Superhydrophilic Coating of Pine Wood by Plasma Functionalization of Self-Assembled Polystyrene Spheres
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
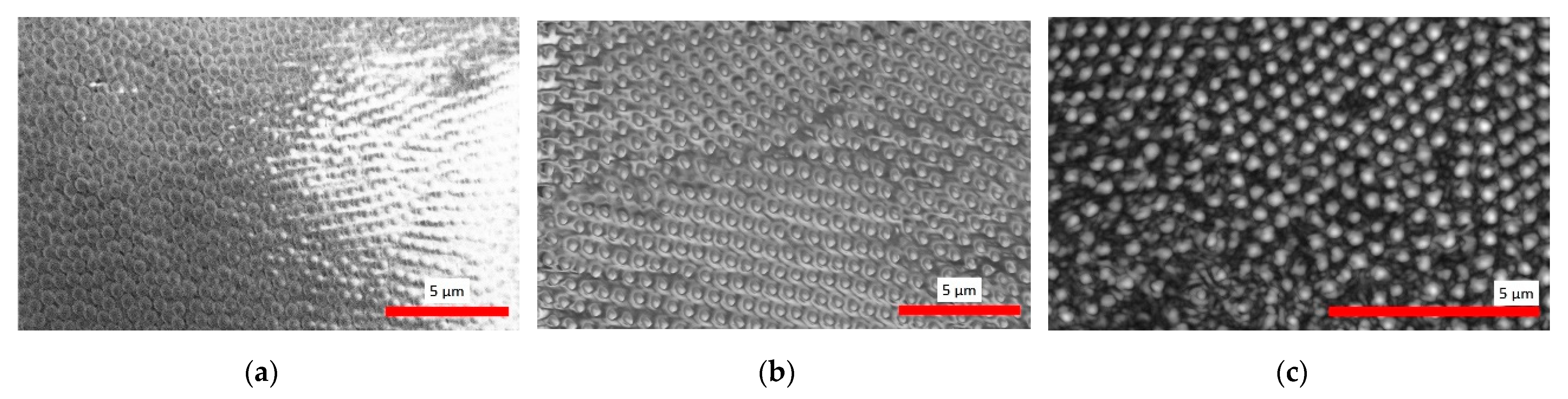
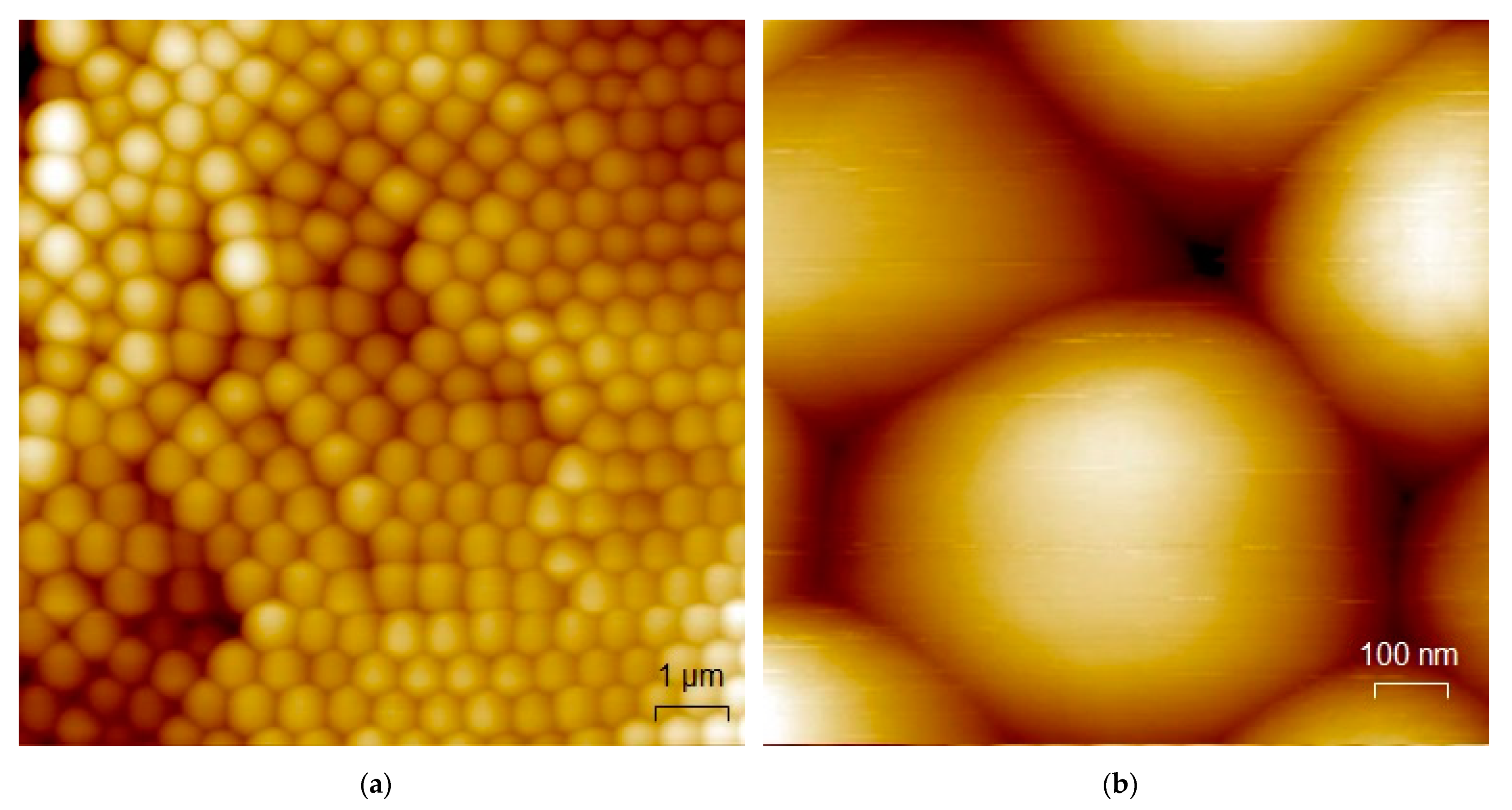
3.1. Microscopic Characterization
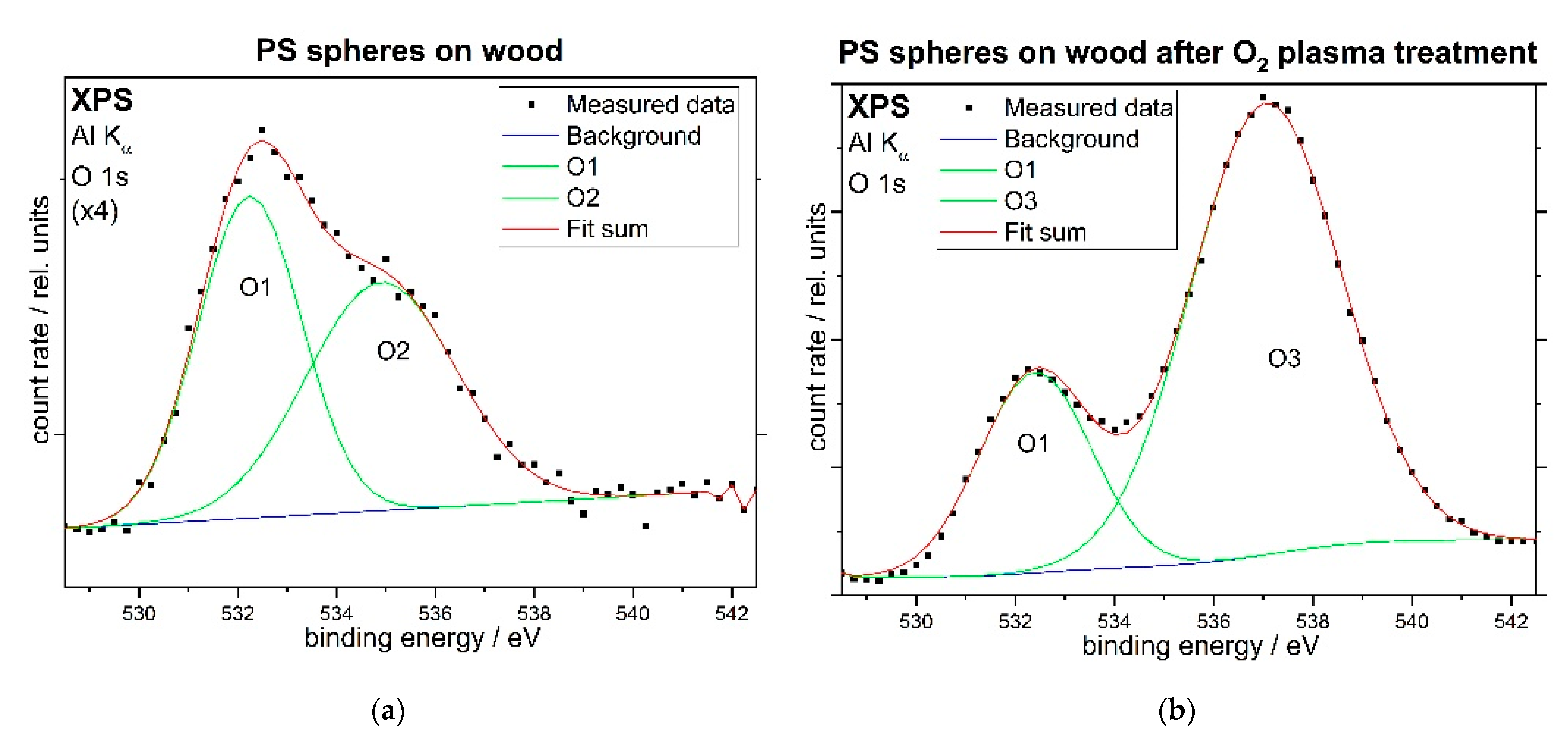
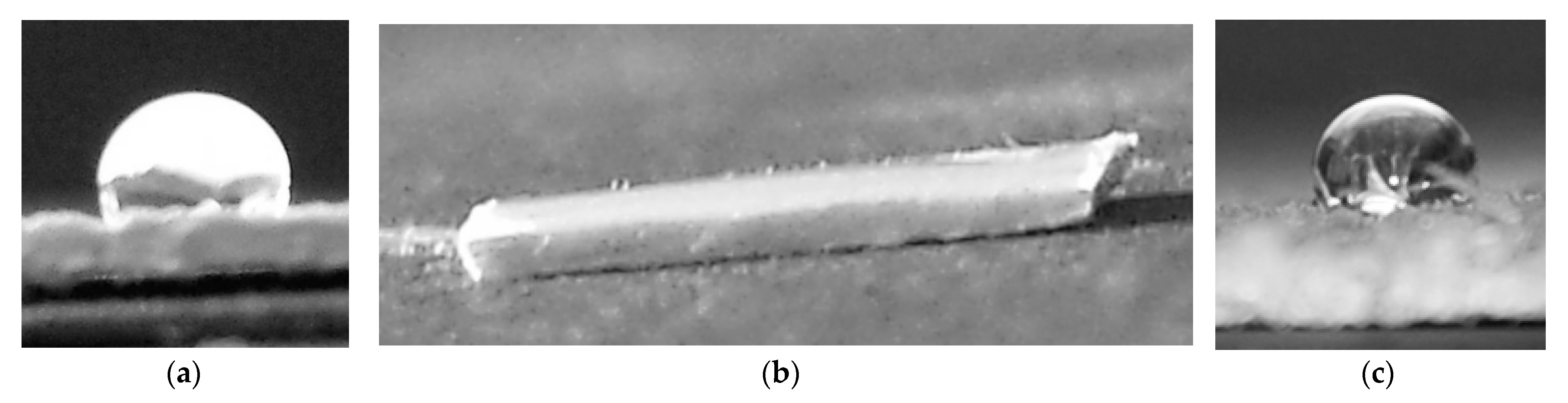
3.2. Spectroscopic Characterization
- Deposition of the self-assembling units
- Fixation of this colloidal film
- Simultaneous plasma etching and functionalization
- Attaching surfactant molecules
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagayama, K.; Dimitrov, A.S. Fabrication and Application of Particle-Crystalline Films. ACS Symp. Ser. 1996, 648, 468–489. [Google Scholar]
- Dimitrov, A.S.; Nagayama, K. Continuous Convective Assembling of Fine Particles into Two-Dimensional Arrays on Solid Surfaces. Langmuir 1996, 12, 1303–1311. [Google Scholar] [CrossRef]
- Sato, T.; Hasko, D.G.; Ahmed, H. Nanoscale colloidal particles: Monolayer organization and patterning. J. Vac. Sci. Technol. B 1997, 15, 45–48. [Google Scholar] [CrossRef]
- Jiang, P.; Bertone, J.F.; Hwang, K.S.; Colvin, V.L. Single-Crystal Colloidal Multilayers of Controlled Thickness. Chem. Mater. 1999, 11, 2132–2140. [Google Scholar] [CrossRef]
- Pillai, S.; Hemmersam, A.G.; Mukhopadhyay, R.; Meyer, R.L.; Moghimi, S.M.; Besenbacher, F.; Kingshott, P. Tunable 3D and 2D polystyrene nanoparticle assemblies using surface wettability, low volume fraction and surfactant effects. Nanotechnology 2009, 20, 025604. [Google Scholar] [CrossRef] [PubMed]
- Masson, J.-F.; Murray-Méthot, M.-P.; Live, L.S. Nanohole arrays in chemical analysis: Manufacturing methods and applications. Analyst 2010, 135, 1483–1489. [Google Scholar] [CrossRef]
- Singh, G.; Pillai, S.; Arpanaei, A.; Kingshott, P. Multicomponent colloidal crystals that are tunable over large areas. Soft Matter 2011, 7, 3290–3294. [Google Scholar] [CrossRef]
