Abstract
The problem of real treatment of teeth hypersensitivity is still important and unsolved. The main goal of the experiment was to calculate the possible toxic effects on the fibroblasts cells CCL-1™ (NCTC clone 929) caused by original preparation to reduce tooth surfaces’ hypersensitivity, compared to the marketable preparation Seal & Protect (Dentsply). The assessment was made through measuring lactate dehydrogenase (LDH assay). Lactate dehydrogenase releases from the cell’s cytoplasm to the culture medium as a result of cell membrane damage and lysis of the cells. The measurement is based on an assessment of the ability of LDH to oxidize lactic acid to pyruvic acid, which is dependent on the increase of the release level. The increase of LDH activity in the supernatants of cell cultures shows a relationship with the percentage of dead cells (increased cytotoxicity correlates with the increasing content of dead cells). In the LDH assay, both formulations evaluated after 24 h obtained results which were located below the control values. After seven days, the mean values obtained in cytotoxicity assay LDH are measurable and lower for the original formulation in comparison to the commercial one at the dilution of 1:5. At the dilution with 1:10 ratio, they are comparable and within the range of accepted values. At the maximal dilution of 1:15, the results are higher for the experimental formulation in comparison to the marketable formulation. The polymerization process is beneficial for the cytotoxicity test results in case of both tested preparations. Average values of cytotoxicity of both preparations attain an acceptable level of less than 22.6 ± 8.1%, reliant on the degree of dilution and the remark time. Original formulation is characterized by a greater homogeneity of results. The marketable preparation has a larger diversity of effects, dependent on the time of observation and attenuation; however, the cytotoxicity values are lower when paralleled to the experimental formulation in the test conducted after seven days. This should not have a disastrous effect on the pulp, as the values of both as the values of both preparations are within expected ranges. The obtained results allows to assume that will be possible to introduce the original formulation to the stage of clinical trials in the future.
1. Introduction
The treatment of dentin hypersensitivity in each case should be individualized and should be carried out both at the dental surgery and by the patient at home, under professional supervision of the doctor [1]. The therapeutic success while using professional compounds is subject to their chemical composition. For this reason, their multidirectional impact seems to be preferable, which depends on the activity of their individual ingredients. Complex physical-chemical-biological characteristics are also an important condition for the application of such formulations. In this group of therapeutically relevant formulations, it may be important that the components have the ability to form nanoemulsions encapsulating polydimethylsiloxane oils or other neutralizing therapeutic ingredients [2,3].
Some of them should be present in order for those compounds to be accepted for application in the clinical setting. Taking into account the above conditions, these preparations should be neutral with respect to living pulp without causing its irritation, should relatively quickly bring the desired therapeutic effects, and be easy to apply, as well as provide long-lasting local effects. Moreover, they cannot provoke excessive pain reactions during application and should not affect the change in tone of hard tooth tissue [4]. Among the materials used for the dental hypersensitivity elimination, resin-based systems play a significant role due to their durability and relatively easy application [5]. Formulations based on composite resin should have appropriate physical and chemical properties, including mechanical resistance to abrasion and high adhesion [6]. Physiochemical properties of resin materials strongly influences their effective usage. But clinical success is dependent on the biological compatibility of the resins, due to the close relation between pulp and dentin [7]. In the scientific literature, there are few reports on the cytotoxicity of compounds that reduce dentin hypersensitivity [8,9]. The cytotoxicity depends on the setting, status of dentin, and contact time of the preparation with the tissues [10]. Before introducing experimental formulation to the human application stage, it is extremely important to conduct studies, e.g., on cell lines.
The main goal of the study was to calculate the potential toxic effects on the fibroblasts cells CCL-1 ™ (NCTC clone 929) caused by original preparation to reduce tooth sufraces’ hypersensitivity, compared to the marketable preparation Seal & Protect (Dentsply). The assessment was made through measuring lactate dehydrogenase (LDH assay).
2. Materials and Methods
The study used an original caring preparation with the composition of our own design. The formulation was compound of PMMAn monomer (2-(7-methyl-1,6-dioxo-2,5-diox-7-octenyl) trimellitic anhydride) (characterized by adhesive properties), carboxylic anhydride, and functional groups which are reactive to dentin. PMMAn does not include a possibly healing fragment, so, in addition, the formula has been enriched with a salutary ingredient—triclosan (≈5%). Methacrylate resin with a photoinitiator system creates ≈50% of the preparation. Moreover, ≈3% of hydroxyapatite nanopowder (HA) and ≈1% of potassium fluoride (KF) was also added. Anhydrous acetone was used as the organic solvent in this preparation, constituting ≈31% of its complete volume. The comparative material was a marketable preparation Seal & Protect (Dentsply) [3].
