RETRACTED: High-Sensitivity Biosensor Based on Glass Resonance PhC Cavities for Detection of Blood Component and Glucose Concentration in Human Urine
Abstract
1. Introduction
2. Optical Biosensor
3. Materials and Methods
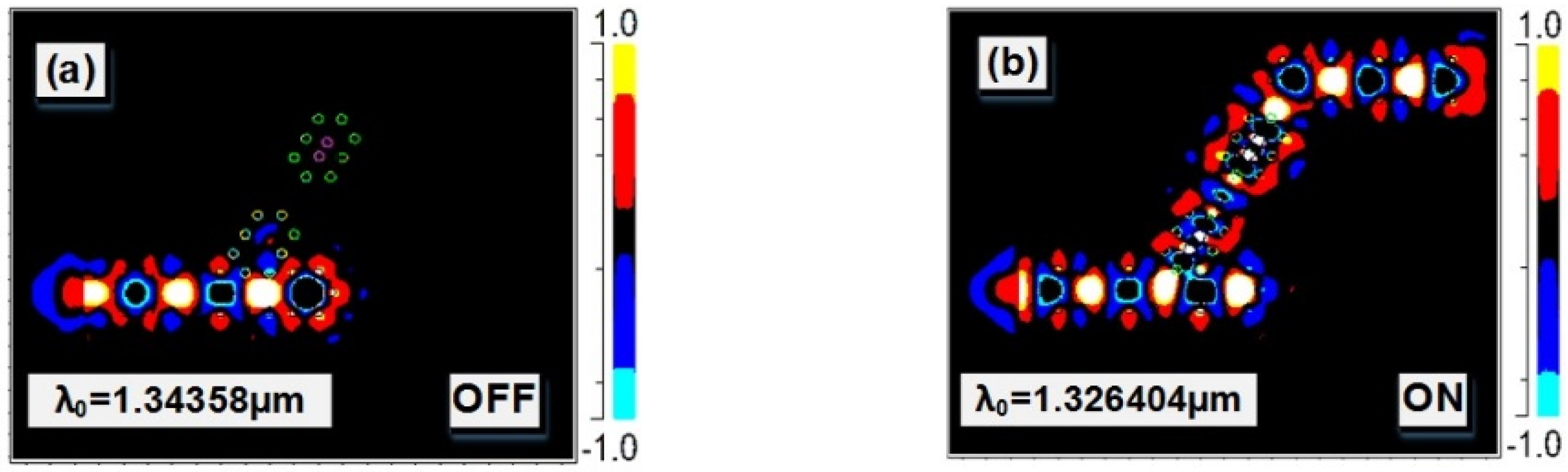
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, C.H.; Park, J.S. An SDN-Based Packet Scheduling Scheme for Transmitting Emergency Data in Mobile Edge Computing Environments. Hum. Cent. Comput. Inf. Sci. 2021, 11, 28. [Google Scholar] [CrossRef]
- Baek, S.; Jeon, J.; Jeong, B.; Jeong, Y.S. Two-Stage Hybrid Malware Detection Using Deep Learning. Human-centric Computing and Information Sciences volume. Hum. Cent. Comput. Inf. Sci. 2021, 11, 27. [Google Scholar] [CrossRef]
- Dai, H.; Li, J.; Kuang, Y.; Liao, J.; Zhang, Q.; Kang, Y. Multiscale Fuzzy Entropy and PSO-SVM Based Fault Diagnoses for Airborne Fuel Pumps. Hum. Cent. Comput. Inf. Sci. 2021, 11, 25. [Google Scholar] [CrossRef]
- Maqsood, M.; Mehmood, I.; Kharel, R.; Muhammad, K.; Lee, J.; Alnumay, W.S. Exploring the Role of Deep Learning in Industrial Applications: A Case Study on Coastal Crane Casting Recognition. Human Centric Computing and Information Sciences. Hum. Cent. Comput. Inf. Sci. 2021, 11, 20. [Google Scholar]
- Salim, M.M.; Shanmuganathan, V.; Loia, V.; Park, J.H. Deep Learning Enabled Secure IoT Handover Authentication for Blockchain Networks. Hum. Cent. Comput. Inf. Sci. 2021, 11, 21. [Google Scholar] [CrossRef]
- Blundo, C.; De Maio, C.; Parente, M.; Siniscalchi, L. Targeted Advertising That Protects the Privacy of Social Networks Users. Hum. Cent. Comput. Inf. Sci. 2021, 11, 18. [Google Scholar] [CrossRef]
- Liu, C.; Li, K.; Li, K. A Game Approach to Multi-Servers Load Balancing with Load-Dependent Server Availability Consideration. IEEE Trans. Cloud Comput. 2021, 9, 1–13. [Google Scholar] [CrossRef]
- Liu, C.; Li, K.; Li, K.; Buyya, R. A New Service Mechanism for Profit Optimizations of a Cloud Provider and Its Users. IEEE Trans. Cloud Comput. 2021, 9, 14–26. [Google Scholar] [CrossRef]
- Xiao, G.; Li, K.; Chen, Y.; He, W.; Albert, Y.; Zomaya, T.L. CASpMV: A Customized and Accelerative SpMV Framework for the Sunway TaihuLight. IEEE Trans. Parallel Distrib. Syst. 2021, 32, 131–146. [Google Scholar] [CrossRef]
- Duan, M.; Li, K.; Li, K.; Tian, Q. A Novel Multi-Task Tensor Correlation Neural Network for Facial Attribute Prediction. ACM Trans. Intell. Syst. Technol. 2021, 12, 1–22. [Google Scholar] [CrossRef]
- Chen, C.; Li, K.; Teo, S.G.; Zou, X.; Li, K.; Zeng, Z. Citywide Traffic Flow Prediction Based on Multiple Gated Spatio-temporal Convolutional Neural Networks. ACM Trans. Knowl. Discov. Data 2020, 14, 42:1–42:23. [Google Scholar] [CrossRef]
- Zhou, X.; Li, K.; Yang, Z.; Gao, Y.; Li, K. Efficient Approaches to k Representative G-Skyline Queries. ACM Trans. Knowl. Discov. Data 2020, 14, 58:1–58:27. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Liu, C.; Luo, X. Characterization of the Complete Mitochondrial Genome of Acanthacorydalis fruhstorferi van der Weele (Megaloptera: Corydalidae). J. Kans. Èntomol. Soc. 2020, 93, 267–281. [Google Scholar] [CrossRef]
