The Influence of the Powder Characteristics on 316L Stainless Steel Coatings Sprayed by Cold Gas Spray
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock Powders
2.2. Cold Gas Spray Conditions
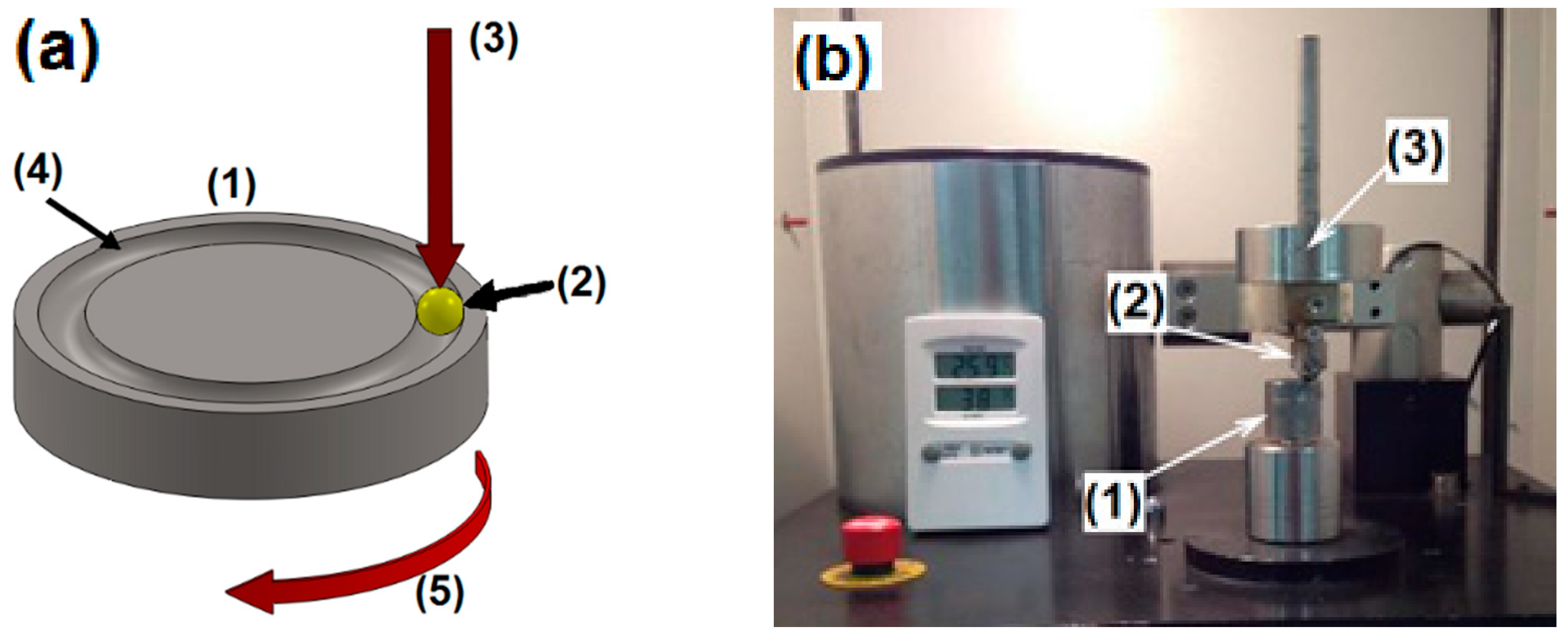
2.3. Powder and Coating Characterization
3. Results and Discussions
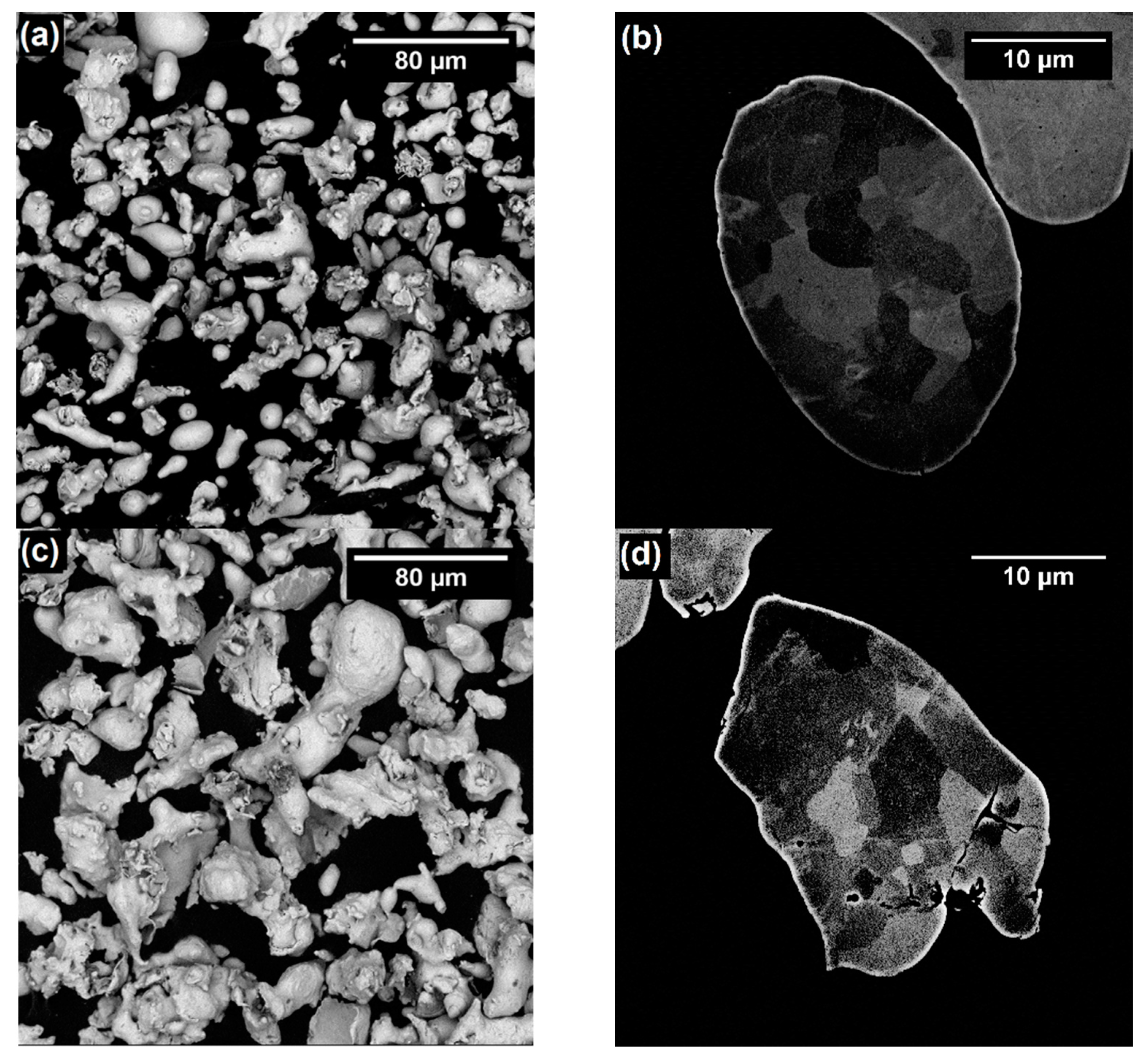
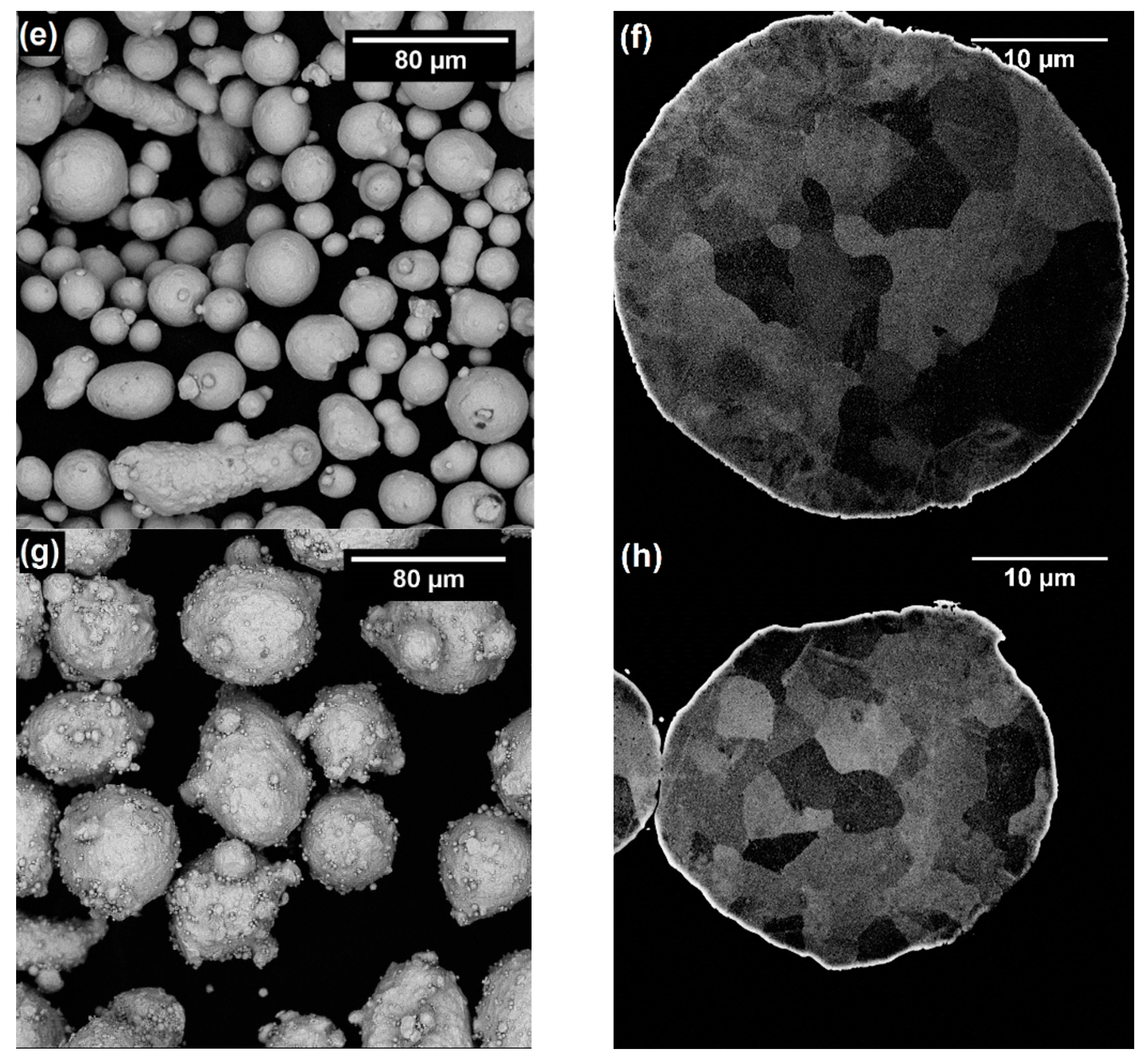
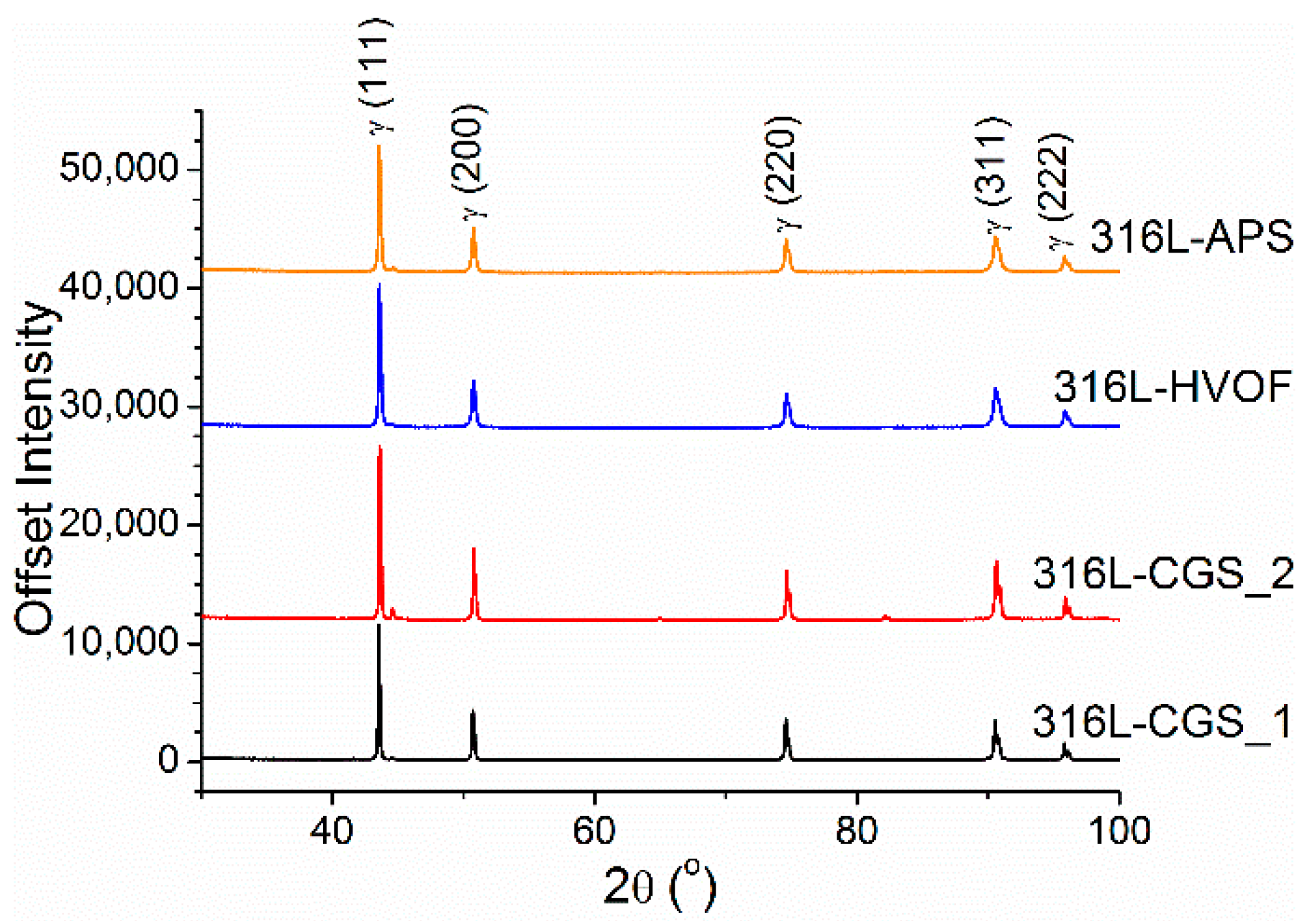
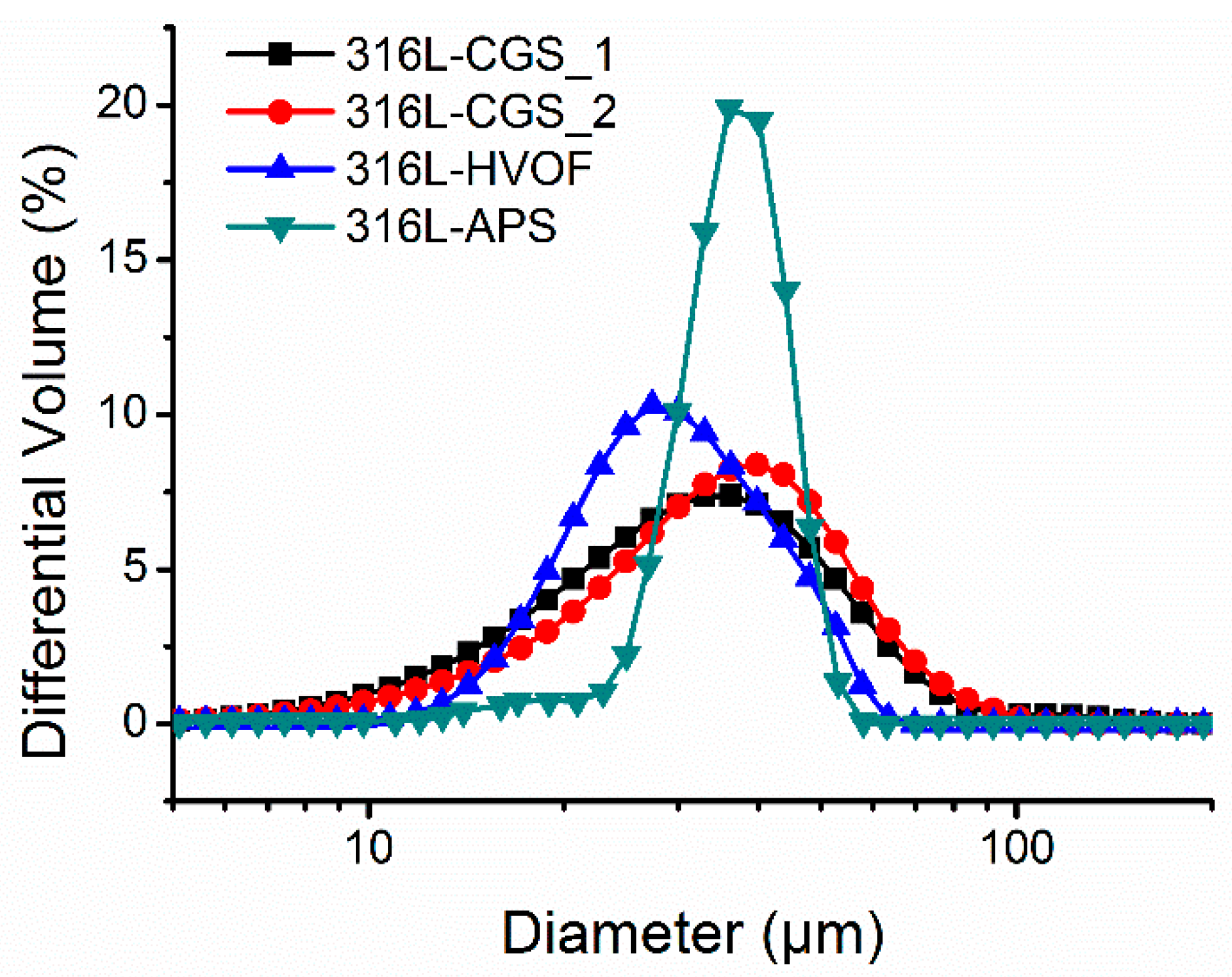
3.1. Characterization of Powders
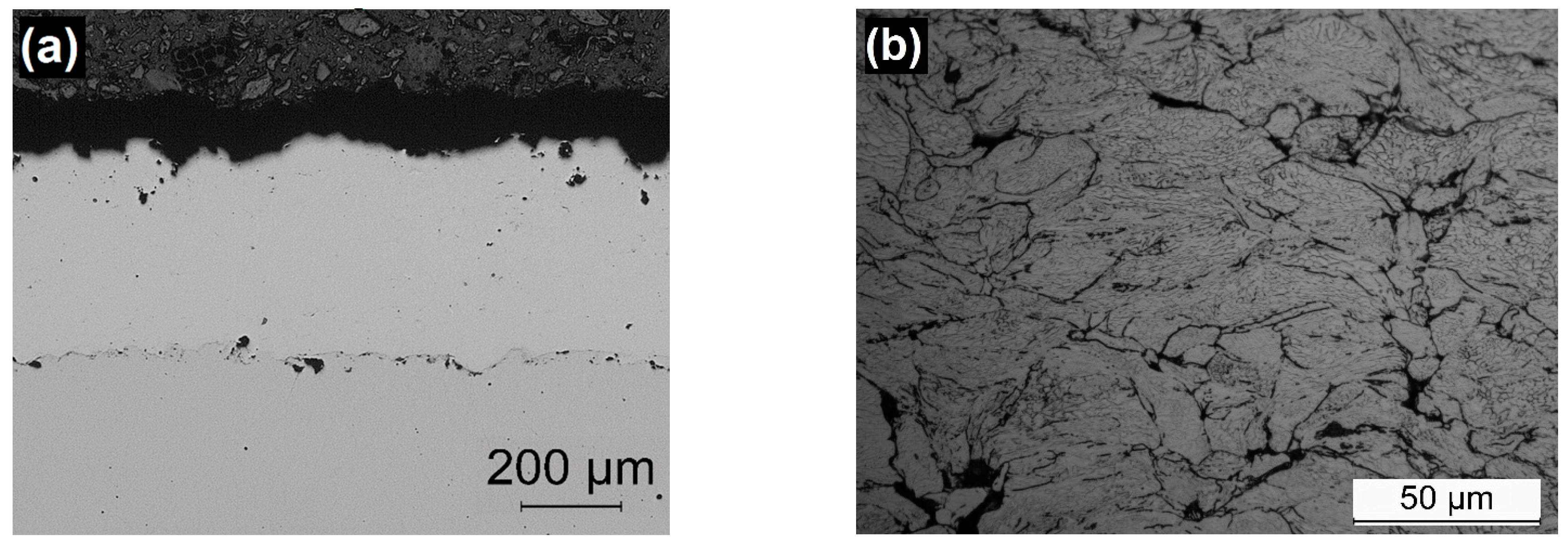
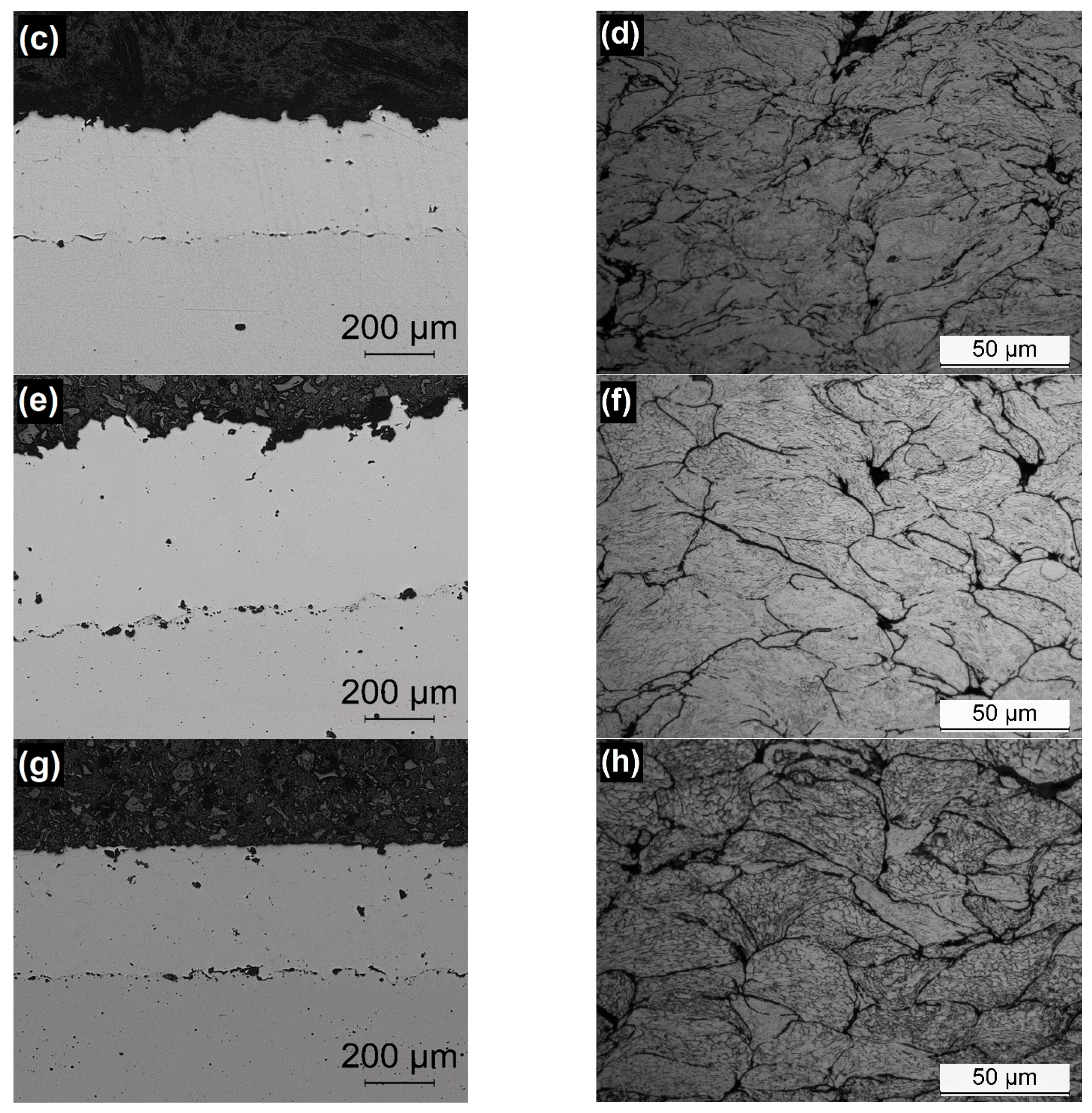
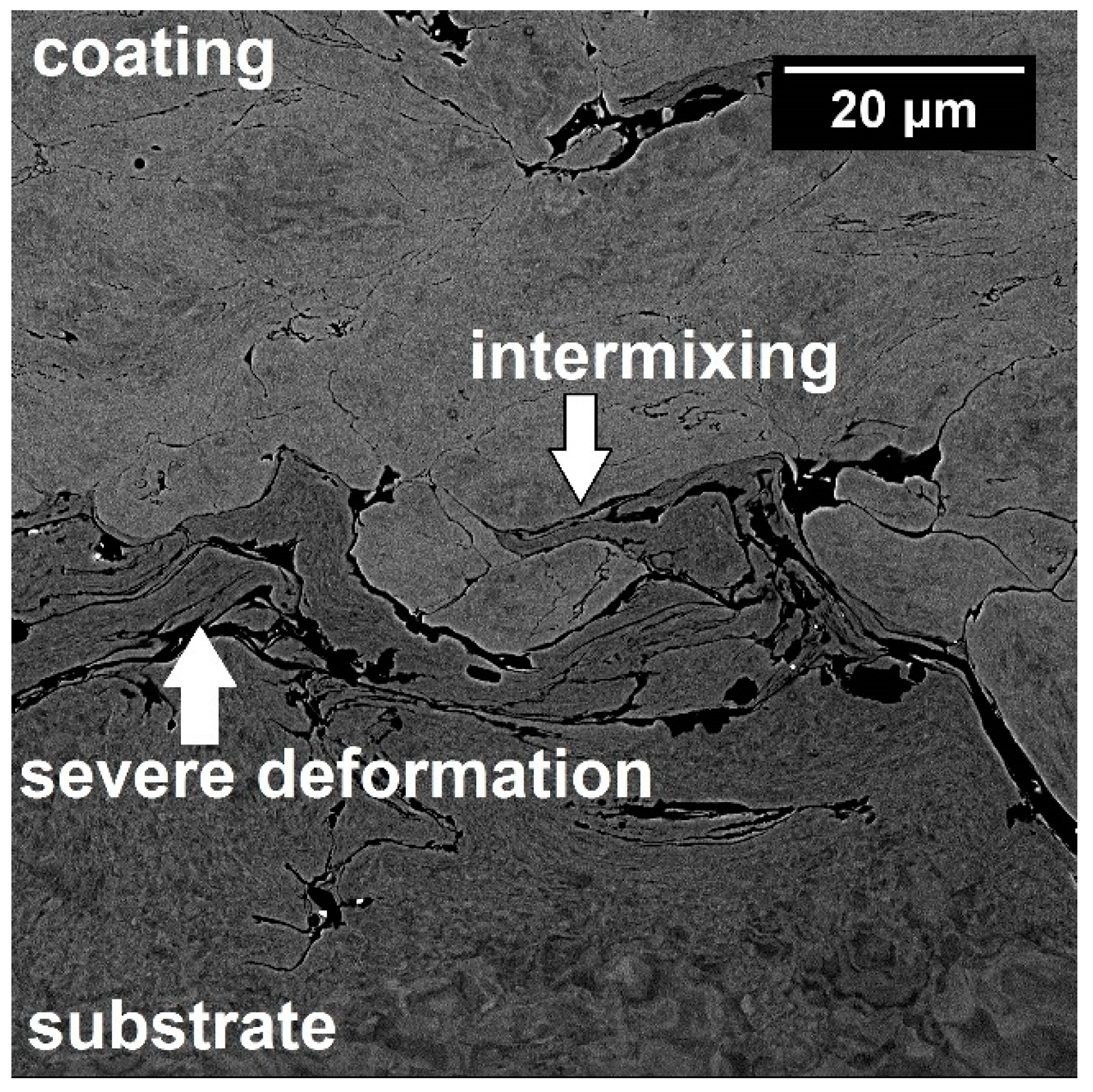
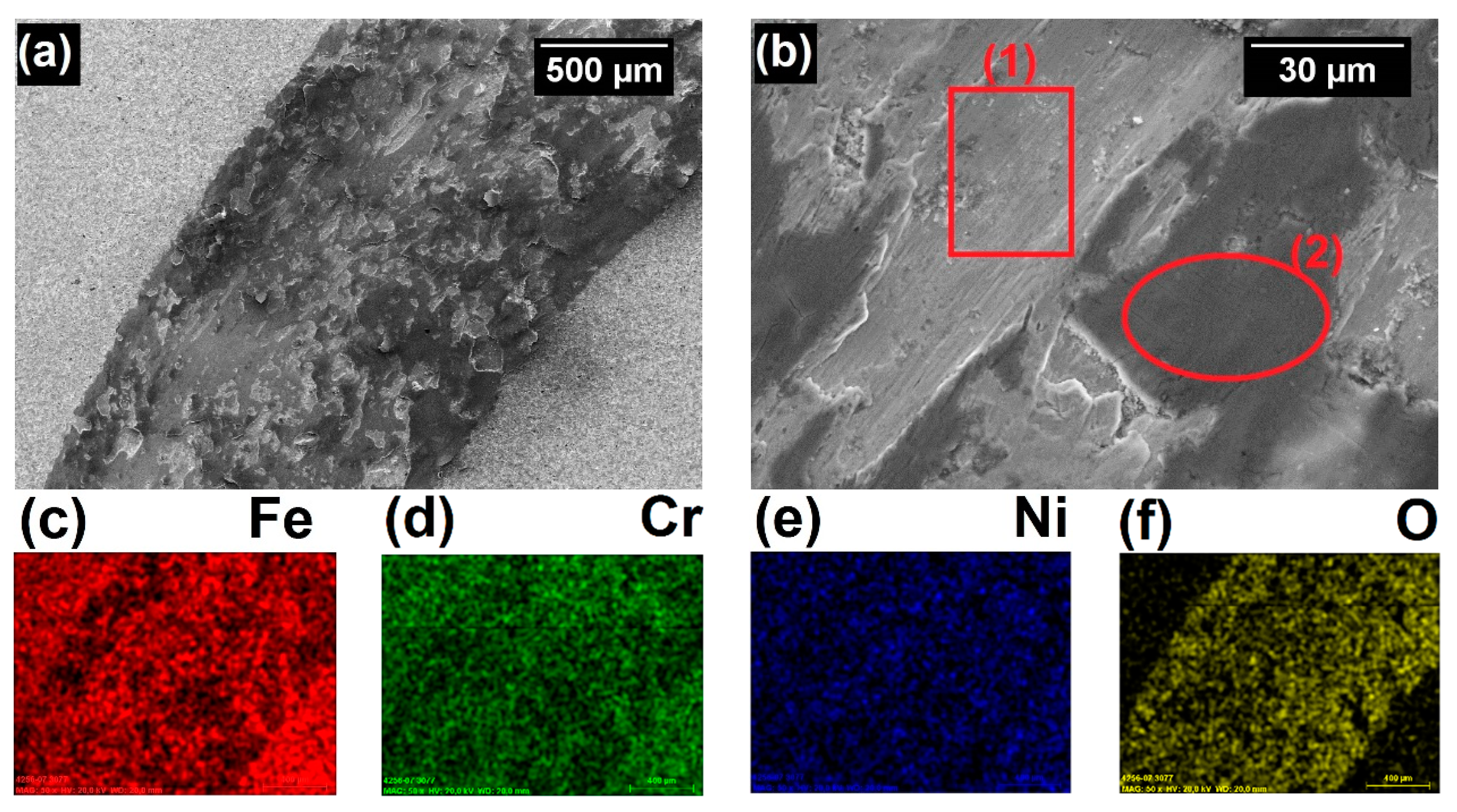
3.2. Characterization of Coatings
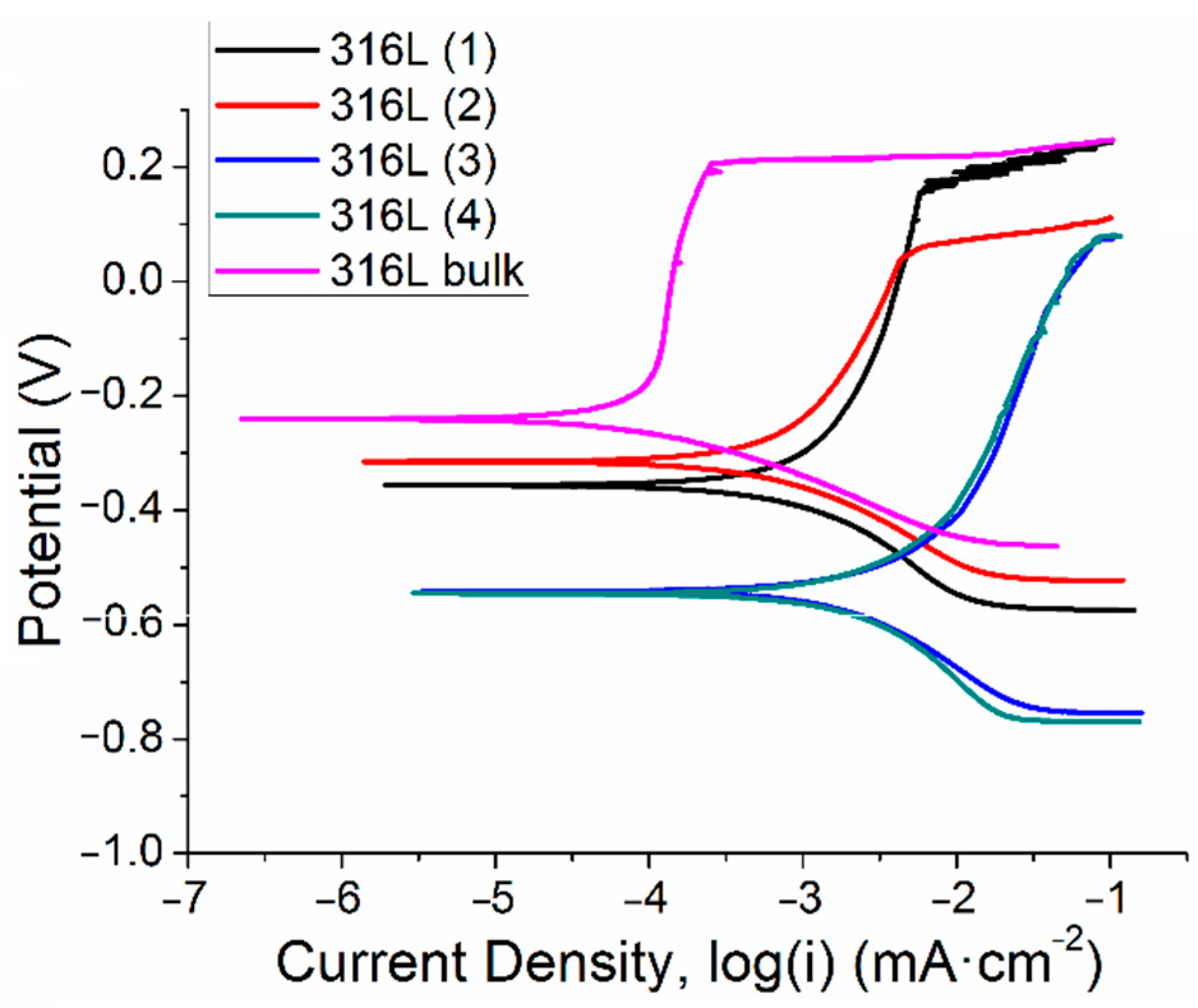
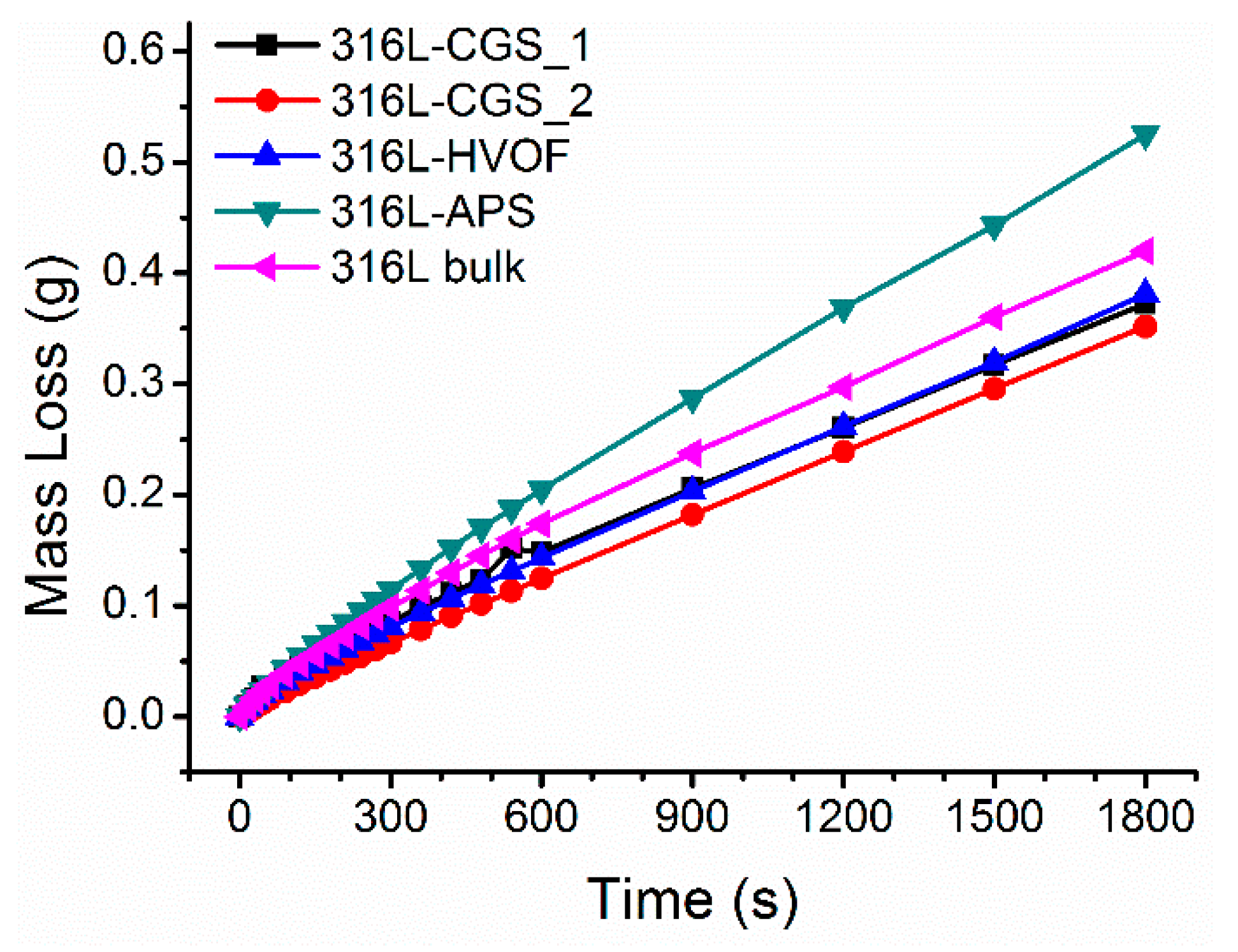
3.3. Corrosion Performance Testing
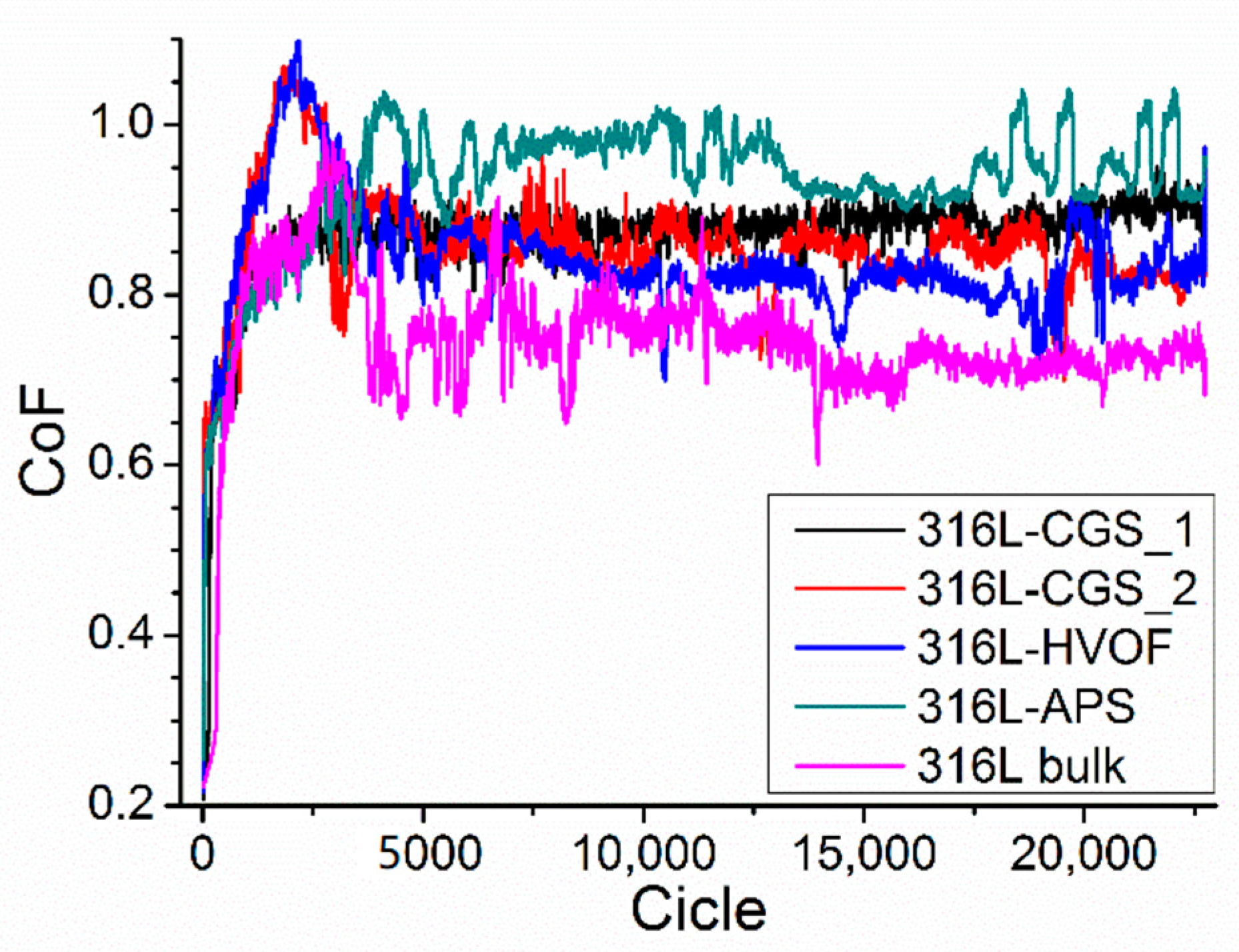
3.4. Tribological Behavior Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schweitzer, P.A. Paint and Coatings: Application and Corrosion Resistance; CRC Press: Boca Raton, FL, USA, 2006; ISBN 9781574447026. [Google Scholar]
- Dogan, H.; Findik, F.; Oztarhan, A. Comparative study of wear mechanism of surface treated AISI 316L stainless steel. Ind. Lubr. Tribol. 2003, 55, 76–83. [Google Scholar] [CrossRef]
- Chavan, N.M.; Kiran, B.; Jyothirmayi, A.; Phani, P.S.; Sundararajan, G. The corrosion behavior of cold sprayed zinc coatings on mild steel substrate. J. Therm. Spray Technol. 2013, 22, 463–470. [Google Scholar] [CrossRef]
