Bodipy-Loaded Micelles Based on Polylactide as Surface Coating for Photodynamic Control of Staphylococcus aureus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of mPEG–(PLA)n Copolymers
2.3. NMR Measurements
2.4. CMC Measurements
2.5. Preparation of BODIPY Loaded Micelles and Study of Release
2.6. Bacterial Strain and Growth Conditions
2.7. Photoinactivation by Suspended Micelles
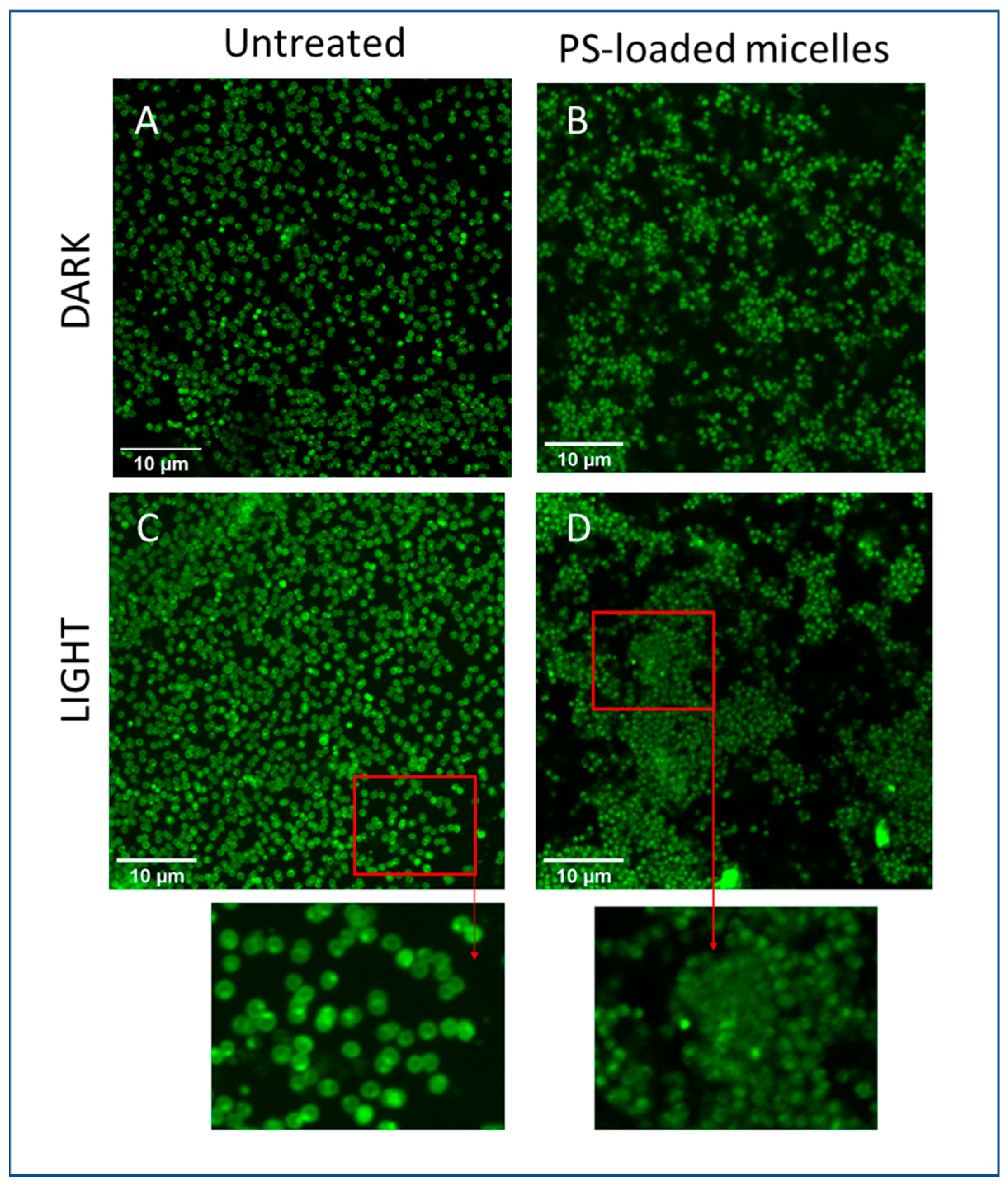
2.8. Photoinactivation Induced by Micelle-Coated Glass
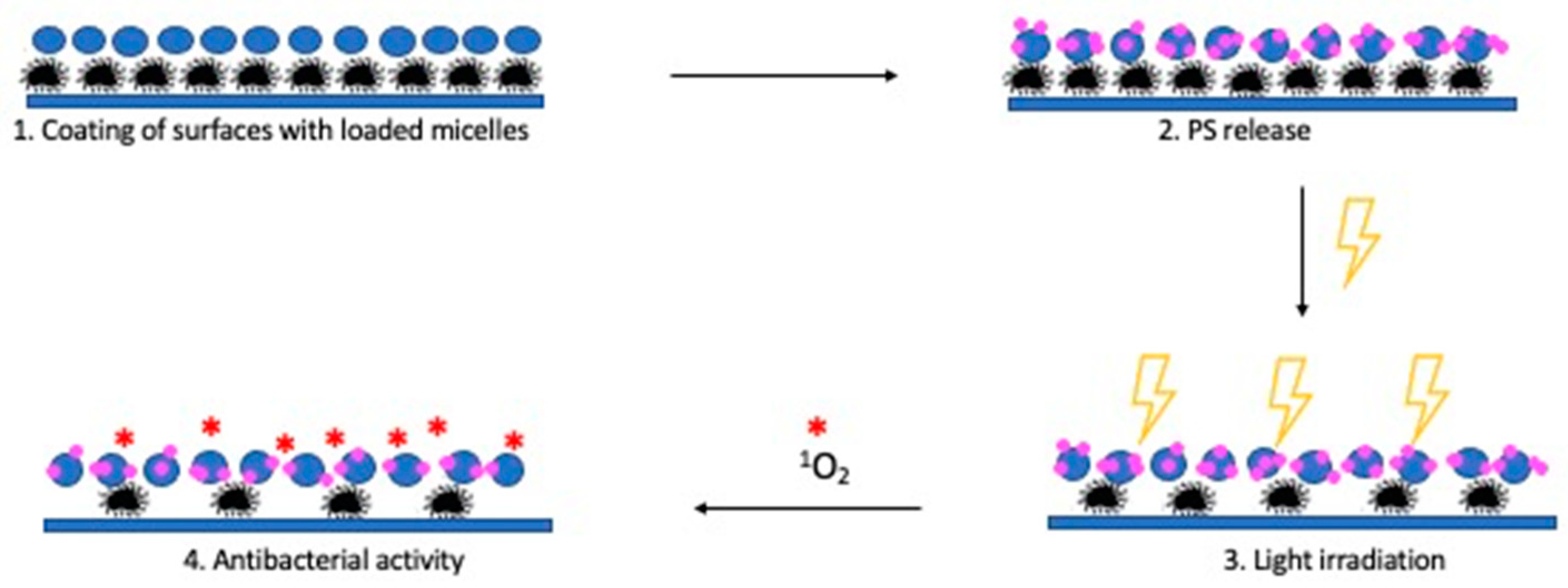
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russel, A.D. Bacterial resistance to disinfectant. J. Infect. Prev. 2002, 3, 22–24. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterial technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef] [PubMed]
- Tamanna, T.; Bulitta, J.B.; Landersdorfer, C.B.; Cashin, V.; Yu, A. Stability and controlled antibiotic release from thin films embedded with antibiotic loaded mesoporous silica nanoparticles. RSC Adv. 2015, 5, 107839–107846. [Google Scholar] [CrossRef]
- Banfi, S.; Caruso, E.; Orlandi, V.T.; Barbieri, P.; Cavallari, S.; Viganò, P.; Clerici, P.; Chiodaroli, L. Antibacterial activity of leaf extracts from Combretum micranthum and Guiera senegalensis (Combretaceae). Res. J. Microbiol. 2014, 9, 66–81. [Google Scholar] [CrossRef][Green Version]
- Takahashi, H.; Caputo, G.A.; Vemparala, S.; Kuroda, K. Synthetic Random Copolymers as a Molecular Platform to Mimic Host-Defense Antimicrobial Peptides. Bioconjug. Chem. 2017, 28, 1340–1350. [Google Scholar] [CrossRef]
- Caruso, E.; Malacarne, M.C.; Banfi, S.; Gariboldi, M.B.; Orlandi, V.T. Cationic diarylporphyrins: In vitro versatile anticancer and antibacterial photosensitizers. J. Photochem. Photobiol. B 2019, 197, 111548. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—what we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Abrahamse, H. Can light-based approaches overcome antimicrobial resistance? Drug Dev. Res. 2018, 80, 48–67. [Google Scholar] [CrossRef]
