Chitosan Coated Luteolin Nanostructured Lipid Carriers: Optimization, In Vitro-Ex Vivo Assessments and Cytotoxicity Study in Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines
2.3. Experimental Design
2.4. Formulation of LTN-CS-NLCs
2.5. Characterization
2.5.1. Particle Characterization
2.5.2. Entrapment Efficiency (%EE) and Drug Loading (%DL)
2.6. In Vitro Gastrointestinal Stability Study
2.7. LTN Release Study
2.8. Mucoadhesion Study
2.9. Antioxidant Activity
2.10. Cytotoxicity Study
2.11. DAPI for Nuclear Staining
2.12. Intestinal Permeation Study
2.13. Statistical Analysis
3. Results and Discussion
3.1. Optimization
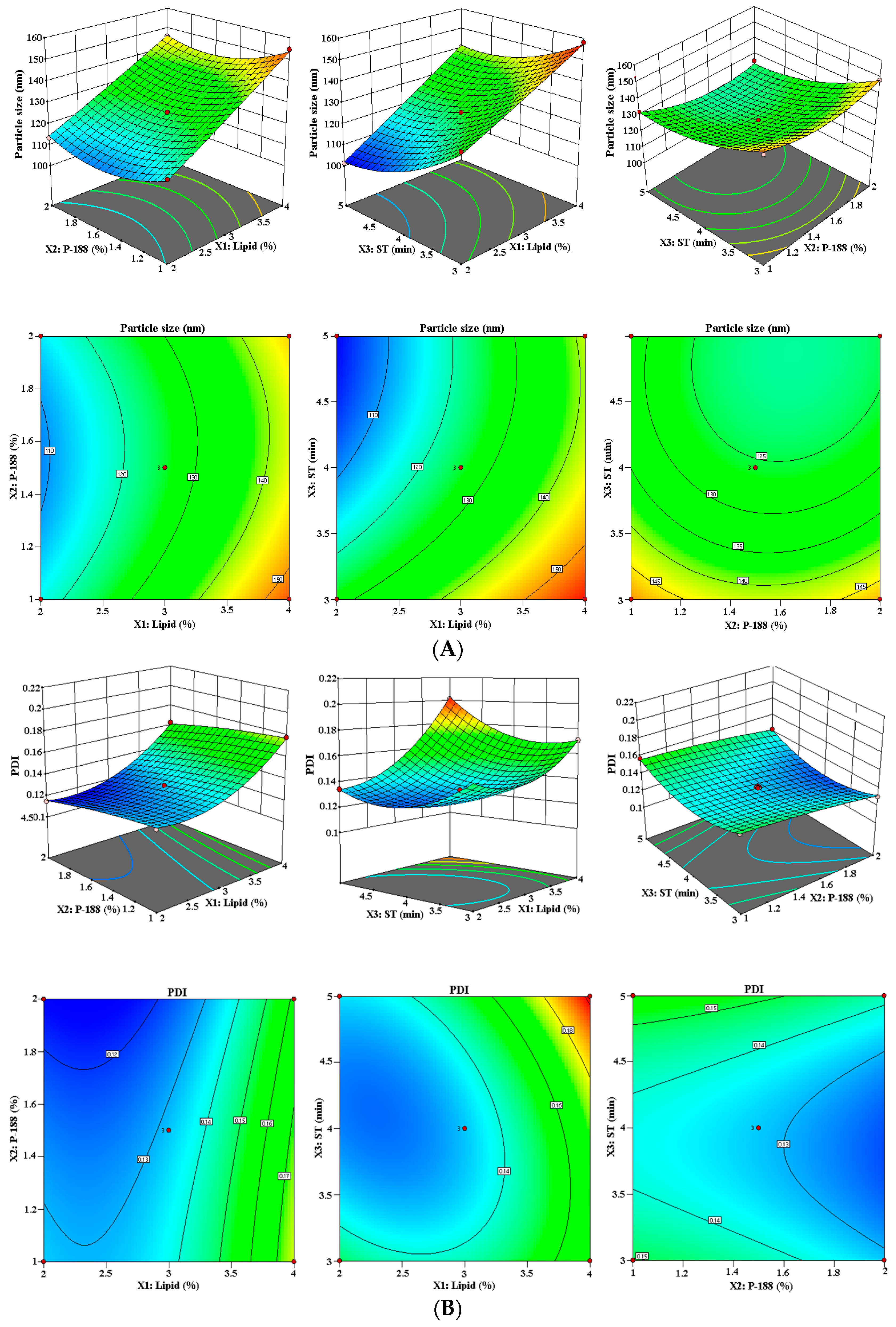
3.1.1. Effect of Lipid (X1), P-188 (X2), and ST (X3) on PS (Y1)
3.1.2. Effect of Lipid (X1), P-188 (X2), and ST (X3) on PDI (Y2)
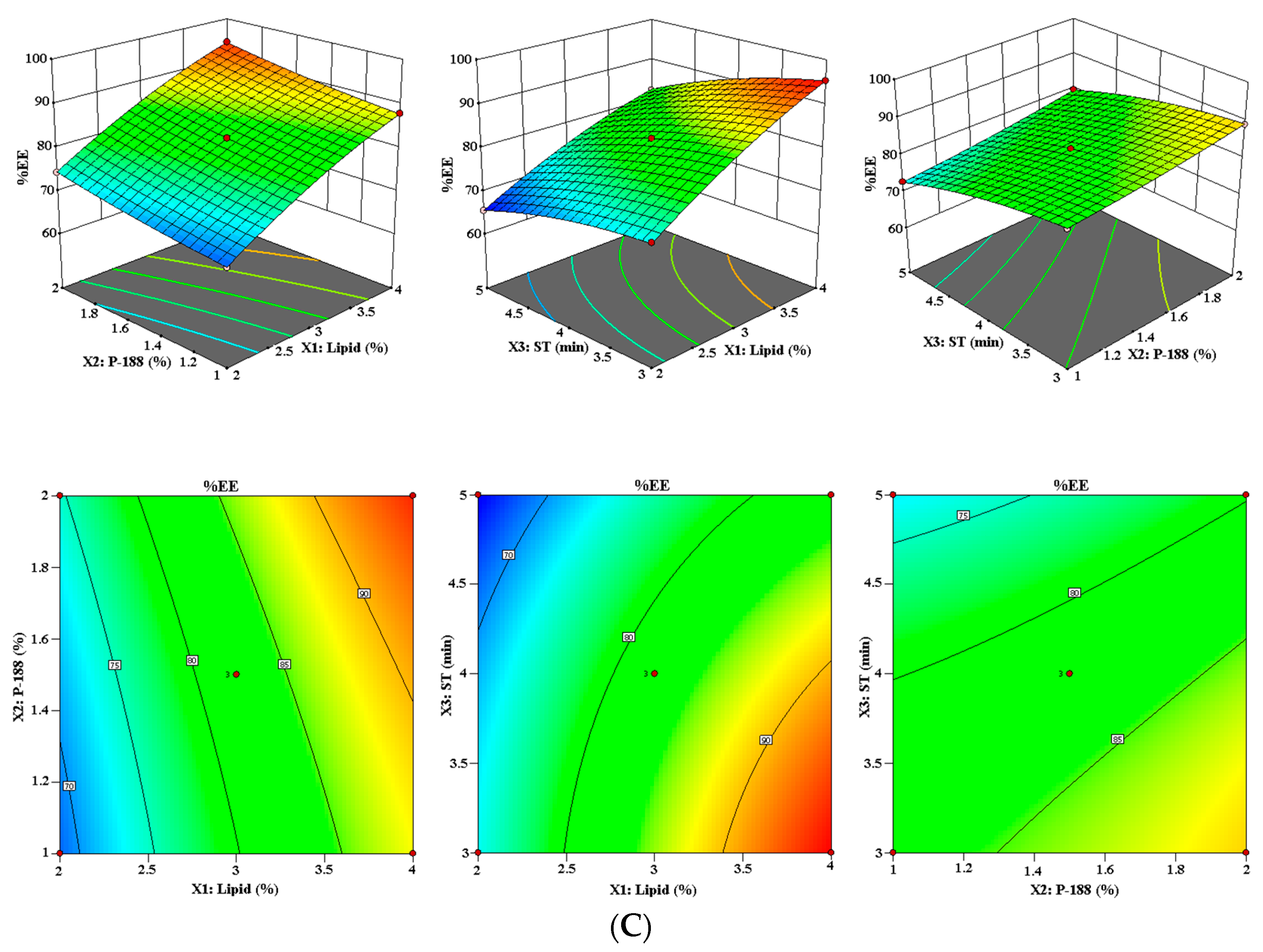
3.1.3. Effect of Lipid (X1), P-188 (X2), and ST (X3) on %EE (Y3)
3.1.4. Optimization by Point Prediction
3.2. Characterization
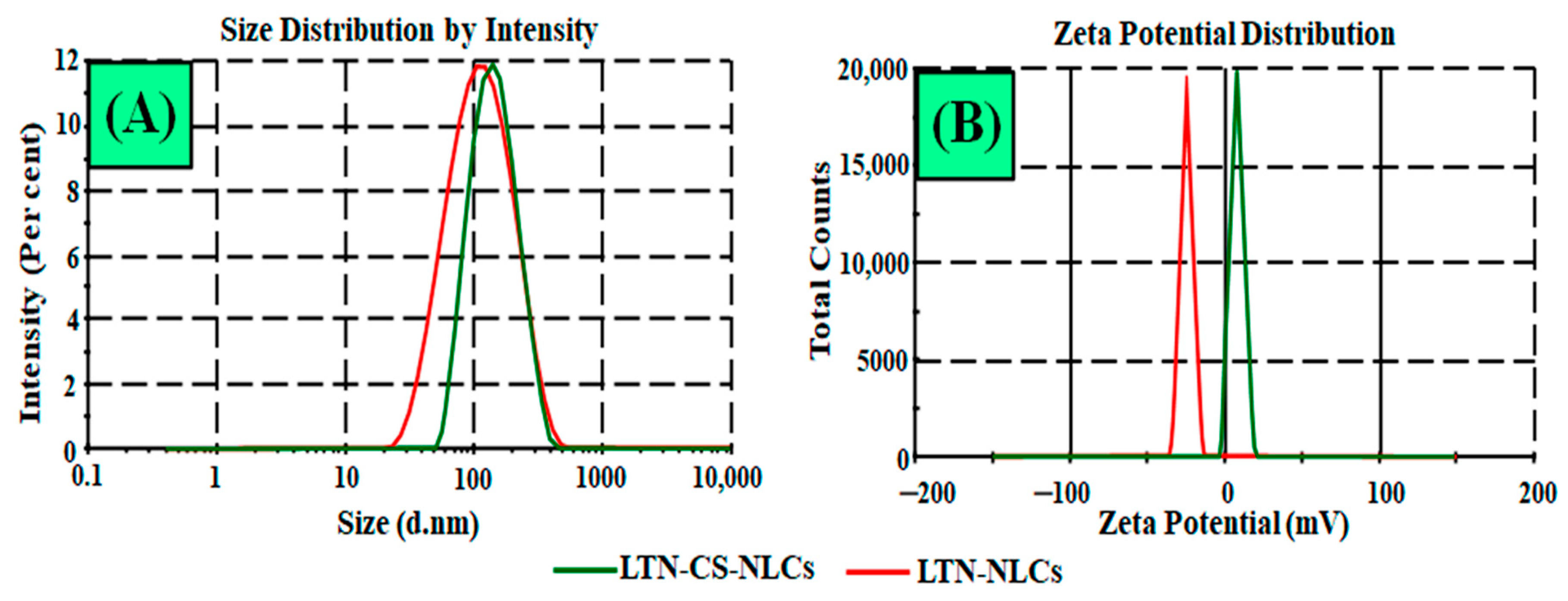
3.2.1. Particle Characterization
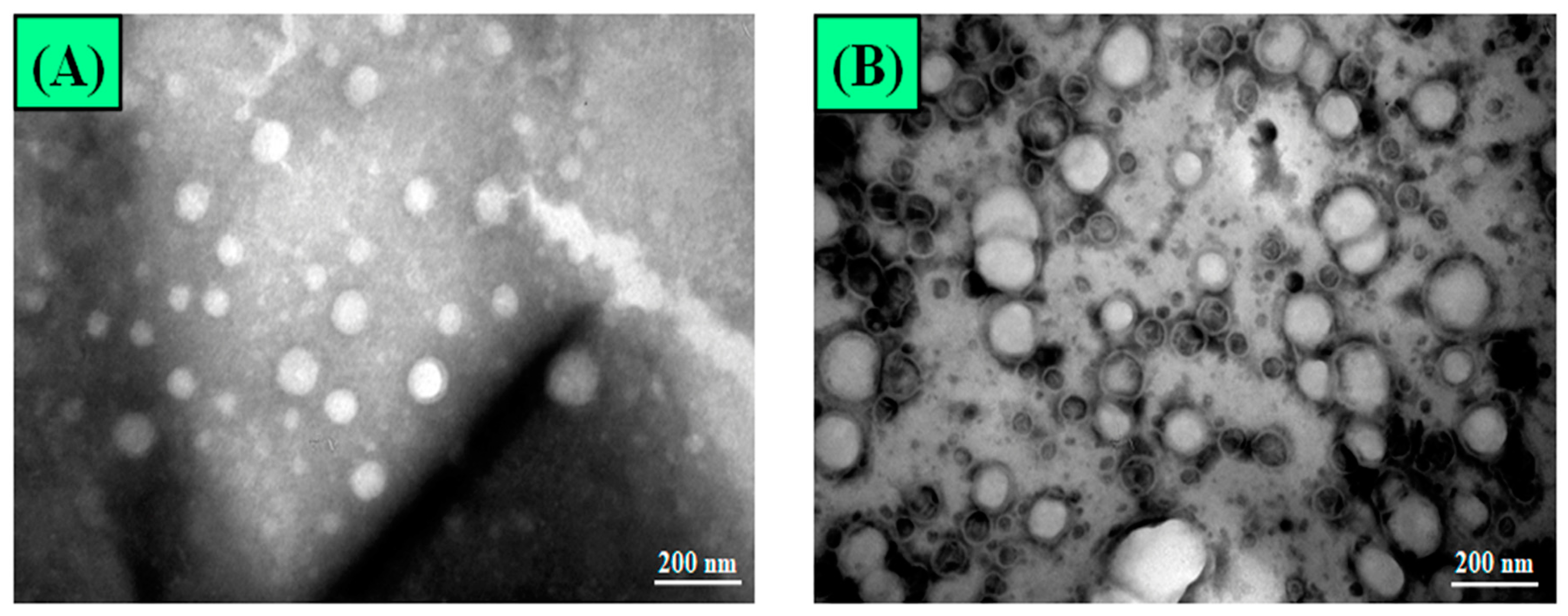
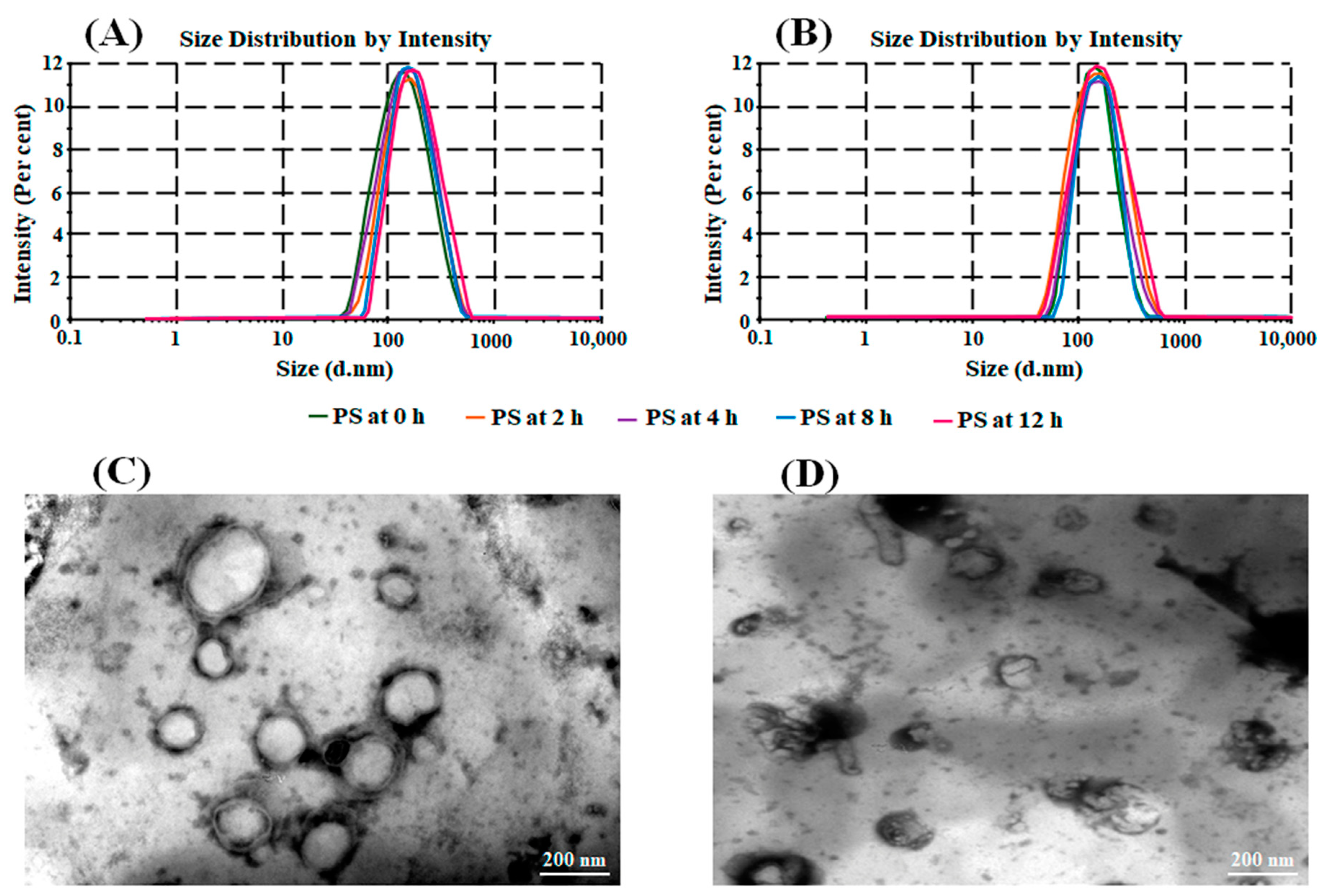
3.2.2. Surface Morphology
3.2.3. %EE and %DL
3.3. In Vitro Gastrointestinal Stability Study
3.4. LTN Release Study
3.5. Mucoadhesion Study
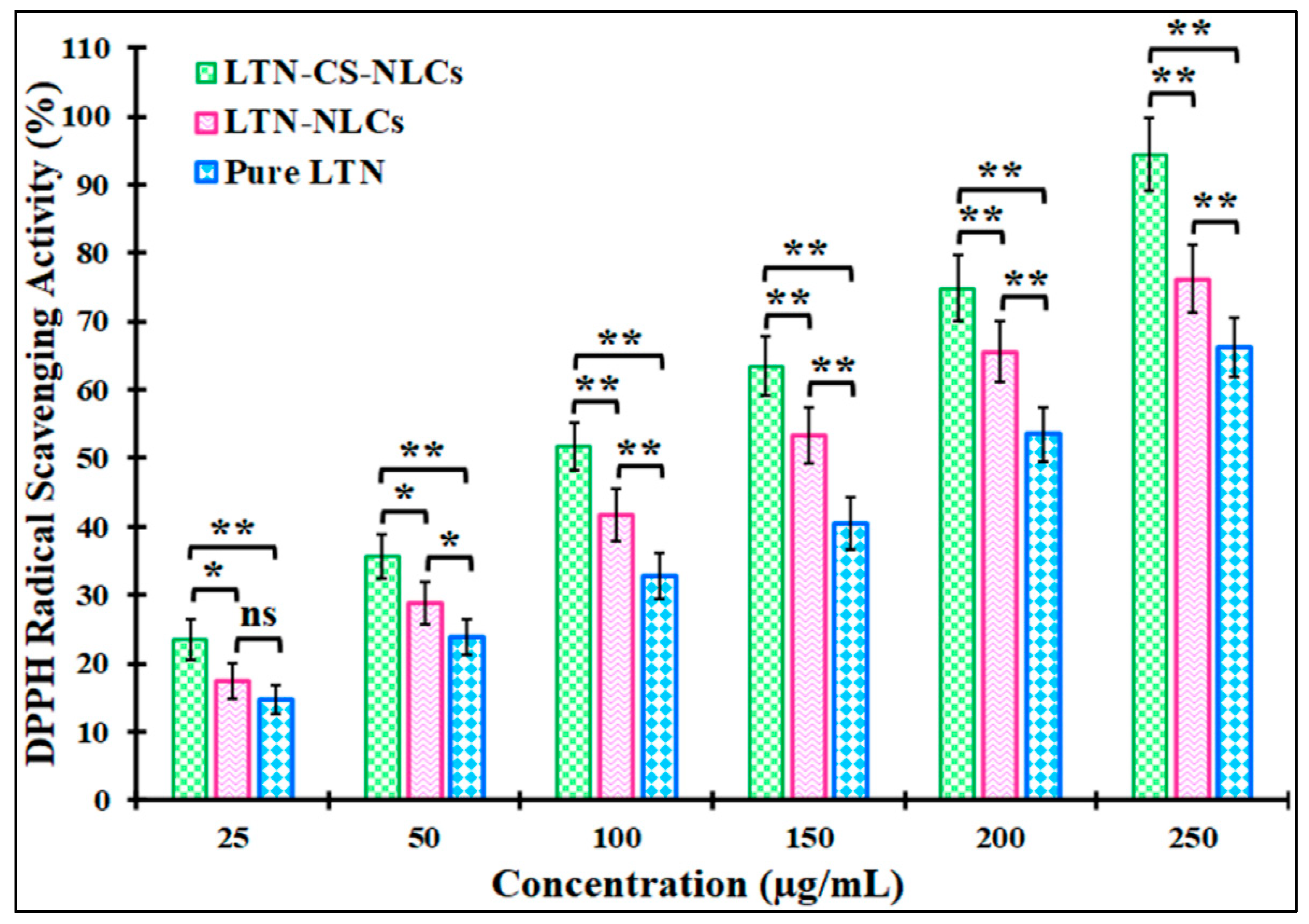
3.6. Antioxidant Activity
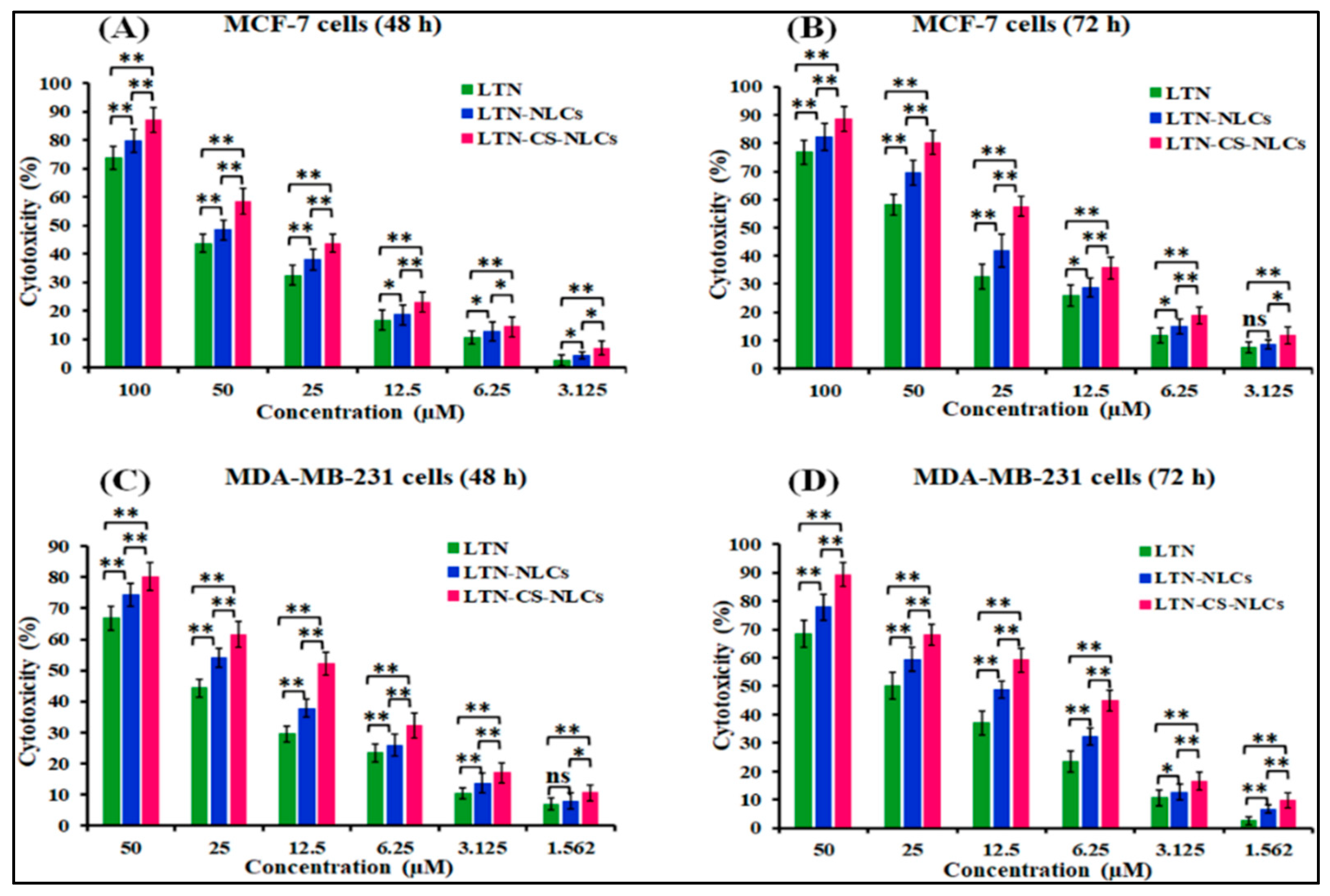
3.7. Cytotoxicity Study
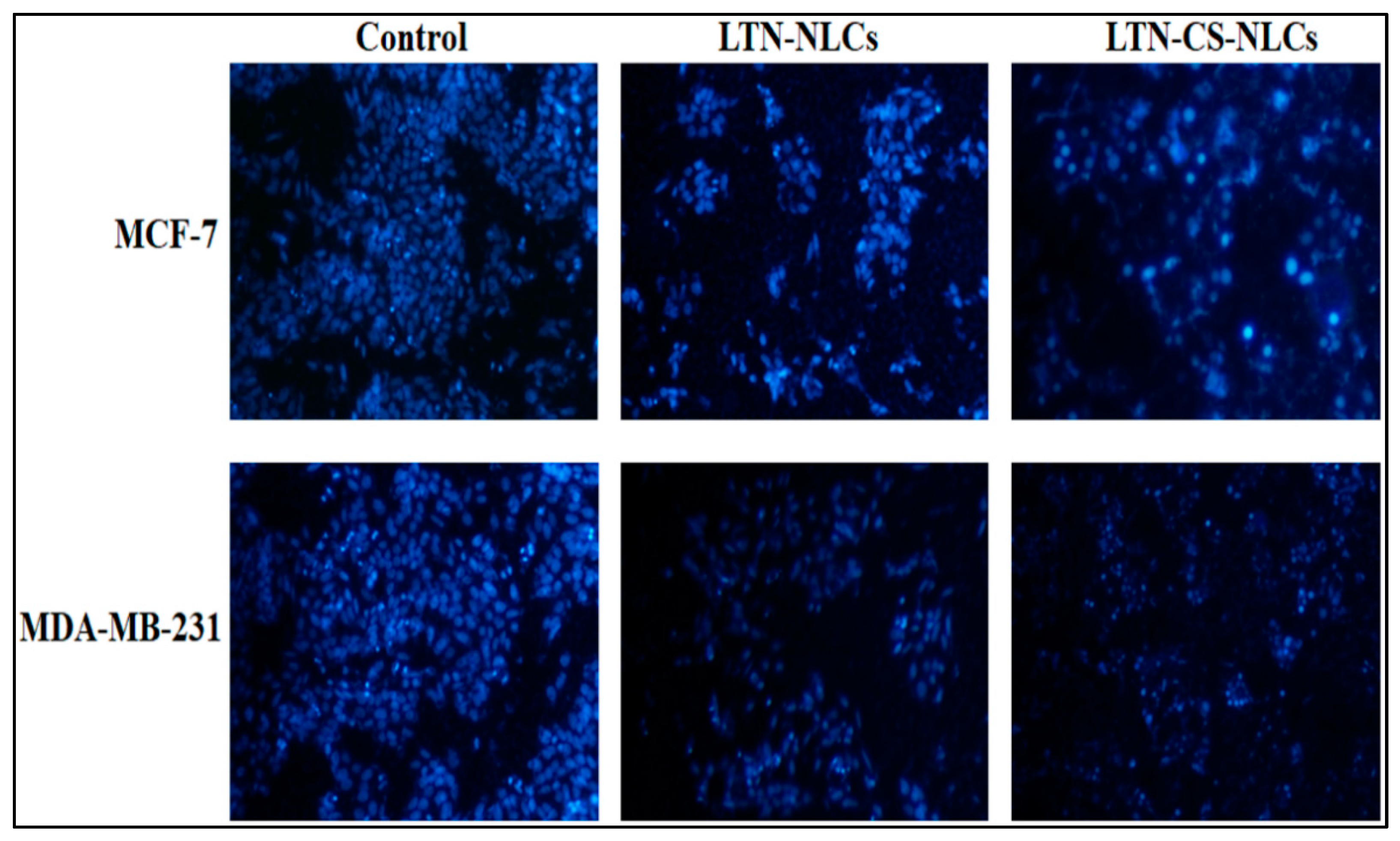
3.8. DAPI for Nuclear Staining
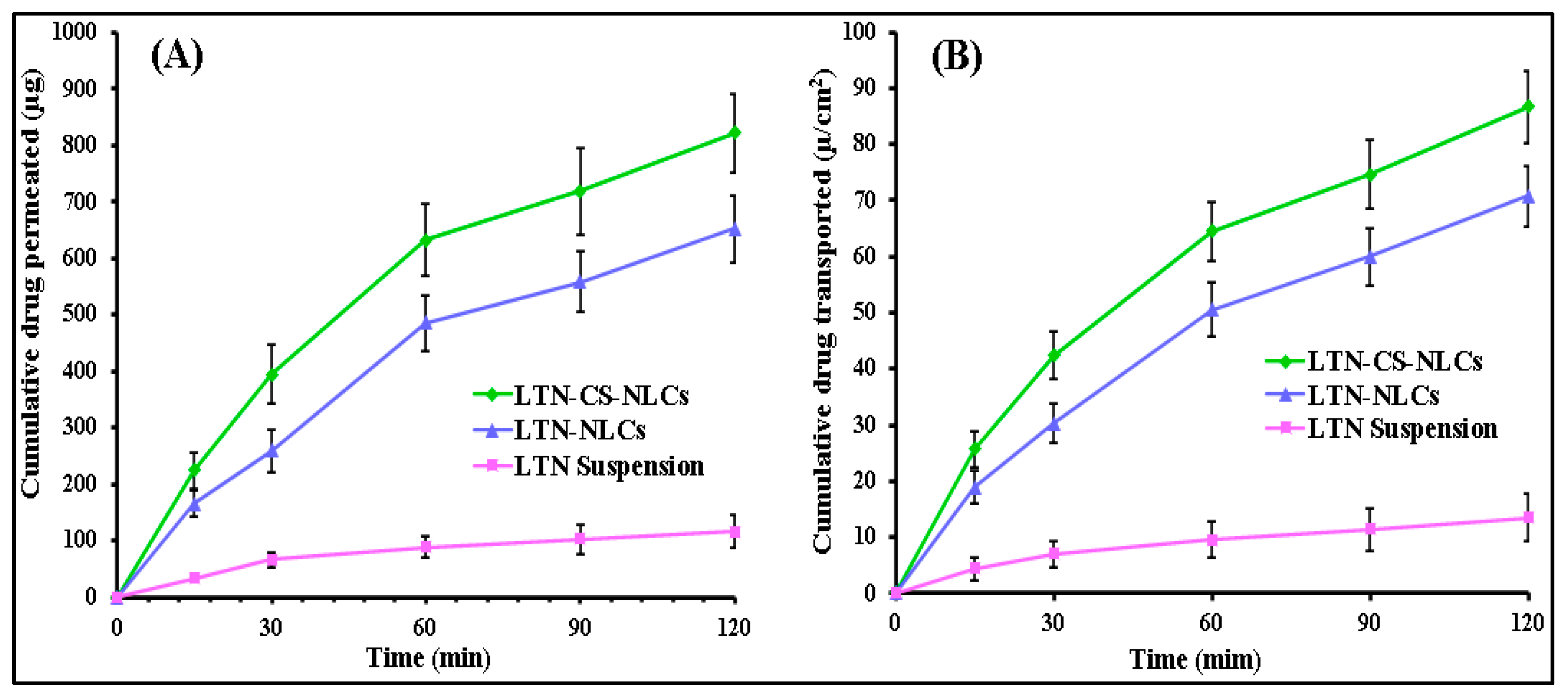
3.9. Ex Vivo Intestinal Permeation Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Jain, V.K.; Rizwanullah, M.; Ahmad, J.; Jain, K. PI3K/AKT/mTOR pathway inhibitors in triple-negative breast cancer: A review on drug discovery and future challenges. Drug Discov. Today 2019, 24, 2181–2191. [Google Scholar] [CrossRef]