- Asher, S.A.; Alexeev, V.L.; Goponenko, A.V.; Sharma, A.C.; Lednev, I.K.; Wilcox, C.S.; Finegold, D.N. Photonic Crystal Carbohydrate Sensors: Low Ionic Strength Sugar Sensing. J. Am. Chem. Soc. 2003, 125, 3322–3329. [Google Scholar] [CrossRef]
- Holtz, J.H.; Asher, S.A. Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature 1997, 389, 829–832. [Google Scholar] [CrossRef]
- Xia, Y.; Gates, B.; Yin, Y.; Lu, Y. Monodispersed Colloidal Spheres: Old Materials with New Applications. Adv. Mater. 2000, 12, 693–713. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Baumberg, J.J.; Birkin, P.R.; Ghanem, M.A.; Netti, M.C. Highly Ordered Macroporous Gold and Platinum Films Formed by Electrochemical Deposition through Templates Assembled from Submicron Diameter Monodisperse Polystyrene Spheres. Chem. Mater. 2002, 14, 2199–2208. [Google Scholar] [CrossRef]
- Carstens, T.; Prowald, A.; El Abedin, S.Z.; Endres, F. Electrochemical synthesis of PEDOT and PPP macroporous films and nanowire architectures from ionic liquids. J. Solid State Electrochem. 2012, 16, 3479–3485. [Google Scholar] [CrossRef]
- Al Zoubi, M.; Al-Salman, R.; Li, Y.; Endres, F. Highly Ordered 3D-Macroporous Poly(Para-Phenylene) Films Made by Electropolymerization of Benzene in an Ionic Liquid. Z. Phys. Chem. 2011, 225, 393–403. [Google Scholar] [CrossRef]
- Nagayama, K. Two-dimensional self-assembly of colloids in thin liquid films. Colloid. Surf. A 1996, 109, 363–374. [Google Scholar] [CrossRef]
- Gasparotto, L.H.S.; Prowald, A.; Borisenko, N.; El Abedin, S.Z.; Garsuch, A.; Endres, F. Electrochemical synthesis of macroporous aluminium films and their behavior towards lithium deposition/stripping. J. Power Sour. 2011, 5, 2879–2883. [Google Scholar] [CrossRef]
- Bhawalkar, S.P.; Qian, J.; Heiber, M.C.; Jia, L. Development of a Colloidal Lithography Method for Patterning Nonplanar Surfaces. Langmuir 2010, 26, 16662–16666. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Barthlott, W. Diversity of structure, morphology and wetting of plant surfaces. Soft Matter 2008, 4, 1943–1963. [Google Scholar] [CrossRef]