- Gerola, A.P.; Costa, P.F.A.; de Morais, F.A.P.; Tsubone, T.M.; Caleare, A.O.; Nakamura, C.V.; Brunaldi, K.; Caetano, W.; Kimura, E.; Hioka, N. Liposome and polymeric micelle-based delivery systems for chlorophylls: Photodamage effects on Staphylococcus aureus. Colloids Surfaces B Biointerfaces 2019, 177, 487–495. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Hu, D.; Deng, Y.; Chen, T.; Jin, Q.; Ji, J. Bacteria-Targeted Supramolecular Photosensitizer Delivery Vehicles for Photodynamic Ablation Against Biofilms. Macromol. Rapid Commun. 2018, 40, 1800763. [Google Scholar] [CrossRef] [PubMed]
- McCoy, P.C.; O’Neil, E.J.; Cowley, J.F.; Carson, L.; De Baroid, A.T.; Gdowski, G.T.; Gorma, S.P.; Jones, D.S. Photodynamic Antimicrobial Polymers for Infection. PLoS ONE 2014, 9, e108500. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, L.L.; Pu, W.; Liu, P.; Liu, S.X.; Li, Y.; Liu, X.L.; Lu, Z.X.; Zheng, L.Y.; Cao, Q.E. A hydrogel directly assembled from a copper metal-organic polyhedron for antimicrobial application. Chem. Commun. 2019, 55, 2206–2209. [Google Scholar] [CrossRef]
- Henke, P.; Kirakci, K.; Kubat, P.; Fraiberk, M.; Forstovà, J.; Mosinger, J. Antibacterial, antiviral, and oxygen-sensing nanoparticles prepared from electrospun materials. ACS Appl. Mater. Interfaces 2016, 8, 25127–25136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, C.L.; Peng, H.Y.; Cong, H.P.; Qian, H.S. Near-infrared photocatalytic upconversion nanoparticles/TiO2 nanofibers assembled in large scale by electrospinning. Part. Part. Syst. Charact. 2016, 33, 248–253. [Google Scholar] [CrossRef]