2.1. Measurement of Lactate Dehydrogenase (LDH Assay)
The cytotoxic effect of the verified preparations (marketable and original) on the fibroblasts cells CCL-1 ™ (NCTC clone 929) was evaluated by measuring level of the lactate dehydrogenase (LDH). Lactate dehydrogenase releases from the cell’s cytoplasm to the culture medium as a result of cell membrane damage and lysis of the cells. The measurement is based on an assessment of the ability of LDH to oxidize lactic acid to pyruvic acid, which is dependent on the increase of the release level. The increase of LDH activity in the supernatants of cell cultures shows a relationship with the percentage of dead cells (increased cytotoxicity correlates with the increasing content of dead cells).
Cytotoxicity assay was based on binary reactions. First one is the reduction of NAD+ to NADH/H+ catalyzed by LDH conversion of lactate to pyruvate. Subsequent reaction is a transfer of H/H+ NADH/H+ to tetrazolium salt INT (2-[4-iodophenyl-3-[4-nitrophenyl]-5-phenyltetrazole chloride) catalyzed by diaphoresis. The salt is reduced to formazan. Soluble formazan shows an absorption maximum at a wavelength of 550 nm, while yellow solution of tetrazolium salt INT does not exhibit absorption for that wavelength (Figure 1).
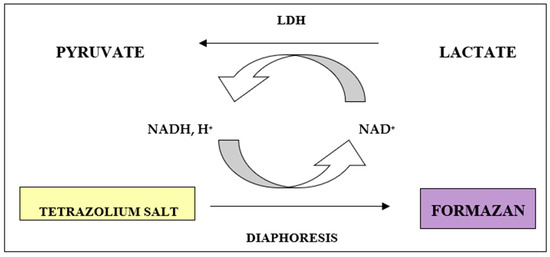
Figure 1.
Reactions underlying the lactate dehydrogenase (LDH) assay from measuring lactate dehydrogenase activity released from cells that have lysed.
The determination was achieved in 96-well plates according to the procedures specified by the producer (Storage Plate 96-Well Flat Bottom Ltd., a Non-Treated Costar-Corning Incorporated, New York, NY, USA), which was prepared before the study (Figure 2).
Figure 2.
Extraction of an unpolymerized marketable preparation in a sterile 96-well plates according to the procedures specified by the manufacturer (Storage Plate 96-Well Flat Bottom Ltd., a Non-Treated Costar-Corning Incorporated).
The extraction of the fibroblasts cells CCL-1 ™ (NCTC clone 929 (from American Type Culture Collection, Manassas, VA, USA) to the culture medium (DMEM) scheduled for 24 h and 7 days. The extraction was performed in sterile polypropylene plates (Storage 96-Well Plate Flat Bottom Ltd., Non-Treated Costar-Corning Incorporated). Before the examination, new tiles were rinsed twice with distilled water, dried at 50 °C in an incubator, placed in a petri dish, and transferred to an autoclave (121 °C/20 min/2 atmospheres of absolute pressure relative to vacuum).
2.2. Extraction of Non-Polar Formulation
Twenty-five microliters of the tested preparations (original and marketable) was added to separately plate. In order to obtain the desired dilution, volume presented below were used:
- 1:5 dilution of 25 µL + 100 µL of culture fluid (DMEM);
- 1:10 dilution of 25 µL + 225 µL of culture fluid (DMEM);
- 1:15 dilution of 25 µL + 350 µL of culture fluid (DMEM).
The solvents were vaporized for 3 days at 37 °C in an incubator with the plates secured against uncontrolled evaporation of the liquid. Subsequently, the preparations were extracted. Extractions were implemented in an incubator at 37 °C with the plates secured against uncontrolled evaporation of the liquid. The following mixture volumes: 5 µL; 2.5 µL; and 1.6 µL were used to obtain subsequent dilutions.
2.3. Extraction of the Polymerized Formulations
Twenty-five milliliters of the tested preparations (original and marketable) were polymerized before the extraction with a polymerization lamp halogen—HILUX 200 (600 mW/cm2) (Visible Light Curing Express, Benlioglu Dental, Ankara, Turkey) designed for the dental applications.