- Łatka, L.; Pawłowski, L.; Winnicki, M.; Sokołowski, P.; Malachowska, A.; Kozerski, S. Review of functionally graded thermal sprayed coatings. Appl. Sci. 2020, 10, 5153. [Google Scholar] [CrossRef]
- Cortés, R.; Garrido, M.Á.; Poza, P.; Martos, A.; Dosta, S.; García, I. Cold sprayed coatings for repairing damaged metallic structures. Key Eng. Mater. 2019, 813, 74–79. [Google Scholar] [CrossRef]
- Adachi, S.; Ueda, N. Wear and corrosion properties of cold-sprayed AISI 316L coatings treated by combined plasma carburizing and nitriding at low temperature. Coatings 2018, 8, 456. [Google Scholar] [CrossRef]
- Dikici, B.; Yilmazer, H.; Ozdemir, I.; Isik, M. The effect of post-heat treatment on microstructure of 316L cold-sprayed coatings and their corrosion performance. J. Therm. Spray Technol. 2016, 25, 704–714. [Google Scholar] [CrossRef]
- Yin, S.; Cizek, J.; Yan, X.; Lupoi, R. Annealing strategies for enhancing mechanical properties of additively manufactured 316L stainless steel deposited by cold spray. Surf. Coatings Technol. 2019, 370, 353–361. [Google Scholar] [CrossRef]
- Assadi, H.; Kreye, H.; Gärtner, F.; Klassen, T. Cold spraying – A materials perspective. Acta Mater. 2016, 116, 382–407. [Google Scholar] [CrossRef]
- Rokni, M.R.; Nutt, S.R.; Widener, C.A.; Champagne, V.K.; Hrabe, R.H. Review of relationship between particle deformation, coating microstructure, and properties in high-pressure cold spray. J. Therm. Spray Technol. 2017, 26, 1308–1355. [Google Scholar] [CrossRef]
