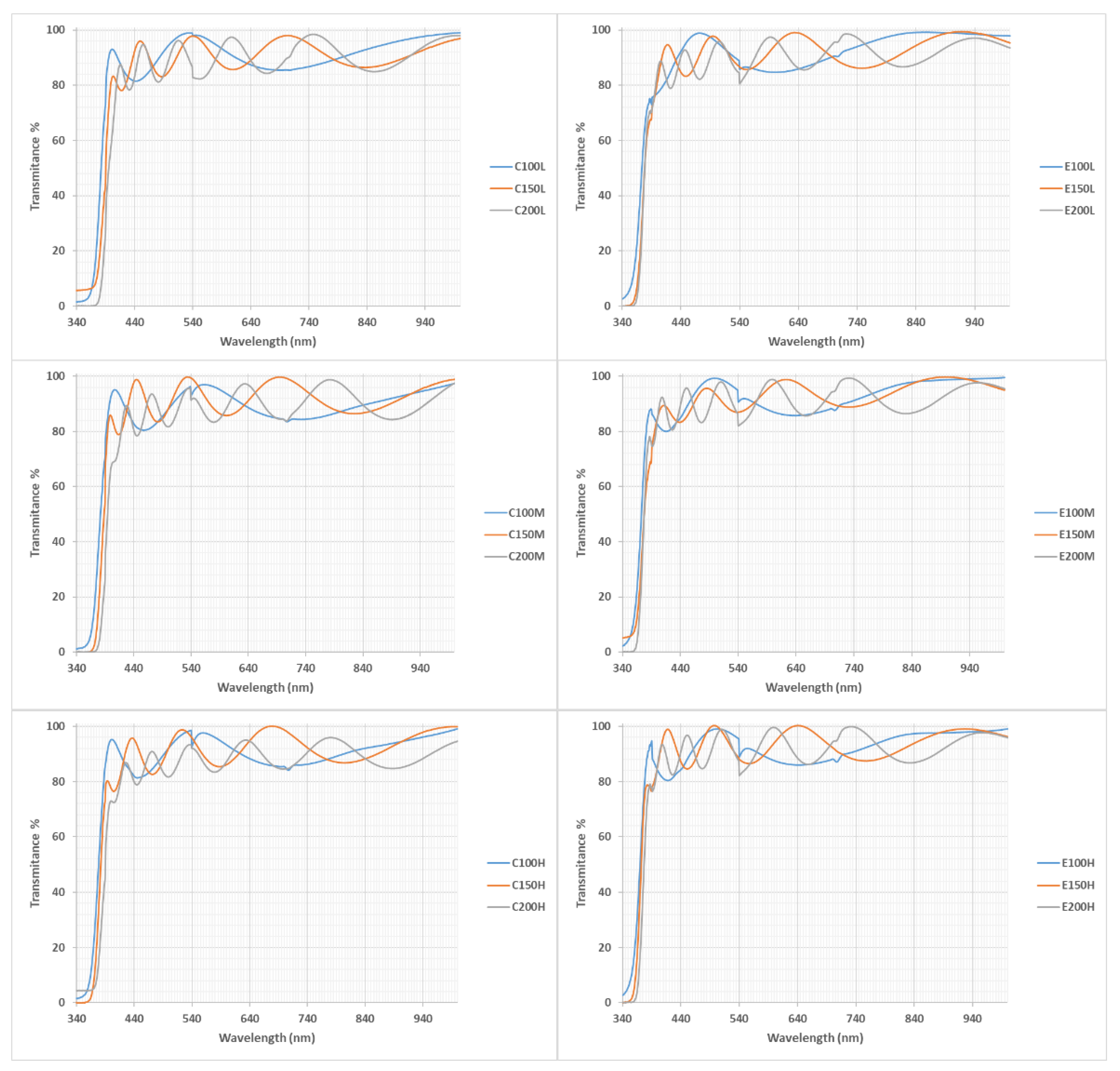
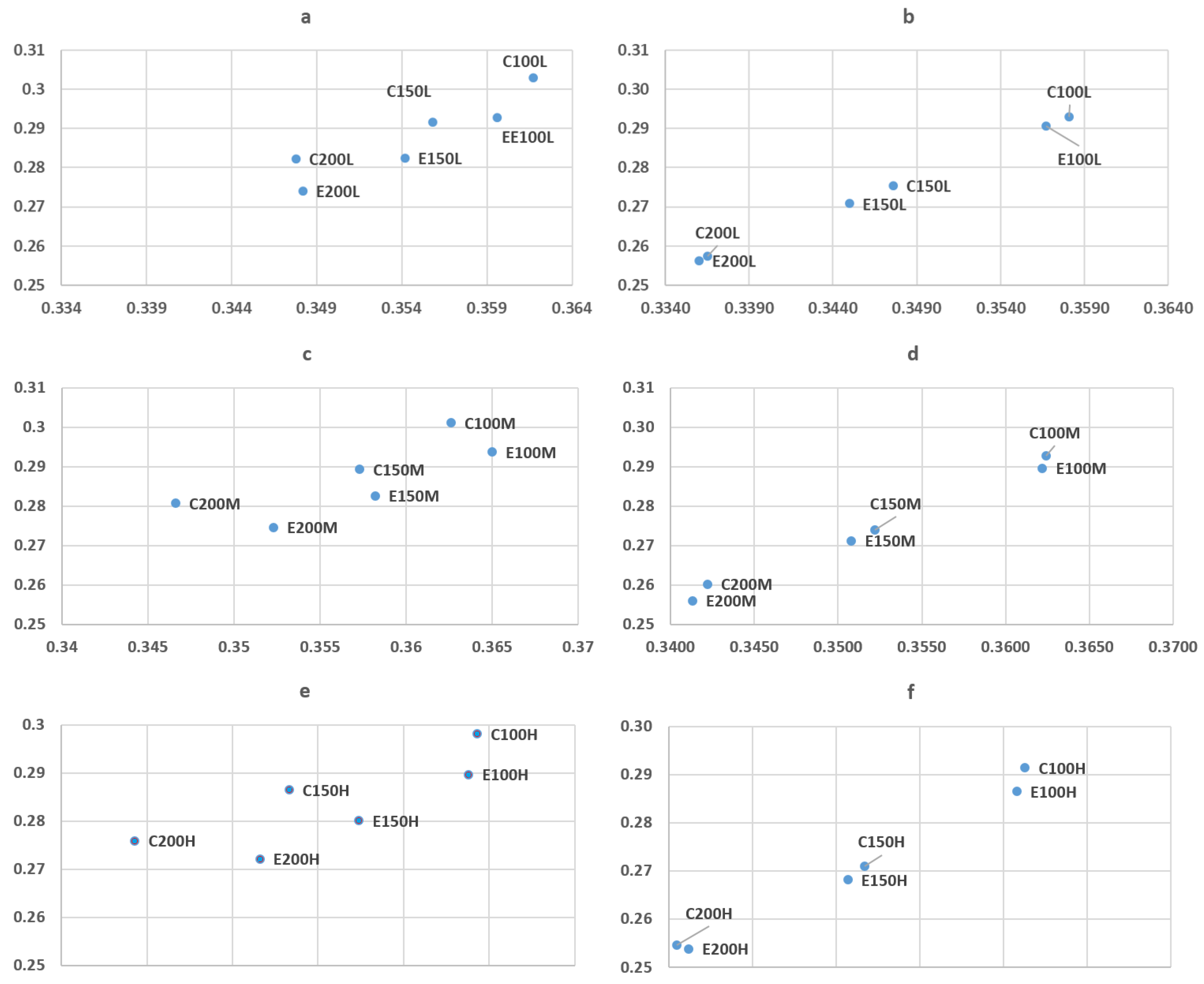
3.2. Film Thickness and Optical Properties
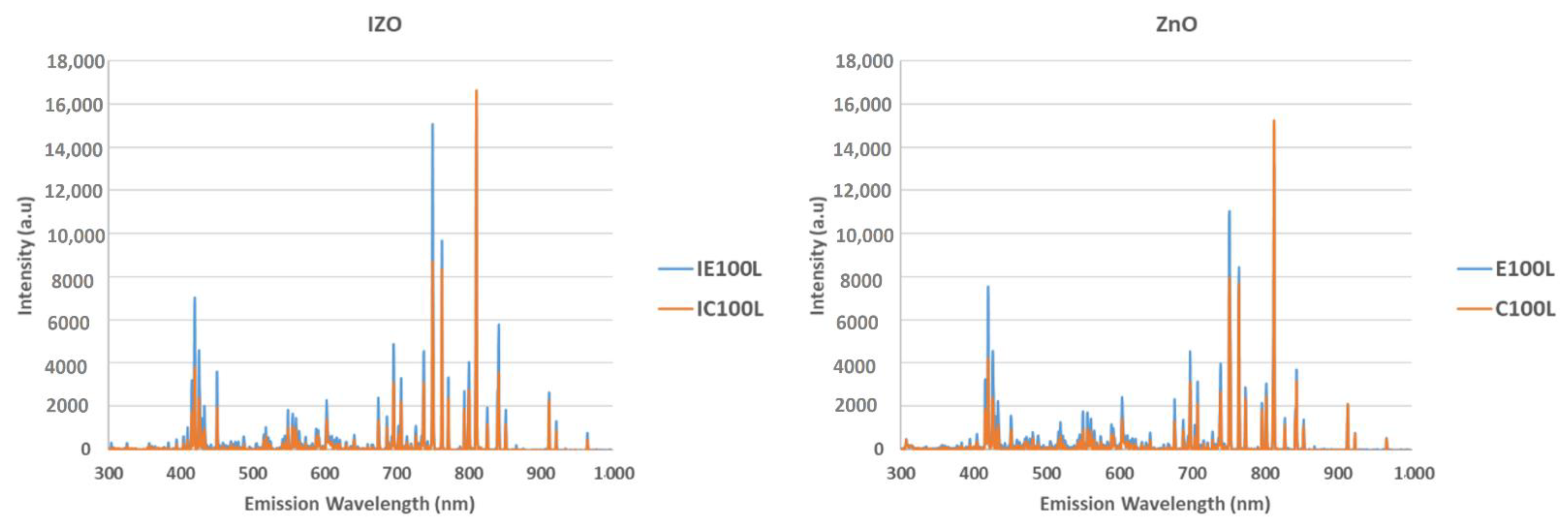
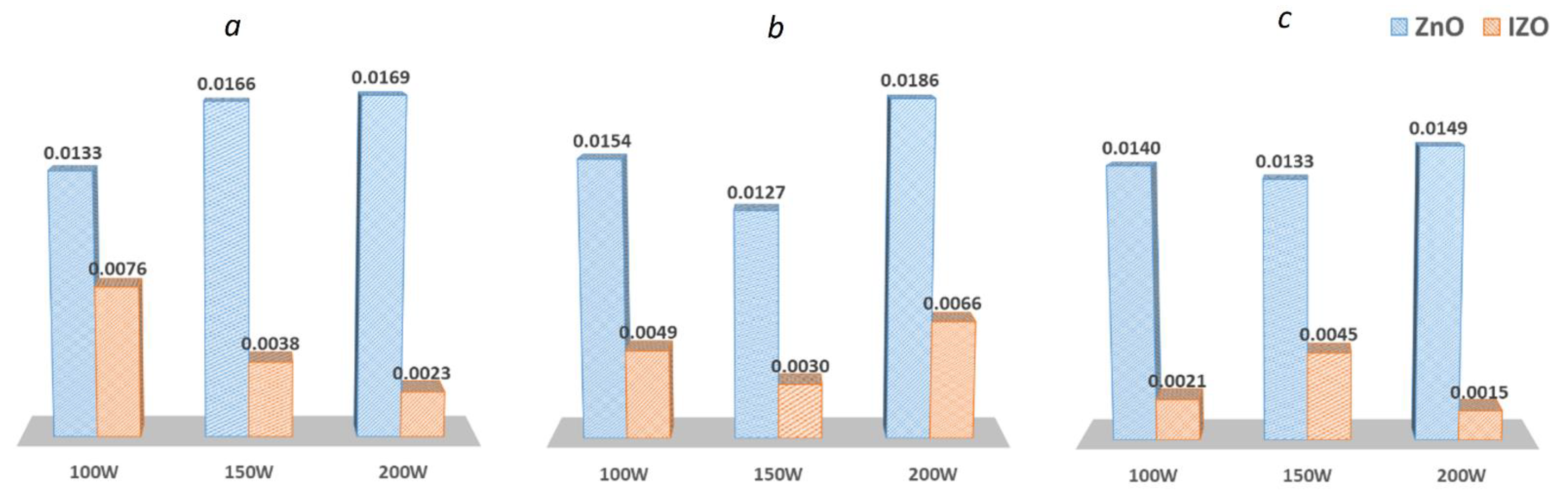
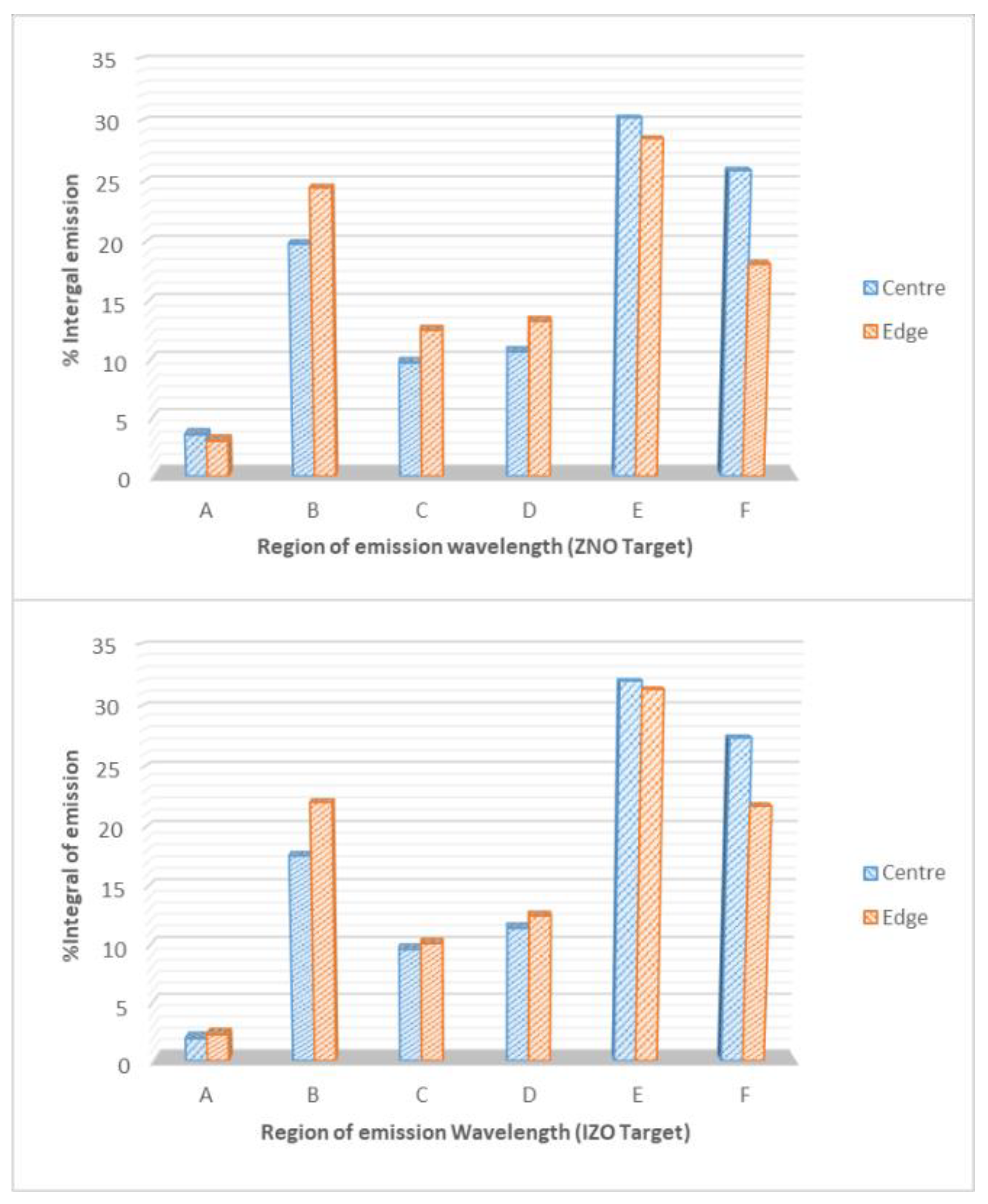
3.3. Analysis of the Plasma Emissions
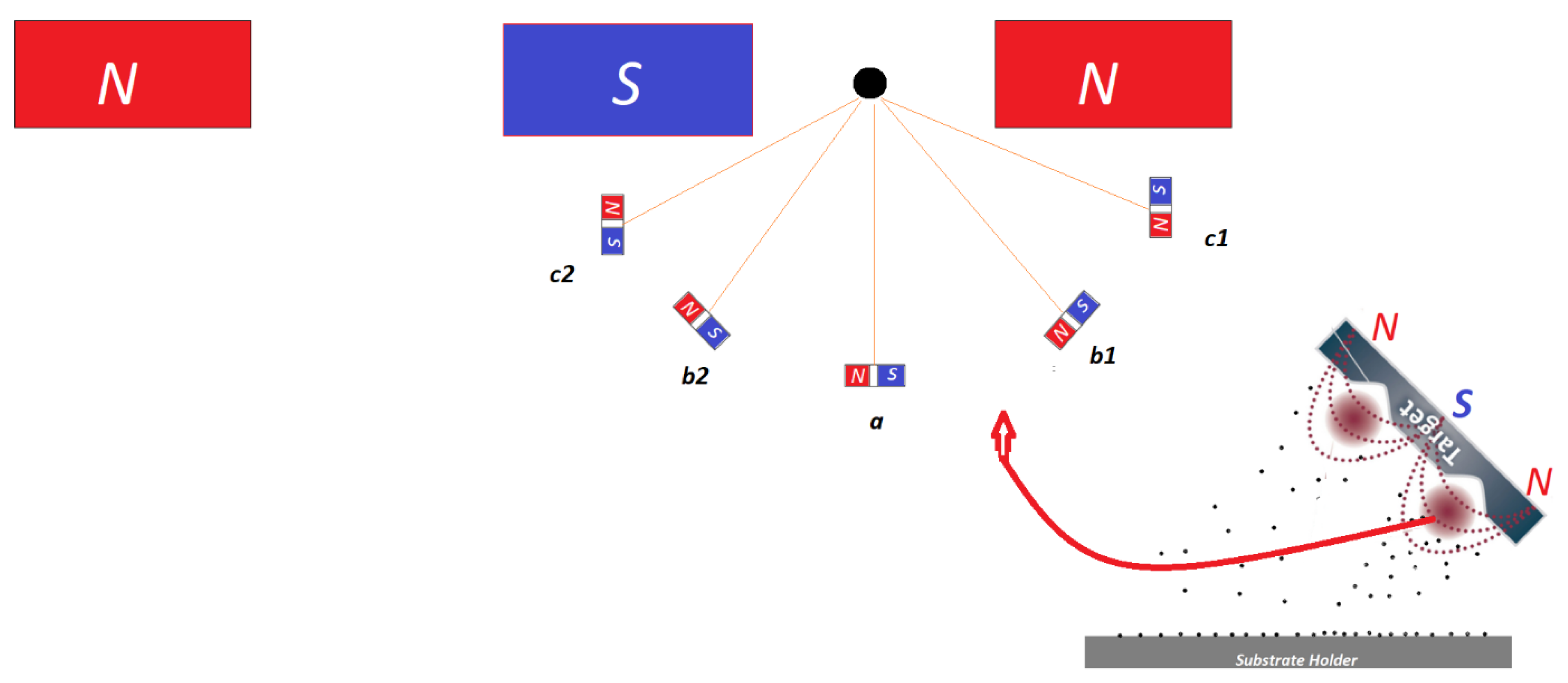
- Firstly, hydrogen ions (along with argon ions) bombard the surface of the target material, resulting in ejection of surface atoms from the surface of the target, while some of these ions will also integrate into the target material.
- Secondly, the integration of the hydrogen into the surface of the target material as discussed and based on referenced literature may lead to formation of regions at the target surface possessing complex magnetic behaviour.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ginley, D.S.; Hosono, H.; Paine, D.C. Handbook of Transparent Conductors; Springer: Berlin, Germany, 2011; pp. 353–425. [Google Scholar]
- Ginley, D.S.; Bright, C. Transparent Conducting Oxides. MRS Bull. 2000, 25, 15–18. [Google Scholar] [CrossRef]
- Hosono, H. Recent progress in transparent oxide semiconductors: Materials and device application. Thin Solid Films 2007, 515, 6000–6014. [Google Scholar] [CrossRef]
- Ellmer, K. Past achievements and future challenges in the development of optically transparent electrodes. Nat. Photonics 2012, 6, 809–817. [Google Scholar] [CrossRef]
- Granqvist, C.G. Transparent conductors as solar energy materials: A panoramic review. Sol. Energy Mater. Sol. Cells 2007, 91, 1529–1598. [Google Scholar] [CrossRef]
- King, P.; Veal, T.D. Conductivity in transparent oxide semiconductors. J. Phys. Condens. Matter 2011, 23, 334214. [Google Scholar] [CrossRef]
