Thin Films of Plasma-Polymerized n-Hexane and ZnO Nanoparticles Co-Deposited via Atmospheric Pressure Plasma Jet
Abstract
1. Introduction
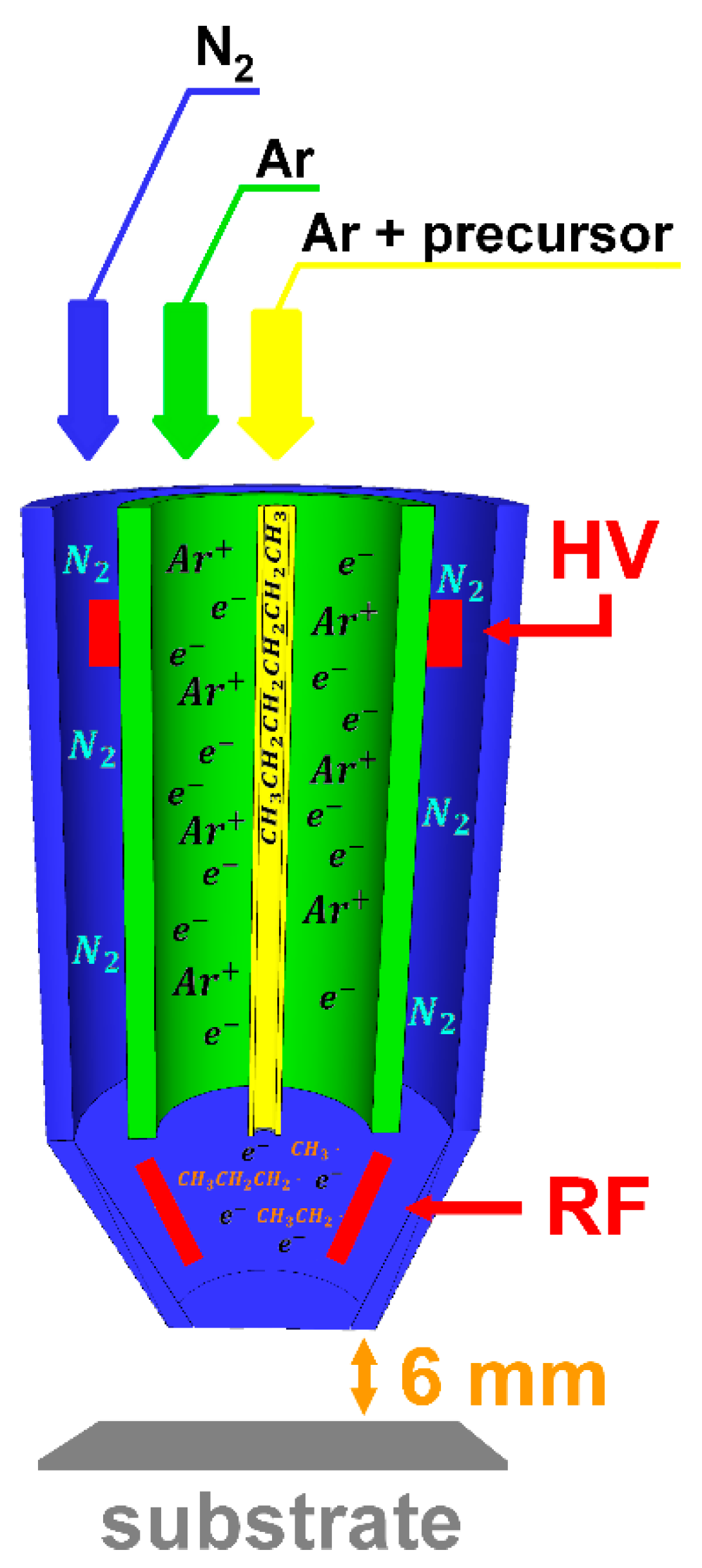
2. Materials and Methods
3. Results and Discussion
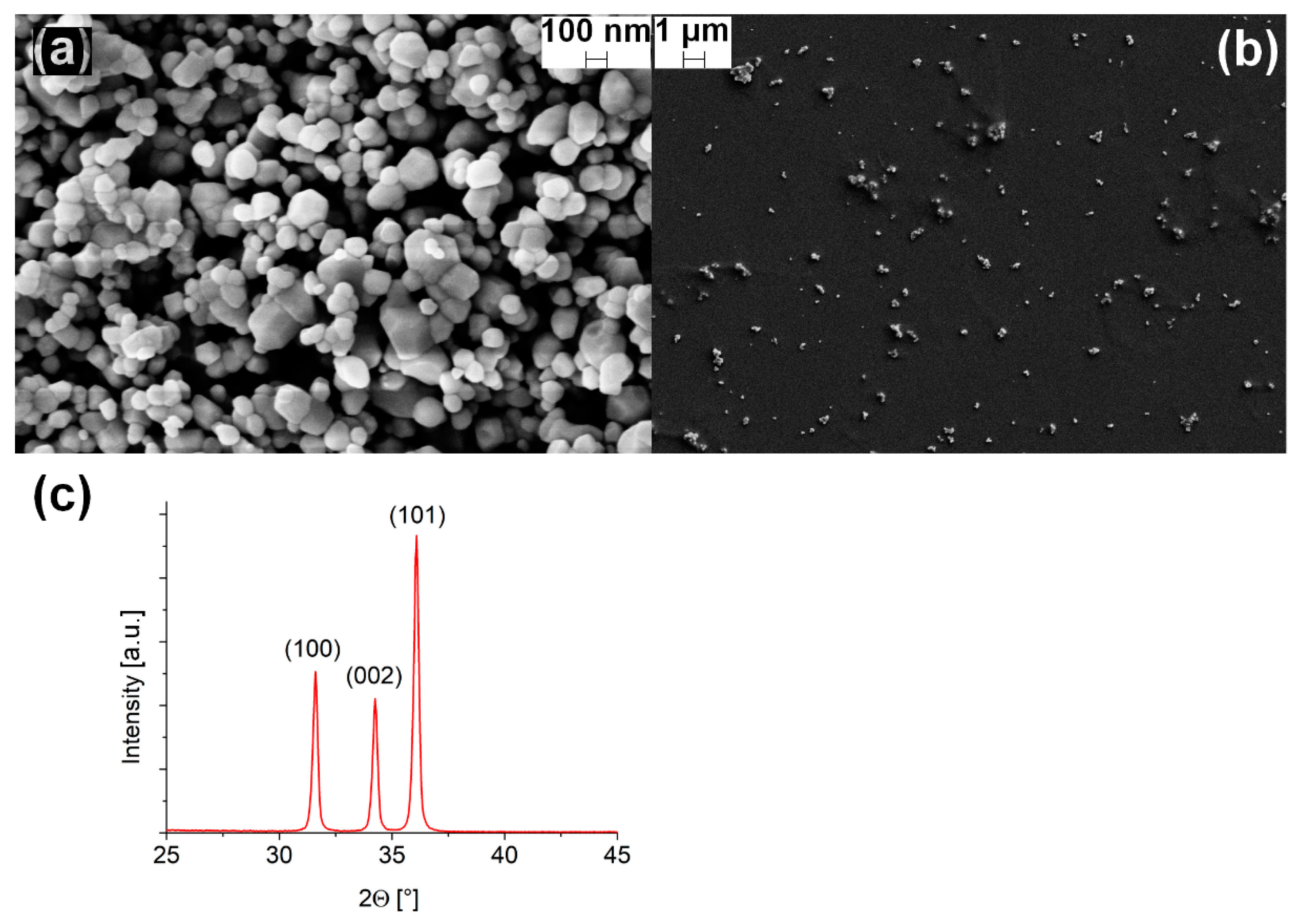
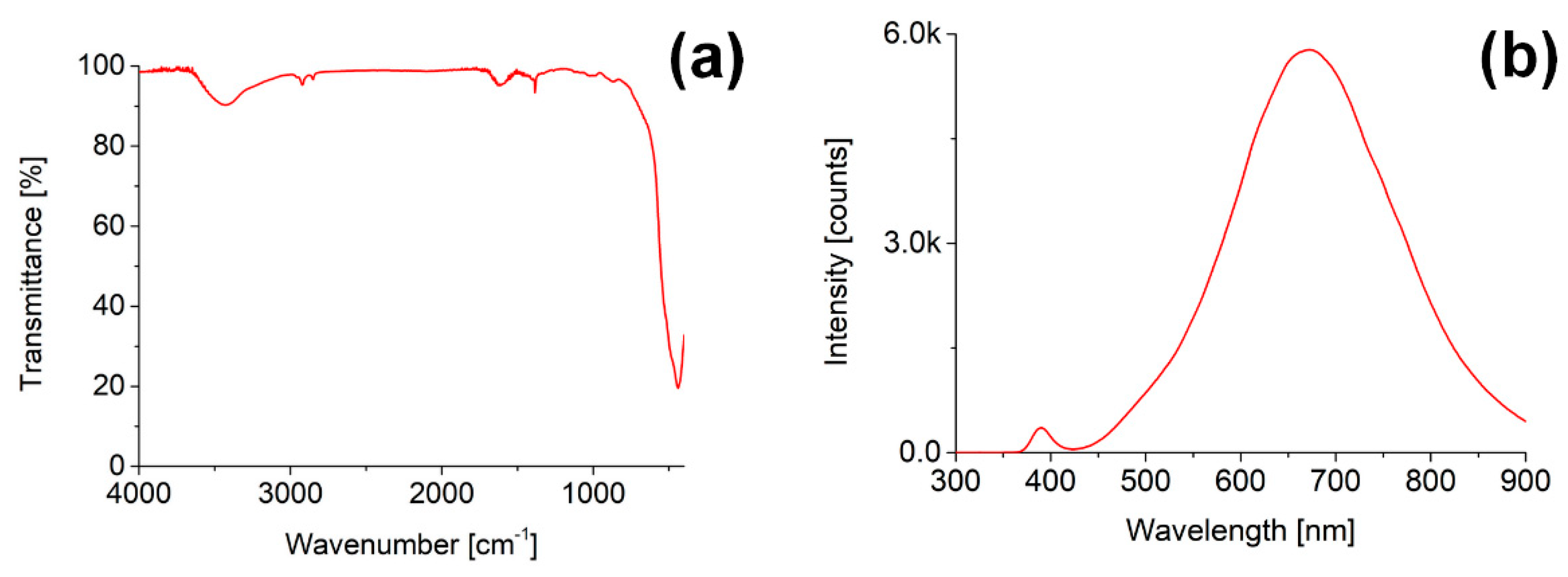
3.1. ZnO Nanoparticles
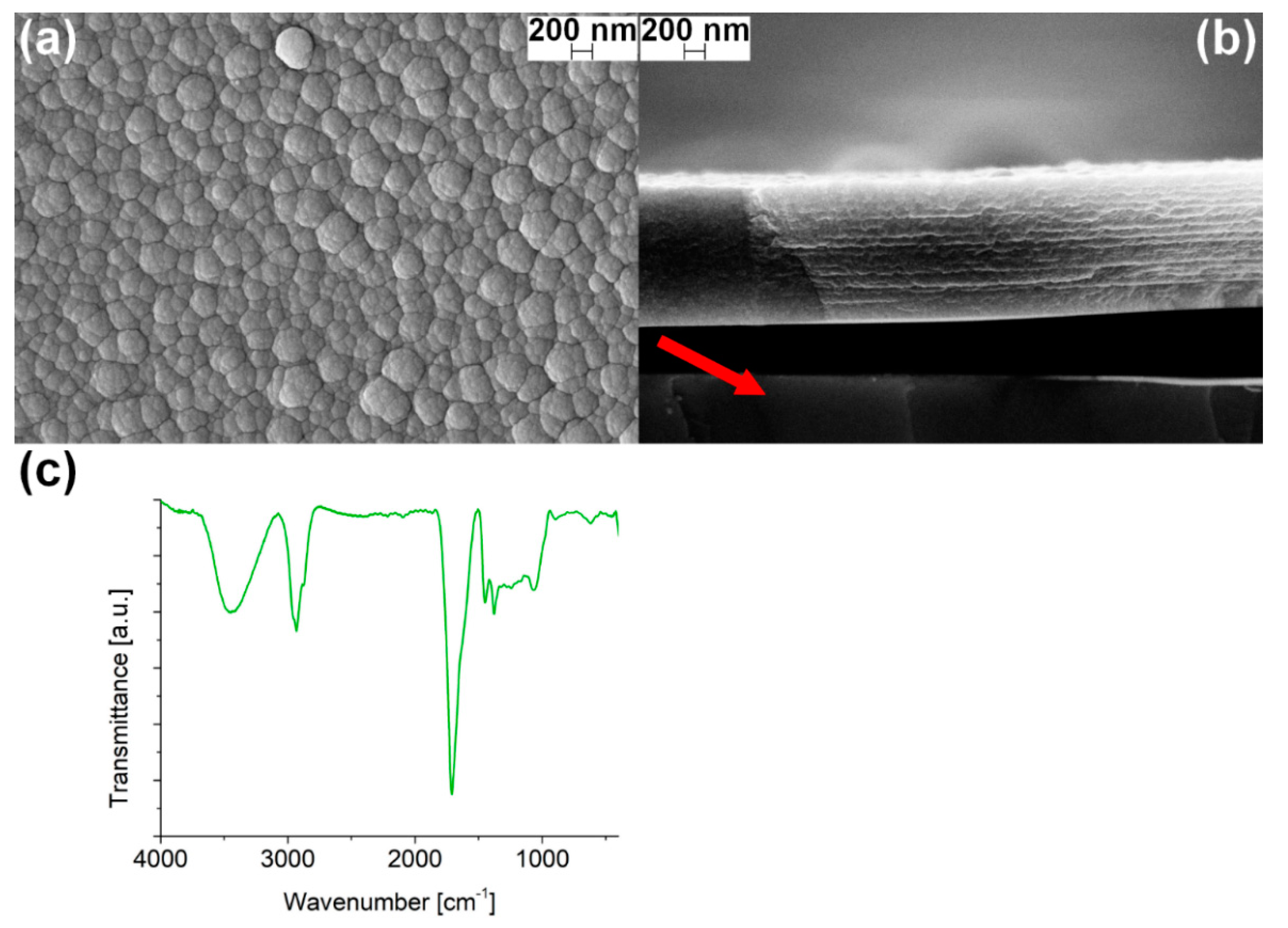
3.2. PPH Coating
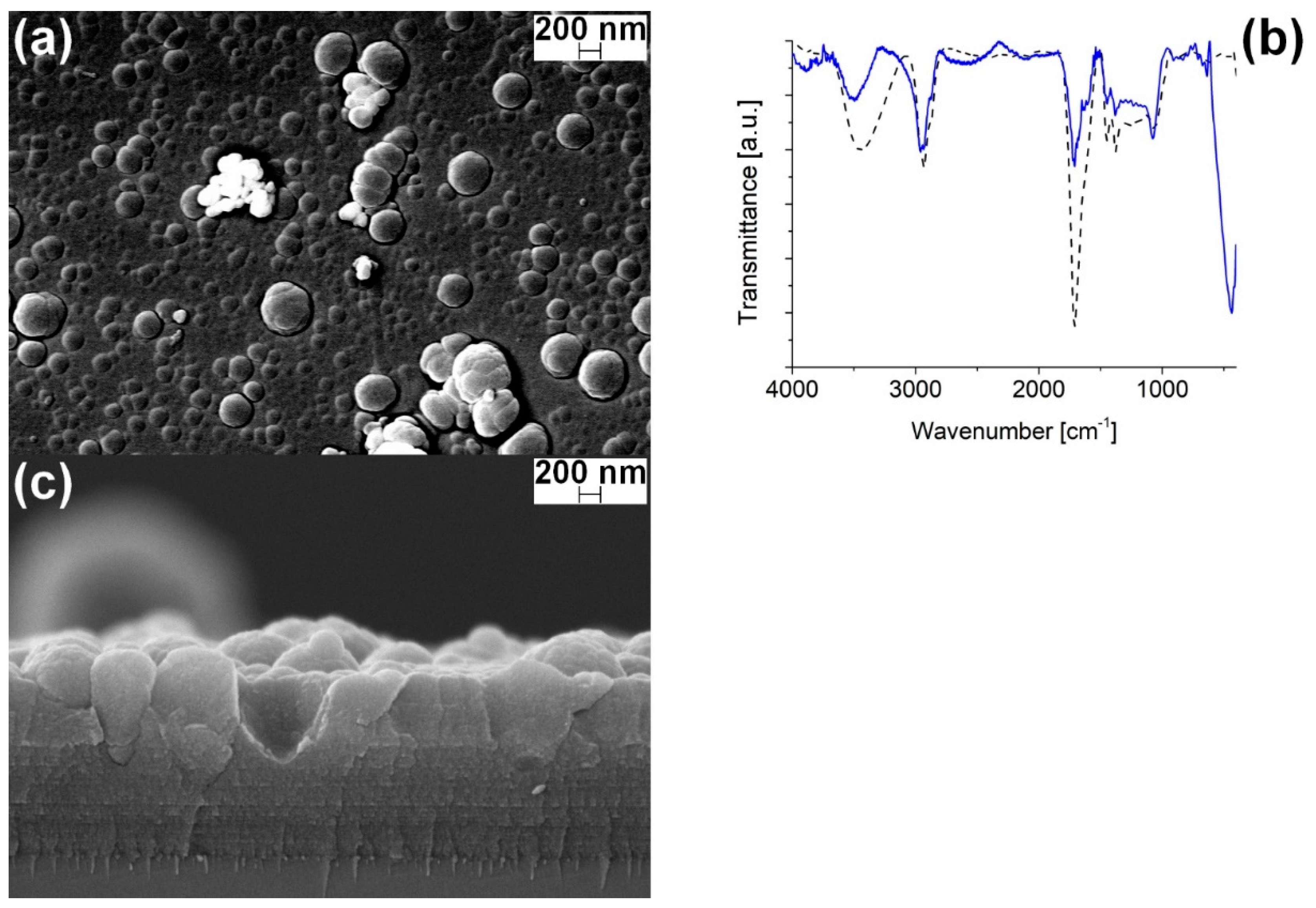
3.3. ZnO-PPH Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Al-Jumaili, A.; Prasad, K.; Bazaka, K.; Mulvey, P.; Warner, J.; Jacob, M.V. Pulse Plasma Deposition of Terpinen-4-ol: An Insight into Polymerization Mechanism and Enhanced Antibacterial Response of Developed Thin Films. Plasma Chem. Plasma Process. 2019, 339–355. [Google Scholar] [CrossRef]
- Gerchman, D.; Bones, B.; Pereira, M.B.; Takimi, A.S. Thin film deposition by plasma polymerization using D-limonene as a renewable precursor. Prog. Org. Coat. 2019, 129, 133–139. [Google Scholar] [CrossRef]
- Demaude, A.; Poleunis, C.; Goormaghtigh, E.; Viville, P.; Lazzaroni, R.; Delcorte, A.; Gordon, M.; Reniers, F. Atmospheric Pressure Plasma Deposition of Hydrophilic/Phobic Patterns and Thin Film Laminates on Any Surface. Langmuir 2019, 35, 9677–9683. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Dinh, D.K.; Abbas, Q.; Imran, M.; Sattar, H.; Ul Ahmad, A. Controlled Surface Wettability by Plasma Polymer Surface Modification. Surfaces 2019, 2, 349–371. [Google Scholar] [CrossRef]
- Muzammil, I.; Li, Y.; Lei, M. Tunable wettability and pH-responsiveness of plasma copolymers of acrylic acid and octafluorocyclobutane. Plasma Process. Polym. 2017, 14, 1–10. [Google Scholar] [CrossRef]
- Patelli, A.; Mussano, F.; Brun, P.; Genova, T.; Ambrosi, E.; Michieli, N.; Mattei, G.; Scopece, P.; Moroni, L. Nanoroughness, Surface Chemistry, and Drug Delivery Control by Atmospheric Plasma Jet on Implantable Devices. ACS Appl. Mater. Interfaces 2018, 10, 39512–39523. [Google Scholar] [CrossRef] [PubMed]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic materials for biomedical applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef]
- Siow, K.S.; Kumar, S.; Griesser, H.J. Low-pressure plasma methods for generating non-reactive hydrophilic and hydrogel-like bio-interface coatings—A review. Plasma Process. Polym. 2015, 12, 8–24. [Google Scholar] [CrossRef]
- Hiratsuka, A.; Karube, I. Plasma polymerized films for sensor devices. Electroanalysis 2000, 12, 695–702. [Google Scholar] [CrossRef]
