Testing New Coatings for Outdoor Bronze Monuments: A Methodological Overview
Abstract
1. Introduction
- Archaeological metals are characterised mainly by long burial in soil [15], waterlogged [12,15,16] or underwater [17]; they bear information on very long-term corrosion of metals [18,19]; the equilibrium state reached during burial may be broken when excavated, giving rise to new corrosion process if not properly treated [20].
- A large variety of historic objects (such as scientific instruments, fine arts, historic pieces, ethnographic specimens, etc.) is conserved indoors (museums, monumental buildings, collections); the main preservation strategy in this environment is preventive conservation [4,21], such as humidity control; critical parameter to consider are dangers from “off-gassing” materials used to build display cases and rooms, as well as air pollution introduced by visitors [21].
2. Treatments in Use
3. The Ideal Treatment from a Conservation Perspective
4. Critical Issues on Testing New Coatings
4.1. Lack of Reference Standards
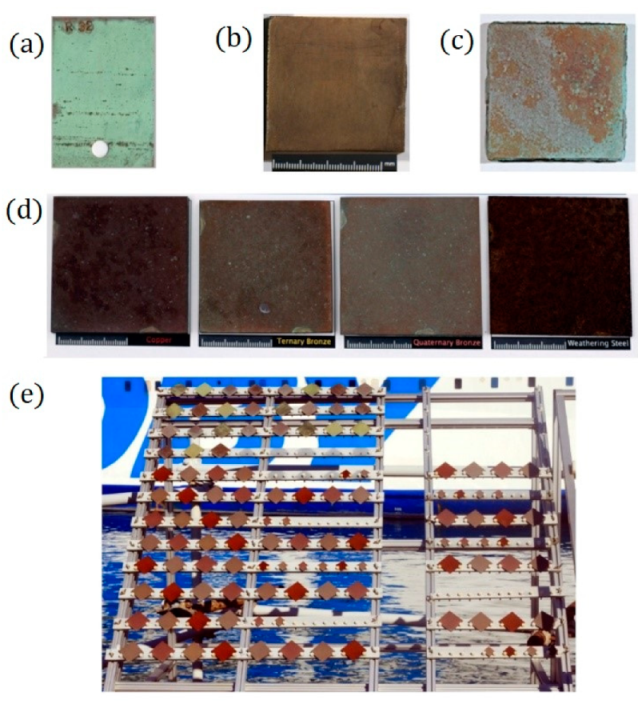
4.2. Testing Surfaces
4.3. Application Methods
4.4. Ageing Techniques
4.5. Analytical Techniques
5. Final Comments
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ankersmit, B.; Griesser-Stermscheg, M.; Selwyn, L.; Sutherland, S. Rust Never Sleeps: Recognizing Metals and Their Corrosion Products; Parks Canada: Gatineau, QC, Canada, 2008.
- Selwyn, L. Metals and Corrosion: A Handbook for the Conservation Professional; Canadian Conservation Institute: Ottawa, ON, Canada, 2004. [Google Scholar]
- Watkinson, D. Conservation, corrosion science and evidence-based preservation strategies for metallic heritage artefacts. In Corrosion and Conservation of Cultural Heritage Metallic Artefacts; EFS Series; Woodhead Publishing: Cambridge, UK, 2013; pp. 9–36. [Google Scholar] [CrossRef]
- Watkinson, D. Preservation of metallic cultural heritage. In Shreir’s Corrosion; Elsevier: Amsterdam, The Netherlands, 2010; pp. 3307–3340. [Google Scholar] [CrossRef]
- Cano, E.; Lafuente, D. Corrosion inhibitors for the preservation of metallic heritage artefacts. In Corrosion and Conservation of Cultural Heritage Metallic Artefacts; EFS Series; Woodhead Publishing: Cambridge, UK, 2013; pp. 570–594. [Google Scholar] [CrossRef]
- Alunno-Rossetti, V.; Marabelli, M. Analyses of the patinas of a gilded horse of StMark’s Basilica in Venice: Corrosion mechanism and conservation problems. Stud. Conserv. 1976, 21, 161–170. [Google Scholar] [CrossRef]
- Baboian, R. A corrosion scientist’s summary of Dialogue 89. In Dialogue/89: The Conservation of Bronze Sculpture in the Outdoor Environment: A Dialogue among Conservators, Curators, Environmental Scientists, and Corrosion Engineers; Drayman-Weisser, T., Ed.; National Association of Corrosion Engineers: Houston, UK, 1992; pp. 379–386. [Google Scholar]
- Marabelli, M.; Napolitano, G. Nuovi sistemi protettivi applicabili su opere o manufatti in bronzo esposti all’aperto (New Protective Systems Applied to Outdoor Bronze Statuary or Artifacts). Mater. E Strutt. Probl. Conserv. 1991, 1, 51–58. [Google Scholar]
- Leoni, M. Un particolare fenomeno corrosivo sulla Fiorenza del Giambologna. OPD Restauro 1991, 3, 52–56. [Google Scholar]
- Scott, D.A.; Podany, J.; Considine, B.B. (Eds.) Ancient & Historic Metals: Conservation and Scientific Research—Proceedings of a Symposium Organized by the J. Paul Getty Museum and the Getty Conservation Institute November 1991; Getty Conservation Institute: Marina del Rey, CA, USA, 1994. [Google Scholar]
- Dillmann, P.; Watkinson, D.; Angelini, E.; Adriaens, A. Introduction: Conservation versus laboratory investigation in the preservation of metallic heritage artefacts. In Corrosion and Conservation of Cultural Heritage Metallic Artefacts; EFS Series; Woodhead Publishing: Cambridge, UK, 2013; pp. 1–5. [Google Scholar] [CrossRef]
- Matthiesen, H.; Gregory, D.; Jensen, P.; Sørensen, B. Environmental monitoring at Nydam, a waterlogged site with weapon sacrifices from the Danish Iron age. I: A comparison of methods used and results from undisturbed conditions. J. Wetl. Archaeol. 2004, 4, 55–74. [Google Scholar] [CrossRef]