- Dolansky, J.; Henke, P.; Malà, Z.; Zàrskà, L.; Kubàt, P.; Mosinger, J. Antibacterial nitric-oxide- and singlet oxygen-releasing polystyrene nanoparticles responsive to light and temperature triggers. Nanoscale 2018, 10, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; Liu, X.Q.; Kantrow, S.P.; Lancaster, J.R. The biological lifetime of nitric oxide: Implications for the perivascular dynamics of NO and O2. Proc. Natl. Acad. Sci. USA 2001, 98, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Bregnhoj, M.; Westberg, M.; Jensen, F.; Ogilby, P.R. Solvent-dependent singlet oxygen lifetimes: Temperature effects implicate tunneling and charge-transfer interactions. Phys. Chem. Chem. Phys. 2016, 18, 22946–22961. [Google Scholar] [CrossRef]
- Felgenträger, A.; Maisch, T.; Späth, A.; Schröder, J.A.; Bäumler, W. Singlet oxygen generation in porphyrin-doped polymeric surface coating enables antimicrobial effects on Staphylococcus aureus. Phys. Chem. Chem. Phys. 2014, 16, 20598–20607. [Google Scholar] [CrossRef]
- Izzo, L.; Pappalardo, D. “Tree-Shaped” Copolymers Based on Poly(ethylene glycol) and Atactic or Isotactic Polylactides: Synthesis and Characterization. Macromol. Chem. Phys. 2010, 211, 2171–2178. [Google Scholar] [CrossRef]
- Garofalo, C.; Capuano, G.; Sottile, R.; Tallerico, R.; Adami, R.; Reverchon, E.; Carbone, E.; Izzo, L.; Pappalardo, D. Different Insight into Amphiphilic PEG-PLA Copolymers: Influence of Macromolecular Architecture on the Micelle Formation and Cellular Uptake. Biomacromolecules 2014, 15, 403–415. [Google Scholar] [CrossRef]
- Adami, R.; Liparoti, S.; Izzo, L.; Pappalardo, D.; Reverchon, E. PLA-PEG copolymers micronization by Supercritical Assisted Atomization. J. Supercrit. Fluids 2012, 72, 15–21. [Google Scholar] [CrossRef]