- Koparkar, K.A. Plasma polymerized thin film sensor: Synthesis and application. Sens. Transducers 2012, 143, 10–31. [Google Scholar]
- Lima, R.R.; Hernandez, L.F.; Carvalho, A.T.; Carvalho, R.A.M.; da Silva, M.L.P. Corrosion resistant and adsorbent plasma polymerized thin film. Sens. Actuators B Chem. 2009, 141, 349–360. [Google Scholar] [CrossRef]
- Thomas, D.G. The exciton spectrum of zinc oxide. J. Phys. Chem. Solids 1960, 15, 86–96. [Google Scholar] [CrossRef]
- Mang, A.; Reimann, K.; Rübenacke, S. Band gaps, crystal-field splitting, spin-orbit coupling, and exciton binding energies in ZnO under hydrostatic pressure. Solid State Commun. 1995, 94, 251–254. [Google Scholar] [CrossRef]
- Cantwell, G.; Harsch, W.C.; Jogai, B. Valence-band ordering in zno. Phys. Rev. B—Condens. Matter Mater. Phys. 1999, 60, 2340–2344. [Google Scholar] [CrossRef]
- Chen, Y.; Bagnall, D.M.; Koh, H.J.; Park, K.T.; Hiraga, K.; Zhu, Z.; Yao, T. Plasma assisted molecular beam epitaxy of ZnO on c-plane sapphire: Growth and characterization. J. Appl. Phys. 1998, 84, 3912–3918. [Google Scholar] [CrossRef]
- Ghamsari, M.S.; Alamdari, S.; Razzaghi, D.; Arshadi Pirlar, M. ZnO nanocrystals with narrow-band blue emission. J. Lumin. 2019, 205, 508–518. [Google Scholar] [CrossRef]
- Jagadish, C.; Pearton, S. Zinc Oxide Bulk, Thin Films and Nanostructures; Elsevier Science: Amsterdam, The Netherlands, 2006; ISBN 9780080447223. [Google Scholar]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Empizo, M.J.F.; Fukuda, K.; Arita, R.; Minami, Y.; Yamanoi, K.; Shimizu, T.; Sarukura, N.; Vargas, R.M.; Salvador, A.A.; Sarmago, R.V. Photoluminescence properties of a single ZnO microstructure for potential scintillator applications. Opt. Mater. (Amst). 2014, 38, 256–260. [Google Scholar] [CrossRef]
- Demidenko, V.A.; Gorokhova, E.I.; Khodyuk, I.V.; Khristich, O.A.; Mikhrin, S.B.; Rodnyi, P.A. Scintillation properties of ceramics based on zinc oxide. Radiat. Meas. 2007, 42, 549–552. [Google Scholar] [CrossRef][Green Version]
- Bourret-Courchesne, E.D.; Derenzo, S.E.; Weber, M.J. Development of ZnO:Ga as an ultra-fast scintillator. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2009, 601, 358–363. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Yanagida, T.; Sekiwa, H.; Yokota, Y.; Chani, V.; Yoshikawa, A. Scintillation characteristic of in,Ga-Doped ZnO thin films with different dopant concentrations. Jpn. J. Appl. Phys. 2011, 50. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, Q.; Shao, Z. Magnetron sputtering for ZnO:Ga scintillation film production and its application research status in nuclear detection. Crystals 2019, 9, 263. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Hwang, D.-K.; Oh, M.-S.; Hong, K.-P.; Em, V.T.; Choi, H.-W.; Park, S.-J. Growth and Characterization of Gallium-Doped ZnO Films for α-Particle Scintillators. J. Electrochem. Soc. 2008, 155, H909. [Google Scholar] [CrossRef]
