Euler’s Numerical Method for Ions Rejection Reassessment of a Defect-Free Synthesized Nanofiltration Membrane with Ultrathin Titania Film as the Selective Layer
Abstract
:1. Introduction
2. Mathematical Modeling
2.1. Model Assumptions
- All the solutions used are assumed to be ideal.
- The effective charge density of the membrane is identical at all points of the NF membrane under study.
- The nanofiltration membrane consists of a bundle of straight cylindrical pores, all identical, each having a uniform radius and depth (with ).
- The NPs layer thickness is negligible towards the substrate thickness.
- All the solute particles in the solution are transportable.
- The electric potential inside the membrane and the Na2SO4, MgSO4, NaCl, CaCl2, and MgCl2 solutions are defined as centrifugal averaged quantities.
- The Donnan equilibrium is applied not only at the interface of membrane/feed-solution but also at the interface of membrane/permeate solution.
2.2. Model Equations
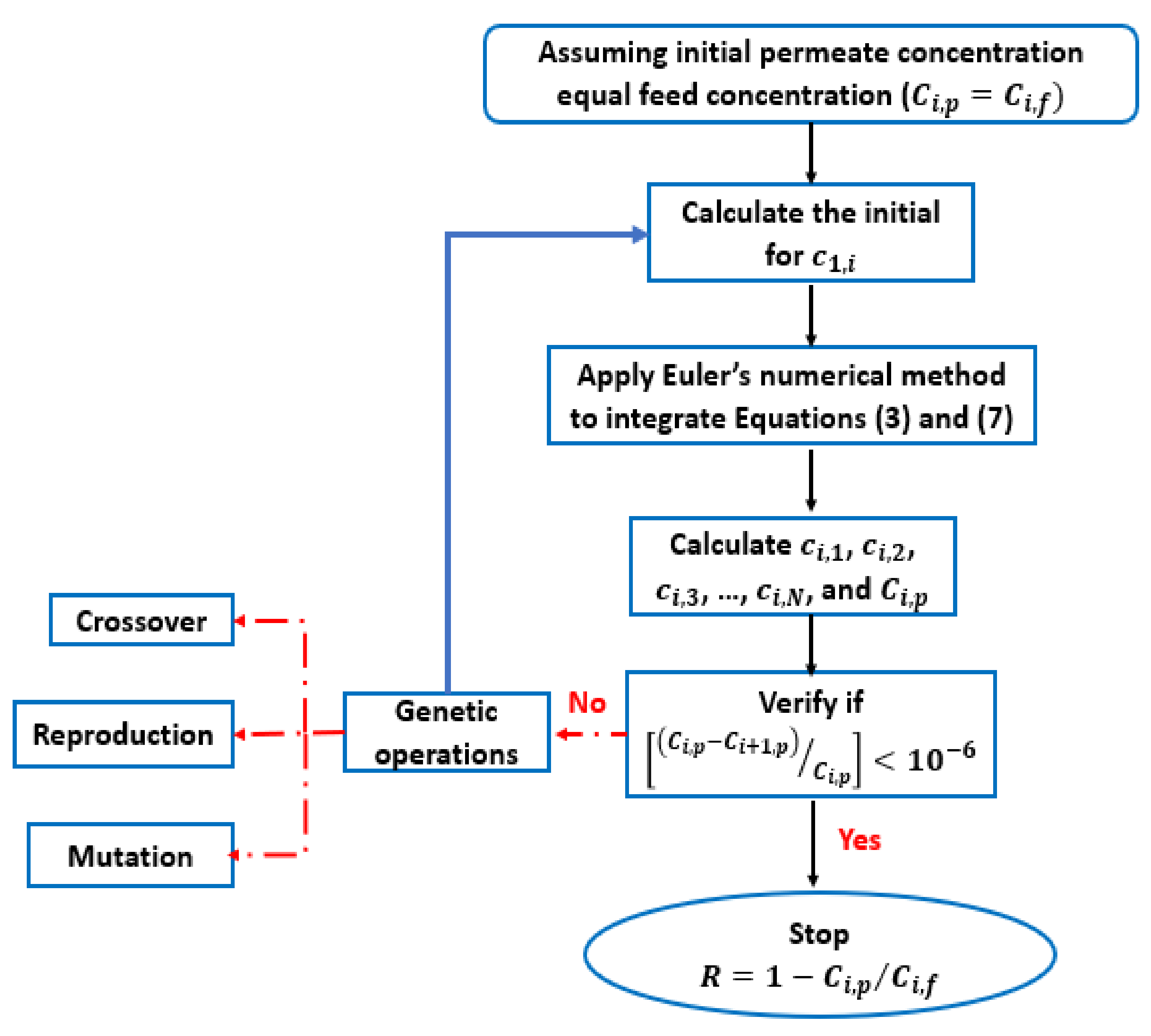
2.3. Description of The Computation Procedure
- Using Equation (10), the feed concentration Ci,f enables the initial concentration at the fees–solution/membrane interface ci,1 calculation, and even the practical integration of both Equations (3) and (7).