- Solga, A.; Cerman, Z.; Striffler, B.F.; Spaeth, M.; Barthlott, W. The dream of staying clean: Lotus and biomimetic surfaces. Bioinspir. Biomim. 2007, 2, S126–S134. [Google Scholar] [CrossRef]
- Nosonovsky, M. Multiscale Roughness and Stability of Superhydrophobic Biomimetic Interfaces. Langmuir 2007, 23, 3157–3161. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Jung, Y.C.; Barthlott, W. Fabrication of artificial Lotus leaves and significance of hierarchical structure for superhydrophobicity and low adhesion. Soft Matter 2009, 5, 1386–1393. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing Superoleophobic Surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Mammen, L.; Butt, H.-J.; Vollmer, D. Candle Soot as a Template for a Transparent Robust Superamphiphobic Coating. Science 2012, 335, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Allard, D.; Lange, B.; Fleischhaker, F.; Zentel, R.; Wulf, M. Opaline effect pigments by spray induced self-assembly on porous substrates. Soft Mater. 2005, 3, 121–131. [Google Scholar] [CrossRef]
- Wang, C.; Lin, X.; Schäfer, C.G.; Hirsemann, S.; Ge, J. Spray Synthesis of Photonic Crystal Based Automotive Coatings with Bright and Angular-Dependent Structural Colors. Adv. Function. Mater. 2020, 134, 2008601. [Google Scholar] [CrossRef]
- Lange, B.; Fleischhaker, F.; Zentel, R. Functional 3D photonic films from polymer beads. Phys. Status Solidi A 2007, 204, 3618–3635. [Google Scholar] [CrossRef]
- Sprafke, A.N.; Schneevoigt, D.; Seidel, S.; Schweizer, S.L.; Wehrspohn, R.B. Automated spray coating process for the fabrication of large-area artificial opals on textured substrates. Opt. Express 2013, 21, A528. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, G.; Dong, J.; Wang, H.; Guo, Y.; Zhuo, X.; Li, Y. Facile, Scalable Spray-Coating of Stable Emulsion for Transparent Self-Cleaning Surface of Cellulose-Based Materials. ACS Sustain. Chem. Eng. 2018, 6, 11335–11344. [Google Scholar] [CrossRef]