- Di Mauro, A.; Fragalà, M.E.; Privitera, V.; Impellizzeri, G. ZnO for application in photocatalysis: From thin films to nanostructures. Mater. Sci. Semicond. Process. 2017, 69, 44–51. [Google Scholar] [CrossRef]
- Di Mauro, A.; Cantarella, M.; Nicotra, G.; Pellegrino, G.; Gulino, A.; Brundo, M.V.; Privitera, V.; Impellizzeri, G. Novel synthesis of ZnO/PMMA nanocomposites for photocatalytic applications. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Bharat, T.C.; Shubham; Mondal, S.; Gupta, H.S.; Singh, P.K.; Das, A.K. Synthesis of Doped Zinc Oxide Nanoparticles: A Review. Mater. Today Proc. 2019, 11, 767–775. [Google Scholar] [CrossRef]
- Podasca, V.E.; Buruiana, T.; Buruiana, E.C. UV-cured polymeric films containing ZnO and silver nanoparticles with UV-vis light-assisted photocatalytic activity. Appl. Surf. Sci. 2016, 377, 262–273. [Google Scholar] [CrossRef]
- Neal, J.S.; Devito, D.M.; Armstrong, B.L.; Hong, M.; Kesanli, B.; Yang, X.; Giles, N.C.; Howe, J.Y.; Ramey, J.O.; Wisniewski, D.J.; et al. Investigation of ZnO-based polycrystalline ceramic scintillators for use as α -particle detectors. IEEE Trans. Nucl. Sci. 2009, 56, 892–898. [Google Scholar] [CrossRef]
- Yanagida, T.; Fujimoto, Y.; Yoshikawa, A.; Yokota, Y.; Miyamoto, M.; Sekiwa, H.; Kobayashi, J.; Tokutake, T.; Kamada, K.; Maeo, S. Scintillation properties of in doped ZnO with different in concentrations. IEEE Trans. Nucl. Sci. 2010, 57, 1325–1328. [Google Scholar] [CrossRef]
- Burešová, H.; Procházková, L.; Turtos, R.M.; Jarý, V.; Mihóková, E.; Beitlerová, A.; Pjatkan, R.; Gundacker, S.; Auffray, E.; Lecoq, P.; et al. Preparation and luminescence properties of ZnO:Ga—Polystyrene composite scintillator. Opt. Express 2016, 24, 15289. [Google Scholar] [CrossRef]
- Rodnyi, P.A.; Chernenko, K.A.; Gorokhova, E.I.; Kozlovskii, S.S.; Khanin, V.M.; Khodyuk, I.V. Novel scintillation material ZnO transparent ceramics. IEEE Trans. Nucl. Sci. 2012, 59, 2152–2155. [Google Scholar] [CrossRef]
- Neal, J.S.; Boatner, L.A.; Giles, N.C.; Halliburton, L.E.; Derenzo, S.E.; Bourret-Courchesne, E.D. Comparative investigation of the performance of ZnO-based scintillators for use as α-particle detectors. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2006, 568, 803–809. [Google Scholar] [CrossRef]