- Tului, M.; Bartuli, C.; Bezzon, A.; Marino, A.L.; Marra, F.; Matera, S.; Pulci, G. Amorphous steel coatings deposited by cold-gas spraying. Metals 2019, 9, 678. [Google Scholar] [CrossRef]
- Villa, M.; Dosta, S.; Guilemany, J.M. Optimization of 316L stainless steel coatings on light alloys using Cold Gas Spray. Surf. Coatings Technol. 2013, 235, 220–225. [Google Scholar] [CrossRef]
- Sousa, B.C.; Gleason, M.A.; Haddad, B.; Champagne, V.K.; Nardi, A.T.; Cote, D.L. Nanomechanical characterization for cold spray: From feedstock to consolidated material properties. Metals 2020, 10, 1195. [Google Scholar] [CrossRef]
- Beckers, D.; Ellendt, N.; Fritsching, U.; Uhlenwinkel, V. Impact of process flow conditions on particle morphology in metal powder production via gas atomization. Adv. Powder Technol. 2020, 31, 300–311. [Google Scholar] [CrossRef]
- Lagutkin, S.; Achelis, L.; Sheikhaliev, S.; Uhlenwinkel, V.; Srivastava, V. Atomization process for metal powder. Mater. Sci. Eng. A 2004, 383, 1–6. [Google Scholar] [CrossRef]
- Suri, P.; Koseski, R.P.; German, R.M. Microstructural evolution of injection molded gas- and water-atomized 316L stainless steel powder during sintering. Mater. Sci. Eng. A 2005, 402, 341–348. [Google Scholar] [CrossRef]
- Jeandin, M.; Rolland, G.; Descurninges, L.L.; Berger, M.H. Which powders for cold spray? Surf. Eng. 2014, 30, 291–298. [Google Scholar] [CrossRef]