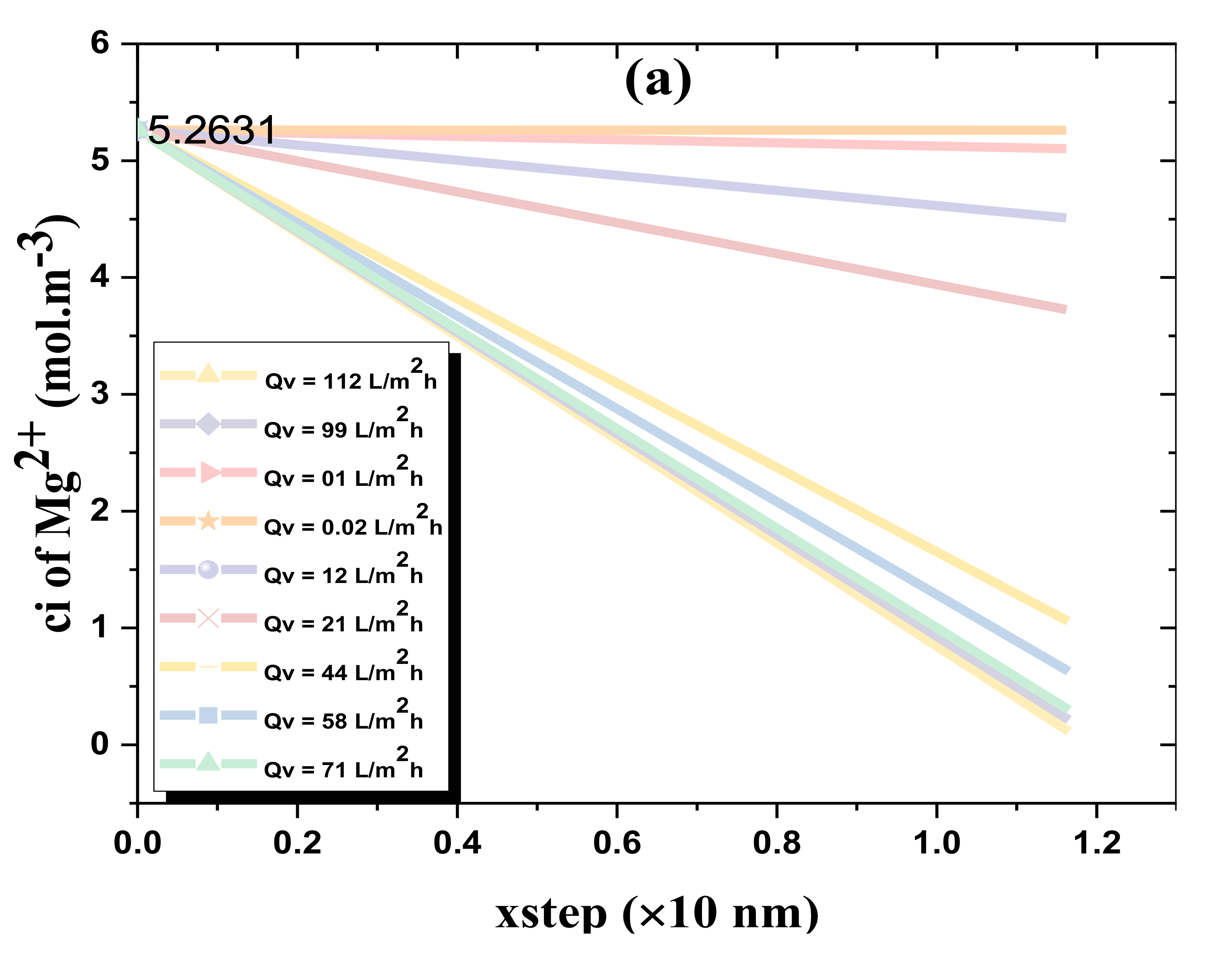
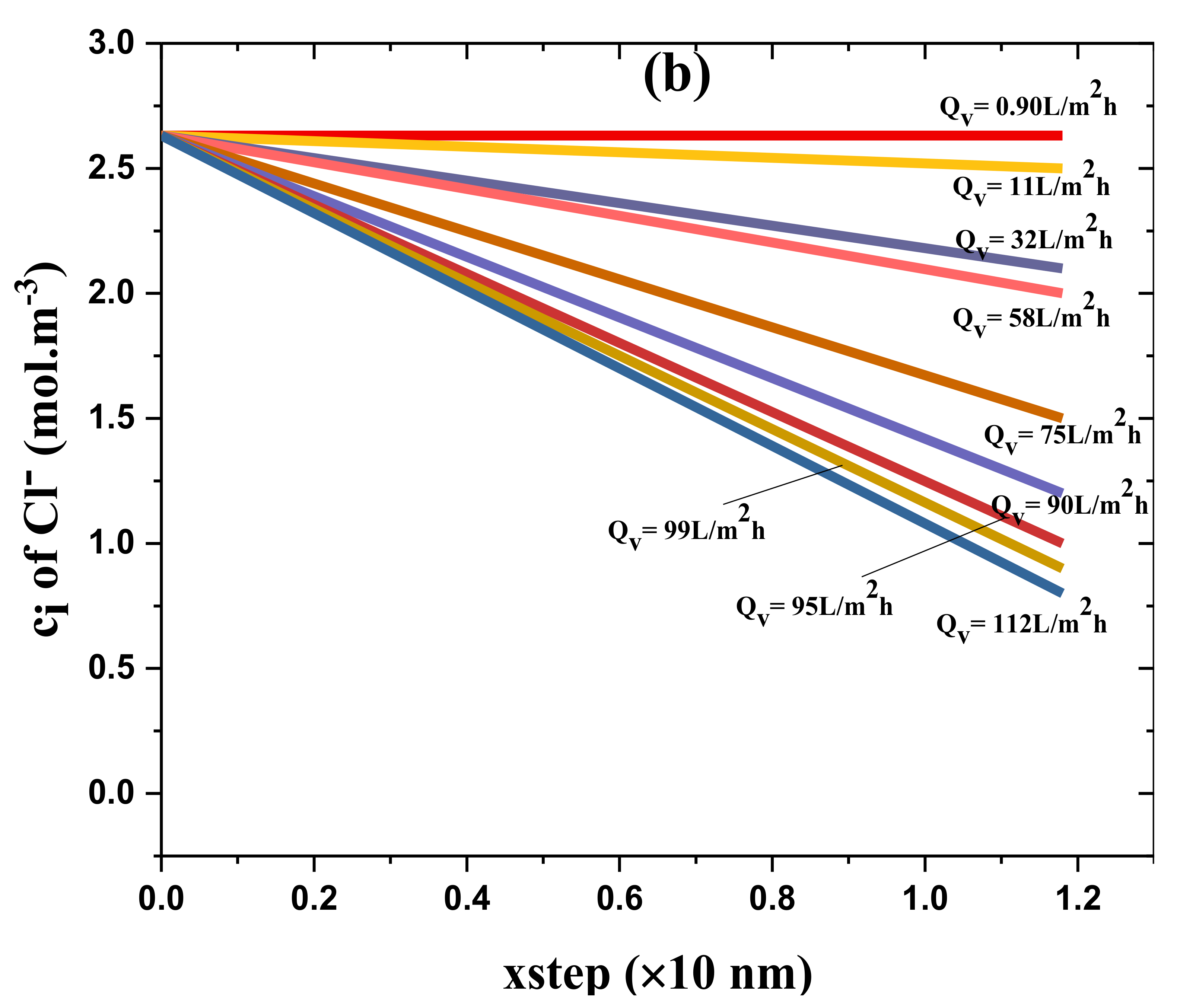
- Using the Euler numerical method, ci,1, ci,2, ci,3, ci,4, …, and ci,N are estimated (integrating Equations (3) and (7)).