- Rodnyi, P.A.; Stryganyuk, G.B.; Khodyuk, I.V. Luminescence of a ZnO:Ga crystal upon excitation in vacuum UV region. Opt. Spectrosc. 2008, 104, 210–212. [Google Scholar] [CrossRef]
- Katagiri, M.; Sakasai, K.; Matsubayashi, M.; Nakamura, T.; Kondo, Y.; Chujo, Y.; Nanto, H.; Kojima, T. Scintillation materials for neutron imaging detectors. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2004, 529, 274–279. [Google Scholar] [CrossRef]
- Gorokhova, E.I.; Anan’eva, G.V.; Demidenko, V.A.; Rodnyĭ, P.A.; Khodyuk, I.V.; Bourret-Courchesne, E.D. Optical, luminescence, and scintillation properties of ZnO and ZnO:Ga ceramics. J. Opt. Technol. 2008, 75, 741. [Google Scholar] [CrossRef]
- Simpson, P.J.; Tjossem, R.; Hunt, A.W.; Lynn, K.G.; Munné, V. Superfast timing performance from ZnO scintillators. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2003, 505, 82–84. [Google Scholar] [CrossRef]
- Du, X.W.; Fu, Y.S.; Sun, J.; Han, X.; Liu, J. Complete UV emission of ZnO nanoparticles in a PMMA matrix. Semicond. Sci. Technol. 2006, 21, 1202–1206. [Google Scholar] [CrossRef]
- Hong, R.Y.; Qian, J.Z.; Cao, J.X. Synthesis and characterization of PMMA grafted ZnO nanoparticles. Powder Technol. 2006, 163, 160–168. [Google Scholar] [CrossRef]
- Kulyk, B.; Kapustianyk, V.; Tsybulskyy, V.; Krupka, O.; Sahraoui, B. Optical properties of ZnO/PMMA nanocomposite films. J. Alloys Compd. 2010, 502, 24–27. [Google Scholar] [CrossRef]
- Shanshoof, H.M.; Yahaya, M.; Yunus, W.M.M.; Abdullah, I.Y. Polymer-ZnO nanocomposites foils and thin films for UV protection. AIP Conf. Proc. 2014, 1614, 136–141. [Google Scholar] [CrossRef]
- Tang, E.; Cheng, G.; Ma, X. Preparation of nano-ZnO/PMMA composite particles via grafting of the copolymer onto the surface of zinc oxide nanoparticles. Powder Technol. 2006, 161, 209–214. [Google Scholar] [CrossRef]
- Mahamumi, S.; Bendre, B.; Leppert, V.; Smith, C.; Cooke, D.; Risbud, S.; Lee, H.W. ZnO Nanoparticles Embedded in Polymeric Matrices. Nanostruct. Mater. 1996, 7, 659–666. [Google Scholar] [CrossRef]
- Norberg, N.S.; Gamelin, D.R. Influence of surface modification on the luminescence of colloidal ZnO nanocrystals. J. Phys. Chem. B 2005, 109, 20810–20816. [Google Scholar] [CrossRef] [PubMed]
- Favaro, M.; Zanazzi, E.; Patelli, A.; Carturan, S.; Ceccato, R.; Mulloni, V.; Bortolotti, M.; Quaranta, A. Aluminum doped zinc oxide coatings at low temperature by atmospheric pressure plasma jet. Thin Solid Films 2020, 708. [Google Scholar] [CrossRef]