- Caruso, E.; Banfi, S.; Barbieri, P.; Leva, B.; Orlandi, V.T. Synthesis and antibacterial activity of novel cationic BODIPY photosensitizers. J. Photochem. Photobiol. B 2012, 114, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, V.T.; Rybtke, M.; Caruso, E.; Banfi, S.; Tolker-Nielsen, T.; Barbieri, P. Antimicrobial and anti-biofilm effect of a novel BODIPY photosensitizer against Pseudomonas aeruginosa PAO1. Biofuling 2014, 30, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Caruso, E.; Ferrara, S.; Ferruti, P.; Manfredi, A.; Ranucci, E.; Orlandi, V.T. Enhanced photoinduced antibacterial activity of a BODIPY photosensitizer in the presence of polyamidoamines. Lasers Med. Sci. 2018, 33, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Banfi, S.; Nasini, G.; Zaza, S.; Caruso, E. Synthesis and Photo-Physical properties of a series of BODIPY dyes. Tetrahedron 2013, 69, 4845–4856. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Martegani, E.; Bolognese, F.; Trivellin, N.; Paldrychova, M.; Matatkova, O.; Baj, A.; Caruso, E. Photodynamic therapy by diaryl-porphyrins to control Candida albicans growth. Cosmetics 2020, 7, 31. [Google Scholar] [CrossRef]
- Meiners, J.C.; Quintel-Ritzi, A.; Mlynek, J.; Elbs, H.; Kraisch, G. Adsorption of block-copolymer micelles from a selective solvent. Macromolecules 1997, 30, 4945–4951. [Google Scholar] [CrossRef]
- Farinha, J.P.S.; D’Oliveira, J.M.R.; Martinoho, J.M.; Xu, R.; Winnik, M.A. Structure in tethered chains: Polymeric micelles and chains anchored on polystyrene latex spheres. Langmuir 1998, 14, 2291–2296. [Google Scholar] [CrossRef]
- Li, Y.; Pi, Q.; You, H.; Li, J.; Wang, P.; Yang, X.; Wu, Y. A smart multi-functional coating based on anti-pathogen micelles tethered with copper nanoparticles via a biosynthesis method using L-vitamin C. RSC Adv. 2018, 8, 18272–18283. [Google Scholar] [CrossRef]