- Calnan, S.; Tiwari, A.N. High mobility transparent conducting oxides for thin film solar cells. Thin Solid Films 2010, 518, 1839–1849. [Google Scholar] [CrossRef]
- Khachatryan, H.; Kim, D.J.; Kim, M.; Kim, H.-K. Roll-to-Roll fabrication of ITO thin film for flexible optoelectronics appli-cations: The role of post-annealing. Mater. Sci. Semicond. Process 2018, 88, 51–56. [Google Scholar] [CrossRef]
- Morales-Masis, M.; De Nicolas, S.M.; Holovsky, J.; De Wolf, S.; Ballif, C. Low-Temperature High-Mobility Amorphous IZO for Silicon Heterojunction Solar Cells. IEEE J. Photovoltaics 2015, 5, 1340–1347. [Google Scholar] [CrossRef]
- Martins, R.; Almeida, P.; Barquinha, P.; Pereira, L.; Pimentel, A.; Ferreira, I.; Fortunato, E. Electron transport and optical characterization amorphous indium zinc oxide films. J. Non Cryst. Solids 2006, 352, 1471–1474. [Google Scholar] [CrossRef]
- Taylor, M.P.; Readey, D.W.; Van Hest, M.F.A.M.; Teplin, C.W.; Alleman, J.L.; Dabney, M.S.; Gedvilas, L.M.; Keyes, B.M.; To, B.; Perkins, J.D.; et al. The remarkable thermal stability of amorphous In-Zn-O transparent conductors. Adv. Funct. Mater. 2008, 18, 3169–3178. [Google Scholar] [CrossRef]
- Park, Y.R.; Nam, E.-K.; Boo, J.-H.; Jung, D.-G.; Suh, S.-J.; Kim, Y.-S. Hydrogenated In-doped ZnO thin films for the new anode material of organic light-emitting devices: Synthesis and application test. Bull. Korean Chem. Soc. 2007, 28, 2396–2400. [Google Scholar]
- Park, Y.R.; Kim, J.; Kim, Y.S. Growth and characteristics of hydrogenated In-doped ZnO thin films by pulsed DC magnetron sputtering. Appl. Surf. Sci. 2009, 256, 1589–1594. [Google Scholar] [CrossRef]
- Bädeker, K. The electrical conductivity and thermoelectric power of some heavy metal compounds. Ann. Phys. 1907, 327, 749–766. [Google Scholar] [CrossRef]