- Fanelli, F.; Mastrangelo, A.M.; Fracassi, F. Aerosol-assisted atmospheric cold plasma deposition and characterization of superhydrophobic organic-inorganic nanocomposite thin films. Langmuir 2014, 30, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, F.; Bosso, P.; Mastrangelo, A.M.; Fracassi, F. Thin film deposition at atmospheric pressure using dielectric barrier discharges: Advances on three-dimensional porous substrates and functional coatings. Jpn. J. Appl. Phys. 2016, 55. [Google Scholar] [CrossRef]
- Liguori, A.; Traldi, E.; Toccaceli, E.; Laurita, R.; Pollicino, A.; Focarete, M.L.; Colombo, V.; Gherardi, M. Co-Deposition of Plasma-Polymerized Polyacrylic Acid and Silver Nanoparticles for the Production of Nanocomposite Coatings Using a Non-Equilibrium Atmospheric Pressure Plasma Jet. Plasma Process. Polym. 2016, 13, 623–632. [Google Scholar] [CrossRef]
- Choukourov, A.; Melnichuk, I.; Shelemin, A.; Solař, P.; Hanuš, J.; Slavínská, D.; Biederman, H. Plasma Polymerization on Mesoporous Surfaces: N-Hexane on Titanium Nanoparticles. J. Phys. Chem. C 2015, 119, 28906–28916. [Google Scholar] [CrossRef]
- Sohbatzadeh, F.; Shakerinasab, E.; Eshghabadi, M.; Ghasemi, M. Characterization and performance of coupled atmospheric pressure argon plasma jet with n-hexane electrospray for hydrophobic layer coatings on cotton textile. Diam. Relat. Mater. 2019, 91, 34–45. [Google Scholar] [CrossRef]
- Nateq, M.H.; Ceccato, R. Enhanced sol-gel route to obtain a highly transparent and conductive aluminum-doped zinc oxide thin film. Materials 2019, 12, 1744. [Google Scholar] [CrossRef]
- Yang, S.H.; Liu, C.H.; Su, C.H.; Chen, H. Atmospheric-pressure plasma deposition of SiOx films for super-hydrophobic application. Thin Solid Films 2009, 517, 5284–5287. [Google Scholar] [CrossRef]
- Hegemann, D.; Hanselmann, B.; Blanchard, N.; Amberg, M. Plasma-Substrate Interaction during Plasma Deposition on Polymers. Contrib. Plasma Phys. 2014, 54, 162–169. [Google Scholar] [CrossRef]
- Friedrich, J. Mechanisms of plasma polymerization—Reviewed from a chemical point of view. Plasma Process. Polym. 2011, 8, 783–802. [Google Scholar] [CrossRef]
- Lutterotti, L.; Chateigner, D.; Ferrari, S.; Ricote, J. Texture, residual stress and structural analysis of thin films using a combined X-ray analysis. Thin Solid Films 2004, 450, 34–41. [Google Scholar] [CrossRef]