- Bertholon, R. The location of the original surface: A review of the conservation literature. In Metal 2001, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Santiago, Chile, 2–6 April 2001; MacLeod, I.D., Theile, J.M., Degrigny, C., Eds.; Western Australian Museum: Perth, Australia, 2004; pp. 167–179. [Google Scholar]
- Bernard, M.C.; Joiret, S. Understanding corrosion of ancient metals for the conservation of cultural heritage. Electrochim. Acta 2009, 54, 5199–5205. [Google Scholar] [CrossRef]
- Selwyn, L. Overview of archaeological iron: The corrosion problem, key factors affecting treatment, and gaps in current knowledge. In Metal 2004, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Canberra, Australia, 4–8 October 2004; Ashton, J., Hallam, D., Eds.; National Museum of Australia: Canberra, Australia, 2004; pp. 294–306. [Google Scholar]
- Goodburn-Brown, D. Surface studies on metals from waterlogged sites. In Metal 95, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Semur en Auxois, France, 25–28 September 1995; MacLeod, I.D., Pennec, S.L., Robbiola, L., Eds.; James & James: London, UK, 1997; pp. 61–66. [Google Scholar]
- Memet, J.B. The corrosion of metallic artefacts in seawater: Descriptive analysis. In Corrosion of Metallic Heritage Artefacts; Dillmann, P., Béranger, G., Piccardo, P., Matthiesen, H., Eds.; European Federation of Corrosion (EFC) Series; Woodhead Publishing: Cambridge, UK, 2007; pp. 152–169. [Google Scholar] [CrossRef]
- Robbiola, L.; Blengino, J.-M.; Fiaud, C. Morphology and mechanisms of formation of natural patinas on archaeological Cu–Sn alloys. Corros. Sci. 1998, 40, 2083–2111. [Google Scholar] [CrossRef]
- Moore, J.D. Long-term corrosion processes of iron and steel shipwrecks in the marine environment: A review of current knowledge. J. Marit. Archaeol. 2015, 10, 191–204. [Google Scholar] [CrossRef]
- Rémazeilles, C.; Langlet-Marzloff, V.; Creus, J.; Lotte, G.; Deshayes, C.; Baleux, F.; Robbiola, L. Remarkable corrosion resumption of archaeological bronzes, induced by the oxidation of ternary Cu–Sn–S phases in atmosphere, after long-term burial with sulfides. Corros. Sci. 2020, 175, 108865. [Google Scholar] [CrossRef]
- Thickett, D. Frontiers of preventive conservation. Stud. Conserv. 2018, 63 (Suppl. 1), 262–267. [Google Scholar] [CrossRef]
- Lins, A.; Power, T. The corrosion of bronze monuments in polluted urban sites: A report on the stability of copper mineral species at different pH levels. In Ancient & Historic Metals: Conservation and Scientific Research—Proceedings of a Symposium Organized by the J. Paul Getty Museum and the Getty Conservation Institute November 1991; Getty Conservation Institute: Marina del Rey, CA, USA, 1994; pp. 119–151. [Google Scholar]
- Matero, F.G. Conservation of architectural metalwork: Historical approaches to the surface treatment of iron. In Ancient & Historic Metals: Conservation and Scientific Research—Proceedings of a Symposium Organized by the J. Paul Getty Museum and the Getty Conservation Institute November 1991; Getty Conservation Institute: Marina del Rey, CA, USA, 1994; pp. 198–228. [Google Scholar]
- Grissom, C.A. The conservation of outdoor zinc sculpture. In Ancient & Historic Metals: Conservation and Scientific Research—Proceedings of a Symposium Organized by the J. Paul Getty Museum and the Getty Conservation Institute November 1991; Getty Conservation Institute: Marina del Rey, CA, USA, 1994; pp. 279–304. [Google Scholar]
- Strandberg, H. Perspectives on Bronze Sculpture Conservation. Modelling Copper and Bronze Corrosion. Ph.D. Thesis, University of Gothenburg, Gothenburg, Sweden, 1997. [Google Scholar]
- Shashoua, Y.; Matthiesen, H. Protection of iron and steel in large outdoor industrial heritage objects. Corros. Eng. Sci. Technol. 2010, 45, 357–361. [Google Scholar] [CrossRef]
- Kreislova, K.; Knotkova, D.; Geiplova, H. Atmospheric corrosion of historical industrial structures. In Corrosion and Conservation of Cultural Heritage Metallic Artefacts; Dillmann, P., Watkinson, D., Angelini, E., Adriaens, A., Eds.; European Federation of Corrosion (EFC) Series; Woodhead Publishing: Cambridge, UK, 2013; pp. 311–343. [Google Scholar] [CrossRef]
- Thickett, D.; Stanley, B. The use and mis-use of renaissance wax. In Metal 2019, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Neuchâtel, Switzerland, 2–6 September 2019; Chemello, C., Brambilla, L., Joseph, E., Eds.; ICOM-CC and HE-Arc CR: Neuchatel, Switzerland, 2019; pp. 232–239. ISBN 978-92-9012-457-3. [Google Scholar]