- Sun, J.; Du, H.; Chen, Z.; Wang, L.; Shen, G. MXene Quantum Dot within Natural 3D Watermelon Peel Matrix for Biocompatible Flexible Sensing Platform. Nano Res. 2021, 3, 1–7. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, H.; Guo, X.; Peng, L.; Ding, Y.; Tang, J.; Guo, F. MK-FSVM-SVDD: A Multiple Kernel-based Fuzzy SVM Model for Predicting DNA-binding Proteins via Support Vector Data Description. Curr. Bioinform. 2021, 16, 274–283. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, F.; Chen, H.; Tan, H.; Yang, L.; Zhang, L.; Xie, J.; Sun, J.; Huang, X.; Huang, Y. Postnatal Exposure to DINP Was Associated with Greater Alterations of Lipidomic Markers for Hepatic Steatosis Than Dehp in Postweaning Mice. Sci. Total Environ. 2020, 758, 143631. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Meng, L.; Chen, X.; Chen, X.; Su, L.; Yuan, L.; Shi, W.; Huang, G. A Strategy to Significantly Improve the Classification Accuracy of LIBS Data: Application for the Determination of Heavy Metals in Tegillarca Granosa. Plasma Sci. Technol. 2021, 23, 085503. [Google Scholar] [CrossRef]
- Yan, Y.; Feng, L.; Shi, M.; Cui, C.; Liu, Y. Effect of Plasma-Activated Water on the Structure and in Vitro Digestibility of Waxy and Normal Maize Starches during Heat-Moisture Treatment. Food Chem. 2019, 306, 125589. [Google Scholar] [CrossRef]
- Shi, M.; Wang, F.; Lan, P.; Zhang, Y.; Zhang, M.; Yan, Y.; Liu, Y. Effect of Ultrasonic Intensity on Structure and Properties of Wheat Starch-Monoglyceride Complex and Its Influence on Quality of Norther-Style Chinese Steamed Bread. LWT 2020, 138, 110677. [Google Scholar] [CrossRef]
- Prather, D.W.; Shi, S.; Murakowski, J.; Schneider, G.J.; Sharkawy, A.; Chen, C.; Miao, B.; Martin, R. Self-Collimation in Photonic Crystal Structures: A New Paradigm for Applications and Device Development. J. Phys. D Appl. Phys. 2007, 40, 2635–2651. [Google Scholar] [CrossRef]
- Duan, M.; Li, K.; Ouyang, A.; Win, K.N.; Li, K.; Tian, Q. EGroupNet: A Feature-Enhanced Network for Age Estimation with Novel Age Group Schemes. ACM Trans. Multim. Comput. Commun. Appl. 2020, 16, 1–23. [Google Scholar] [CrossRef]
- Yang, W.; Li, K.; Li, K. A Pipeline Computing Method of SpTV for Three-Order Tensors on CPU and GPU. ACM Trans. Knowl. Discov. Data 2019, 13, 1–27. [Google Scholar] [CrossRef]
- Zhou, X.; Li, K.; Yang, Z.; Xiao, G.; Li, K. Progressive Approaches for Pareto Optimal Groups Computation. IEEE Trans. Knowl. Data Eng. 2019, 31, 521–534. [Google Scholar] [CrossRef]
- Mei, J.; Li, K.; Tong, Z.; Li, Q.; Li, K. Profit Maximization for Cloud Brokers in Cloud Computing. IEEE Trans. Parallel Distrib. Syst. 2019, 30, 190–203. [Google Scholar] [CrossRef]