After extraction of the polymerized and unpolymerized preparations, pH was measured (pH-indicator strip) in the obtained extracts. Neutralization took place in the pH range of pH 7.2–7.3.
Measurements were repeated six times.
On the basis of LDH activity measured in the supernatants of the tested samples, controls, and in the cell lysate, the percentage of the total enzyme release in the test batch was calculated, which reflects by the percentage of dead cells.
Cytotoxicity (% CT) was calculated according to the following formula:
where:
CT (%) = [(AB − ANK)/(AWK − ANK)] × 100%,
AB—absorbance of the tested sample,
AWK—absorbance of high control, that is the value of the total LDH release (maximal release of LDH, when added to a cell culture solution of 1% Triton X-100), and
ANK—absorbance of control low, that is the value of the spontaneous release of LDH (spontaneous release of LDH in the native cell culture).
2.4. Statistical Analysis
Descriptive parameters (arithmetic mean, expected value, standard deviation, minimum and maximum values, the median and quartiles) were estimated. To verify the distribution the Shapiro-Wilk test was done. A parametric test of significance: ANOVA test of three means) was used to assess the differences caused by dilution, while, in order to check the differences due to formulations, we used a test for two means. If a significant difference was observed, a test of homogeneity of 3 means was performed, comparing means in pairs with the usage of Tukey’s least significant difference NIR algorithm. Both tests for two means and a test for two variances made it possible to properly choose the test variant for two means. Analysis was done with Statisitica 6.0 (StatSoft/SUM License).
3. Results
The results, including arithmetic mean, expected value, and standard deviation (SD) for the original and marketable preparation and each dilution (1:5, 1:10, 1:15), achieved after 24 h and 7 days, are presented in Table 1 and Table 2 and Figure 2, Figure 3 and Figure 4. In the first point of the examination, a comparison of the mean percentage of cytotoxicity in both original and marketable preparations achieved from the LDH assay after 24 h and 7 days was completed. The results of the examinations for 3 means, obtained according to the dilution of preparations (1:5, 1:10, 1:15 ratios), are shown in Table 1 and Table 2. The next stage was a comparison of mean cytotoxicity values obtained for the original and marketable formulations in the LDH assay for particular dilution values of those preparations. The results of the tests for 2 means are presented in Figure 2, Figure 3 and Figure 4; p values are shown over the columns which reflect the compared means.

Table 1.
The results including arithmetic mean, expected value, standard deviation (SD), for the original formulations and, respectively, dilution (1:5, 1:10, 1:15), achieved after 24 h and 7 days.

Table 2.
The results, including arithmetic mean, expected value, standard deviation (SD), for the marketable preparation Seal & Protect (Dentsply) and, respectively, dilution (1:5, 1:10, 1:15), achieved after 24 h and 7 days.
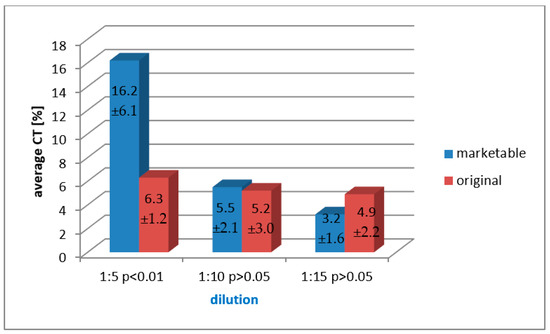
Figure 3.
A combined, dilution-related comparison of arithmetic mean cytotoxicity values of both marketable and original formulation, obtained in the LDH assay for the unpolymerized preparations after 7 days of analysis.
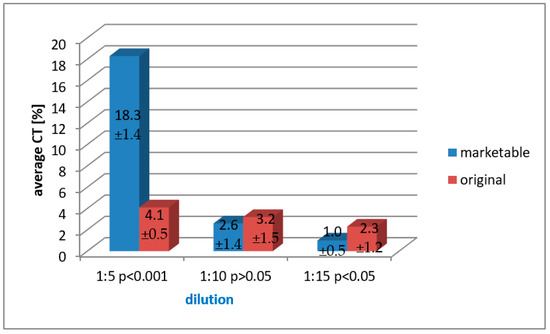
Figure 4.
A paired, dilution-related comparison of arithmetic mean cytotoxicity values of both marketable and original formulation, obtained in the LDH assay for the polymerized preparations after 24 h of testing.