- Gullman, J.; Törnblom, M. Principles of Bronze Sculpture–Its Making and Unmaking: A Study of Outdoor Bronze Sculpture Conservation; The Central Board of National Antiquities (Riksantikvarieämbetet): Stockholm, Sweden, 1994; ISBN 91-7192-943-6. [Google Scholar]
- Knotkova, D.; Boschek, P.; Kreislova, K. Effect of acidification on atmospheric corrosion of structural metals in Europe. Water Air Soil Pollut. 1995, 85, 2661–2666. [Google Scholar] [CrossRef]
- Leygraf, C.; Odnevall Wallinder, I.; Tidblad, J.; Graedel, T. Atmospheric Corrosion, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Matteini, M.; Lalli, C.; Tosini, I. Controllo dei componenti solubili delle patine mediante cromatografia ionica e assorbimento atomico. OPD Restauro 1991, 3, 40–46. [Google Scholar]
- Graedel, T.E. Copper patinas formed in the atmosphere—II. A qualitative assessment of mechanisms. Corros. Sci. 1987, 27, 721–740. [Google Scholar] [CrossRef]
- Robbiola, L.; Fiaud, C.; Pennec, S. New model of outdoor bronze corrosion and its implications for conservation. In Proceedings of the ICOM Committee for Conservation Tenth Triennial Meeting, Washington, DC, USA, 22–27 August 1993; pp. 796–802. [Google Scholar]
- Fischer, W.R.; Wagner, B.D.; Siedlarek, H.; Füßinger, B.; Hänßel, I.; von der Bank, N. The influence of chloride ions and light on the corrosion behaviour of copper alloys in aqueous environments with special regard to bronze disease. In Metal 95, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Semur en Auxois, France, 25–28 September 1995; MacLeod, I.D., Pennec, S., Robbiola, L., Eds.; James & James: London, UK, 1997; pp. 89–94. [Google Scholar]
- Chiavari, C.; Rahmouni, K.; Takenouti, H.; Joiret, S.; Vermaut, P.; Robbiola, L. Composition and electrochemical properties of natural patinas of outdoor bronze monuments. Electrochim. Acta 2007, 52, 7760–7769. [Google Scholar] [CrossRef]
- Bernardi, E.; Chiavari, C.; Lenza, B.; Martini, C.; Morselli, L.; Ospitali, F.; Robbiola, L. The atmospheric corrosion of quaternary bronzes: The leaching action of acid rain. Corros. Sci. 2009, 51, 159–170. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J. The effects of UV and visible light on the corrosion of bronze covered with an oxide film in aqueous solution. Corros. Sci. 2019, 154, 144–158. [Google Scholar] [CrossRef]
- Chang, T.; Maltseva, A.; Volovitch, P.; Odnevall Wallinder, I.; Leygraf, C. A mechanistic study of stratified patina evolution on Sn-bronze in chloride-rich atmospheres. Corros. Sci. 2020, 166, 108477. [Google Scholar] [CrossRef]
- Joseph, E.; Simon, A.; Prati, S.; Wörle, M.; Job, D.; Mazzeo, R. Development of an analytical procedure for evaluation of the protective behaviour of innovative fungal patinas on archaeological and artistic metal artefacts. Anal. Bioanal. Chem. 2011, 399, 2899–2907. [Google Scholar] [CrossRef]
- Contreras-Vargas, J.; Cama-Villafranca, J. The metal patina and surface layer of El Caballito: Calling things by their name. In Metal 2019, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Neuchâtel, Switzerland, 2–6 September 2019; Chemello, C., Brambilla, L., Joseph, E., Eds.; ICOM-CC and HE-Arc CR: Neuchatel, Switzerland, 2019; pp. 322–328. ISBN 978-92-9012-457-3. [Google Scholar]
- Dajnowski, A.; Ross, T.; Craig, A.B.; Dajnowski, B. New trends in art conservation, the use of lasers to clean as well as generate an augmented reality representation of an iconic public monument in bronze: The Alma Mater. Stud. Conserv. 2015, 60 (Suppl. 1), S65–S72. [Google Scholar] [CrossRef]
- Di Carlo, G.; Giuliani, C.; Riccucci, C.; Pascucci, M.; Messina, E.; Fierro, G.; Lavorgna, M.; Ingo, G.M. Artificial patina formation onto copper-based alloys: Chloride and sulphate induced corrosion processes. Appl. Surf. Sci. 2017, 421, 120–127. [Google Scholar] [CrossRef]
- Chiavari, C.; Bernardi, E.; Martini, C.; Morselli, L.; Ospitali, F.; Robbiola, L.; Textier, A. Predicting the corrosion behaviour of outdoor bronzes: Assessment of artificially exposed and real outdoor samples. In Metal 2010, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Charleston, SC, USA, 11–15 October 2010; Mardikian, P., Chemello, C., Watters, C., Hull, P., Eds.; Clemson University: Clemson, SC, USA, 2011; pp. 218–226. [Google Scholar]