- Kamaruzman, N.I.; Aziz, N.A.; Poh, C.L.; Chowdhury, E.H. Oncogenic signaling in tumorigenesis and applications of siRNA nanotherapeutics in breast cancer. Cancers 2019, 11, 632. [Google Scholar] [CrossRef]
- Sharma, A.; Jain, N.; Sareen, R. Nanocarriers for diagnosis and targeting of breast cancer. Biomed Res. Int. 2013, 2013, 960821. [Google Scholar] [CrossRef] [PubMed]
- Rizwanullah, M.; Alam, M.; Mir, S.R.; Rizvi, M.M.A.; Amin, S. Polymer-lipid hybrid nanoparticles: A next-generation nanocarrier for targeted treatment of solid tumors. Curr. Pharm. Des. 2020, 26, 1206–1215. [Google Scholar] [CrossRef]
- Du, M.; Ouyang, Y.; Meng, F.; Ma, Q.; Liu, H.; Zhuang, Y.; Pang, M.; Cai, T.; Cai, Y. Nanotargeted agents: An emerging therapeutic strategy for breast cancer. Nanomedicine 2019, 14, 1771–1786. [Google Scholar] [CrossRef]
- Haider, N.; Fatima, S.; Taha, M.; Rizwanullah, M.; Firdous, J.; Ahmad, R.; Mazhar, F.; Khan, M.A. Nanomedicines in Diagnosis and Treatment of Cancer: An Update. Curr. Pharm. Des. 2020, 26, 1216–1231. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2020, 9, e109. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.A.; Rahaman, A.; Muhammad Aadil, R.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019, 43, e12974. [Google Scholar]
- Lv, P.C.; Li, H.Q.; Xue, J.Y.; Shi, L.; Zhu, H.L. Synthesis and biological evaluation of novel luteolin derivatives as antibacterial agents. Eur. J. Med. Chem 2009, 44, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Liu, M.; Fu, Y.; Zhang, J.; Liu, W.; Li, J.; Li, X.; Li, Y.; Wang, T. Antimicrobial mechanism of luteolin against Staphylococcus aureus and Listeria monocytogenes and its antibiofilm properties. Microb. Pathog. 2020, 142, 104056. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Alshehri, S.; Imam, S.S.; Altamimi, M.A.; Hussain, A.; Shakeel, F.; Elzayat, E.; Mohsin, K.; Ibrahim, M.; Alanazi, F. Enhanced dissolution of luteolin by solid dispersion prepared by different methods: Physicochemical characterization and antioxidant activity. ACS Omega 2020, 5, 6461–6471. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lu, Y.; Xie, J.; Zhang, E.; Zhu, H.; Du, H.; Wang, K.; Song, B.; Yang, C.; Shi, Y.; et al. Zwitterionic micelles efficiently deliver oral insulin without opening tight junctions. Nat Nanotechnol. 2020, 15, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Wang, L.; You, X.; Wu, J. Nano and microscale delivery platforms for enhanced oral peptide/protein bioavailability. Biomater. Sci. 2020, 8, 5804–5823. [Google Scholar] [CrossRef] [PubMed]
- Rizwanullah, M.; Amin, S.; Ahmad, J. Improved pharmacokinetics and antihyperlipidemic efficacy of rosuvastatin-loaded nanostructured lipid carriers. J. Drug Target. 2017, 25, 58–74. [Google Scholar]
- Eid, R.K.; Ashour, D.S.; Essa, E.A.; El Maghraby, G.M.; Arafa, M.F. Chitosan coated nanostructured lipid carriers for enhanced in vivo efficacy of albendazole against Trichinella spiralis. Carbohydr. Polym. 2020, 232, 115826. [Google Scholar] [CrossRef]
- Ling Tan, J.S.; Roberts, C.J.; Billa, N. Mucoadhesive chitosan-coated nanostructured lipid carriers for oral delivery of amphotericin B. Pharm. Dev. Technol. 2019, 24, 504–512. [Google Scholar] [CrossRef]
- Ling, J.T.S.; Roberts, C.J.; Billa, N. Antifungal and Mucoadhesive Properties of an Orally Administered Chitosan-Coated Amphotericin B Nanostructured Lipid Carrier (NLC). AAPS PharmSciTech. 2019, 20, 136. [Google Scholar] [CrossRef]
- Liu, M.; Zhong, X.; Yang, Z. Chitosan functionalized nanocochleates for enhanced oral absorption of cyclosporine A. Sci. Rep. 2017, 7, 41322. [Google Scholar] [CrossRef]
- Gartziandia, O.; Herran, E.; Pedraz, J.L.; Carro, E.; Igartua, M.; Hernandez, R.M. Chitosan coated nanostructured lipid carriers for brain delivery of proteins by intranasal administration. Colloids Surf. B Biointerfaces 2015, 134, 304–313. [Google Scholar] [CrossRef]