- Sun, W.; Tan, A.W.; Wu, K.; Yin, S.; Yang, X.; Marinescu, I.; Liu, E. Post-process treatments on supersonic cold sprayed coatings: A review. Coatings 2020, 10, 123. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Tokoro, R.; Tanno, M.; Ogawa, K. Acta Materialia Elucidation of cold-spray deposition mechanism by auger electron spectroscopic evaluation of bonding interface oxide fi lm. Acta Mater. 2019, 164, 39–49. [Google Scholar] [CrossRef]
- Sun, W.; Wei, A.; Tan, Y.; Marinescu, I.; Quan, W.; Liu, E. Adhesion, tribological and corrosion properties of cold-sprayed CoCrMo and Ti6Al4V coatings on 6061-T651 Al alloy. Surf. Coat. Technol. 2017, 326, 291–298. [Google Scholar] [CrossRef]
- Ko, K.H.; Choi, J.O.; Lee, H. Intermixing and interfacial morphology of cold-sprayed Al coatings on steel. Mater. Lett. 2014, 136, 45–47. [Google Scholar] [CrossRef]
- Bae, G.; Kumar, S.; Yoon, S.; Kang, K.; Na, H.; Kim, H.; Lee, C. Bonding features and associated mechanisms in kinetic sprayed titanium coatings. Acta Mater. 2009, 57, 5654–5666. [Google Scholar] [CrossRef]
- Yin, S.; Cizek, J.; Cupera, J.; Hassani, M.; Luo, X.; Jenkins, R.; Xie, Y.; Li, W.; Lupoi, R. Formation conditions of vortex-like intermixing interfaces in cold spray. Mater. Des. 2021, 200, 1–10. [Google Scholar] [CrossRef]
- Schmidt, T.; Gaertner, F.; Kreye, H. New developments in cold spray based on higher gas and particle temperatures. J. Therm. Spray Technol. 2006, 15, 488–494. [Google Scholar] [CrossRef]
- Fukanuma, H.; Ohno, N.; Sun, B.; Huang, R. In-flight particle velocity measurements with DPV-2000 in cold spray. Surf. Coatings Technol. 2006, 201, 1935–1941. [Google Scholar] [CrossRef]