- Robbiola, L.; Chiavari, C.; Bernardi, E.; Bignozzi, M.C.; Martini, C. General understanding of outdoor bronze corrosion: An overview. In Métal à Ciel Ouvert: La Sculpture Métallique D’extérieur du XIXe au début du XXe Siècle: Identification, Conservation, Restauration; International Institute for Conservation of Historic and Artistic Works; Section Française: Champs-sur-Marne, France, 2014; pp. 146–153. ISBN 9782905430182. [Google Scholar]
- Bozzini, B.; Alemán, B.; Amati, M.; Boniardi, M.; Caramia, V.; Giovannelli, G.; Gregoratti, L.; Kazemian Abyaneh, M. Novel insight into bronze disease gained by synchrotron-based photoelectron spectro-microscopy, in support of electrochemical treatment strategies. Stud. Conserv. 2017, 62, 465–473. [Google Scholar] [CrossRef]
- Selwyn, L.S.; Binnie, N.E.; Poitras, J.; Laver, M.E.; Downham, D.A. Outdoor bronze statues: Analysis of metal and surface samples. Stud. Conserv. 1996, 41, 205–228. [Google Scholar] [CrossRef]
- Petiti, C.; Toniolo, L.; Gulotta, D.; Mariani, B.; Goidanich, S. Effects of cleaning procedures on the long-term corrosion behavior of bronze artifacts of the cultural heritage in outdoor environment. Environ. Sci. Pollut. Res. 2020, 27, 13081–13094. [Google Scholar] [CrossRef] [PubMed]
- Agnoletti, S.; Brambilla, L.; Brini, A.; Cagnini, A.; Camaiti, M.; Celi, C.; Cetarini, L.; De Lapi, R.; Galeotti, M.; Goidanich, S.; et al. Formulati e metodologie per la pulitura e la protezione di superfici metalliche. Arkos 2011, 28, 34–37. [Google Scholar]
- Chiavari, G.; Colucci, A.; Mazzeo, R.; Ravanelli, M. Organic content evaluation of corrosion patinas in outdoor bronze monuments. Chromatographia 1999, 49, 35–41. [Google Scholar] [CrossRef]
- Letardi, P.; Salvadori, B.; Galeotti, M.; Cagnini, A.; Porcinai, S.; Santagostino Barbone, A.; Sansonetti, A. An in situ multi-analytical approach in the restoration of bronze artefacts. Microchem. J. 2016, 125, 151–158. [Google Scholar] [CrossRef]
- Degrigny, C. Survey of European experiences on cleaning procedures. In Monumenti in Bronzo All’aperto: Esperienze di Conservazione a Confronto; Letardi, P., Trentin, I., Cutugno, G., Eds.; Nardini Editore: Florence, Italy, 2004; pp. 87–93. [Google Scholar]
- Römich, H. New Conservation Methods for Outdoor Bronze Sculptures. 1993–1995. Available online: https://cordis.europa.eu/project/id/EV5V0107 (accessed on 25 January 2021).
- Pilz, M.; Römich, H. Sol-gel derived coatings for outdoor bronze conservation. J. Sol-Gel Sci. Technol. 1997, 8, 1071–1075. [Google Scholar] [CrossRef]
- Norbert, B. Conservation notes: Maintenance of outdoor bronze sculpture. Int. J. Mus. Manag. Curatorship 1988, 7, 71–75. [Google Scholar] [CrossRef]
- Brostoff, L.B. Coating Strategies for the Protection of Outdoor Bronze Art and Ornamentation. Ph.D. Thesis, University of Amsterdam-Van ’t Hoff Institute for Molecular Sciences (HIMS), Amsterdam, The Neederlands, 2003. [Google Scholar]
- Mazzeo, R.; Bittner, S.; Farron, G.; Fontinha, R.; Job, D.; Joseph, E.; Letardi, P.; Mach, M.; Prati, S.; Salta, M.; et al. Development and evaluation of new treatments for outdoor bronze monuments. In Proceedings of the International Conference Conservation Science 2007, Milan, Italy, 10–11 May 2007; Townsend, J.H., Toniolo, L., Cappitelli, F., Eds.; Archetype: Washington, DC, USA, 2008; pp. 40–48, ISBN 978-1904982340. [Google Scholar]
- Joseph, E.; Letardi, P.; Mazzeo, R.; Prati, S.; Vandini, M. Innovative treatments for the protection of outdoor bronze monuments. In Metal 07, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Amsterdam, The Neederlands, 17–21 September 2007; Degrigny, C., van Langh, R., Joosten, I., Ankersmit, B., Eds.; Rijksmuseum: Amsterdam, The Neederlands, 2007; Volume 5, pp. 71–77. [Google Scholar]
- Albini, M.; Letardi, P.; Mathys, L.; Brambilla, L.; Schröter, J.; Junier, P.; Joseph, E. Comparison of a bio-based corrosion inhibitor versus benzotriazole on corroded copper surfaces. Corros. Sci. 2018, 143, 84–92. [Google Scholar] [CrossRef]
- Faraldi, F.; Cortese, B.; Caschera, D.; Di Carlo, G.; Riccucci, C.; de Caro, T.; Ingo, G.M. Smart conservation methodology for the preservation of copper-based objects against the hazardous corrosion. Thin Solid Films 2017, 622, 130–135. [Google Scholar] [CrossRef]