In the case of unpolymerized solutions evaluated through the LDH assay after 7 days, upper cytotoxicity values were obtained for the marketable formulation when compared to the experimental formulation with the dilution ratio of 1:5. Other associations did not prove to have any statistical significance (Figure 3).
In the case of polymerized solutions assessed through the LDH assay after 24 h, upper cytotoxicity values with very high statistical significance (p < 0.001) were achieved for the commercial preparation when compared to the original formulation diluted with 1:5 ratio. In the case of comparison of the averages for the dilution of 1:15, higher values with statistical significance were found for the experimental formulation when paralleled to the marketable compound. Comparisons for the dilution of 1:10 did not show any statistical significance (Figure 4).
In the case of polymerized solutions assessed through the LDH assay after 7 days, upper cytotoxicity values with very high statistical significance (p < 0.001) were obtained for the marketable preparation when compared to the original formulation diluted with 1:5 and 1:10 ratios. The comparison of the averages for the dilution of 1:15 of both marketable and original solutions was not performed as the obtained results were in the range below the control value (Figure 5).
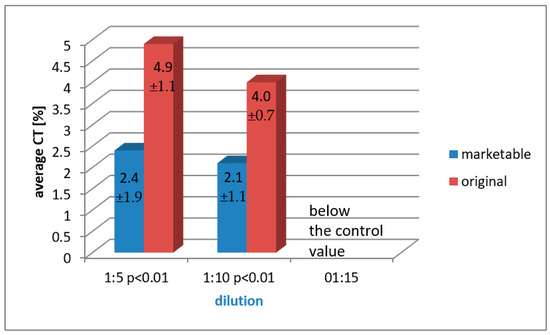
Figure 5.
A paired, dilution-related comparison of arithmetic mean cytotoxicity values of both marketable and original formulation, obtained in the LDH assay for the polymerized preparations after 7 days of analysis.
Summary of Result
Connotation of the results achieved in the LDH evaluate for both marketable and original products show that, in case of unpolymerized formulations evaluated after 24 h, all obtained values were within the range below the control value.
After seven days, the average values of cytotoxicity are detectable and lower for the original formulation in comparison to the marketable solution at a dilution of 1:5. At the dilution of 1:10, they are comparable but, in both cases, are within the acceptable range. At a maximal dilution of 1:15, they are higher.
However, after the polymerization, the parameters obtained with the LDH assay were significantly higher for the marketable preparation at a dilution of 1:5 when compared with the original solution. An increase in dilution reduced those parameters. Average cytotoxicity values of the original preparation were similar, regardless of the dilution, and generally slightly higher when compared to the marketable one.
After seven days, the cytotoxicity of the original formulation increased at a dilution of 1:5 and 1:10 in comparison with the marketable formulation. At a maximal dilution of 1:15, results for both examined formulations were within the range below the control values.
In the LDH assay, both formulations evaluated after 24 h obtained results which were located below the control values. After seven days, the mean values obtained in cytotoxicity assay LDH are measurable and lower for the original formulation in comparison to the marketable one at the dilution of 1:5. At the dilution with 1:10 ratio, they are comparable and within the range of accepted values. At the maximal dilution of 1:15, the results are higher for the original formulation in comparison to the marketable formulation.
The polymerization process is beneficial for the cytotoxicity test results in case of both tested preparations. Average values of cytotoxicity of both preparations attain an adequate rate of less than 22.6% ± 8.1 and are contingent on the degree of dilution and the remark period.
Original formulation is characterized by an inordinate regularity of effects. The marketable formulation has a better diversity of results, depending on the time of examination and dilution; however, the cytotoxicity values are lower when equated with the original formulation in the test conducted after seven days (LDH assay, dilution 1:15). This should not have a disastrous effect on the pulp, as the values of both preparations are within expected ranges.