- Bortolotti, M.; Lutterotti, L.; Pepponi, G. Combining XRD and XRF analysis in one Rietveld-like fitting. Powder Diffr. 2017, 32, S225–S230. [Google Scholar] [CrossRef]
- Sengupta, J.; Sahoo, R.K.; Bardhan, K.K.; Mukherjee, C.D. Influence of annealing temperature on the structural, topographical and optical properties of sol-gel derived ZnO thin films. Mater. Lett. 2011, 65, 2572–2574. [Google Scholar] [CrossRef]
- Becheri, A.; Dürr, M.; Lo Nostro, P.; Baglioni, P. Synthesis and characterization of zinc oxide nanoparticles: Application to textiles as UV-absorbers. J. Nanopart. Res. 2008, 10, 679–689. [Google Scholar] [CrossRef]
- Morfa, A.J.; Gibson, B.C.; Karg, M.; Karle, T.J.; Greentree, A.D.; Mulvaney, P.; Tomljenovic-Hanic, S. Single-photon emission and quantum characterization of zinc oxide defects. Nano Lett. 2012, 12, 949–954. [Google Scholar] [CrossRef]
- Shi, W.S.; Agyeman, O.; Xu, C.N. Enhancement of the light emissions from zinc oxide films by controlling the post-treatment ambient. J. Appl. Phys. 2002, 91, 5640–5644. [Google Scholar] [CrossRef]
- Kumar, V.; Swart, H.C.; Ntwaeaborwa, O.M.; Kroon, R.E.; Terblans, J.J.; Shaat, S.K.K.; Yousif, A.; Duvenhage, M.M. Origin of the red emission in zinc oxide nanophosphors. Mater. Lett. 2013, 101, 57–60. [Google Scholar] [CrossRef]
- Kondyurin, A.; Polonskyi, O.; Nosworthy, N.; Matousek, J.; Hlidek, P.; Biederman, H.; Bilek, M.M.M. Covalent attachment and bioactivity horseradish peroxidase on plasma-polymerized hexane coatings. Plasma Process. Polym. 2008, 5, 727–736. [Google Scholar] [CrossRef]
- Sundell, B.J.; Shaver, A.T.; Liu, Q.; Nebipasagil, A.; Pisipati, P.; Mecham, S.J.; Riffle, J.S.; Freeman, B.D.; McGrath, J.E. Synthesis, oxidation and crosslinking of tetramethyl bisphenol F (TMBPF)-based polymers for oxygen/nitrogen gas separations. Polymer (Guildf) 2014, 55, 5623–5634. [Google Scholar] [CrossRef]
- Cruz, S.M.; Viana, J.C. Melt blending and characterization of carbon nanoparticles-filled thermoplastic polyurethane elastomers. J. Elastomers Plast. 2015, 47, 647–665. [Google Scholar] [CrossRef]
- Han, B.; Cheng, A.; Ji, G.; Wu, S.S.; Shen, J. Effect of organophilic montmorillonite on polyurethane/montmorillonite nanocomposites. J. Appl. Polym. Sci. 2004, 91, 2536–2542. [Google Scholar] [CrossRef]
- Guo, L.; Yang, S.; Yang, C.; Yu, P.; Wang, J.; Ge, W.; Wong, G.K.L. Highly monodisperse polymer-capped ZnO nanoparticles: Preparation and optical properties. Appl. Phys. Lett. 2000, 76, 2901–2903. [Google Scholar] [CrossRef]