- Webber, S.E. Polymer micelles: An example of self-assembling polymers. J. Phys. Chem. B 1998, 102, 2618–2626. [Google Scholar] [CrossRef]
- Karymov, M.A.; Prochazka, K.; Mendenhall, J.M.; Martin, T.J.; Munk, P.; Webber, S.E. Chemical Attachment of Polystyrene-block-poly(methacrylic acid) Micelles on a Silicon Nitride Surface. Langmuir 1996, 12, 4748–4753. [Google Scholar] [CrossRef]
- Emoto, K.; Nagasaki, Y.; Kataoka, K. Coating of Surfaces with Stabilized Reactive Micelles from Poly(ethylene glycol)-Poly(DL-lactic acid) Block Copolymer. Langmuir 1999, 15, 5212–5218. [Google Scholar] [CrossRef]
- Izzo, L.; Gorrasi, G.; Sorrentino, A.; Tagliabue, A.; Mella, M. Controlling Drug Release of Anti-inflammatory Molecules Through a pH-Sensitive, Bactericidal Polymer Matrix: Towards a Synergic and Combined Therapy. Lect. Notes Bioeng. 2019, 151–163. [Google Scholar] [CrossRef]
- Mella, M.; Tagliabue, A.; Mollica, L.; Izzo, L. Monte Carlo study on the effect on macroion charge distribution on the ionization and adsorption of weak polyelectrolytes and concurrent counterion release. J. Colloids Interface Sci. 2020, 560, 667–680. [Google Scholar] [CrossRef]
- Tagliabue, A.; Izzo, L.; Mella, M. Impact of charge correlation, chain rigidity, and chemical specific interactions on the behaviour of weak polyelectrolytes in solution. J. Phys. Chem. B 2019, 123, 8872–8888. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.; Matrella, S.; Mella, M.; Benvenuto, G.; Vigliotta, G. Escherichia coli as a Model for the Description of the Antimicrobial Mechanism of a Cationic Polymer Surface: Cellular Target and Bacterial Contrast Response. ACS Appl. Mat. Interfaces 2019, 11, 15332–15343. [Google Scholar] [CrossRef]