- Masi, G.; Aufray, M.; Balbo, A.; Bernardi, E.; Bignozzi, M.C.; Chiavari, C.; Esvan, J.; Gartner, N.; Grassi, V.; Josse, C.; et al. B-IMPACT project: Eco-friendly and non-hazardous coatings for the protection of outdoor bronzes. IOP Conf. Ser. Mater. Sci. Eng. 2020, 949, 012097. [Google Scholar] [CrossRef]
- Letardi, P.; Ramirez-Barat, B.; Albini, M.; Traverso, P.; Cano, E.; Joseph, E. Copper alloys and weathering steel used in outdoor monuments: Available online: Weathering in an urban-marine environment. In Metal 2016, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, New Delhi, India, 26–30 September 2016; Menon, R., Chemello, C., Pandya, A., Eds.; ICOM Committee for Conservation: Paris, France, 2016; pp. 320–328. ISBN 978-92-9012-418-4. [Google Scholar]
- Letardi, P. Laboratory and field test on patinas and protective coating systems for outdoor bronze Monuments. In Metal 2004, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Canberra, Australia, 4–8 October 2004; Ashton, J., Hallam, D., Eds.; National Museum of Australia: Canberra, Australia, 2004; pp. 379–387. [Google Scholar]
- Franceschi, E.; Letardi, P.; Luciano, G. Colour measurements on patinas and coating system for outdoor bronze monuments. J. Cult. Herit. 2006, 7, 166–170. [Google Scholar] [CrossRef]
- Sansonetti, A.; Colella, M.; Letardi, P.; Salvadori, B.; Striova, J. Laser cleaning of a nineteenth-century bronze sculpture: In situ multi-analytical evaluation. Stud. Conserv. 2015, 60, S28–S33. [Google Scholar] [CrossRef]
- Ramírez Barat, B.; Crespo, A.; García, E.; Díaz, S.; Cano, E. An EIS study of the conservation treatment of the bronze sphinxes at the Museo Arqueológico Nacional (Madrid). J. Cult. Herit. 2017, 24, 93–99. [Google Scholar] [CrossRef]
- Bruni, T.; Mariani, B.; Salvadori, B.; Letardi, P. A multi-analytical approach to evaluate surface treatments on copper-alloy artefacts: A case study applied to the restoration of the memorial of redipuglia. In Metal 2019, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Neuchâtel, Switzerland, 2–6 September 2019; Chemello, C., Brambilla, L., Joseph, E., Eds.; ICOM-CC and HE-Arc CR: Neuchatel, Switzerland, 2019; pp. 92–99. ISBN 978-92-9012-457-3. [Google Scholar]
- Giuntoli, G.; Rosi, L.; Frediani, M.; Sacchi, B.; Salvadori, B.; Porcinai, S.; Frediani, P. Novel coatings from renewable resources for the protection of bronzes. Prog. Org. Coat. 2014, 77, 892–903. [Google Scholar] [CrossRef]
- Kosec, T.; Škrlep, L.; Švara Fabjan, E.; Sever Škapin, A.; Masi, G.; Bernardi, E.; Chiavari, C.; Josse, C.; Esvan, J.; Robbiola, L. Development of multi-component fluoropolymer based coating on simulated outdoor patina on quaternary bronze. Prog. Org. Coat. 2019, 131, 27–35. [Google Scholar] [CrossRef]
- Wolfe, J.; Grayburn, R. A review of the development and testing of Incralac lacquer. J. Am. Inst. Conserv. 2017, 56, 225–244. [Google Scholar] [CrossRef]
- Wolfe, J.; Grayburn, R.; Khanjian, H.; Heginbotham, A.; Phenix, A. Deconstructing Incralac: A formulation study of acrylic coatings for the protection of outdoor bronze sculpture. In Proceedings of the ICOM-CC 18th Triennial Conference, Copenhagen, Denmark, 4–8 September 2017; Bridgland, J., Ed.; International Council of Museums: Paris, France, 2017. [Google Scholar]
- Ramirez-Barat, B.; Letardi, P.; Cano, E. An overview of the use of EIS measurements for the assessment of patinas and coatings in the conservation of metallic cultural heritage. In Metal 2019, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Neuchâtel, Switzerland, 2–6 September 2019; Chemello, C., Brambilla, L., Joseph, E., Eds.; ICOM-CC and HE-Arc CR: Neuchatel, Switzerland, 2019; pp. 83–91. ISBN 978-92-9012-457-3. [Google Scholar]
- Swartz, N.; Lasseter Clare, T. On the protective nature of wax coatings for culturally significant outdoor metalworks: Microstructural flaws, oxidative changes, and barrier properties. J. Am. Inst. Conserv. 2015, 54, 181–201. [Google Scholar] [CrossRef]
- Jáuregui-Gónzalez, D.A.; López-Arriaga, M.; Rodríguez-Gómez, F.J.; Contreras-Vargas, J.; Roncagliolo-Barrera, P. Influence of the application and preparation of wax coatings on artificially patinated bronze surfaces. In Metal 2016, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, New Delhi, India, 26–30 September 2016; Menon, R., Chemello, C., Pandya, A., Eds.; ICOM Committee for Conservation: Paris, France, 2016; pp. 170–177. ISBN 978-92-9012-418-4. [Google Scholar]