- Kaithwas, V.; Dora, C.P.; Kushwah, V.; Jain, S. Nanostructured lipid carriers of olmesartan medoxomil with enhanced oral bioavailability. Colloids Surf. B Biointerfaces 2017, 154, 10–20. [Google Scholar] [CrossRef]
- Dalmoro, A.; Bochicchio, S.; Nasibullin, S.F.; Bertoncin, P.; Lamberti, G.; Barba, A.A.; Moustafine, R.I. Polymer-lipid hybrid nanoparticles as enhanced indomethacin delivery systems. Eur. J. Pharm. Sci. 2018, 121, 16–28. [Google Scholar]
- Son, G.H.; Lee, B.J.; Cho, C.W. Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles. J. Pharm. Investig. 2017, 47, 287–296. [Google Scholar] [CrossRef]
- Khalifa, A.Z.M.; Rasool, B.K.A. Optimized Mucoadhesive Coated Niosomes as a Sustained Oral Delivery System of Famotidine. AAPS Pharm. Sci. Tech. 2017, 18, 3064–3075. [Google Scholar] [CrossRef] [PubMed]
- Fakhria, A.; Gilani, S.J.; Imam, S.S. Formulation of thymoquinone loaded chitosan nano vesicles: In-vitro evaluation and in-vivo anti-hyperlipidemic assessment. J. Drug Deliv. Sci. Tech. 2019, 50, 339–346. [Google Scholar] [CrossRef]
- Zarai, Z.; Boujelbene, E.; Ben Salem, N.; Gargouri, Y.; Sayari, A. Antioxidant and antimicrobial activities of various solvent extracts, piperine and piperic acid from Piper nigrum. LWT Food Sci. Technol. 2013, 50, 634–641. [Google Scholar] [CrossRef]
- Alshehri, S.; Imam, S.S.; Rizwanullah, M.; Fakhri, K.U.; Rizvi, M.; Alam, M.; Mahdi, W.; Kazi, M. Effect of Chitosan Coating on PLGA Nanoparticles for Oral Delivery of Thymoquinone: In Vitro, Ex Vivo, and Cancer Cell Line Assessments. Coatings 2021, 11, e6. [Google Scholar]
- Al-Joudi, F.S.; Alias, I.Z.; Samsudin, A.R. The effects of chemotherapeutic drugs on viabilty, apoptosis, and survivin expression in MCF-7 cells. Acta Histochem. Cytochem. 2005, 38, 323–330. [Google Scholar] [CrossRef][Green Version]