- From the estimated ci,N* value, and applying Equation (10), the permeate concentration Ci,p is calculated.
- Finally, the solute particle rejection (R) can be calculated using Equation (11).
2.4. Euler Numerical Method and Ion Transport Inside the Membrane Active Layer
3. Experimental Section
3.1. Materials
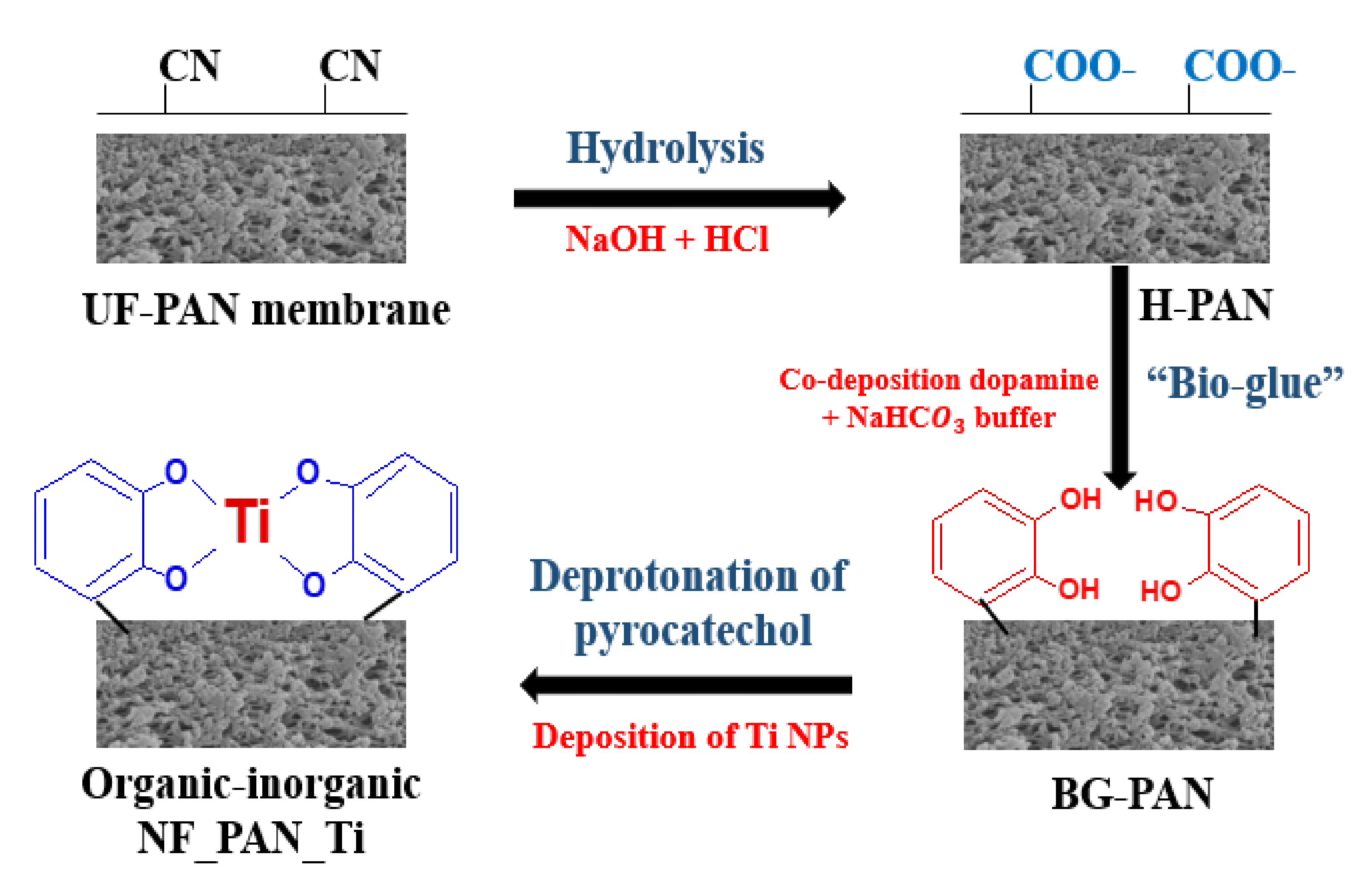
3.2. Novel Organic-Inorganic Nanofiltration Membrane NF_PAN_Ti Preparation
3.3. NF_PAN_Ti Membrane Characterization
3.4. Filtration Performance of Organic-Inorganic NF_PAN_Ti Membrane
3.5. Long Test Stability on NF_PAN_Ti Membrane
3.6. Validation of the Predicted Results with Experimental Data
4. Results and Discussion
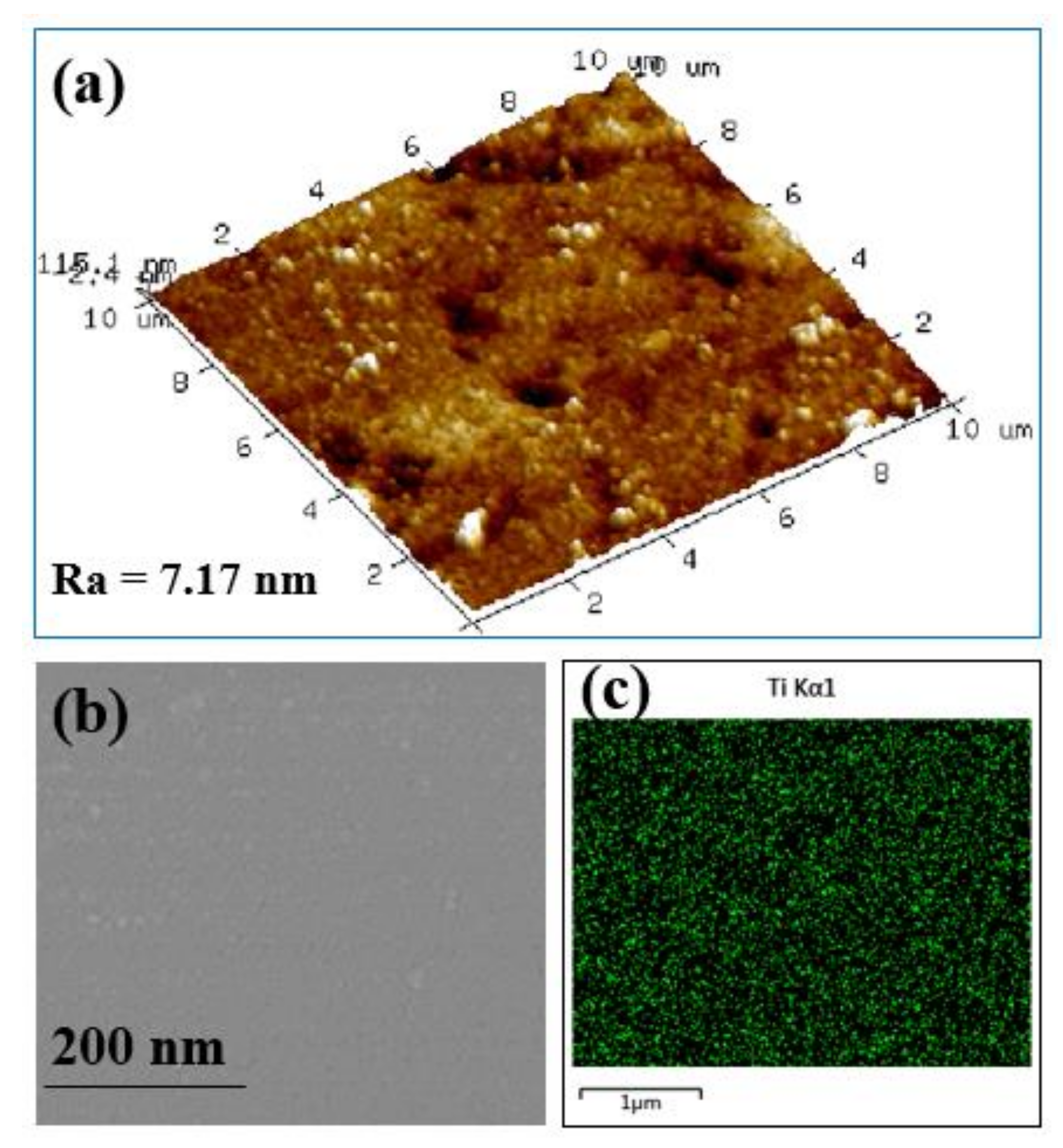
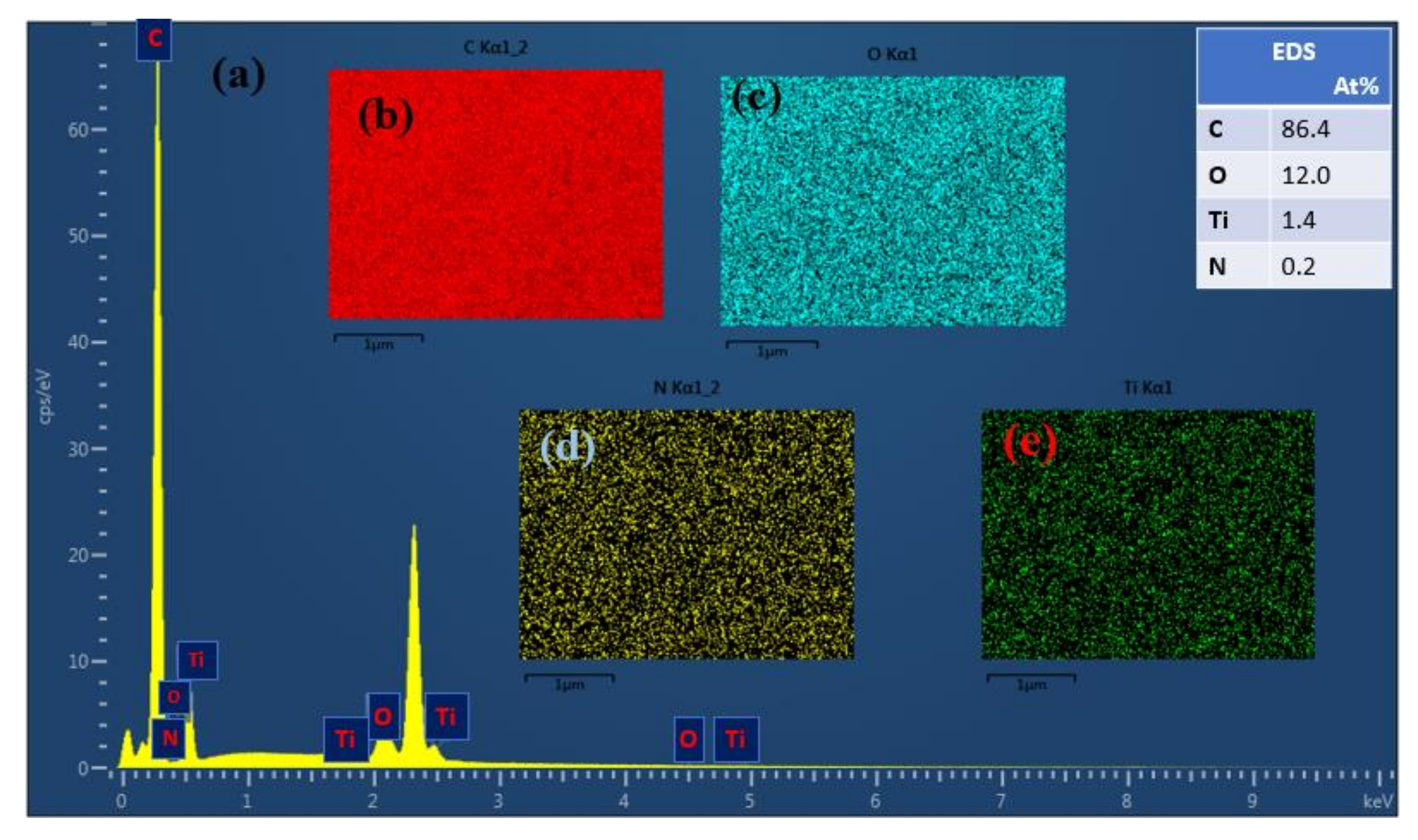
4.1. NF_PAN_Ti Structures Characterization
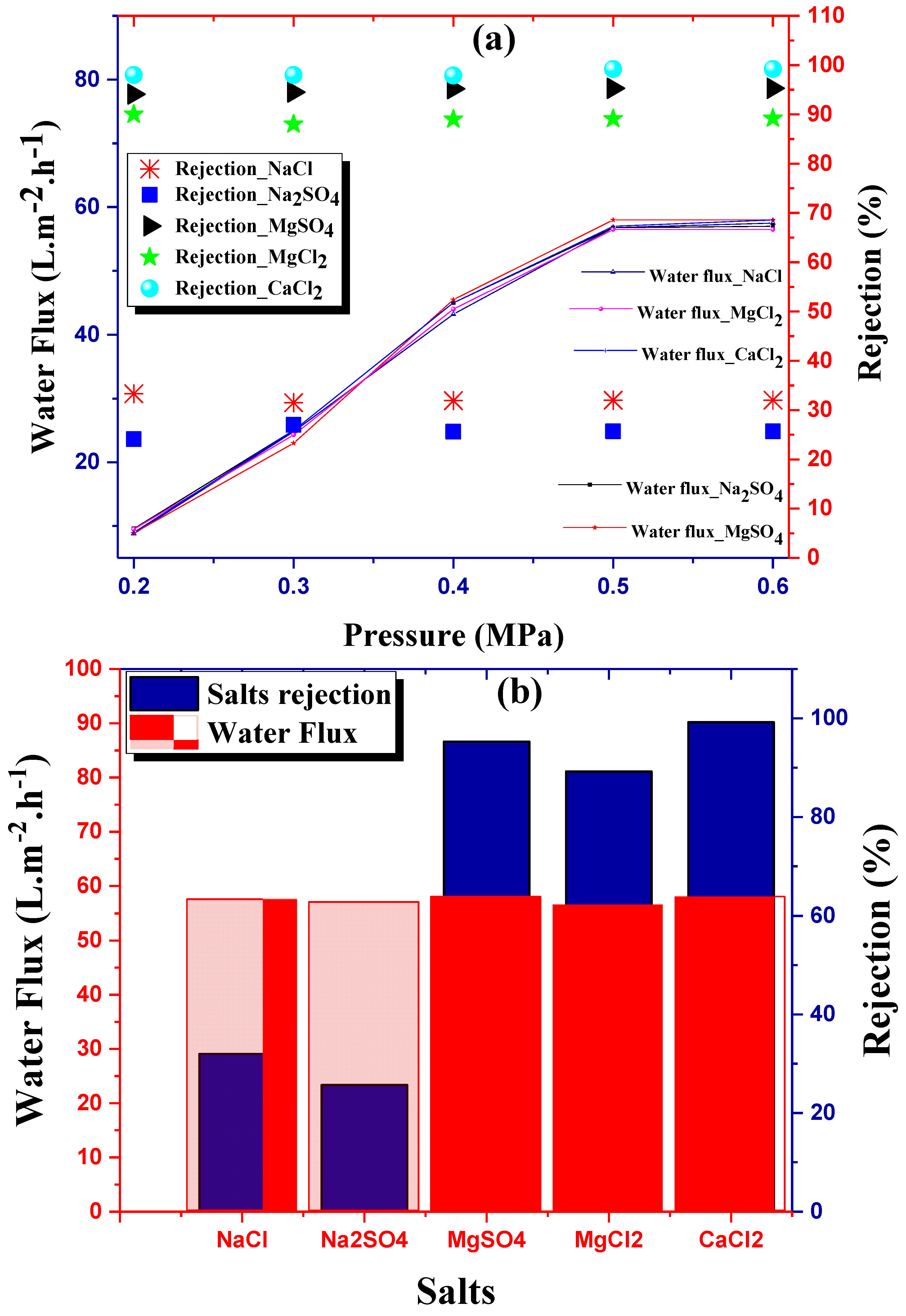
4.2. Flux Behavior and Experimental Salts Rejection
4.3. Model Reassessment of Salts Rejection
4.4. Membrane Long-Term Stability
4.5. Validation of the Predicted Results with Experimental Data
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| concentration of ion i within pore, mol·m−3 | |
| bulk feed concentration, mol·m−3 | |
| ionic solute bulk solution concentration, mol·m−3 | |
| uncharged solute bulk permeate concentration, mol·m−3 | |
| / | (Un)charged solute pore diffusion coefficient, m2·s−1 (=) |
| solute bulk diffusion coefficient, m2·s−1 | |
| I | ionic strength (mol·m−3) |
| uncharged solute flux, pore area basis, mol·m−2·s−1 | |
| k | feed-side mass transfer coefficient, m/s |
| P | Pressure N/m2 |
| effective pore radius, m | |
| V | solvent velocity, m/s |
| effective charge density, mol/m3 | |
| valence of ion i | |
| activity coefficient of ion i within pore, dimensionless | |
| bulk activity coefficient of ion i, dimensionless | |
| applied pressure, N·m−2 | |
| effective pressure driving force, N·m−2 | |
| membrane thickness, m | |
| osmotic pressure difference, N·m−2 | |
| Donnan potential, V | |
| Bulk/pore dielectric constant, dimensionless | |
| η | solvent viscosity within pores, N·s·m−2 |
| λ | ratio of ionic or uncharged solute radius to pore radius, dimensionless |
| ratio of effective membrane charge density to bulk feed concentration, di-mensionless () | |
| steric partition coefficient of ion i, dimensionless | |