- Minami, T. New n-type transparent conducting oxides. MRS Bull. 2000, 25, 38–44. [Google Scholar] [CrossRef]
- Mollwo, E. The effect of hydrogen on the conductivity and luminescence of zinc oxide crystals. Z. Phys. 1954, 138, 478–488. [Google Scholar] [CrossRef]
- Thomas, D.G.; Lander, J.J. Hydrogen as a Donor in Zinc Oxide. J. Chem. Phys. 1956, 25, 1136–1142. [Google Scholar] [CrossRef]
- Van de Walle, C.G. Hydrogen as a Cause of Doping in Zinc Oxide. Phys. Rev. Lett. 2000, 85, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Kohan, F.; Ceder, G.; Morgan, D.; Van de Walle, C.G. First-principles study of native point defects in ZnO. Phys. Rev. B 2000, 61, 15019. [Google Scholar] [CrossRef]
- Hofmann, D.M.; Hofstaetter, A.; Leiter, F.; Zhou, H.; Henecker, F.; Meyer, B.K.; Orlinskii, S.B.; Schmidt, J.; Baranov, P.G. Hydrogen: A Relevant Shallow Donor in Zinc Oxide. Phys. Rev. Lett. 2002, 88, 045504. [Google Scholar] [CrossRef]
- Cox, S.F.J.; Davis, E.A.; Cottrell, S.P.; King, P.J.C.; Lord, J.S.; Gil, J.M.; Alberto, H.V.; Vilão, R.C.; Duarte, J.P.; De Campos, N.A.; et al. Experimental Confirmation of the Predicted Shallow Donor Hydrogen State in Zinc Oxide. Phys. Rev. Lett. 2001, 86, 2601–2604. [Google Scholar] [CrossRef]
- Van de Walle, C.G. Hydrogen as a shallow centre in semiconductors and oxides. Phys. Status Solidi b 2003, 235, 89–95. [Google Scholar] [CrossRef]
- Hosono, H.; Ueda, K. Transparent Conductive Oxides. In Springer Handbook of Electronic and Photonic Materials; Springer: Berlin, Germany, 2017. [Google Scholar] [CrossRef]
- Yan, X.; Tian, L.; Tan, X.; Zhou, M.; Liu, L.; Chen, X. Modifying oxide nanomaterials’ properties by hydrogenation. MRS Commun. 2016, 6, 192–203. [Google Scholar] [CrossRef]