4. Discussion
With improvement and appearance of new materials and formulations used in the dentistry, in addition to evaluating their effectiveness, attempts are made to research the possibly unsafe effects they have on the soft tissue of the mouth, i.e., pulp, mucosa, and the soft tissue of periodontium [11,12,13]. Non-light-curing or incomplete light-curing of methacrylate-based composites results in the release of resin matrix components, called residual monomers, i.e., unpolymerized monomers. The release of these non-light-cured resin materials has been associated with various adverse effects. Regarding cytotoxic effects specifically, it is known that isolated monomers cause several biological effects on cells, such as damage to the cell membrane, inhibition of metabolic enzyme activity, cell-cycle delay and interruption, and gene mutation [14]. In order to analyze cellular cytotoxicity, bonding systems are used for tests passed in synchronization with various protocols of research. Cultures are treated with multiple active extracts or individual components of those compounds. Extractions of polymerized substances were tested in an integer of experimentations [15,16,17]. To puzzle over the question of what cell lines are most suitable for cytotoxicity tests of dental bonding systems raises continuous controversy. Some researchers prefer mouse 3T3 cells, which have a similarly increasing cytotoxic effect when compared to that of the human fibroblast cell line. The following compounds have been studied: Scotchbond, SingleBond (3M ESPE), Prime & Bond NT (Dentsply De Trey), Xeno III (Dentsply De Trey), and Clearfil Protect Bond (Kuraray), which in several arrangements enclose possibly toxic ingredients. HEMA is considered to be cytotoxic even at low concentrations (0.5 mmol/L) [18]. Huang and Chang and Huang et al. obtained similar effects using extracts from bonding systems in a study conducted immediately after the process of photopolymerization and after 16 weeks since photopolymerization of those systems [16,17]. In many trials, bonding agents and their effect on the cultures in relation to their dilution were analyzed. Non-diluted extracts had a greater lethal effect on the cells than thinned ones [19,20,21]. Non-polymerized media have a sturdier cytotoxic effect than polymerized ones [20]. In subsequent experiments, dental materials were examined, taking account the effect of each substance on the cell survival. Kaga et al. compared the cytotoxicity of polymerized and unpolymerized materials, and the two major components of HEMA and TEGDMA. This study showed a higher cell toxicity for HEMA [21].
In the literature, there are considerations about the discrepancy of the results of research conducted over the cytotoxicity in vitro and in vivo observations. These differences are explained by the fact that traditional in vitro assays are based on direct observation of the impact of materials and ingredients on cell cultures, and little attention is paid to their role in dentin [21]. Because the cytotoxic effect induced by a resin material in in vitro conditions can be reduced by diluting the irritant during diffusion through the biological barriers which exist in in vivo conditions, two strategies of evaluation of cytotoxicity in vitro were adopted. One is a study of cytotoxic concentrations of each substance in the material, cell culture, and then a comparison of those concentrations is made with the values for these substances that can be achieved in vivo and a subsequent assessment of the cytotoxic effect is conducted [22]. The second method involves the use of an in vitro dentin barrier and a measurement of the pressure and flow of the liquid which mimics the in vivo conditions [23]. Some studies used a combination of both. Individual components were tested by a barrier made of dentin materials in order to evaluate which of them can cause a reaction of pulp. However, the results depended mainly on the type of material being evaluated. It is usually accepted that the resin materials of the older generation, including polymethyl methacrylate (PMMA), are much more toxic than the newer generation of materials containing bis-GMA. In her studies, Postek-Stefańska showed that Syntac binding compound (Ivoclar Vivadent, Liechtenstein) and the composite material Tetric (Ivoclar Vivadent, Lichtenstein) exerts cytotoxic activity on cultures of human fibroblasts of in vitro pulp, while the toxicity of the unpolymerized binder system was proven to be greater than of the material. Moreover, higher toxicity of Adhesive Syntac material (Ivoclar Vivadent) was demonstrated in comparison to Syntac Primer (Ivoclar Vivadent). According to the author, glutaraldehyde is responsible for that effect [24]. It was also found that the combination of the individual components of the bonding system increased the toxicity in comparison with the ingredients used alone [24]. Probably the same principle can be applied to the preparations which remove hypersensitivity of dentin. Engelmann et al. evaluated the impact of monomers, including TEGDMA, on the metabolism of immortalized murine embryonic cells (3T3 fibroblasts) [11]. Co-monomer TEGDMA caused a reduction or removal of glutathione from the cells. Glutathione is an important antioxidant which is involved in detoxification processes, removal of free radicals and reactive oxygen species, and inactivation of xenobiotics. Reducing the concentration of glutathione in the cell may significantly damage its self-defense potential and plays a key role in triggering of the apopotosis mechanism, genetically programmed aging and cell death. Unpolymerized monomers may leak out of the material causing irritation of pulp [12]. Total polymerization of materials in a clinical environment is not possible due to the presence of oxygen in the environment (the phenomenon of oxygen inhibition). Monomers, such as HEMA and TEGDMA, are released into the oral environment mainly in the first 24 h after polymerization and then at a later stage due to the gradual degradation and erosion of the material [12].