- Jodoin, B.; Ajdelsztajn, L.; Sansoucy, E.; Zúñiga, A.; Richer, P.; Lavernia, E.J. Effect of particle size, morphology, and hardness on cold gas dynamic sprayed aluminum alloy coatings. Surf. Coatings Technol. 2006, 201, 3422–3429. [Google Scholar] [CrossRef]
- Adachi, S.; Ueda, N. Effect of cold-spray conditions using a nitrogen propellant gas on AISI 316L stainless steel-coating microstructures. Coatings 2017, 7, 87. [Google Scholar] [CrossRef]
- Standard Test Method for Particle Size Distribution of Metal Powders and Related Compounds by Laser Scattering; ASTM B822-02; ASTM International: West Conshohocken, PA, USA, 2002.
- Standard Test Method for Apparent Density of Powders using the Hall Flowmeter Funnel; ASTM B212-99; ASTM International: West Conshohocken, PA, USA, 1999.
- Standard Test Method for Flow Rate of Metal Powders; ASTM B213-03; ASTM International: West Conshohocken, PA, USA, 2003.
- Standard Guide for Metallographic Preparation of Thermal Sprayed Coatings; ASTM E1920-03; ASTM International: West Conshohocken, PA, USA, 2003.
- Standard Guide for Preparation of Metallographic Specimens; ASTM E3-01; ASTM International: West Conshohocken, PA, USA, 2001.
- ASTM B487-85 - Standard Test Method for Measurement of Metal and Oxide Coating Thickness by Microscopical Examination of a Cross Section; ASTM International: West Conshohocken, PA, USA, 2002.
- Standard test Methods for Determining Area Percentage Porosity in Thermal Sprayed Coatings; ASTM E2109-01; ASTM International: West Conshohocken, PA, USA, 2002.