- Li, T.; Ong, C.S.; Herng, T.S.; Yi, J.; Bao, N.; Xue, J.M.; Feng, Y.P.; Ding, J. Surface ferromagnetism in hydrogenated-ZnO film. Appl. Phys. Lett. 2011, 98. [Google Scholar] [CrossRef]
- Park, J.K.; Kwon, H.-J.; Lee, C.E. NMR Observation of Mobile Protons in Proton-Implanted ZnO Nanorods. Sci. Rep. 2016, 6, 23378. [Google Scholar] [CrossRef] [PubMed]
- Robaina, O.V.; Cabrera, A.F.; Meyer, M.; Romano, R.M.; Cruz, A.F.; Torres, C.E.R. Room-Temperature Ferromagnetism Induced by High-Pressure Hydrogenation of ZnO. J. Phys. Chem. C 2019, 123, 19851–19861. [Google Scholar] [CrossRef]
- Esquinazi, P.; Hergert, W.; Spemann, D.; Setzer, A.; Ernst, A. Defect-Induced Magnetism in Solids. IEEE Trans. Magn. 2013, 49, 4668–4674. [Google Scholar] [CrossRef]
- Salimian, A.; Hasnath, A.; Anguilano, L.; Onwukwe, U.; Aminishahsavarani, A.; Sachez, C.; Upadhyaya, H. Highly Conductive Zinc Oxide Based Transparent Conductive Oxide Films Prepared Using RF Plasma Sputtering Under Reducing Atmosphere. Coatings 2020, 10, 472. [Google Scholar] [CrossRef]
- Salimian, A.; Haghpanahan, R.; Hasnath, A.; Upadhyaya, H. Optical analysis of RF sputtering plasma through colour char-acterization. Coatings 2019, 9, 315. [Google Scholar] [CrossRef]
- Hautier, G.; Miglio, A.; Waroquiers, D.; Rignanese, G.-M.; Gonze, X. How Does Chemistry Influence Electron Effective Mass in Oxides? A High-Throughput Computational Analysis. Chem. Mater. 2014, 26, 5447–5458. [Google Scholar] [CrossRef]
- Wallinga, J.; Arnoldbik, W.M.; Vredenberg, A.M.; Schropp, R.E.I.; Van Der Weg, W.F. Reduction of Tin Oxide by Hydrogen Radicals. J. Phys. Chem. B 1998, 102, 6219–6224. [Google Scholar] [CrossRef]