| 𝜓 | potential within the pore (V) |
References
- Wang, Y.; Zucker, I.; Boo, C.; Elimelech, M. Removal of Emerging Wastewater Organic Contaminants by Polyelectrolyte Multilayer Nanofiltration Membranes with Tailored Selectivity. ACS ES&T Eng. 2020. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Chua, Y.T.; Lin, C.X.C.; Kleitz, F.; Zhao, X.S.; Smart, S. Nanoporous organosilica membrane for water desalination. Chem. Commun. 2013, 49, 4534. [Google Scholar] [CrossRef] [PubMed]
- Oatley-Radcliffe, D.L.; Walters, M.; Ainscough, T.J.; Williams, P.M.; Mohammad, A.W.; Hilal, N. Nanofiltration membranes and processes: A review of research trends over the past decade. J. Water Process. Eng. 2017, 19, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Elcik, H.; Celik, S.O.; Cakmakci, M.; Ozkaya, B. Performance of nanofiltration and reverse osmosis membranes for arsenic removal from drinking water. Desalinisation Water Treat. 2015, 57, 20422–20429. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, H.-C.; Liang, H.-Q.; Wan, L.; Xu, Z.-K. Novel nanofiltration membrane with ultrathin zirconia film as selective layer. J. Membr. Sci. 2016, 500, 265–271. [Google Scholar] [CrossRef]
- Nicomel, N.R.; Leus, K.; Folens, K.; Van Der Voort, P.; Du Laing, G. Technologies for Arsenic Removal from Water: Current Status and Future Perspectives. Int. J. Environ. Res. Public Heal. 2015, 13, 62. [Google Scholar] [CrossRef]
- Hafiz, M.; Hawari, A.H.; Alfahel, R.; Hassan, M.K.; Altaee, A. Comparison of Nanofiltration with Reverse Osmosis in Reclaiming Tertiary Treated Municipal Wastewater for Irrigation Purposes. Membranes 2021, 11, 32. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Dimitriou, E.; Nuez, I. Retrofitting assessment of a full-scale brackish water reverse osmosis desalination plant with a feed capacity of 600 m3/d. Desalinisation Water Treat. 2019, 144, 72–78. [Google Scholar] [CrossRef]
- Higgins, C.J.; Duranceau, S.J. Removal of Enantiomeric Ibuprofen in a Nanofiltration Membrane Process. Membranes 2020, 10, 383. [Google Scholar] [CrossRef]
- Zakmout, A.; Sadi, F.; Portugal, C.A.M.; Crespo, J.G.; Velizarov, S. Tannery Effluent Treatment by Nanofiltration, Reverse Osmosis and Chitosan Modified Membranes. Membranes 2020, 10, 378. [Google Scholar] [CrossRef]