- Kim, J.B.; Lee, S.Y.; Lee, J.M.; Kim, S.-H. Designing Structural-Color Patterns Composed of Colloidal Arrays. ACS Appl. Mater. Int. 2019, 11, 14485–14509. [Google Scholar] [CrossRef]
- Bermel, P.; Luo, C.; Zeng, L.; Kimerling, L.C.; Joannopoulos, J.D. Improving thin-film crystalline silicon solar cell efficiencies with photonic crystals. Opt. Express 2007, 15, 16986–17000. [Google Scholar] [CrossRef]
- Üpping, J.; Salzer, R.; Otto, M.; Beckers, T.; Steidl, L.; Zentel, R.; Carius, R.; Wehrspohn, R.B. Transparent conductive oxide photonic crystals on textured substrates. Photonic. Nanostruct. 2011, 9, 31–34. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Y.; Shen, X.; Sun, Q.; Jin, C. Candle soot nanoparticles decorated wood for efficient solar vapor generation. Sustain. Energy Fuels 2019, 4, 354–361. [Google Scholar] [CrossRef]
- Vogel, N.; Retsch, M.; Fustin, C.-A.; del Campo, A.; Jonas, U. Advances in Colloidal Assembly: The Design of Structure and Hierarchy in Two and Three Dimensions. Chem. Rev. 2015, 115, 6265–6311. [Google Scholar] [CrossRef] [PubMed]
- Edwards, E.; Wang, D.; Moehwald, H. Hierarchical Organization of Colloidal Particles: From Colloidal Crystallization to Supraparticle Chemistry. Macromol. Chem. Phys. 2007, 208, 439–445. [Google Scholar] [CrossRef]
- Seo, K.; Kim, M.; Kim, D.H. Candle-based process for creating a stable superhydrophobic surface. Carbon 2014, 68, 583–596. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Castano, C.E.; Bressler, A.H.; Abolghasemibizaki, M.; Mohammadi, R. Rapid synthesis of inherently robust and stable superhydrophobic carbon soot coatings. Appl. Surf. Sci. 2016, 369, 341–347. [Google Scholar] [CrossRef]
- Chang, H.; Tu, K.; Wang, X.; Liu, J. Fabrication of mechanically durable superhydrophobic wood surfaces using polydimethylsiloxane and silica nanoparticles. RSC Adv. 2015, 5, 30647–30653. [Google Scholar] [CrossRef]
- Lin, X.; Park, S.; Choi, D.; Heo, J.; Hong, J. Mechanically Durable Superhydrophobic PDMS-Candle Soot Composite Coatings with High Biocompatibility. J. Ind. Eng. Chem. 2019, 74, 79–85. [Google Scholar] [CrossRef]