The process of polymerization, time of observation, and diluting favorably affect the obtained intensity of cytotoxicity of the two tested preparations. Original formulation has more stable effects, while the marketable preparation yields grander differences in terms of observation period, dilution, and the used assay, but there are slightly lower figures when compared with the experimental results of cytotoxicity assay after 24 h at a dilution of 1:10 and 1:15 and after seven days. LDH activity assay is a direct measurement test as it measures the leakage of lactate dehydrogenase resulting from the damage to the cell membrane and has a lower tendency to cause intra- and inter-experimental variations. Hensten-Pettersen et al. confirmed that biocompatibility studies on the cytotoxicity of dental materials carried out on the same cell lines may produce different results depending on the used technique or even sensitivity of a given cell line [25].
5. Conclusions
The process of polymerization, time of observation, and diluting favorably affect the obtained intensity of cytotoxicity of the two tested preparations.
Original formulation is characterized by a greater uniformity of results. The marketable preparation has a grander diversity of results in correlation with the conditions; however, the cytotoxicity values are lower when compared to the original formulation in the test conducted after seven days. This should not have a disastrous effect on the dental pulp in long period of time, as the values of both formulations are within expected ranges.
The polymerization process of resins’ composite materials is beneficial for the cytotoxicity test results in case of both tested preparations.
The obtained results allow us to assume that it will be possible to introduce the original formulation to the stage of clinical trials in the future.
Author Contributions
Conceptualization, M.T. and J.P.; methodology, M.T., A.M., and A.D.; formal analysis, J.P. and T.H.; investigation, J.P. and A.M.; resources, A.T. and T.H.; data curation, writing—original draft preparation, J.P. and M.T.; writing—review and editing, A.T. and M.T.; supervision, M.T. and A.D.; project administration, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data regarding the results can be found in Department of Conservative Dentistry with Endodontics of Silesian Medical University in Katowice. Information on cells used in the research available are at: https://www.lgcstandards-atcc.org/products/all/CCL-1.aspx?geo_country=pl#generalinformation (accessed on 12 February 2021).
Acknowledgments
The authors thank Mirosław Gibas from Technical University of Silesia (Gliwice, Poland) who prepared the original formulation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Swift, E.J. Causes, prevention and treatment of dentin hypersensitivity. Compend. Contin. Educ. Dent. 2004, 5, 95–109. [Google Scholar]
- Gupta, S.; Singh, P.; Moghadas, B.; Grim, B.J.; Kodibagkar, V.D. Synthesis of PEG and Quaternary Ammonium Grafted Silicone Copolymers as Nanoemulsifiers. ACS Appl. Polym. Mater. 2020, 2, 1856–1864. [Google Scholar] [CrossRef]
- Tanasiewicz, M.; Gibas, M.; Skucha-Nowak, M.; Twardawa, H.; Machorowska-Pieniążek, A. Concept of experimental preparation for treating dentin hypersensitivity. Open Med. 2016, 11, 387–393. [Google Scholar] [CrossRef]
- Walters, P.A. Dentinal hypersensivity. A. Review. J. Dent. Pract. 2005, 6, 563–569. [Google Scholar]
- Mason, S.; Kingston, R.; Shneyer, L.; Harding, M. Clinical study to monitor dentinal hypersensitivity with episodic use of a desensitising dentifrice. BDJ Open 2017, 3, 17011. [Google Scholar] [CrossRef]
- Khosravani, M.R. Mechanical behavior of restorative dental composites under various loading conditions. J. Mech. Behav. Biomed. Mater. 2019, 93, 151–157. [Google Scholar] [CrossRef]