- Cai, Y.-H.; Yang, X.J.; Schäfer, A.I. Removal of Naturally Occurring Strontium by Nanofiltration/Reverse Osmosis from Groundwater. Membranes 2020, 10, 321. [Google Scholar] [CrossRef]
- Marszałek, A.; Puszczało, E. Effect of Photooxidation on Nanofiltration Membrane Fouling During Wastewater Treatment from the Confectionery Industry. Water 2020, 12, 793. [Google Scholar] [CrossRef] [Green Version]
- Marecka-Migacz, A.; Mitkowski, P.T.; Antczak, J.; Różański, J.; Prochaska, K. Assessment of the Total Volume Membrane Charge Density through Mathematical Modeling for Separation of Succinic Acid Aqueous Solutions on Ceramic Nanofiltration Membrane. Processes 2019, 7, 559. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Nymann, M.C.; Christensen, M.L.; Quist-Jensen, C.A. Industrial Wastewater Treatment by Nanofiltration—A Case Study on the Anodizing Industry. Membranes 2020, 10, 85. [Google Scholar] [CrossRef]
- Cooray, T.; Wei, Y.; Zhang, J.; Zheng, L.; Zhong, H.; Weragoda, S.; Weerasooriya, R. Drinking-Water Supply for CKDu Affected Areas of Sri Lanka, Using Nanofiltration Membrane Technology: From Laboratory to Practice. Water 2019, 11, 2512. [Google Scholar] [CrossRef] [Green Version]
- Shao, W.; Liu, C.; Yu, T.; Xiong, Y.; Hong, Z.; Xie, Q. Constructing Positively Charged Thin-Film Nanocomposite Nanofiltration Membranes with Enhanced Performance. Polymers 2020, 12, 2526. [Google Scholar] [CrossRef]
- Thi, H.Y.N.; Nguyen, B.T.D.; Kim, J.F. Sustainable Fabrication of Organic Solvent Nanofiltration Membranes. Membranes 2020, 11, 19. [Google Scholar] [CrossRef]
- Lesimple, A.; Ahmed, F.E.; Hilal, N. Remineralization of desalinated water: Methods and environmental impact. Desalination 2020, 496, 114692. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.; Ang, W.; Chung, Y.T.; Oatleyradcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Van der Bruggen, B. Chemical modification of polyethersulfone nanofiltration membranes: A review. J. Appl. Polym. Sci. 2009, 114, 630–642. [Google Scholar] [CrossRef]