- Tu, K.; Wang, X.; Kong, L.; Chang, H.; Liu, J. Fabrication of robust, damage-tolerant superhydrophobic coatings on naturally micro-grooved wood surfaces. RSC Adv. 2016, 6, 701–707. [Google Scholar] [CrossRef]
- Tu, K.; Kong, L.; Wang, X.; Liu, J. Semitransparent, durable superhydrophobic polydimethylsiloxane/SiO2 nanocomposite coatings on varnished wood. Holzforschung 2016, 70, 1039–1045. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Y.; Chu, Q.; Ma, C.; Cai, L.; Shen, Z.; Chen, H. Facile Construction of Superhydrophobic Surfaces by Coating Fluoroalkylsilane/Silica Composite on a Modified Hierarchical Structure of Wood. Polymers 2020, 12, 813. [Google Scholar] [CrossRef]
- Tu, K.; Wang, X.; Kong, L.; Guan, H. Facile preparation of mechanically durable, self-healing and multifunctional superhydrophobic surfaces on solid wood. Mater. Des. 2018, 140, 30–36. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Niu, H.; Zhao, Y.; Xu, Z.; Lin, T. A Waterborne Coating System for Preparing Robust, Self-healing, Superamphiphobic Surfaces. Adv. Funct. Mater. 2017, 27, 1604261. [Google Scholar] [CrossRef]
- Wu, X.; Liu, M.; Zhong, X.; Liu, G.; Wyman, I.; Wang, Z.; Wang, J. Smooth Water-Based Antismudge Coatings for Various Substrates. ACS Sustain. Chem. Eng. 2017, 5, 2605–2613. [Google Scholar] [CrossRef]
- Bao, W.; Jia, Z.; Cai, L.; Liang, D.; Li, J. Fabrication of a superamphiphobic surface on the bamboo substrate. Eur. J. Wood Wood Prod. 2018, 76, 1595–1603. [Google Scholar] [CrossRef]
- Bao, W.; Zhang, M.; Jia, Z.; Jiao, Y.; Cai, L.; Liang, D.; Li, J. Cu thin films on wood surface for robust superhydrophobicity by magnetron sputtering treatment with perfluorocarboxylic acid. Eur. J. Wood Wood Prod. 2019, 77, 115–123. [Google Scholar] [CrossRef]