- Masi, G.; Bernardi, E.; Martini, C.; Vassura, I.; Skrlep, L.; Švara Fabjan, E.; Gartner, N.; Kosec, T.; Josse, C.; Esvan, J.; et al. An innovative multi-component fluoropolymer-based coating on outdoor patinated bronze for cultural heritage: Durability and reversibility. J. Cult. Herit. 2020, 45, 122–134. [Google Scholar] [CrossRef]
- Texier, A.; Geffroy, A.M.; Syvilay, D.; Brocard-Rosa, T. Les cires microcristallines dans la protection de la statuaire en cuivre et alliages de cuivre exposée en extérieur. In Métal à Ciel Ouvert: La Sculpture Métallique D’extérieur du XIXe au Début du XXe Siècle: Identification, Conservation, Restauration; International Institute for Conservation of Historic and Artistic Works; Section Française: Champs-sur-Marne, France, 2014; pp. 163–175. ISBN 9782905430182. [Google Scholar]
- Kosec, T.; Legat, A.; Milošev, I. The comparison of organic protective layers on bronze and copper. Prog. Org. Coat. 2010, 69, 199–206. [Google Scholar] [CrossRef]
- Chiavari, C.; Bernardi, E.; Cauzzi, D.; Volta, S.; Chiara Bignozzi, M.; Lenza, B.; Montalbani, S.; Robbiola, L.; Martini, C. Influence of natural patinas of outdoor quaternary bronzes on conservation treatments. In Metal 2013, Proceedings of the Interim Meeting of the ICOM-CC Metal Working Group, Edinburgh, UK, 16–20 September 2013; Hyslop, E., Gonzalez, V., Troalen, L., Wilson, L., Eds.; Historic Scotland: Edinburgh, UK; pp. 163–172. ISBN 978-1-84917-132-8.
- Otieno-Alego, V.; Hallam, D.; Heath, G.; Creagh, D. Electrochemical evaluation of the anti-corrosion performance of waxy coatings for outdoor bronze conservation. In Metal 98, Proceedings of the International Conference on Metals Conservation, Draguignan-Figanières, France, 27–29 May 1998; Mourey, W., Robbiola, L., Eds.; James & James: London, UK, 1998; pp. 309–314. [Google Scholar]
- Otieno-Alego, V.; Hallam, D.; Viduka, A.; Heath, G.; Creagh, D. Electrochemical impedance studies of the corrosion resistance of wax coatings on artificially patinated bronze. In Metal 98, Proceedings of the International Conference on Metals Conservation, Draguignan-Figanières, France, 27–29 May 1998; Mourey, W., Robbiola, L., Eds.; James & James: London, UK, 1998; pp. 315–319. [Google Scholar]
- Mohamed, W.A.; Rifai, M.M.; Abdel Ghany, N.A.; Elmetwaly, M.S. Testing coating systems for bare and patinated outdoor bronze sculptures. In Metal 2016, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, New Delhi, India, 26–30 September 2016; Menon, R., Chemello, C., Pandya, A., Eds.; ICOM Committee for Conservation: Paris, France, 2016; pp. 161–169. [Google Scholar]
- Otieno-Alego, V.; Creagh, D.; Hallam, D.; Viduka, A.; Heath, G. In situ and laboratory studies of the ageing of protective wax coatings on metal surfaces of museum objects and outdoor statues. In Ageing Studies and Lifetime Extension of Materials; Mallinson, L.G., Ed.; Springer: Boston, MA, USA, 2001; pp. 609–618. [Google Scholar] [CrossRef]
- Popoola, L.T. Organic green corrosion inhibitors (OGCIs): A critical review. Corros. Rev. 2019, 37, 71–102. [Google Scholar] [CrossRef]
- Varvara, S.; Caniglia, G.; Izquierdo, J.; Bostan, R.; Găină, L.; Bobis, O.; Souto, R.M. Multiscale electrochemical analysis of the corrosion control of bronze in simulated acid rain by horse-chestnut (Aesculus hippocastanum L.) extract as green inhibitor. Corros. Sci. 2020, 165, 108381. [Google Scholar] [CrossRef]
- Faltermeier, R.B. A corrosion inhibitor test for copper-based artifacts. Stud. Conserv. 1999, 44, 121–128. [Google Scholar] [CrossRef]
- Boccia Paterakis, A. Conservation: Preservation versus analysis? Stud. Conserv. 1996, 41 (Suppl. 1), 143–148. [Google Scholar] [CrossRef]
- Zucchi, F. 24-Sol-gel coatings for the preservation of metallic heritage artefacts. In Corrosion and Conservation of Cultural Heritage Metallic Artefacts; EFS Series; Woodhead Publishing: Cambridge, UK, 2013; pp. 540–551. [Google Scholar] [CrossRef]
- Fassina, V. CEN TC 346 conservation of cultural heritage-update of the activity after a height year period. In Engineering Geology for Society and Territory; Lollino, G., Giordan, D., Marunteanu, C., Christaras, B., Yoshinori, I., Margottini, C., Eds.; Springer: Cham, Switzerland, 2015; Volume 8. [Google Scholar] [CrossRef]