- Dadashpour, M.; Firouzi-Amandi, A.; Pourhassan-Moghaddam, M.; Maleki, M.J.; Soozangar, N.; Jeddi, F.; Nouri, M.; Zarghami, N.; Pilehvar-Soltanahmadi, Y. Biomimetic synthesis of silver nanoparticles using Matricaria chamomilla extract and their potential anticancer activity against human lung cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 902–912. [Google Scholar] [CrossRef]
- Saifi, Z.; Rizwanullah, M.; Mir, S.R.; Amin, S. Bilosomes nanocarriers for improved oral bioavailability of acyclovir: A complete characterization through in vitro, ex-vivo and in vivo assessment. J. Drug Deliv. Sci. Tech. 2020, 57, 101634. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Ahmad, J.; Amin, S. Nanostructured lipid carriers: A novel platform for chemotherapeutics. Curr. Drug Deliv. 2016, 13, 4–26. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Zidan, A.S.; Khan, M.A. Non-destructive methods of characterization of risperidone solid lipid nanoparticles. Eur. J. Pharm. Biopharm. 2010, 76, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Patil, T.S.; Deshpande, A.S. Design, development, and characterisation of clofazimine-loaded mannosylated nanostructured lipid carriers: 33-Box-Behnken design approach. Mater. Technol. 2020, 36, 1–16. [Google Scholar] [CrossRef]
- Fonte, P.; Nogueira, T.; Gehm, C.; Ferreira, D.; Sarmento, B. Chitosan-coated solid lipid nanoparticles enhance the oral absorption of insulin. Drug Deliv. Transl. Res. 2011, 1, 299–308. [Google Scholar] [CrossRef]
- Venishetty, V.K.; Chede, R.; Komuravelli, R.; Adepu, L.; Sistla, R.; Diwan, P.V. Design and evaluation of polymer coated carvedilol loaded solid lipid nanoparticles to improve the oral bioavailability: A novel strategy to avoid intraduodenal administration. Colloids Surf. B Biointerfaces 2012, 95, 1–9. [Google Scholar]