- Barrella, M.C.; Di Capua, A.; Adami, R.; Reverchon, E.; Mella, M.; Izzo, L. Impact of intermolecular drug-copolymer interactions on size and drug release kinetics from pH-responsive polymersomes. Supramol. Chem. 2017, 29, 796–807. [Google Scholar] [CrossRef]
- Villani, S.; Adami, A.; Reverchon, E.; Ferretti, A.M.; Ponti, A.; Lepretti, M.; Caputo, I.; Izzo, L. pH-sensitive polymersomes: Controlling swelling via copolymer structure and chemical composition. J. Drug Target. 2017, 25, 899–909. [Google Scholar] [CrossRef]
- Tagliabue, A.; Izzo, L.; Mella, M. Out of equilibrium self-assembly of Janus nanoparticles: Steering it from disordered amorphous to 2D patterned aggregates. Langmuir 2016, 32, 12934–12946. [Google Scholar] [CrossRef] [PubMed]
- Matrella, S.; Vitiello, C.; Mella, M.; Vigliotta, G.; Izzo, L. The Role of Charge Density and Hydrophobicity on the Biocidal Properties of Self-Protonable Polymeric Materials. Macromol. Biosci. 2015, 15, 927–940. [Google Scholar] [CrossRef]
- Mella, M.; Mollica, L.; Izzo, L. Influence of charged intramolecular hydrogen bonds in weak polyelectrolytes: A Monte Carlo study of flexible and extendible polymeric chains in solution and near charged spheres. J. Polymer Sci. Part B Polymer Phys. 2015, 53, 650–663. [Google Scholar] [CrossRef]
- Izzo, L.; Gorrasi, G. Effect of molecular architecture on physical properties of tree-shaped and star-shaped poly(methylmethacrylate)-based copolymers. J. Macromol. Sci. B 2014, 53, 474–485. [Google Scholar] [CrossRef]
- Vigliotta, G.; Mella, M.; Rega, D.; Izzo, L. Modulating antimicrobial activity by synthesis: Dendritic copolymers based on nonquaternized 2-(dimethylamino)ethyl methacrylate by Cu-mediated ATRP. Biomacromolecules 2012, 13, 833–841. [Google Scholar] [CrossRef]