- Argyropoulos, V.; Boyatzis, S.; Giannoulaki, M.; Polikreti, K. 22-The role of standards in conservation methods for metals in cultural heritage. In Corrosion and Conservation of Cultural Heritage Metallic Artefacts; EFS Series; Woodhead Publishing: Cambridge, UK, 2013; pp. 478–517. [Google Scholar] [CrossRef]
- Letardi, P.; Stifanese, R.; Traverso, P. A difficult balance between simple and effective: Some specific issues in the evaluation of protective coatings for cultural heritage metallic surfaces. Eurocorr 2017. [Google Scholar] [CrossRef]
- Contreras-Vargas, J.; Peñuelas-Guerrero, G.; López-Arriaga, I.M.; García-Abajo, Á. Metal patina, patina loss and repatination criteria: The case of “El Caballito”. In Métal à Ciel Ouvert: La Sculpture Métallique D’extérieur du XIXe au Début du XXe Siècle: Identification, Conservation, Restauration; International Institute for Conservation of Historic and Artistic Works; Section Française: Champs-sur-Marne, France, 2014; pp. 206–213. ISBN 9782905430182. [Google Scholar]
- Masi, G.; Balbo, A.; Esvan, J.; Monticelli, C.; Avila, J.; Robbiola, L.; Bernardi, E.; Bignozzi, M.C.; Asensio, M.C.; Martini, C.; et al. X-ray photoelectron spectroscopy as a tool to investigate silane-based coatings for the protection of outdoor bronze: The role of alloying elements. Appl. Surf. Sci. 2018, 433, 468–479. [Google Scholar] [CrossRef]
- Salvadori, B.; Cagnini, A.; Galeotti, M.; Porcinai, S.; Goidanich, S.; Vicenzo, A.; Celi, C.; Frediani, P.; Rosi, L.; Frediani, M.; et al. Traditional and innovative protective coatings for outdoor bronze: Application and performance comparison. J. Appl. Polym. Sci. 2017, 135, 46011. [Google Scholar] [CrossRef]
- Chiavari, C.; Balbo, A.; Bernardi, E.; Martini, C.; Zanotto, F.; Vassura, I.; Bignozzi, M.C.; Monticelli, C. Organosilane coatings applied on bronze: Influence of UV radiation and thermal cycles on the protectiveness. Prog. Org. Coat. 2015, 82, 91–100. [Google Scholar] [CrossRef]
- L’héronde, M.; Bouttemy, M.; Mercier-Bion, F.; Neff, D.; Apchain, E.; Etcheberry, A.; Dillmann, P. Multiscale study of interactions between corrosion products layer formed on heritage Cu objects and organic protection treatments. Heritage 2019, 2, 2640–2651. [Google Scholar] [CrossRef]
- Marušić, K.; Otmačić-Ćurković, H.; Horvat-Kurbegović, Š.; Takenouti, H.; Stupnišek-Lisac, E. Comparative studies of chemical and electrochemical preparation of artificial bronze patinas and their protection by corrosion inhibitor. Electrochim. Acta 2009, 54, 7106–7113. [Google Scholar] [CrossRef]
- Masi, G.; Josse, C.; Esvan, J.; Chiavari, C.; Bernardi, E.; Martini, C.; Bignozzi, M.C.; Monticelli, C.; Zanotto, F.; Balbo, A.; et al. Evaluation of the protectiveness of an organosilane coating on patinated Cu–Si–Mn bronze for contemporary art. Prog. Org. Coat. 2019, 127, 286–299. [Google Scholar] [CrossRef]
- Masi, G.; Esvan, J.; Josse, C.; Chiavari, C.; Bernardi, E.; Martini, C.; Bignozzi, M.C.; Gartner, N.; Kosec, T.; Robbiola, L. Characterization of typical patinas simulating bronze corrosion in outdoor conditions. Mater. Chem. Phys. 2017, 200, 308–321. [Google Scholar] [CrossRef]
- Stifanese, R.; Letardi, P.; Traverso, P. GEMS-genoa experimental marine station: A tool for research activity and technology transfer. In Proceedings of the EUROCORR 2017-The Annual Congress of the European Federation of Corrosion, 20th International Corrosion Congress and Process Safety Congress 2017, Prague, Czech Republic, 3–7 September 2017. [Google Scholar]
- Albini, M.; Comensoli, L.; Brambilla, L.; Domon Beuret, E.; Kooli, W.; Mathys, L.; Letardi, P.; Joseph, E. Innovative biological approaches for metal conservation. Mater. Corros. 2016, 67, 200–206. [Google Scholar] [CrossRef]
- Chang, T.; Herting, G.; Goidanich, S.; Sánchez Amaya, J.M.; Arenas, M.A.; Le Bozec, N.; Jin, Y.; Leygraf, C.; Odnevall Wallinder, I. The role of Sn on the long-term atmospheric corrosion of binary Cu–Sn bronze alloys in architecture. Corros. Sci. 2019, 149, 54–67. [Google Scholar] [CrossRef]
- Aucouturier, M.; Darque-Ceretti, E. The surface of cultural heritage artefacts: Physicochemical investigations for their knowledge and their conservation. Chem. Soc. Rev. 2007, 36, 1605–1621. [Google Scholar] [CrossRef]
- Madariaga, J.M. Analytical chemistry in the field of cultural heritage. Anal. Methods 2015, 7, 4848–4876. [Google Scholar] [CrossRef]