- De Wit, J. Electrical properties of In2O3. J. Solid State Chem. 1973, 8, 142–149. [Google Scholar] [CrossRef]
- Dewit, J.H.W.; Vanunen, G.; Lahey, M. Electron concentration and mobility in In2O3. J. Phys. Chem. Solids 1997, 38, 819–824. [Google Scholar] [CrossRef]
- Gordon, M.H.; Kruger, C.H. Non-equilibrium effects of diluent addition in recombining argon plasma. Phys. Fluids B Plasma Phys. 1993, 5, 1014. [Google Scholar] [CrossRef]
- Meulenbroeks, R.F.G.; Van Beek, A.J.; Van Helvoort, A.J.G.; Van De Sanden, M.C.M.; Schram, D.C. Argon-hydrogen plasma jet investigated by active and passive spectroscopic means. Phys. Rev. E 1994, 49, 4397–4406. [Google Scholar] [CrossRef]
- Mason, R.S.; Miller, P.D.; Mortimer, I.P. Anomalous loss of ionization in argon-hydrogen plasma studied by fast flow glow discharge mass spectrometry. Phys. Rev. E 1997, 55, 7462–7472. [Google Scholar] [CrossRef]
- Tabares, F.L.; Tafalla, D. Sputtering of metallic walls in Ar/H2 direct current glow discharges at room temperature. J. Vac. Sci. Technol. A 1996, 14, 3087–3091. [Google Scholar] [CrossRef]
- Budtz-Jorgensen, C.V.; Kringhoj, P.; Bottiger, J. The critical role of hydrogen for physical sputtering with Ar–H2 glow dis-charges. Surf. Coat. Technol. 1999, 116, 938–943. [Google Scholar] [CrossRef]
- Smithwick, R.W., III; Lynch, D.W.; Franklin, J.C. Relative ion yields measured with a high-resolution glow discharge mass spectrometer operated with an argon/hydrogen mixture. J. Am. Soc. Mass Spectrom. 1993, 4, 278–285. [Google Scholar] [CrossRef]
- Knewstubb, P.F.; Tickner, A.W. Mass Spectrometry of Ions in Glow Discharges. I. Apparatus and Its Application to the Positive Column in Rare Gases. J. Chem. Phys. 1962, 36, 674–683. [Google Scholar] [CrossRef]