- Wang, J.; Hu, J.; Wen, Y.; Song, Y.; Jiang, L. Hydrogen-Bonding-Driven Wettability Change of Colloidal Crystal Films: From Superhydrophobicity to Superhydrophilicity. Chem. Mater. 2006, 18, 4984–4986. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowksi, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and superhydrophilic surfaces and materials. Soft Matter 2011, 7, 9804–9828. [Google Scholar] [CrossRef]
- Svec, F.; Levkin, P.A.; Frechet, J.M.J. Superhydrophobic and Superhydrophilic Materials, Surfaces and Methods. EP2283067A2, 9 May 2008. [Google Scholar]
- Son, J.; Kundu, S.; Verma, L.K.; Sakhuja, M.; Danner, A.J.; Bhatia, C.S.; Yang, H. A practical superhydrophilic self cleaning and antireflective surface for outdoor photovoltaic applications. Sol. Energ. Mat. Sol. C. 2012, 98, 46–51. [Google Scholar] [CrossRef]
- Mantanis, G.I.; Young, R.A. Wetting of wood. Wood Sci. Technol. 1997, 31, 339. [Google Scholar] [CrossRef]
- De Meijer, M. A review of interfacial aspects in wood coatings: Wetting, surface energy, substrate penetration and adhesion. In Proceedings of the COST E18 Final Seminar, Paris, France, 26–27 May 2005. [Google Scholar]
- Petrič, M.; Knehtl, B.; Krause, A.; Militz, H.; Pavlič, M.; Pétrissans, M.; Rapp, A.; Tomažič, M.; Welzbacher, C.; Gérardin, P. Wettability of waterborne coatings on chemically and thermally modified pine wood. J. Coat. Technol. Res. 2007, 4, 203–206. [Google Scholar] [CrossRef]
- Stehr, M.; Gardner, D.J.; Wålinder, M.E.P. Dynamic Wettability of Different Machined Wood Surfaces. J. Adhes. 2001, 76, 185–200. [Google Scholar] [CrossRef]
- Davalos, J.F.; Qiao, P.; Trimble, B.S. Fiber-Reinforced Composite and Wood Bonded Interfaces: Part 1. Durability and Shear Strength. J. Composit. Technol. Res. 2000, 22, 224–231. [Google Scholar]
- Wood, J.R.; Bader, M.G. Void control for polymer-matrix composites (1): Theoretical and experimental methods for determining the growth and collapse of gas bubbles. Compos. Manuf. 2014, 5, 139–147. [Google Scholar] [CrossRef]
- NanoScan Lab—Scienta Omicron. Available online: https://scientaomicron.com/en/system-solutions/electron-spectroscopy/NanoScan-Lab (accessed on 23 December 2020).