- Glavas, L.; Olsén, P.; Odelius, K.; Albertsson, A.-C. Achieving Micelle Control through Core Crystallinity. Biomacromolecules 2013, 14, 4150–4156. [Google Scholar] [CrossRef] [PubMed]
- Caruso, E.; Gariboldi, M.B.; Sangion, A.; Gramatica, P.; Banfi, S. Synthesis, photodynamic activity, and quantitative structure-activity relationship modelling of a series of BODIPYs. J. Photochem. Photobiol. B 2017, 167, 269–281. [Google Scholar] [CrossRef]
- Álvarez, A.; Fernández, L.; Gutiérrez, D.; Iglesias, B.; Rodríguez, A.; García, P. Methicillin-Resistant Staphylococcus aureus in Hospitals: Latest Trends and Treatments Based on Bacteriophages. J. Clin. Microbiol. 2019, 57, e01006-19. [Google Scholar] [CrossRef]
- Ghorbani, J.; Rahban, B.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef]
- Haaber, J.; Thorup Cohn, M.; Frees, D.; Andersen, T.J.; Ingmer, H. Planktonic Aggregates of Staphylococcus aureus Protect against Common Antibiotics. PLoS ONE 2012, 7, e41075. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, J.; Zhang, X.; Li, Z.; Tang, Y. Cationic Oligo(thiophene ethynylene) with Broad-Spectrum and High Antibacterial Efficiency under White Light and Specific Biocidal Activity against S. aureus in Dark. ACS Appl. Mater. Interfaces 2016, 8, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ergene, C.; Lee, J.Y.; Shirley, D.J.; Carone, B.R.; Caputo, G.A.; Palermo, E.F. Sequence and Dispersity Are Determinants of Photodynamic Antibacterial Activity Exerted by Peptidomimetic Oligo(thiophene)s. ACS Appl. Mater. Interfaces 2019, 11, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
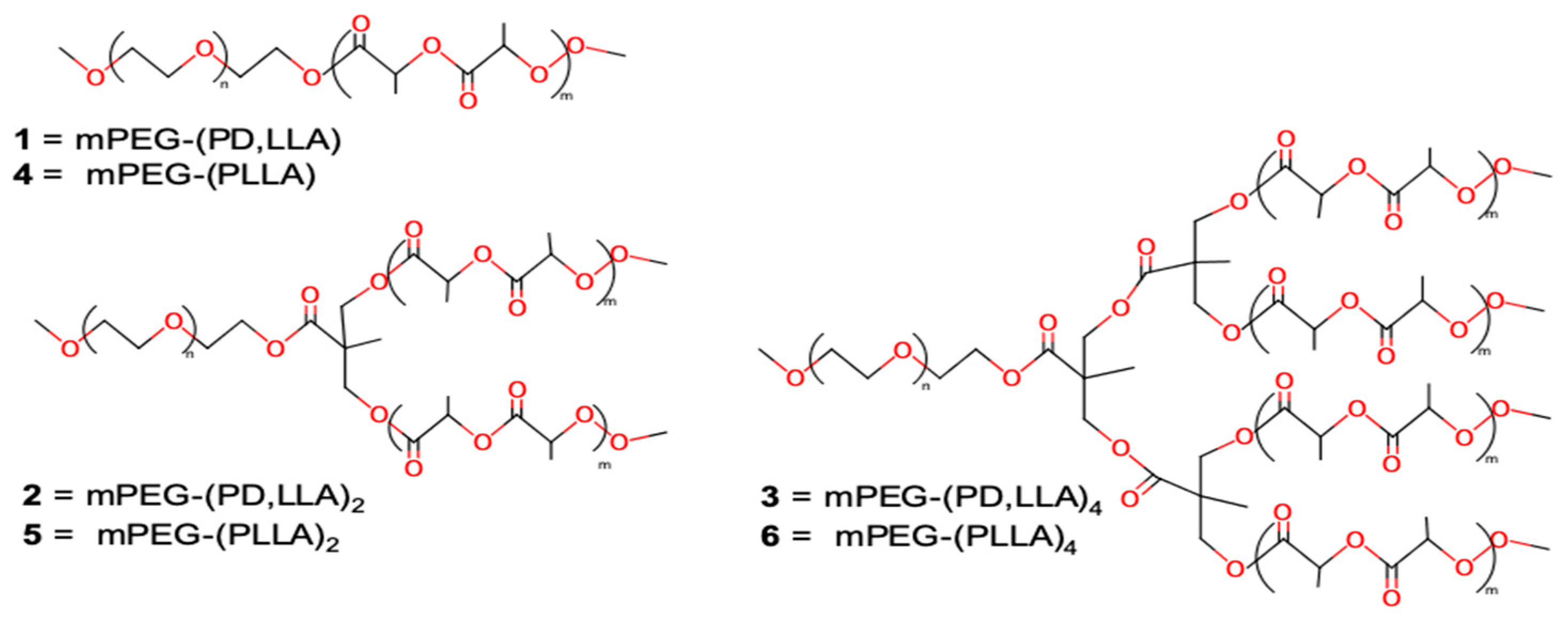
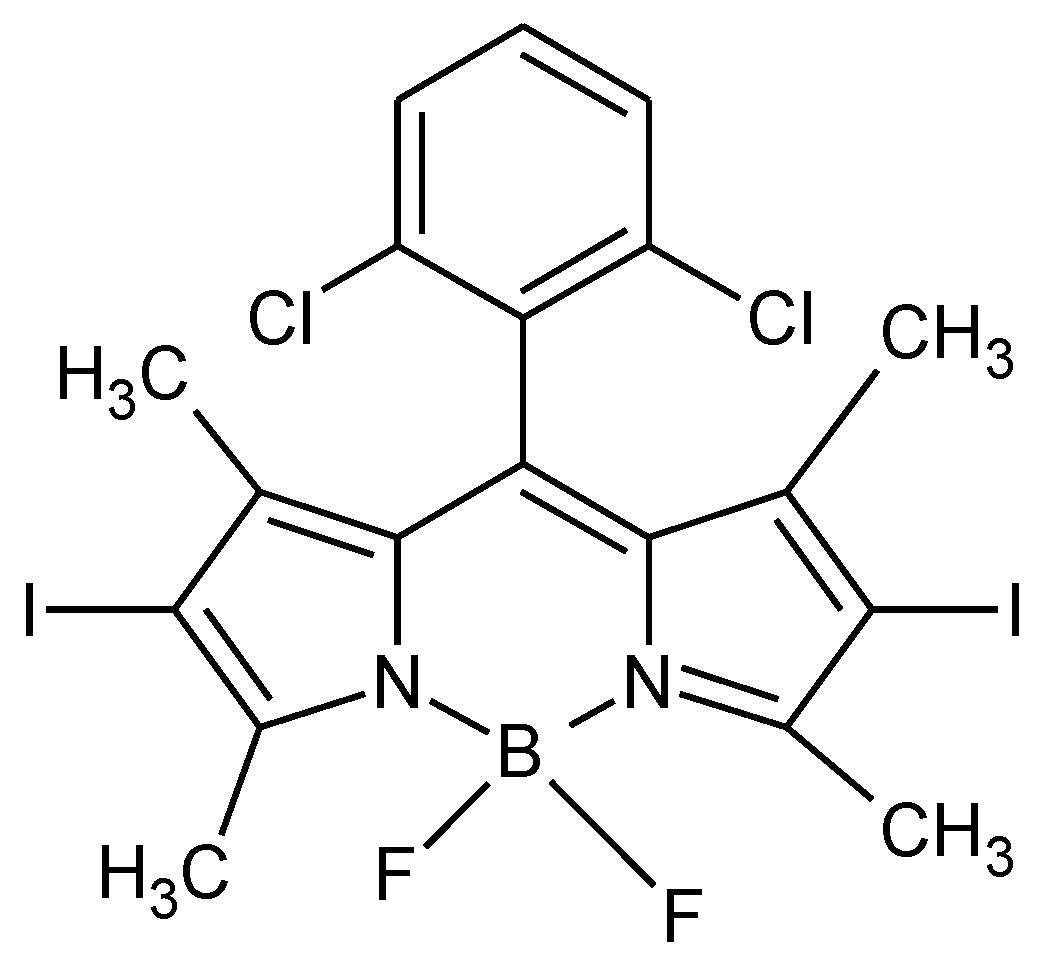



| Entry | Copolymer 1 | LA unit/arm | Mn 2 (kDa) | CMC (M) | CMC (μg/mL) | ∆G 3 (kJ/mol) | BODIPY Loading 4 (%) |