- Luo, S.; Kohiki, S.; Okada, K.; Kohno, A.; Tajiri, T.; Arai, M.; Ishii, S.; Sekiba, D.; Mitome, M.; Shoji, F. Effects of Hydrogen in Working Gas on Valence States of Oxygen in Sputter-Deposited Indium Tin Oxide Thin Films. ACS Appl. Mater. Interfaces 2010, 2, 663–668. [Google Scholar] [CrossRef][Green Version]
- Bales, G.S.; Bruinsma, R.; Eklund, E.A.; Karunasiri, R.P.U.; Rudnick, J.; Zangwill, A. Growth and Erosion of Thin Solid Films. Science 1990, 249, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Horkel, M.; van Aeken, K.; Eisenmenger-Sittner, C.; Depla, D.; Mahieu, S.; Leroy, W.P. Experimental determination and simulation of the angular disruption of the metal flux during magnetron sputter deposition. J. Phys. D Appl. Phys. 2010, 43. [Google Scholar] [CrossRef]
- Hippler, R.; Hubicka, Z.; Cada, M.; Ksirova, P.; Wulff, H.; Helm, C.A.; Stranak, V. Angular dependence of plasma parameters and film properties during high power impulse magnetron sputtering for deposition of Ti and TiO2 layers. J. Appl. Phys. 2017, 121, 171906. [Google Scholar] [CrossRef]
- Hofer, W.O. Angular, energy, and mass distribution of sputtered particles. In Sputtering by Particle Bombardment III; Behrisch, R., Wirrmaack, K., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; Volume 64, p. 15. [Google Scholar]
- Urbassek, H.M.; Hofer, W.O. Sputtering of molecules and clusters. Mat. Fys. Medd. 1993, 43, 97–125. [Google Scholar]
- Sundqvist, B.U.R. Desorption of organic molecules from solid and liquid surfaces induced by particle impact. In Sputtering by Particle Bombardment III; Behrisch, R., Wirrmaack, K., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; Volume 64, p. 257. [Google Scholar]