- Jetson, R.; Yin, K.; Donovan, K.; Zhu, Z. Effects of surface modification on the fluorescence properties of conjugated polymer/ZnO nanocomposites. Mater. Chem. Phys. 2010, 124, 417–421. [Google Scholar] [CrossRef]
- Chen, C.; He, H.; Lu, Y.; Wu, K.; Ye, Z. Surface passivation effect on the photoluminescence of ZnO nanorods. ACS Appl. Mater. Interfaces 2013, 5, 6354–6359. [Google Scholar] [CrossRef]
- Clementi, C.; Rosi, F.; Romani, A.; Vivani, R.; Brunetti, B.G.; Miliani, C. Photoluminescence properties of zinc oxide in paints: A study of the effect of self-absorption and passivation. Appl. Spectrosc. 2012, 66, 1233–1241. [Google Scholar] [CrossRef]
- Zanazzi, E.; Favaro, M.; Ficorella, A.; Pancheri, L.; Dalla Betta, G.F.; Quaranta, A. Real-Time Optical Response of Polysiloxane/Quantum Dot Nanocomposites under 2 MeV Proton Irradiation: Luminescence Enhancement of Polysiloxane Emission through Quantum Dot Sensitization. Phys. Status Solidi Appl. Mater. Sci. 2020, 217. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Tam, K.H.; Hsu, Y.F.; Ding, L.; Ge, W.K.; Zhong, Y.C.; Wong, K.S.; Chan, W.K.; Tam, H.L.; et al. Defect emissions in ZnO nanostructures. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H. Optical properties of ZnO nanostructures. Small 2006, 2, 944–961. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Alves, H.; Hofmann, D.M.; Kriegseis, W.; Meyer, B.K.; Kaczmarczyk, G.; Hoffmann, A. Behind the weak excitonic emission of ZnO quantum dots: ZnO/Zn(OH) 2 core-shell structure. Appl. Phys. Lett. 2002, 80, 210–212. [Google Scholar] [CrossRef]
- Fanelli, F.; Mastrangelo, A.M.; De Vietro, N.; Fracassi, F. Preparation of multifunctional superhydrophobic nanocomposite coatings by aerosol-assisted atmospheric cold plasma deposition. Nanosci. Nanotechnol. Lett. 2015, 7, 84–88. [Google Scholar] [CrossRef]
- Chichibu, S.F.; Onuma, T.; Kubota, M.; Uedono, A.; Sota, T.; Tsukazaki, A.; Ohtomo, A.; Kawasaki, M. Improvements in quantum efficiency of excitonic emissions in ZnO epilayers by the elimination of point defects. J. Appl. Phys. 2006, 99. [Google Scholar] [CrossRef]
- Camarda, P.; Messina, F.; Vaccaro, L.; Agnello, S.; Buscarino, G.; Schneider, R.; Popescu, R.; Gerthsen, D.; Lorenzi, R.; Gelardi, F.M.; et al. Luminescence mechanisms of defective ZnO nanoparticles. Phys. Chem. Chem. Phys. 2016, 18, 16237–16244. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.; Ucer, K.B.; Williams, R.T. Picosecond excitonic luminescence in ZnO and other wide-gap semiconductors. Radiat. Meas. 2004, 38, 501–505. [Google Scholar] [CrossRef]