- Ramalingam, P.; Ko, Y.T. Improved oral delivery of resveratrol from N-trimethyl chitosan-g-palmitic acid surface-modified solid lipid nanoparticles. Colloids Surf. B Biointerfaces 2016, 139, 52–61. [Google Scholar] [CrossRef]
- Shi, L.L.; Lu, J.; Cao, Y.; Liu, J.Y.; Zhang, X.X.; Zhang, H.; Cui, J.H.; Cao, Q.R. Gastrointestinal stability, physicochemical characterization and oral bioavailability of chitosan or its derivative-modified solid lipid nanoparticles loading docetaxel. Drug. Dev. Ind. Pharm. 2017, 43, 839–846. [Google Scholar] [CrossRef]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why is chitosan mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. [Google Scholar]
- Kamel, K.M.; Khalil, I.A.; Rateb, M.E.; Elgendy, H.; Elhawary, S. Chitosan-coated cinnamon/oregano-loaded solid lipid nanoparticles to augment 5-fluorouracil cytotoxicity for colorectal cancer: Extract standardization, nanoparticle optimization, and cytotoxicity evaluation. J. Agric. Food Chem. 2017, 65, 7966–7981. [Google Scholar] [CrossRef]
- Suganya, K.S.U.; Govindaraju, K.; V. Ganesh Kumar, V.G.; Prabhu, D.; Arulvasu, C.; Dhas, T.S.; Karthick, V.; Changmai, N. Anti-proliferative effect of biogenic gold nanoparticles against breast cancer cell lines (MDA-MB-231 & MCF-7). Appl. Sur. Sci. 2016, 371, 415–424. [Google Scholar]

| Factors | Levels Used, Actual (Coded Factor) | ||
|---|---|---|---|
| Independent variables | Low (−1) | Medium (0) | High (+1) |
| X1 = Concentration of lipid (%) | 2 | 3 | 4 |
| X2 = Concentration of P-188 (%) | 1 | 1.5 | 2 |
| X3 = Sonication time (min) | 3 | 4 | 5 |
| Dependent Variables | Goal | ||
| Y1 = Particle size (PS; nm) | Minimize | ||
| Y2 = Polydispersity index (PDI) | Minimize | ||
| Y3 = Entrapment efficiency (EE; %) | Maximize | ||
| Runs | Independent Variables | Dependent Variables | |||||||
|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | Y3 | ||||