- Letardi, P.; Beccaria, A.M.; Marabelli, M.; D’Ercoli, G. Application of electrochemical impedence measurements as a tool for the characterization and protection state of bronze works of art. In Metal 98, Proceedings of the International Conference on Metals Conservation, Draguignan-Figanières, France, 27–29 May 1998; Mourey, W., Robbiola, L., Eds.; James & James: London, UK, 1998; pp. 303–308. [Google Scholar]
- Catelli, E.; Sciutto, G.; Prati, S.; Jia, Y.; Mazzeo, R. Characterization of outdoor bronze monument patinas: The potentialities of near-infrared spectroscopic analysis. Environ. Sci. Pollut. Res. 2018, 25, 24379–24393. [Google Scholar] [CrossRef]
- Letardi, P. Electrochemical measurements in the conservation of metallic heritage artefacts: An overview. In Corrosion and Conservation of Cultural Heritage Metallic Artefacts; Dillmann, P., Watkinson, D., Angelini, E., Adriaens, A., Eds.; (EFC) Series; Woodhead Publishing: Cambridge, UK, 2013; pp. 126–148. ISBN 9781782421542. [Google Scholar] [CrossRef]



| Metal | Mineral | Formula | Approximate Date of First Widespread Use | Typical Colours of Corrosion Products |
|---|---|---|---|---|
| aluminium | gibbsite | Al(OH)3 | 1800–1900 A.D. (Europe/USA) | colourless or white |
| copper | chalcocite | Cu2S | ~7000 B.C. (Near East) for native copper~ 5000 B.C. (Near East) for smelted copper | Cu(I): red, black, colourless Cu(II): green, blue |
| gold | (native) | Au | 5000–4000 B.C. (Balkans) | – |
| iron | hematite | Fe2O3 | 1000–0 B.C. (Near East) | Fe(I,III): black Fe(III): red, yellow, orange |
| lead | galena | PbS | 6000–5000 B.C. (Near East/Balkans) | white, red yellow |
| nickel | pentlandite | (Ni,Fe)9S8 | 2000–1000 B.C. (Near East) for copper/nickel alloys | green |
| silver | argentite | Ag2S | 4000–3000 B.C. (Balkans/Near East) | black, white |
| tin | cassiterite | SnO2 | 4000–3000 B.C. (Near East) | black, white |
| zinc | smithsonite | ZnCO3 | 100–200 A.D. (Rome) for copper/zinc alloys 900–1000 A.D. (India) for zinc metal | colourless or white |
| Construction Step | Description |
|---|---|
| Forming and Shaping | production by pouring liquid metals into moulds (casting) and by mechanical deformation (forging, rolling; working such as milling, turning, spinning, grinding, stamping, cutting, drilling |
| Assembling | fitting components by welding, soldering, brazing, rivetting, bolting, crinping, gluing |
| Finishing | Completing surface appearance by plating, burnishing, polishing, etching, sand-blasting, painting, lacquering, engraving, chasing, embossing, enameling, patinating |
| Level | Properties | Characteristic Features | |
|---|---|---|---|
| Substrate | Alloy | elemental composition metallurgy | principal alloying element casting, rolling, structure |
| Initial surface finish | polishing, blasting foundry patina | surface roughness colour, composition | |
| Surface layer (patina) | origin composition stratigraphy texture appearance electrochemical properties | natural weathering/accelerated weathering/artificial principal inorganic and organic compounds layers, thickness porosity, morphology, surface roughness colour, gloss corrosion rate | |
| Treatment | composition application method texture appearance electrochemical properties | layers, intrinsic features drying time, adhesion, thickness porosity, morphology, surface roughness colour, gloss corrosion rate, inhibition efficacy | |
| weathering stability | UV and light stability stability against condensed moisture water repellent effect coating quality removability/retreatability | ||
| Exposure condition | rural/marine–rural/industrial/marine–industrial | atmospheric pollutants meteorology precipitation quality wet and dry deposition | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Letardi, P. Testing New Coatings for Outdoor Bronze Monuments: A Methodological Overview. Coatings 2021, 11, 131. https://doi.org/10.3390/coatings11020131
Letardi P. Testing New Coatings for Outdoor Bronze Monuments: A Methodological Overview. Coatings. 2021; 11(2):131. https://doi.org/10.3390/coatings11020131
Chicago/Turabian StyleLetardi, Paola. 2021. "Testing New Coatings for Outdoor Bronze Monuments: A Methodological Overview" Coatings 11, no. 2: 131. https://doi.org/10.3390/coatings11020131
APA StyleLetardi, P. (2021). Testing New Coatings for Outdoor Bronze Monuments: A Methodological Overview. Coatings, 11(2), 131. https://doi.org/10.3390/coatings11020131