- Standard Test Method for Microindentation Hardness of Materials; ASTM E384-99; ASTM International: West Conshohocken, PA, USA, 2000.
- Standard Test Method for Adhesion or Cohesion Strength of Thermal Spray Coatings; ASTM C633-17; ASTM International: West Conshohocken, PA, USA, 2017.
- Standard Test Method for Measuring Abrasion using the Dry Sand Rubber wheel Apparatus; ASTM G65-00; ASTM International: West Conshohocken, PA, USA, 2000.
- Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus; ASTM G99-04; ASTM International: West Conshohocken, PA, USA, 2004.
- Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements; ASTM G59-97; ASTM International: West Conshohocken, PA, USA, 2003.
- Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements; ASTM G102-89; ASTM International: West Conshohocken, PA, USA, 1999.
- Hoeges, S.; Zwiren, A.; Schade, C. Additive manufacturing using water atomized steel powders. Met. Powder Rep. 2017, 72, 111–117. [Google Scholar] [CrossRef]
- Sklyar, M.O.; Turichin, G.A.; Klimova, O.G.; Zotov, O.G.; Topalov, I.K. Microstructure of 316L stainless steel components produced by direct laser deposition. Steel Transl. 2016, 46, 883–887. [Google Scholar] [CrossRef]
- Standard Aspecification for Chromium and Chromium-Nickel Stainless Steel Plate, Sheet, and Strip for Pressure Vessels and for General Applications; ASTM A240/A240M-04a; ASTM International: West Conshohocken, PA, USA, 2004.