- Seah, M.Q.; Lau, W.J.; Goh, P.S.; Tseng, H.-H.; Wahab, R.A.; Ismail, A.F. Progress of Interfacial Polymerization Techniques for Polyamide Thin Film (Nano)Composite Membrane Fabrication: A Comprehensive Review. Polymers 2020, 12, 2817. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Z.; Gao, C. Ultrathin Graphene Nanofiltration Membrane for Water Purification. Adv. Funct. Mater. 2013, 23, 3693–3700. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane materials for water purification: Design, development, and application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Gryta, M. Fouling in direct contact membrane distillation process. J. Memb. Sci. 2008, 325, 383–394. [Google Scholar] [CrossRef]
- Boffa, V. Fabrication of ultramicroporous silica membranes for pervaporation and gas separation. In Molecules at Work: Selfassembly, Nanomaterials, Molecular Machinery; Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 177–205. [Google Scholar]
- Xu, R.; Wang, J.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Reverse osmosis performance of organosilica membranes and comparison with the pervaporation and gas permeation properties. AIChE J. 2012, 59, 1298–1307. [Google Scholar] [CrossRef]
- Xu, R.; Wang, J.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Development of Robust Organosilica Membranes for Reverse Osmosis. Langmuir 2011, 27, 13996–13999. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, T. Corrosion properties of alumina and titania NF membranes. J. Membr. Sci. 2003, 214, 21–29. [Google Scholar] [CrossRef]
- Song, Z.; Fathizadeh, M.; Huang, Y.; Chu, K.H.; Yoon, Y.; Wang, L.; Xu, W.L.; Yu, M. TiO2 nanofiltration membranes prepared by molecular layer deposition for water purification. J. Membr. Sci. 2016, 510, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Anderson, M.A. ChemInform Abstract: Sol-Gel Route to Synthesis of Microporous Ceramic Membranes: Preparation and Characterization of Microporous TiO2 and ZrO2 Xerogels. ChemInform 2010, 25, 1939–1945. [Google Scholar] [CrossRef]
- Sekulić, J.; Elshof, J.E.T.; Blank, D.H.A. A Microporous Titania Membrane for Nanofiltration and Pervaporation. Adv. Mater. 2004, 16, 1546–1550. [Google Scholar] [CrossRef]
- Marchetti, P.; Solomon, M.F.J.; Szekely, G.; Livingston, A.G. Molecular Separation with Organic Solvent Nanofiltration: A Critical Review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef] [PubMed]