- Gerhard, W.; Villalba, V.; Maggiolo, A.R. A model calculation of the neutral molecule emission by sputtering processes. Eur. Phys. J. B 1975, 22, 31–39. [Google Scholar] [CrossRef]
- Können, G.P.; Tip, A.; De Vries, A.E. On the energy distribution of sputtered dimers. Radiant. Eff. 1974, 21, 269–274. [Google Scholar] [CrossRef]
- Konnen, G.P.; Tip, A.; de Vries, A.E. On the energy distribution of sputtered Clustrs. Radiant. Eff. 1975, 26, 23–29. [Google Scholar] [CrossRef]
- Honda, F.; Lancaster, G.M.; Fukuda, Y.; Rabalais, J.W. SIMS study of the mechanism of cluster formation during ion bombardment of alkali halides. J. Chem. Phys. 1978, 69, 4931–4937. [Google Scholar] [CrossRef]
- Galera, R.; Blais, J.; Bolbach, G. Molecular sputtering and damage induced by kiloelectron ions in organic monolayer—Metal systems. Int. J. Mass Spectrom. Ion Process. 1991, 107, 531–543. [Google Scholar] [CrossRef]
- Haff, P.K.; Watson, C.C.; Yung, Y.L. Sputter ejection of matter from Io. J. Geophys. Res. Space Phys. 1981, 86, 6933–6938. [Google Scholar] [CrossRef]
- King, B.V.; Ziv, A.R.; Lin, S.H.; Tsong, I.S.T. Mass distribution of ejected molecules and clusters in nanocascade sputtering processes. J. Chem. Phys. 1985, 82, 3641–3645. [Google Scholar] [CrossRef]
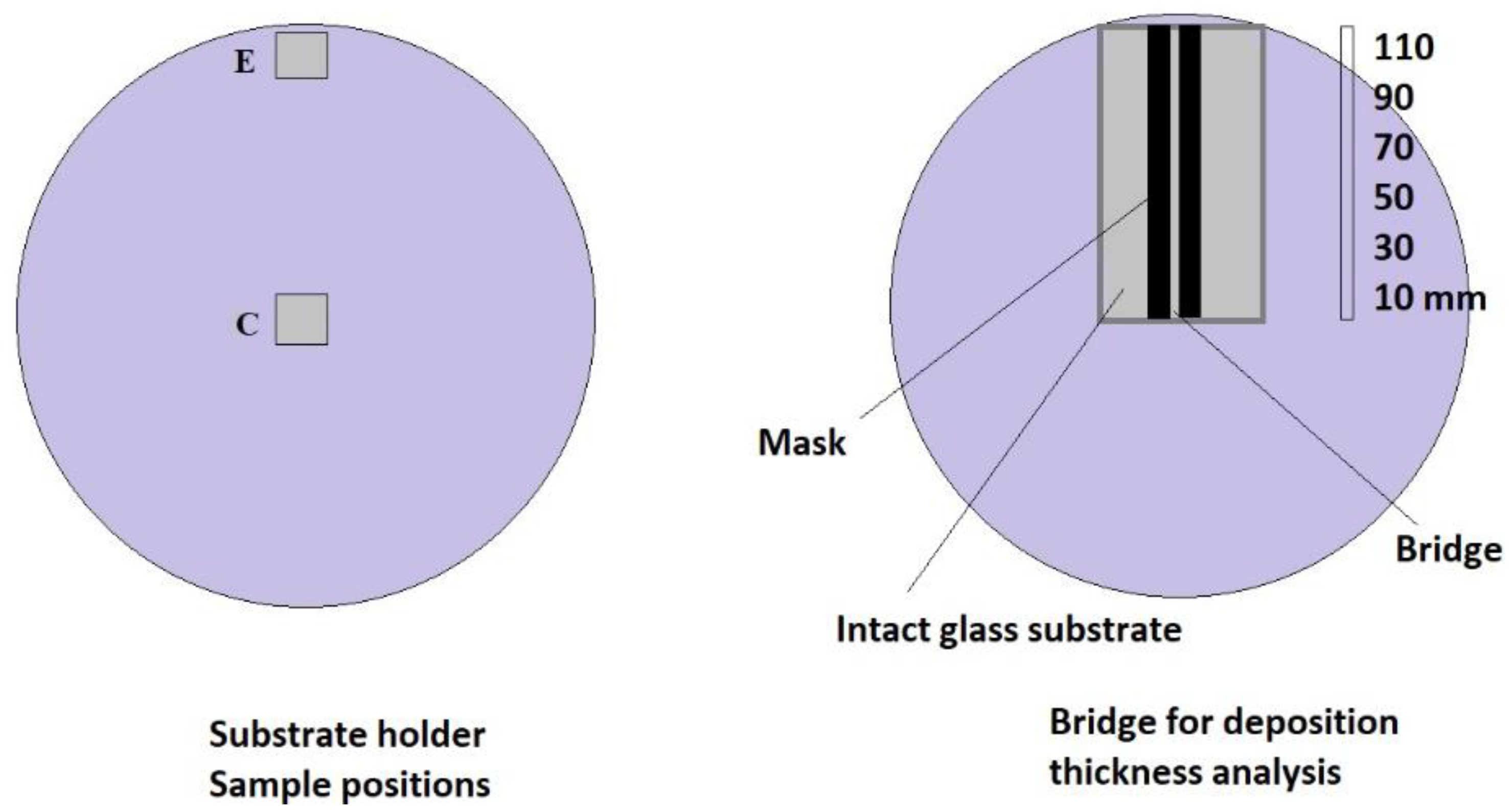
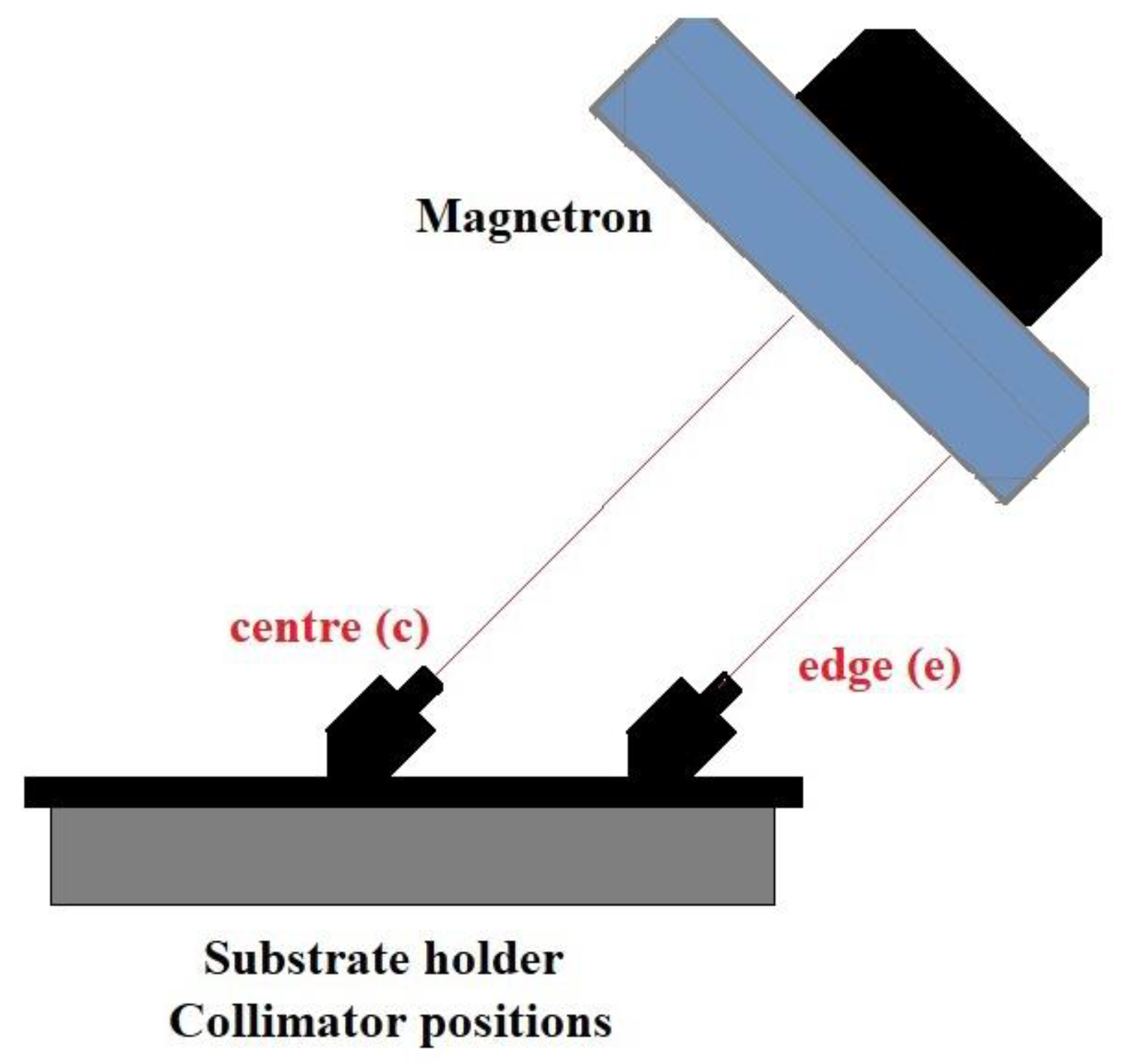


| Sample Name | Position on Substrate Holder | Plasma Power (w) | Gas Flow Rate (sccm) |
|---|---|---|---|
| E100H | E | 100 | 13.9(H) |
| E100M | E | 100 | 10(M) |
| E100L | E | 100 | 6(L) |
| E150H | E | 150 | 13.9(H) |
| E150M | E | 150 | 10(M) |
| E150L | E | 150 | 6(L) |
| E200H | E | 200 | 13.9(H) |
| E200M | E | 200 | 10(M) |
| E200L | E | 200 | 6(L) |
| C100H | C | 100 | 13.9(H) |
| C100M | C | 100 | 10(M) |