| Actual | Predicted | Actual | Predicted | Actual | Predicted | ||||
| F1 | 3 | 1.5 | 4 | 126.84 | 125.72 | 0.131 | 0.132 | 83.46 | 82.36 |
| F2 | 2 | 2 | 4 | 113.43 | 114.44 | 0.115 | 0.116 | 74.45 | 73.57 |
| F3 | 4 | 1 | 4 | 154.73 | 153.73 | 0.178 | 0.177 | 87.96 | 88.84 |
| F4 | 3 | 1.5 | 4 | 124.42 | 125.72 | 0.131 | 0.132 | 82.64 | 81.36 |
| F5 | 3 | 1 | 3 | 148.16 | 149.86 | 0.150 | 0.151 | 83.68 | 81.85 |
| F6 | 2 | 1.5 | 3 | 129.14 | 128.08 | 0.151 | 0.149 | 73.77 | 72.50 |
| F7 | 2 | 1 | 4 | 117.46 | 116.82 | 0.131 | 0.132 | 68.48 | 69.59 |
| F8 | 4 | 1.5 | 3 | 158.04 | 157.35 | 0.171 | 0.172 | 95.37 | 96.19 |
| F9 | 4 | 2 | 4 | 145.75 | 146.39 | 0.163 | 0.161 | 94.29 | 95.19 |
| F10 | 3 | 1.5 | 4 | 122.48 | 125.72 | 0.132 | 0.132 | 84.12 | 82.36 |
| F11 | 3 | 1 | 5 | 132.35 | 132.29 | 0.158 | 0.156 | 72.96 | 72.11 |
| F12 | 4 | 1.5 | 5 | 140.47 | 141.53 | 0.201 | 0.202 | 82.36 | 81.63 |
| F13 | 3 | 2 | 5 | 125.72 | 124.02 | 0.143 | 0.142 | 79.88 | 78.71 |
| F14 | 2 | 1.5 | 5 | 101.25 | 101.94 | 0.134 | 0.133 | 65.55 | 64.59 |
| F15 | 3 | 2 | 3 | 148.35 | 148.41 | 0.131 | 0.132 | 89.13 | 90.28 |
| Model | R2 | Adjusted R2 | Predicted R2 | SD | R2 | Adjusted R2 | Predicted R2 | SD | R2 | Adjusted R2 | Predicted R2 | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Response | Particle Size (Y1) | PDI (Y2) | Encapsulation Efficiency (Y3) | |||||||||
| Linear | 0.9054 | 0.8796 | 0.8384 | 5.6 | 0.6644 | 0.5729 | 0.3886 | 0.014 | 0.9764 | 0.9699 | 0.9546 | 1.52 |
| 2F1 | 0.9176 | 0.8558 | 0.7336 | 6.1 | 0.7414 | 0.5474 | 0.0736 | 0.015 | 0.9823 | 0.9690 | 0.9273 | 1.54 |
| Quadratic | 0.9968 | 0.9909 | 0.9481 | 1.5 | 0.9979 | 0.9941 | 0.9663 | 0.002 | 0.9997 | 0.9992 | 0.9955 | 0.24 |
| Time (h) | Simulated Intestinal Fluids (SIF; pH 6.8) | Simulated Gastric Fluids (SGF; pH 1.2) | ||||
|---|---|---|---|---|---|---|