- Wong, W.; Vo, P.; Irissou, E.; Ryabinin, A.N.; Legoux, J.; Yue, S. Effect of particle morphology and size distribution on cold-sprayed pure titanium coatings. J. Therm. Spray Technol. 2013, 22, 1140–1153. [Google Scholar] [CrossRef]
- Spencer, K.; Zhang, M.X. Optimisation of stainless steel cold spray coatings using mixed particle size distributions. Surf. Coatings Technol. 2011, 205, 5135–5140. [Google Scholar] [CrossRef]
- Chu, X.; Che, H.; Teng, C.; Vo, P.; Yue, S. A multiple particle arrangement model to understand cold spray characteristics of bimodal size 316L/Fe powder mixtures. Surf. Coatings Technol. 2020, 381, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Adrien, J.; Normand, B. Porosity characterization of cold sprayed stainless steel coating using three-dimensional X-ray microtomography. Coatings 2018, 8, 326. [Google Scholar] [CrossRef]
- Mindivan, H.; Kale, A.; Berse, U.; Samur, R. A comparative study of thermal sprayed AISI 316L stainless steel coatings. El-Cezerî J. Sci. Eng. 2017, 4, 127–134. [Google Scholar]
- Zhao, L.; Lugscheider, E. Influence of the spraying processes on the properties of 316L stainless steel coatings. Surf. Coatings Technol. 2002, 162, 6–10. [Google Scholar] [CrossRef]
- Li, Q.; Song, P.; Ji, Q.; Huang, Y.; Li, D.; Zhai, R.; Zheng, B.; Lu, J. Microstructure and wear performance of arc-sprayed Al/316L stainless-steel composite coating. Surf. Coatings Technol. 2019, 374, 189–200. [Google Scholar] [CrossRef]
- Luzin, V.; Spencer, K.; Zhang, M.X. Residual stress and thermo-mechanical properties of cold spray metal coatings. Acta Mater. 2011, 59, 1259–1270. [Google Scholar] [CrossRef]
- Maestracci, R.; Sova, A.; Jeandin, M.; Malhaire, J.M.; Movchan, I.; Bertrand, P.; Smurov, I. Deposition of composite coatings by cold spray using stainless steel 316L, copper and Tribaloy T-700 powder mixtures. Surf. Coatings Technol. 2016, 287, 1–8. [Google Scholar] [CrossRef]
- Sova, A.; Grigoriev, S.; Okunkova, A.; Smurov, I. Cold spray deposition of 316L stainless steel coatings on aluminium surface with following laser post-treatment. Surf. Coatings Technol. 2013, 235, 283–289. [Google Scholar] [CrossRef]
- Xie, Y.; Planche, M.P.; Raoelison, R.; Liao, H.; Suo, X.; Herve, P. Effect of substrate preheating on adhesive strength of SS 316L cold spray coatings. J. Therm. Spray Technol. 2016, 25, 123–130. [Google Scholar] [CrossRef]
- Pukasiewicz, A.G.M.; Sucharski, G.B.; Siqueira, I.B.A.F.; de Andrade, J.; Váz, R.F.; Procopiak, L.A.J. Corrosion resistance of iron-based alloy coatings deposited by HVOF process. In Proceedings of the ITSC 2019 - Proceedings of the International Thermal Spray Conference, Yokohama, Japan, 26–29 May 2019; Azarmi, F., Lau, Y., Veilleux, J., Widener, C., Toma, F., Koivuluoto, H., Balani, K., Li, H., Shinoda, K., Eds.; ASM International: Yokohama, Japan, 2019; pp. 359–368. [Google Scholar]
- Zhang, X.G. Galvanic corrosion. In Uhlig’s Corrosion Handbook; Revie, R.W., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 123–143. ISBN 9780470080320. [Google Scholar]
- Fellah, M.; Labaız, M.; Assala, O.; Iost, A.; Dekhil, L. Tribological behaviour of AISI 316L stainless steel for biomedical applications. Tribology 2013, 7, 135–149. [Google Scholar] [CrossRef]
- Qin, W.; Kang, J.; Li, J.; Yue, W.; Liu, Y.; She, D.; Mao, Q.; Li, Y. Tribological behavior of the 316L stainless steel with heterogeneous lamella structure. Materials 2018, 11, 839. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Li, L.; Li, D.Y. Further look at correlation between ASTM G65 rubber wheel abrasion and pin-on-disc wear tests for data conversion. Tribol.-Mater. Surfaces Interfaces 2013, 7, 109–113. [Google Scholar] [CrossRef]
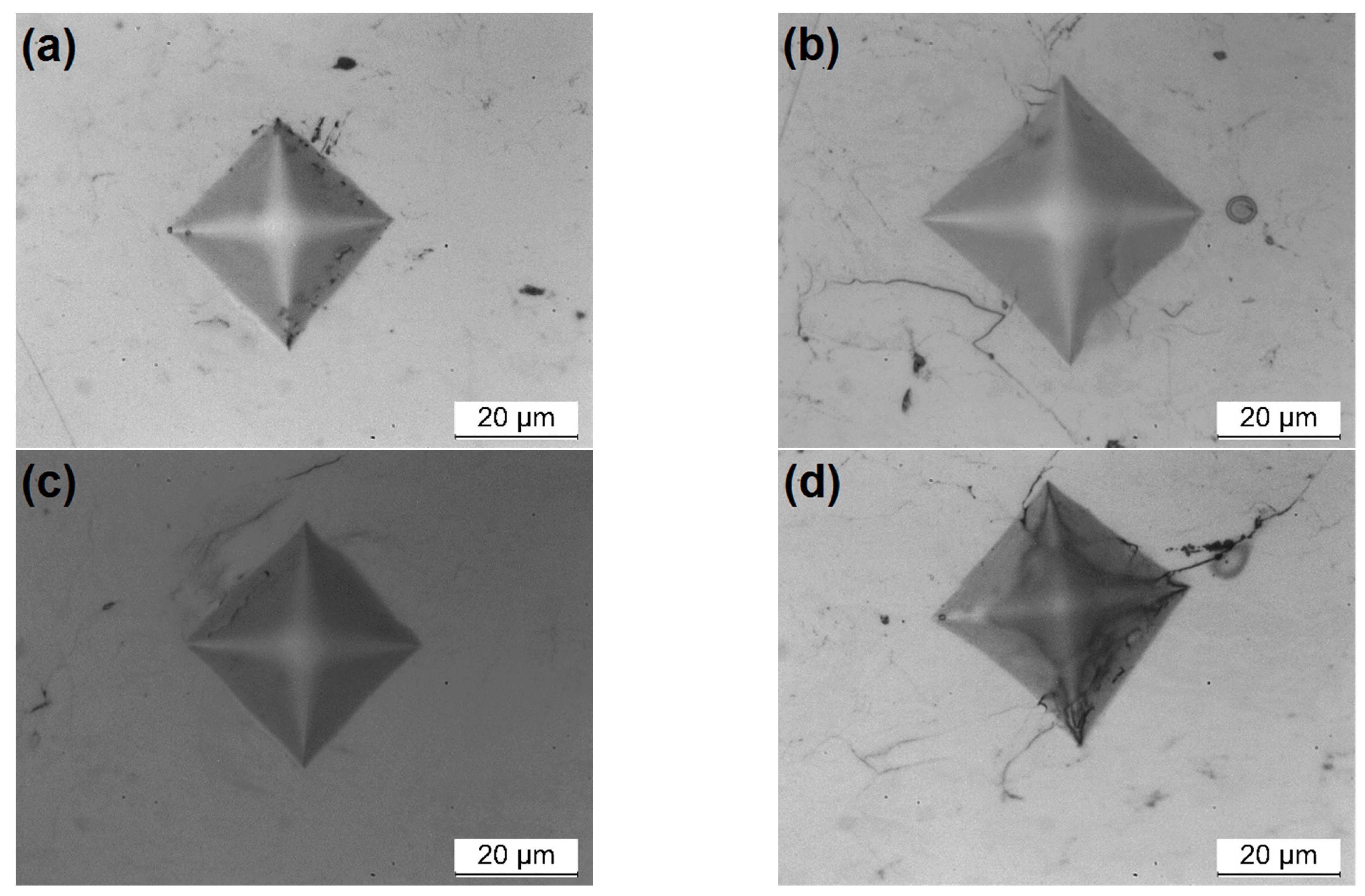