|---|---|---|---|---|---|---|---|
| 1 | mPEG–(PD,LLA) | 37.5 | 10.4 | 6.7 × 10−6 | 70 | −29.50 | – |
| 2 | mPEG–(PD,LLA)2 | 17.4 | 10.0 | 7.1 × 10−6 | 71 | −29.36 | – |
| 3 | mPEG–(PD,LLA)4 | 14.2 | 13.2 | 3.4 × 10−6 | 39 | −31.18 | – |
| 4 | mPEG–(PLLA) | 33.3 | 9.8 | 5.5 × 10−6 | 54 | −29.99 | – |
| 5 | mPEG–(PLLA)2 | 17.4 | 10.0 | 4.4 × 10−7 | 4.4 | −36.24 | 3.01 |
| 6 | mPEG–(PLLA)4 | 13.4 | 12.7 | 2.8 × 10−6 | 36 | −31.66 | 3.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, E.; Orlandi, V.T.; Malacarne, M.C.; Martegani, E.; Scanferla, C.; Pappalardo, D.; Vigliotta, G.; Izzo, L. Bodipy-Loaded Micelles Based on Polylactide as Surface Coating for Photodynamic Control of Staphylococcus aureus. Coatings 2021, 11, 223. https://doi.org/10.3390/coatings11020223
Caruso E, Orlandi VT, Malacarne MC, Martegani E, Scanferla C, Pappalardo D, Vigliotta G, Izzo L. Bodipy-Loaded Micelles Based on Polylactide as Surface Coating for Photodynamic Control of Staphylococcus aureus. Coatings. 2021; 11(2):223. https://doi.org/10.3390/coatings11020223
Chicago/Turabian StyleCaruso, Enrico, Viviana Teresa Orlandi, Miryam Chiara Malacarne, Eleonora Martegani, Chiara Scanferla, Daniela Pappalardo, Giovanni Vigliotta, and Lorella Izzo. 2021. "Bodipy-Loaded Micelles Based on Polylactide as Surface Coating for Photodynamic Control of Staphylococcus aureus" Coatings 11, no. 2: 223. https://doi.org/10.3390/coatings11020223
APA StyleCaruso, E., Orlandi, V. T., Malacarne, M. C., Martegani, E., Scanferla, C., Pappalardo, D., Vigliotta, G., & Izzo, L. (2021). Bodipy-Loaded Micelles Based on Polylactide as Surface Coating for Photodynamic Control of Staphylococcus aureus. Coatings, 11(2), 223. https://doi.org/10.3390/coatings11020223