- Siddique, T.; Dutta, N.K.; Choudhury, N.R. Nanofiltration for Arsenic Removal: Challenges, Recent Developments, and Perspectives. Nanomaterials 2020, 10, 1323. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C. Removal of pollutants from surface water and groundwater by nanofiltration: Overview of possible applications in the drinking water industry. Environ. Pollut. 2003, 122, 435–445. [Google Scholar] [CrossRef]
- Bandini, S.; Vezzani, D. Nanofiltration modeling: The role of dielectric exclusion in membrane characterization. Chem. Eng. Sci. 2003, 58, 3303–3326. [Google Scholar] [CrossRef]
- Bowen, W.R.; Mukhtar, H. Characterisation and prediction of separation performance of nanofiltration membranes. J. Membr. Sci. 1996, 112, 263–274. [Google Scholar] [CrossRef]
- Wong, W.; Wong, H.Y.; Badruzzaman, A.B.M.; Goh, H.H.; Uz-Zaman, A.M.M. Recent advances in exploitation of nanomaterial for arsenic removal from water: A review. Nanotechnology 2016, 28, 042001. [Google Scholar] [CrossRef]
- Bowen, W.R.; Mohammad, A.W. Diafiltration by nanofiltration: Prediction and optimization. AIChE J. 1998, 44, 1799–1812. [Google Scholar] [CrossRef]
- Sigurdardottir, S.B.; DuChanois, R.M.; Epsztein, R.; Pinelo, M.; Elimelech, M. Energy barriers to anion transport in nanofiltration membranes: Role of intra-pore diffusion. J. Membr. Sci. 2020, 603, 117921. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Nguyen, S.; Hoang, T.A.; Yin, G. Tamed-Euler method for hybrid stochastic differential equations with Markovian switching. Nonlinear Anal. Hybrid. Syst. 2018, 30, 14–30. [Google Scholar] [CrossRef]
- Zhang, W. Convergence of the balanced Euler method for a class of stochastic Volterra integro-differential equations with non-globally Lipschitz continuous coefficients. Appl. Numer. Math. 2020, 154, 17–35. [Google Scholar] [CrossRef]
- Gojković, L.; Malijević, S.; Armaković, S. Modeling of fundamental electronic circuits by the Euler method using the Python programming language. Phys. Educ. 2020, 55, 055016. [Google Scholar] [CrossRef]
- Yue, C.; Zhao, L. Strong convergence of the split-step backward Euler method for stochastic delay differential equations with a nonlinear diffusion coefficient. J. Comput. Appl. Math. 2021, 382, 113087. [Google Scholar] [CrossRef]
- Benner, P.; Stillfjord, T.; Trautwein, C. A linear implicit Euler method for the finite element discretization of a controlled stochastic heat equation. 2020. Available online: https://arxiv.org/abs/2006.05370 (accessed on 23 December 2020).