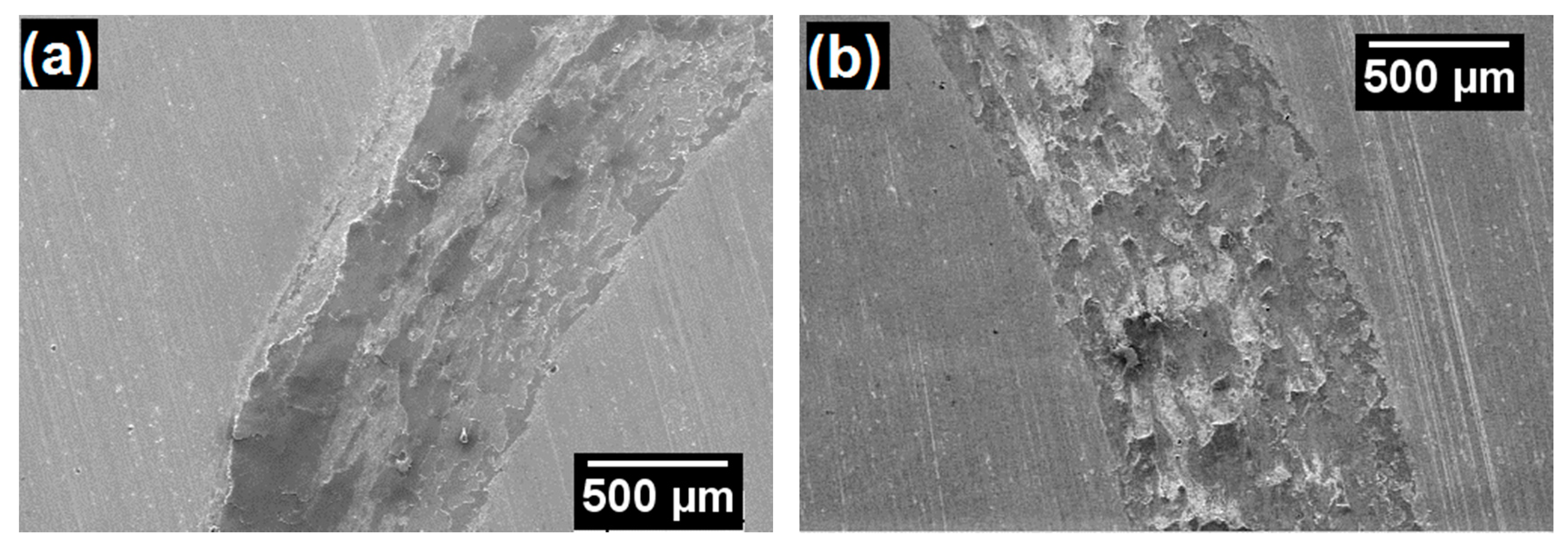
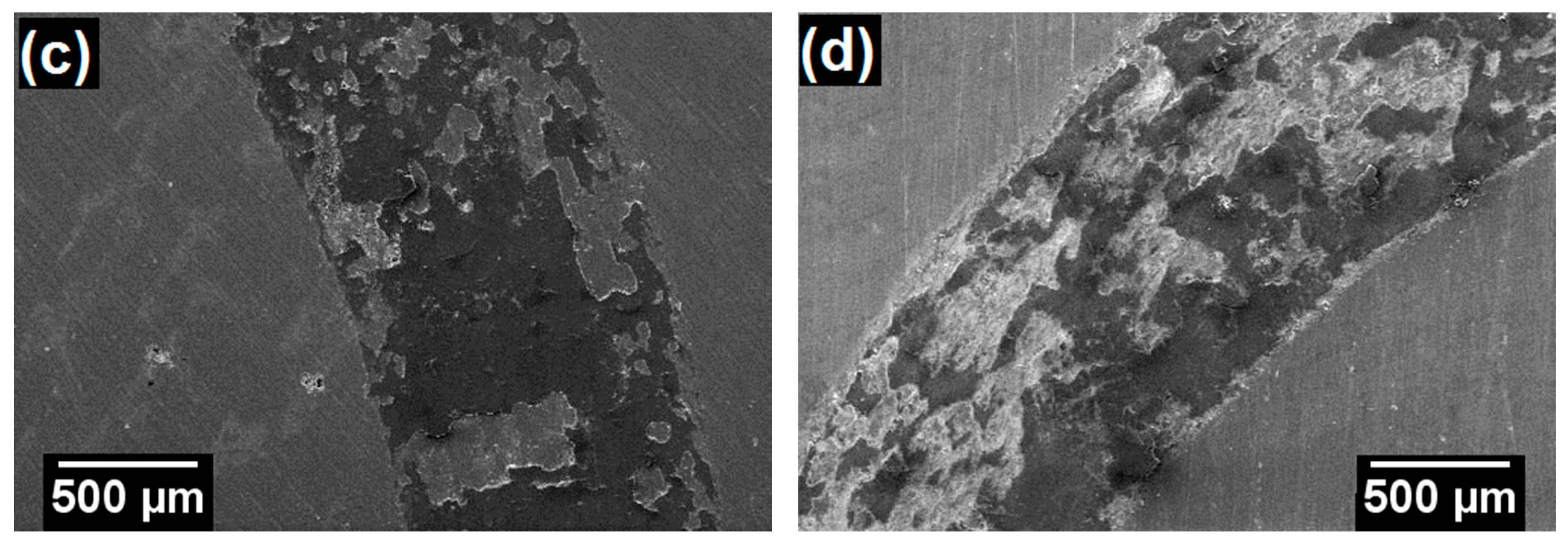
| Powder Identification | Nominal Composition (wt.%) | Particle Size Distribution (μm) | Fabrication Process | |||||
|---|---|---|---|---|---|---|---|---|
| Cr | Ni | Mo | Mn | Si | Fe | |||
| 316L-CGS_1 | 17.6 | - | 2.7 | 0.3 | 0.8 | Bal. | −40 + 15 | Water atomized |
| 316L-CGS_2 | 18 | 14 | 3 | - | 1 | Bal. | −45 + 10 | Water atomized |
| 316L-HVOF | 17 | 12 | 2.5 | - | 2.3 | Bal. | −45 + 11 | Gas atomized |
| 316L-APS | 17 | 12 | 2.5 | 2 | 1 | Bal. | −45 + 20 | Gas atomized |
| Material | Cr | Ni | Mo | Mn | Mg | S | P | Si | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 316L-CGS_1 | 16.03 | 12.27 | 2.57 | 0.52 | 0.1 | 0.5 | 0.5 | 2.0 | Bal. |
| 316L-CGS_2 | 16.32 | 11.19 | 2.16 | 0.09 | 0.1 | 0.5 | 0.5 | 2.0 | Bal. |
| 316L-HVOF | 16.42 | 10.12 | 2.07 | 1.37 | 0.1 | 0.5 | 0.5 | 2.0 | Bal. |
| 316L-APS | 17.07 | 9.55 | 1.83 | 0.55 | 0.1 | 0.5 | 0.5 | 2.0 | Bal. |
| 316L reference [43] | 16.0 18.0 | 10.0 14.0 | 2.00 3.00 | 2.00 max | - | 0.030 max | 0.045 max | 0.75 max | Bal. |
| Material | Particle Size Distribution (µm) | Morphology | Apparent Density (g·cm−3) | Flow Rate (g·s−1) | Powder Feed Rate (g·s−1) | |
|---|---|---|---|---|---|---|
| d10 * | d90 ** | |||||
| 316L-CGS_1 | 16 | 60 | Irregular | 3.03 ± 0.01 | 9.03 ± 0.36 | 0.43 |
| 316L-CGS_2 | 17 | 60 | Irregular | 2.86 ± 0.01 | 8.21 ± 0.49 | 0.41 |
| 316L-HVOF | 19 | 47 | Quasi-spherical | 4.47 ± 0.01 | 17.61 ± 0.40 | 0.55 |
| 316L-APS | 29 | 47 | Quasi-spherical *** | 3.73 ± 0.01 | 13.44 ± 0.65 | 0.50 |
| Material | Hardness (HV0.3) | Thickness (µm) | Porosity (%) | Adherence (MPa) | Deposition Efficiency (%) |
|---|---|---|---|---|---|
| 316L-CGS_1 | 356 ± 23 | 420 ± 26 | 0.1 ± 0.0 | 29.4 ± 4.9 | 97 |
| 316L-CGS_2 | 348 ± 48 | 331 ± 28 | 0.2 ± 0.0 | 19.7 ± 2.4 | 85 |
| 316L-HVOF | 353 ± 44 | 517 ± 21 | 0.2 ± 0.1 | 11.0 ± 1.9 | 92 |
| 316L-APS | 344 ± 25 | 381 ± 12 | 0.4 ± 0.2 | 4.5 ± 2.1 | 80 |
| Material | Current Density icorr (µA·cm−2) | Potential of Corrosion Ecorr (mV) | Polarization Resistance Rp (kΩ) |
|---|---|---|---|
| 316L-CGS_1 | 2.120 | −356.332 | 36.119 |
| 316L-CGS_2 | 0.897 | −315.189 | 45.640 |
| 316L-HVOF | 3.244 | −541.909 | 15.518 |
| 316L-APS | 2.373 | −546.627 | 14.322 |
| 316L bulk | 0.100 | −239.738 | 50.080 |
| Material | Abrasion Rate (mm3·N−1·m−1) | CoF | Friction Wear Rate (mm3·N−1·m−1) |
|---|---|---|---|
| 316L-CGS_1 | 1.70 × 10−4 | 0.869 ± 0.070 | 1.651 × 10−4 |
| 316L-CGS_2 | 1.32 × 10−4 | 0.857 ± 0.073 | 1.251 × 10−4 |
| 316L-HVOF | 1.60 × 10−4 | 0.841 ± 0.077 | 1.619 × 10−4 |
| 316L-APS | 2.25 × 10−4 | 0.934 ± 0.078 | 2.228 × 10−4 |
| 316L bulk | 1.91 × 10−4 | 0.746 ± 0.100 | 1.692 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaz, R.F.; Silvello, A.; Sanchez, J.; Albaladejo, V.; Cano, I.G. The Influence of the Powder Characteristics on 316L Stainless Steel Coatings Sprayed by Cold Gas Spray. Coatings 2021, 11, 168. https://doi.org/10.3390/coatings11020168
Vaz RF, Silvello A, Sanchez J, Albaladejo V, Cano IG. The Influence of the Powder Characteristics on 316L Stainless Steel Coatings Sprayed by Cold Gas Spray. Coatings. 2021; 11(2):168. https://doi.org/10.3390/coatings11020168
Chicago/Turabian StyleVaz, Rodolpho F., Alessio Silvello, Javier Sanchez, Vicente Albaladejo, and Irene García Cano. 2021. "The Influence of the Powder Characteristics on 316L Stainless Steel Coatings Sprayed by Cold Gas Spray" Coatings 11, no. 2: 168. https://doi.org/10.3390/coatings11020168
APA StyleVaz, R. F., Silvello, A., Sanchez, J., Albaladejo, V., & Cano, I. G. (2021). The Influence of the Powder Characteristics on 316L Stainless Steel Coatings Sprayed by Cold Gas Spray. Coatings, 11(2), 168. https://doi.org/10.3390/coatings11020168