| C100L | C | 100 | 6(L) |
| C150H | C | 150 | 13.9(H) |
| C150M | C | 150 | 10(M) |
| C150L | C | 150 | 6(L) |
| C200H | C | 200 | 13.9(H) |
| C200M | C | 200 | 10(M) |
| C200L | C | 200 | 6(L) |
| Centre | Optical Band Gap (eV) | Edge | Optical Band Gap (eV) |
|---|---|---|---|
| C100H | 3.3 | E100H | 3.4 |
| C150H | 3.300 | E150H | 3.4 |
| C200H | 3.200 | E200H | 3.38 |
| C100M | 3.300 | E100M | 3.37 |
| C150M | 3.290 | E150M | 3.3 |
| C200M | 3.320 | E200M | 3.39 |
| C100L | 3.250 | E100L | 3.35 |
| C150L | 3.200 | E150L | 3.39 |
| C200L | 3.220 | E200L | 3.35 |
| Average | 3.264 | 3.37 | |
| SD | 0.047 | 0.032 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salimian, A.; Hasnath, A.; Aminishahsavarani, A.; Upadhyaya, H. Hypothesis on the Influence of the Magnetic Behaviour of Hydrogen Doped Zinc Oxide during Its Plasma Sputtering Process. Coatings 2021, 11, 222. https://doi.org/10.3390/coatings11020222
Salimian A, Hasnath A, Aminishahsavarani A, Upadhyaya H. Hypothesis on the Influence of the Magnetic Behaviour of Hydrogen Doped Zinc Oxide during Its Plasma Sputtering Process. Coatings. 2021; 11(2):222. https://doi.org/10.3390/coatings11020222
Chicago/Turabian StyleSalimian, Ali, Abul Hasnath, Arjang Aminishahsavarani, and Hari Upadhyaya. 2021. "Hypothesis on the Influence of the Magnetic Behaviour of Hydrogen Doped Zinc Oxide during Its Plasma Sputtering Process" Coatings 11, no. 2: 222. https://doi.org/10.3390/coatings11020222
APA StyleSalimian, A., Hasnath, A., Aminishahsavarani, A., & Upadhyaya, H. (2021). Hypothesis on the Influence of the Magnetic Behaviour of Hydrogen Doped Zinc Oxide during Its Plasma Sputtering Process. Coatings, 11(2), 222. https://doi.org/10.3390/coatings11020222