- Voss, T.; Wischmeier, L. Recombination dynamics of surface-related excitonic states in single ZnO nanowires. J. Nanosci. Nanotechnol. 2008, 8, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Tanaka, M.; Nakazato, T.; Tatsumi, T.; Nishikino, M.; Yamatani, H.; Nagashima, K.; Kimura, T.; Murakami, H.; Saito, S.; et al. Temperature dependence of scintillation properties for a hydrothermal-method-grown zinc oxide crystal evaluated by nickel-like silver laser pulses. J. Opt. Soc. Am. B 2008, 25, B118. [Google Scholar] [CrossRef]
- Koida, T.; Chichibu, S.F.; Uedono, A.; Tsukazaki, A.; Kawasakib, M.; Sota, T.; Segawa, Y.; Koinuma, H. Correlation between the photoluminescence lifetime and defect density in bulk and epitaxial ZnO. Appl. Phys. Lett. 2003, 82, 532–534. [Google Scholar] [CrossRef]
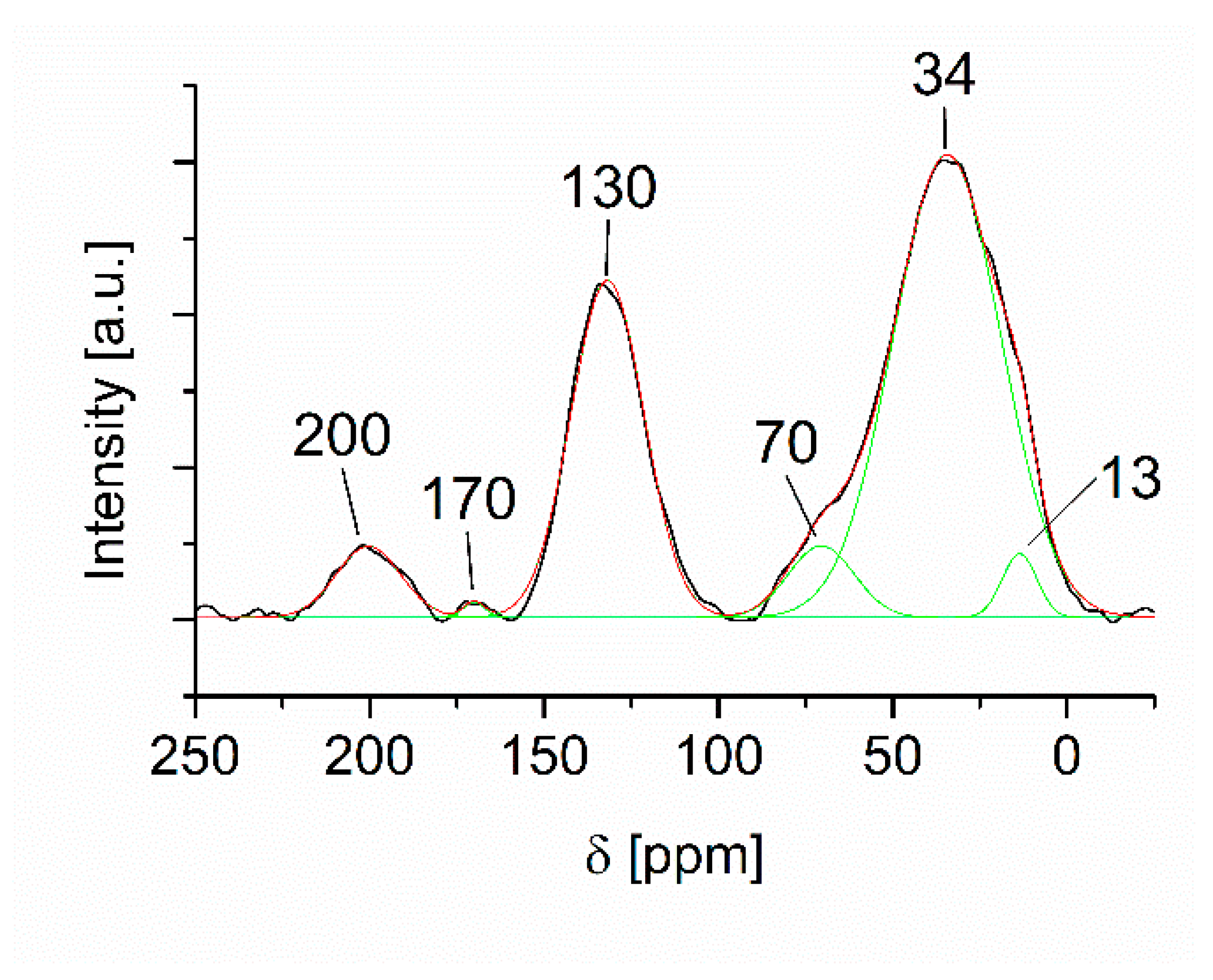
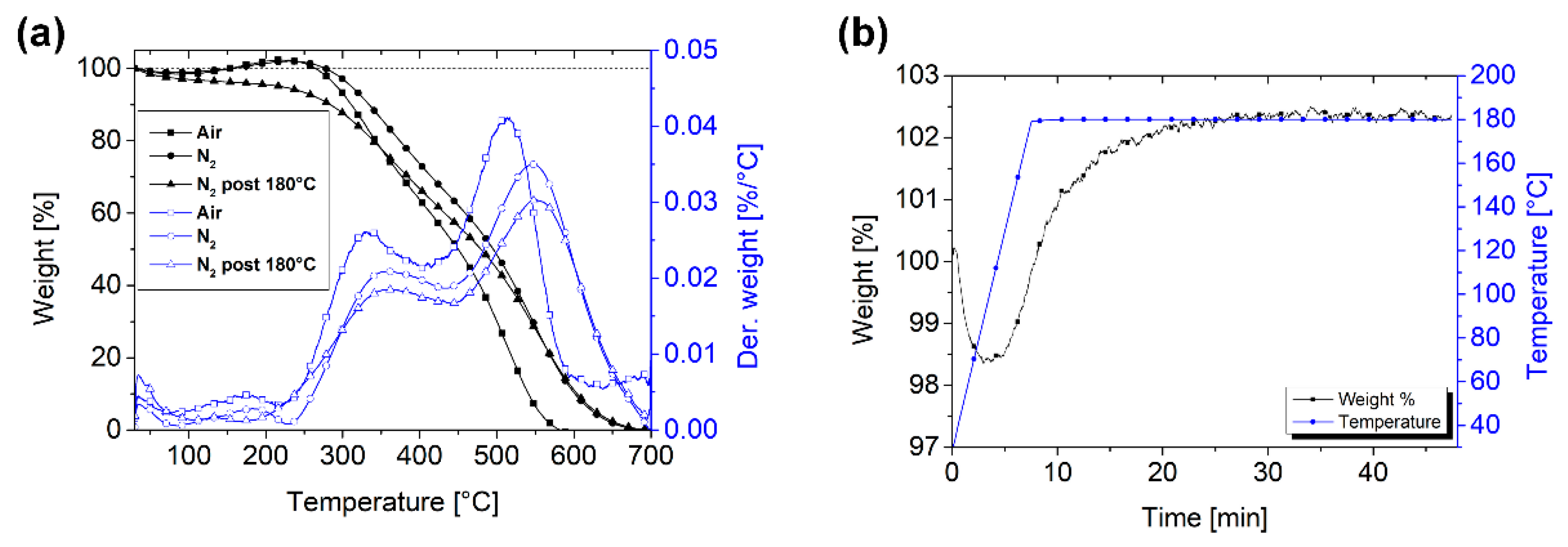
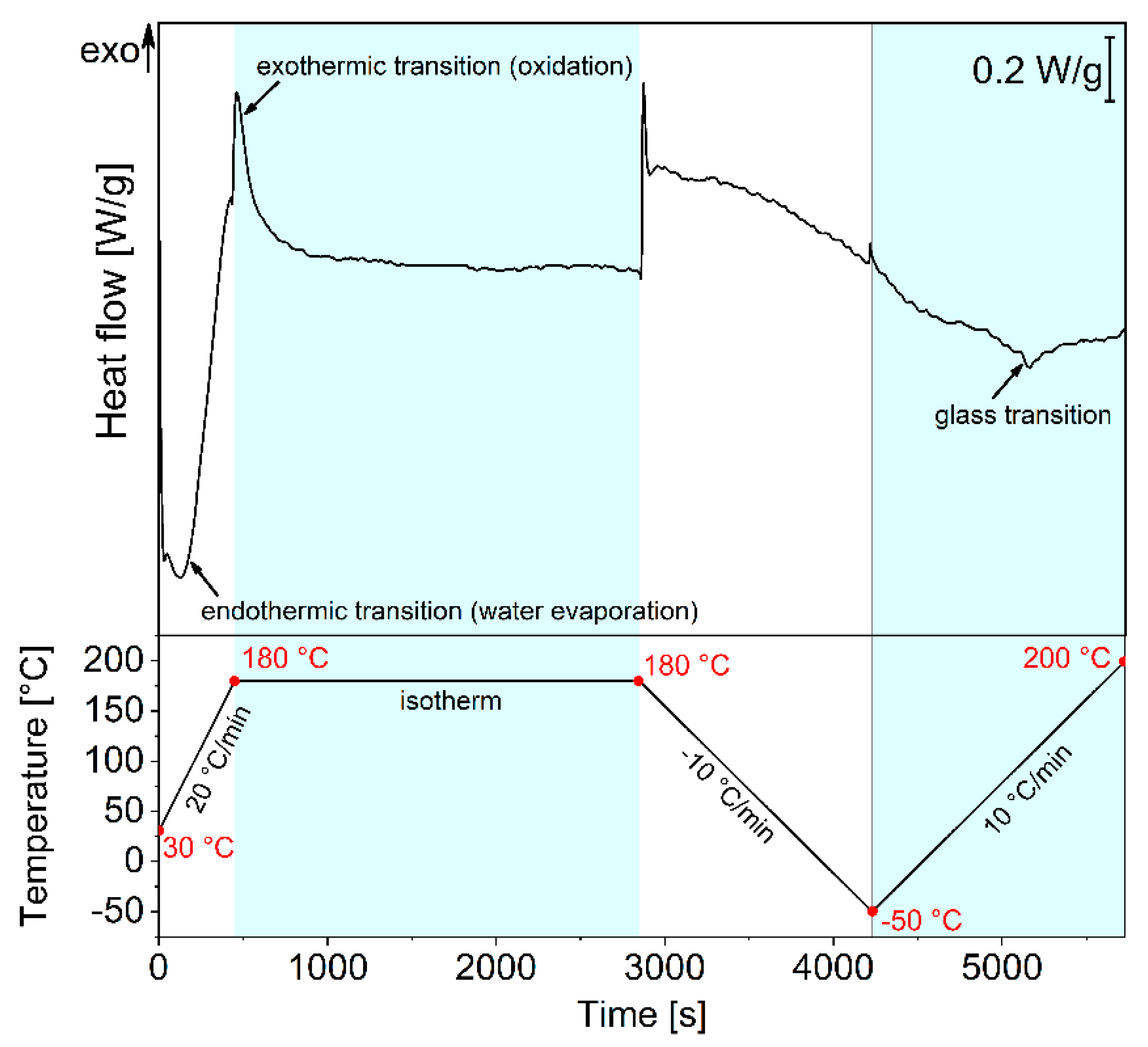
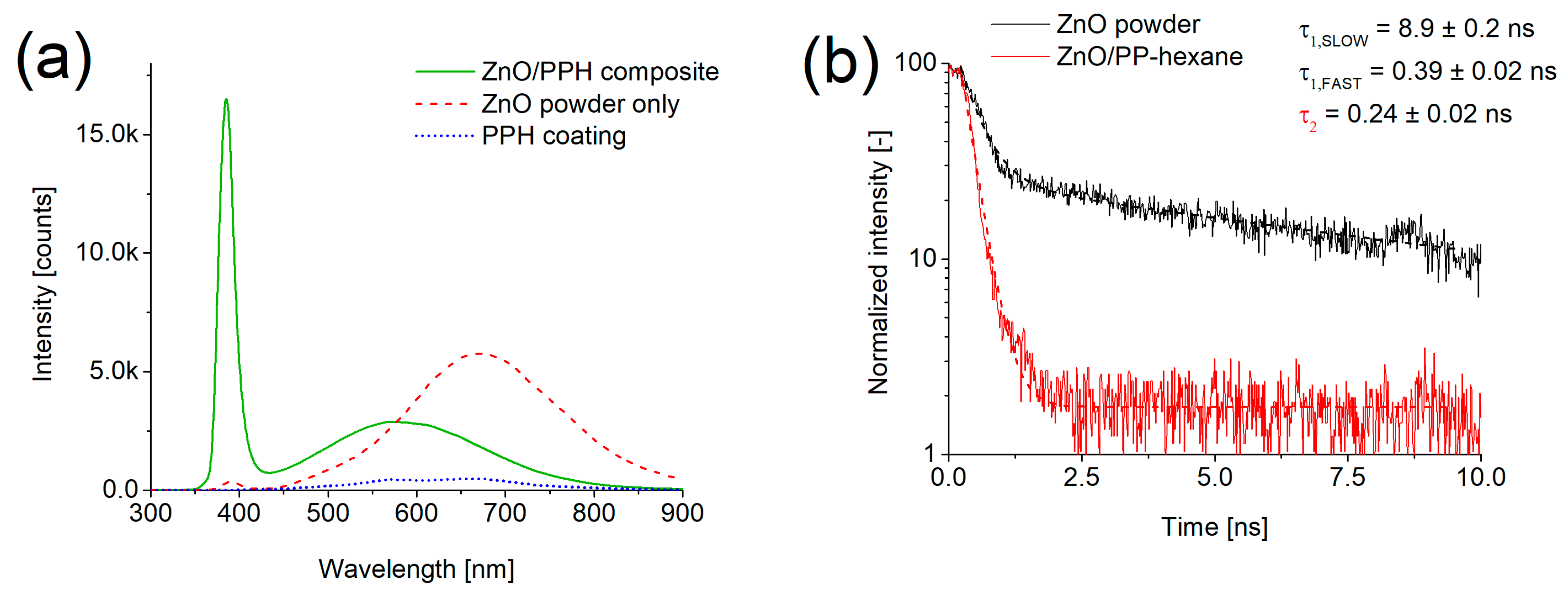
| Deposition Speed [mm/min] | Film Thickness [nm] | Deposition Time [s] | Average Deposition Rate [nm/s] |
|---|---|---|---|
| 300 | 650 ± 10 | 640 | 1.02 ± 0.02 |
| 500 | 230 ± 10 | 380 | 0.61 ± 0.03 |
| 800 | 150 ± 10 | 240 | 0.62 ± 0.04 |
| δ [ppm] | Relative Amount [%] | Assignment |
|---|---|---|
| 200 | 5 | C=O |
| 170 | <1 | O–C=O |
| 130 | 30 | =CH |
| 70 | 3 | C–O–C |
| 34 | 57 | >CH2 |
| 13 | 6 | –CH3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favaro, M.; Patelli, A.; Ceccato, R.; Dirè, S.; Callone, E.; Fredi, G.; Quaranta, A. Thin Films of Plasma-Polymerized n-Hexane and ZnO Nanoparticles Co-Deposited via Atmospheric Pressure Plasma Jet. Coatings 2021, 11, 167. https://doi.org/10.3390/coatings11020167
Favaro M, Patelli A, Ceccato R, Dirè S, Callone E, Fredi G, Quaranta A. Thin Films of Plasma-Polymerized n-Hexane and ZnO Nanoparticles Co-Deposited via Atmospheric Pressure Plasma Jet. Coatings. 2021; 11(2):167. https://doi.org/10.3390/coatings11020167
Chicago/Turabian StyleFavaro, Matteo, Alessandro Patelli, Riccardo Ceccato, Sandra Dirè, Emanuela Callone, Giulia Fredi, and Alberto Quaranta. 2021. "Thin Films of Plasma-Polymerized n-Hexane and ZnO Nanoparticles Co-Deposited via Atmospheric Pressure Plasma Jet" Coatings 11, no. 2: 167. https://doi.org/10.3390/coatings11020167
APA StyleFavaro, M., Patelli, A., Ceccato, R., Dirè, S., Callone, E., Fredi, G., & Quaranta, A. (2021). Thin Films of Plasma-Polymerized n-Hexane and ZnO Nanoparticles Co-Deposited via Atmospheric Pressure Plasma Jet. Coatings, 11(2), 167. https://doi.org/10.3390/coatings11020167