- ISO 4287:1997. Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parameters; International Organisation for Standardization: Geneva, Switzerland, 1997. [Google Scholar]
- Frerichs, M.; Voigts, F.; Maus-Friedrichs, W. Fundamental processes of aluminium corrosion studied under ultra high vacuum conditions. Appl. Surf. Sci. 2006, 253, 950–958. [Google Scholar] [CrossRef]
- Masendorf, R.; Dahle, S.; Wegewitz, L.; Korte, S.; Lilienkamp, G.; Voigts, F.; Maus-Friedrichs, W. On the origin of fatigue corrosion cracking in Al7075. Materialwiss. Werkst. 2013, 44, 311–318. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater Subshell Photoionization Cross-Sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Powell, C.; Jablonski, A. Progress in quantitative surface analysis by X-ray photoelectron spectroscopy: Current status and perspectives. J. Electron. Spectrosc. Relat. Phenom. 2010, 178, 331–346. [Google Scholar] [CrossRef]
- Reilman, R.F.; Msezane, A.; Manson, S.T. Relative intensities in photoelectron spectroscopy of atoms and molecules. J. Electron. Spectrosc. Relat. Phenom. 1976, 8, 389–394. [Google Scholar] [CrossRef]
- Jablonski, A. Database of correction parameters for the elastic scattering effects in XPS. Surf. Interface Anal. 1995, 23, 29–37. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology Electron Inelastic-Mean-Free-Path Database 1.2. Available online: http://www.nist.gov/srd/nist71.cfm (accessed on 29 February 2012).
- Willert, A.; Prowald, A.; El Abedin, S.Z.; Höfft, O.; Endres, F. Electrodeposition of Lithium in Polystyrene Sphere Opal Structures on Copper from an Ionic Liquid. Aust. J. Chem. 2012, 65, 1507–1512. [Google Scholar] [CrossRef]
- Ferrand, P.; Minty, M.J.; Egen, M.; Ahopelto, J.; Zentel, R.; Romanov, S.G.; Torres, C.M.S. Micromoulding of three-dimensional photonic crystals on silicon substrates. Nanotechnology 2003, 14, 323–326. [Google Scholar] [CrossRef][Green Version]
- Wegewitz, L.; Dahle, S.; Höfft, O.; Voigts, F.; Viöl, W.; Endres, F.; Maus-Friedrichs, W. Plasma-oxidation of Ge(100) surfaces using a dielectric barrier discharge investigated by MIES, UPS and XPS. J. Appl. Phys. 2011, 110, 033302. [Google Scholar] [CrossRef]
- Kogelschatz, U. Dielectric-Barrier Discharges: Their History, Discharge Physics, and Industrial Applications. Plasma Chem. Plasma Process. 2003, 23, 1–46. [Google Scholar] [CrossRef]
- Dahle, S.; Hirschberg, J.; Viöl, W.; Maus-Friedrichs, W. Gas purification by the plasma-oxidation of a rotating sacrificial electrode. Plasma Sources Sci. Technol. 2015, 24, 035021. [Google Scholar] [CrossRef]
- Stalder, A.F.; Melchior, T.; Müller, M.; Sage, D.; Blu, T.; Unser, M. Low-bond axisymmetric drop shape analysis for surface tension and contact angle measurements of sessile drops. Colloid. Surf. A 2010, 364, 72–81. [Google Scholar] [CrossRef]