| PS (nm) | PDI | %EE | PS (nm) | PDI | %EE | |
| 0 | 123.84 ± 3.72 | 0.132 ± 0.02 | 83.74 ± 4.68 | 123.84 ± 3.72 | 0.132 ± 0.02 | 83.74 ± 4.68 |
| 2 | 125.21 ± 4.54 | 0.135 ± 0.02 | 82.98 ± 5.14 | 129.97 ± 4.54 | 0.137 ± 0.03 | 81.91 ± 6.14 |
| 4 | 126.46 ± 5.82 | 0.139 ± 0.02 | 82.72 ± 5.36 | 136.78 ± 4.82 | 0.141 ± 0.03 | 80.17 ± 4.98 |
| 8 | 128.25 ± 5.28 | 0.144 ± 0.03 | 82.57 ± 6.35 | 142.54 ± 5.28 | 0.154 ± 0.04 | 77.15 ± 5.46 |
| 12 | 131.38 ± 6.46 | 0.151 ± 0.03 | 81.91 ± 5.77 | 148.04 ± 5.86 | 0.182 ± 0.05 | 73.74 ± 5.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilani, S.J.; Bin-Jumah, M.; Rizwanullah, M.; Imam, S.S.; Imtiyaz, K.; Alshehri, S.; Rizvi, M.M.A. Chitosan Coated Luteolin Nanostructured Lipid Carriers: Optimization, In Vitro-Ex Vivo Assessments and Cytotoxicity Study in Breast Cancer Cells. Coatings 2021, 11, 158. https://doi.org/10.3390/coatings11020158
Gilani SJ, Bin-Jumah M, Rizwanullah M, Imam SS, Imtiyaz K, Alshehri S, Rizvi MMA. Chitosan Coated Luteolin Nanostructured Lipid Carriers: Optimization, In Vitro-Ex Vivo Assessments and Cytotoxicity Study in Breast Cancer Cells. Coatings. 2021; 11(2):158. https://doi.org/10.3390/coatings11020158
Chicago/Turabian StyleGilani, Sadaf Jamal, May Bin-Jumah, Md. Rizwanullah, Syed Sarim Imam, Khalid Imtiyaz, Sultan Alshehri, and Mohd. Moshahid Alam Rizvi. 2021. "Chitosan Coated Luteolin Nanostructured Lipid Carriers: Optimization, In Vitro-Ex Vivo Assessments and Cytotoxicity Study in Breast Cancer Cells" Coatings 11, no. 2: 158. https://doi.org/10.3390/coatings11020158
APA StyleGilani, S. J., Bin-Jumah, M., Rizwanullah, M., Imam, S. S., Imtiyaz, K., Alshehri, S., & Rizvi, M. M. A. (2021). Chitosan Coated Luteolin Nanostructured Lipid Carriers: Optimization, In Vitro-Ex Vivo Assessments and Cytotoxicity Study in Breast Cancer Cells. Coatings, 11(2), 158. https://doi.org/10.3390/coatings11020158