- Bowen, W.R.; Welfoot, J.S. Modelling the performance of membrane nanofiltration—critical assessment and model development. Chem. Eng. Sci. 2002, 57, 1121–1137. [Google Scholar] [CrossRef]
- Van Gestel, T.; Vandecasteele, C.; Buekenhoudt, A.; Dotremont, C.; Luyten, J.; Leysen, R.; Van Der Bruggen, B.; Maes, G. Salt retention in nanofiltration with multilayer ceramic TiO2 membranes. J. Membr. Sci. 2002, 209, 379–389. [Google Scholar] [CrossRef]
- Gholami, S.; López, J.; Rezvani, A.; Cortina, J.L.; Cortina, J.L. Fabrication of thin-film nanocomposite nanofiltration membranes incorporated with aromatic amine-functionalized multiwalled carbon nanotubes. Rejection performance of inorganic pollutants from groundwater with improved acid and chlorine resistance. Chem. Eng. J. 2020, 384, 123348. [Google Scholar] [CrossRef]
- Ghazali, N.F.; Lim, K.M. Mass Transport Models in Organic Solvent Nanofiltration: A Review. J. Adv. Resear. Fluid 2020, 76, 126–138. [Google Scholar] [CrossRef]
- Zilberbrand, M. A nonelectrical mechanism of ion exclusion in thin water films in finely dispersed media. J. Colloid Interface Sci. 1997, 192, 471–474. [Google Scholar] [CrossRef]
- Xu, G.-R.; Liu, X.-Y.; Xu, J.-M.; Li, L.; Su, H.-C.; Zhao, H.-L.; Feng, H.-J. High flux nanofiltration membranes based on layer-by-layer assembly modified electrospun nanofibrous substrate. Appl. Surf. Sci. 2018, 434, 573–581. [Google Scholar] [CrossRef]
- Bagheripour, E.; Moghadassi, A.; Hosseini, S.; Ray, M.; Parvizian, F.; Van Der Bruggen, B. Highly hydrophilic and antifouling nanofiltration membrane incorporated with water-dispersible composite activated carbon/chitosan nanoparticles. Chem. Eng. Res. Des. 2018, 132, 812–821. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Novel antibifouling nanofiltration polyethersulfone membrane fabricated from embedding TiO2 coated multiwalled carbon nanotubes. Sep. Purif. Technol. 2012, 90, 69–82. [Google Scholar] [CrossRef]
- Vatanpour, V.; Esmaeili, M.; Farahani, M.H.D.A. Fouling reduction and retention increment of polyethersulfone nanofiltration membranes embedded by amine-functionalized multi-walled carbon nanotubes. J. Membr. Sci. 2014, 466, 70–81. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Zhang, H.; Li, B.; Pan, J.; Qi, Y.; Shen, J.; Gao, C.; Van Der Bruggen, B. Carboxyl-functionalized graphene oxide polyamide nanofiltration membrane for desalination of dye solutions containing monovalent salt. J. Membr. Sci. 2017, 539, 128–137. [Google Scholar] [CrossRef]
- Miao, J.; Zhang, L.-C.; Lin, H. A novel kind of thin film composite nanofiltration membrane with sulfated chitosan as the active layer material. Chem. Eng. Sci. 2013, 87, 152–159. [Google Scholar] [CrossRef]


| Parameters | Value |
|---|---|
| Faraday’s constant (F) | 96,487 C·mol−1 |
| Operating temperature (T) | 303.15 K |
| pH in the oxidant unit | 10.2 |
| ) | 0.60 MPa |
| Crossflow velocity | |
| Boltzmann constant (k) | 1.38066 × 10−23 J·K−1 |
| Parameter | Unit | Value | Refs |
|---|---|---|---|
| rejection | This study | ||
| rejection | |||
| Water flux | |||
| Membrane geometry | |||
| Membrane surface area | |||
| Membrane thickness | |||
| Equation (27) |
| Particle | References | |||
|---|---|---|---|---|
| 2.03 | 0.121 | 17.82 | [49,50,51] | |
| 0.72 | 0.348 | −21.57 |
| Membranes | Water Flux (L·m−2·h−1) | Rejection (%) | References |
|---|---|---|---|
| 58 | 89.3 | This study | |
| 75 | 80 | [52] | |
| 60 | >90 | [6] | |
| 30 | 95 | [53] | |
| 4.2 | 81.9 | [54] | |
| 24 | 80 | [55] | |
| 20.4 | 96 | [56] | |
| 42 | 90 | [57] | |
| 22.9 | 90.8 | [58] |
| NF_PAN_Ti membrane | |||
|---|---|---|---|
| 24.1 | 24.0191 | 0.33 | |
| 89.3 | 89.2152 | 0.09 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Worou, C.N.; Kang, J.; Shen, J.; Degan, A.; Yan, P.; Wang, W.; Gong, Y.; Chen, Z. Euler’s Numerical Method for Ions Rejection Reassessment of a Defect-Free Synthesized Nanofiltration Membrane with Ultrathin Titania Film as the Selective Layer. Coatings 2021, 11, 184. https://doi.org/10.3390/coatings11020184
Worou CN, Kang J, Shen J, Degan A, Yan P, Wang W, Gong Y, Chen Z. Euler’s Numerical Method for Ions Rejection Reassessment of a Defect-Free Synthesized Nanofiltration Membrane with Ultrathin Titania Film as the Selective Layer. Coatings. 2021; 11(2):184. https://doi.org/10.3390/coatings11020184
Chicago/Turabian StyleWorou, Chabi Noël, Jing Kang, Jimin Shen, Arcadius Degan, Pengwei Yan, Weiqiang Wang, Yingxu Gong, and Zhonglin Chen. 2021. "Euler’s Numerical Method for Ions Rejection Reassessment of a Defect-Free Synthesized Nanofiltration Membrane with Ultrathin Titania Film as the Selective Layer" Coatings 11, no. 2: 184. https://doi.org/10.3390/coatings11020184
APA StyleWorou, C. N., Kang, J., Shen, J., Degan, A., Yan, P., Wang, W., Gong, Y., & Chen, Z. (2021). Euler’s Numerical Method for Ions Rejection Reassessment of a Defect-Free Synthesized Nanofiltration Membrane with Ultrathin Titania Film as the Selective Layer. Coatings, 11(2), 184. https://doi.org/10.3390/coatings11020184