- Dushkin, C.D.; Nagayama, K.; Miwa, T.; Kralchevsky, P.A. Colored multilayers from transparent submicrometer spheres. Langmuir 1993, 9, 3695–3701. [Google Scholar] [CrossRef]
- Wegewitz, L.; Prowald, A.; Meuthen, J.; Dahle, S.; Höfft, O.; Endres, F.; Maus-Friedrichs, W. Plasma chemical and chemical functionalization of polystyrene colloidal systems. Phys. Chem. Chem. Phys. 2014, 16, 18261–18267. [Google Scholar] [CrossRef]
- Marlow, F.; Muldarisnur, M.; Sharifi, P.; Brinkmann, R.; Mendive, C. Opals: Status and prospects. Angew. Chem. Int. Ed. 2009, 48, 6212–6233. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers; John Wiley & Sons Ltd.: Chichester, UK, 1992. [Google Scholar]
- Lin, W.; Klein, J. Control of surface forces through hydrated boundary layers. Curr. Opin. Colloid Interface Sci. 2019, 44, 94–106. [Google Scholar] [CrossRef]



| Marking | Correlation | Binding Energy | Chemical Shift |
|---|---|---|---|
| C0 | aromatic | 284.8 eV | −0.2 eV |
| C1 | aliphatic | 285.0 eV | +0.0 eV |
| C2 | C–O | 286.5 eV | +1.5 eV |
| C3 | C=O, O–C–O | 287.8 eV | +2.8 eV |
| C4 | O–C=O | 288.8 eV | +3.8 eV |
| Sample | Region | Fraction | Binding Energy | FWHM | Rel. Intensity | Correlation | Mark |
|---|---|---|---|---|---|---|---|
| Before plasma | C 1s | 0.94 | 287.1 eV | 2.35 eV | 0.75 | aromatic | C0 |
| 287.4 eV | 2.58 eV | 0.25 | aliphatic | C1 | |||
| O 1s | 0.06 | 532.2 eV | 2.47 eV | 0.50 | Mo–O | O1 | |
| 534.9 eV | 3.55 eV | 0.50 | Mo–CO3 | O2 | |||
| After plasma | C 1s | 0.69 | 288.7 eV | 2.73 eV | 0.23 | aromatic | C0 |
| 288.9 eV | 3.00 eV | 0.54 | aliphatic | C1 | |||
| 290.4 eV | 2.90 eV | 0.05 | C–O | C2 | |||
| 291.8 eV | 3.00 eV | 0.12 | C=O, O–C–O | C3 | |||
| 294.3 eV | 3.00 eV | 0.05 | O–C=O | C4 | |||
| O 1s | 0.31 | 532.4 eV | 2.65 eV | 0.25 | Mo–O | O1 | |
| 537.1 eV | 3.57 eV | 0.75 | C–O | O3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahle, S.; Meuthen, J.; Gustus, R.; Prowald, A.; Viöl, W.; Maus-Friedrichs, W. Superhydrophilic Coating of Pine Wood by Plasma Functionalization of Self-Assembled Polystyrene Spheres. Coatings 2021, 11, 114. https://doi.org/10.3390/coatings11020114
Dahle S, Meuthen J, Gustus R, Prowald A, Viöl W, Maus-Friedrichs W. Superhydrophilic Coating of Pine Wood by Plasma Functionalization of Self-Assembled Polystyrene Spheres. Coatings. 2021; 11(2):114. https://doi.org/10.3390/coatings11020114
Chicago/Turabian StyleDahle, Sebastian, John Meuthen, René Gustus, Alexandra Prowald, Wolfgang Viöl, and Wolfgang Maus-Friedrichs. 2021. "Superhydrophilic Coating of Pine Wood by Plasma Functionalization of Self-Assembled Polystyrene Spheres" Coatings 11, no. 2: 114. https://doi.org/10.3390/coatings11020114
APA StyleDahle, S., Meuthen, J., Gustus, R., Prowald, A., Viöl, W., & Maus-Friedrichs, W. (2021). Superhydrophilic Coating of Pine Wood by Plasma Functionalization of Self-Assembled Polystyrene Spheres. Coatings, 11(2), 114. https://doi.org/10.3390/coatings11020114