- Tanasiewicz, M.; Pawlak, J. Mechanizm działania czynników bioaktywnych wchodzących w skład preparatów osłonowo-protekcyjnych znoszących nadwrażliwość zębiny. TPS-Twój Przegląd Stomatol. 2012, 3, 59–60. [Google Scholar]
- Wataha, J.C. Cytotoxicity of components of resin and other restorative materials. J. Oral Rehabil. 1994, 21, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Camps, J.; Aboutt, I.; van Meerebeek, B.; Franquin, J.C. Efficiency and cytotoxicity of resin-based desensitizing agents. Am. J. Dent. 2002, 15, 300–304. [Google Scholar]
- Sengun, A.; Buyukbas, S.; Hakki, S.S. Cytotoxic effects of dental desensitizers on human gingival fibroblast. J. Biomed. Mater. Res. B Appl. Mater. 2006, 78, 131–137. [Google Scholar] [CrossRef]
- Engelmann, J.; Leyhausen, G.; Leibfritz, D.; Geurtsen, W. Metabolic effects of dental resin components in vitro detected by NMR spectroscop. J. Dent. Res. 2001, 80, 869. [Google Scholar] [CrossRef]
- Geurtsen, W. Biocompatibility of resin modified filling materials. Crit. Rev. Oral Biol. Med. 2000, 11, 333–355. [Google Scholar] [CrossRef]
- Oliveira, D.C.; Silva, C.B.; Muniz, B.V.; Volpato, M.C.; Costa, A.R.; Sinhoreti, M.A. Effect of 4-(N,N-dimethylamino) phenethyl alcohol on degree of conversion and cytotoxicity of photo-polymerized CQ-based resin composites. Braz. Dent. J. 2014, 25, 538–542. [Google Scholar] [CrossRef]
- Longo, D.L.; Paula-Silva, F.W.G.; Faccioli, L.H.; Gaton-Hernandez, P.M.; Queiroz, A.M.; Silva, L.A.B. Cytotoxicity and cytokine expression induced by silorane and methacrylate-based composite resins. J. Appl. Oral Sci. 2016, 24, 338–343. [Google Scholar] [CrossRef]
- Franz, A.; Konig, F.; Lucas, T.; Watts, D.C.; Schelde, A. Cytotoxic effects of dental bonding substances as a function of degree of conversion. Dent. Mater. 2009, 25, 232–239. [Google Scholar] [CrossRef]
- Huang, F.M.; Chang, Y.C. Cytotoxicity of dentin-bonding agents on human pulp cell in vitro. Int. Endod. J. 2002, 35, 905–909. [Google Scholar] [CrossRef]
- Huang, F.M.; Chou, M.Y.; Chang, Y.C. Dentin bonding agents induce c-fos and c-jun protooncogenes expression in human gingival fibroblasts. Biomater 2003, 24, 157–163. [Google Scholar] [CrossRef]
- Koulaouzidou, E.A.; Papazisis, K.T.; Yiannaki, E.; Palagbias, G.; Helvatjoglu-Antoniades, M. Effects of dentin bonding agents on the cell cycle of fibroblastis. J. Enodod. 2009, 35, 275–279. [Google Scholar] [CrossRef]
- Costa, C.A.; Vaerten, M.A.; Edwards, C.A.; Hanks, C.T. Cytotoxic effects of current dental adhesives on immortalized odontoblasty cell Line MDPC-23. Dent. Mater. 1999, 15, 434–441. [Google Scholar] [CrossRef]
- Chen, R.S.; Liu, C.C.; Tseng, W.Y.; Jeng, J.H.; Lin, C.P. Cytotoxicity of three dentin bonding agents on human dental pulp cell. J. Dent. 2003, 31, 223–229. [Google Scholar] [CrossRef]
- Kaga, M.; Noda, M.; Ferracane, J.L.; Nakamura, W.; Oguchi, H.; Sano, H. The in vitro cytotoxicity of eluates from dentin bonding resins and their effects on phosphorylation of L929 cells. Dent. Mater. 2001, 17, 333–339. [Google Scholar] [CrossRef]
- Ponce-Bravo, S.; Ledesma-Montes, C.; Martinez-Rivera, J.L.; Garces-Ortiz, M. Toxicity test of a dental commercial composite. J. Clin. Exp. Dent. 2015, 1, 289–292. [Google Scholar] [CrossRef]
- Wegehaupt, S.J.; Lunghi, N.; Belibasakis, G.N.; Attin, T. Influence of light-curing distance on degree of conversion and cytotoxicity of etch-and-rinse and self-etch adhesives. BMC Oral Health 2017, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Postek-Stefańska, L. Wczesne obserwacje nad wpływem materiałów kompozytowych i ich systemów wiążących na miazgę zębową. Czasopismo Stomatol. 1996, 49, 531–539. [Google Scholar]
- Hensten-Pettersen, A.; Helgeland, K. Sensitivity of different human cell lines in the biologic evaluation of dental resin-based restorative materials. Scan. J. Dent. Res. 1981, 89, 102–107. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).