- Chen, Y.; Li, K.; Yang, W.; Xiao, G.; Xie, X.; Li, T. Performance-Aware Model for Sparse Ma-Trix-Matrix Multiplication on the Sunway TaihuLight Supercomputer. IEEE Trans. Parallel Distrib. Syst. 2019, 30, 923–938. [Google Scholar] [CrossRef]
- Chen, J.; Li, K.; Bilal, K.; Zhou, X.; Li, K.; Philip, S.Y. A Bi-layered Parallel Training Architecture for Large-Scale Convolutional Neural Networks. IEEE Trans. Parallel Distrib. Syst. 2019, 30, 965–976. [Google Scholar] [CrossRef]
- Liu, C.; Li, K.; Liang, J.; Li, K. Service Reliability in an HC: Considering from the Perspective of Scheduling with Load-Dependent Machine Reliability. IEEE Trans. Reliab. 2019, 68, 476–495. [Google Scholar] [CrossRef]
- Chen, C.; Li, K.; Ouyang, A.; Li, K. FlinkCL: An OpenCL-Based in-Memory Computing Architecture on Heteroge-neous CPU-GPU Clusters for Big Data. IEEE Trans. Comput. 2018, 67, 1765–1779. [Google Scholar] [CrossRef]
- Duan, M.; Li, K.; Li, K. An Ensemble CNN2ELM for Age Estimation. IEEE Trans. Inf. Forensics Secur. 2018, 13, 758–772. [Google Scholar] [CrossRef]
- John, S.G.; Joannopoulos, D.; Winn, J.N.; Meade, R.D. Photonic Crystals: Molding the Flow of Light; InPrinceton University of Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Kassa-Baghdouche, L.; Cassan, E. Mid-Infrared Refractive Index Sensing Using Optimized Slotted Photonic Crystal Waveguides. Photon. Nanostruct. Fundam. Appl. 2018, 28, 32–36. [Google Scholar] [CrossRef]
- Nakamura, T.; Takahashi, Y.; Tanaka, Y.; Asano, T.; Noda, S. Improvement in the Quality Factors for Photonic Crystal Nanocavities via Visualization of the Leaky Components. Opt. Express 2016, 24, 9541–9549. [Google Scholar] [CrossRef] [PubMed]
- Kassa-Baghdouche, L.; Boumaza, T.; Cassan, E.; Bouchemat, M. Enhancement of Q-Factor in SiN-Based Planar Photonic Crystal L3 Nanocavity for Integrated Photonics in the Visible-Wavelength Range. Optik 2015, 126, 3467–3471. [Google Scholar] [CrossRef]
- Sani, M.H.; Khosroabadi, S. A Novel Design and Analysis of High-Sensitivity Biosensor Based on Nano-Cavity for Detection of Blood Component, Diabetes, Cancer and Glucose Concentration. IEEE Sens. J. 2020, 20, 7161–7168. [Google Scholar] [CrossRef]
- Jannesari, R.; Ranacher, C.; Consani, C.; Grille, T.; Jakoby, B. Sensitivity Optimization of a Photonic Crystal Ring Resonator for Gas Sensing Applications. Sens. Actuators A Phys. 2017, 264, 347–351. [Google Scholar] [CrossRef]
- Sharma, P.; Sharan, P.; Deshmukh, P. A Photonic Crystal Sensor for Analysis and Detection of Cancer Cells. In Proceedings of the 2015 IEEE International Conference on Pervasive Computing (ICPC), St. Louis, MO, USA, 23–27 March 2015; pp. 1–5. [Google Scholar]
- Zhang, J.; Hodge, W.; Hutnick, C.; Wang, X. Noninvasive Diagnostic Devices for Diabetes through Measuring Tear Glucose. J. Diabetes Sci. Technol. 2011, 5, 166–172. [Google Scholar] [CrossRef]
- Makaram, P.; Owens, D.; Aceros, J. Trends in Nanomaterial-Based Non-Invasive Diabetes Sensing Technologies. Diagnostics 2014, 4, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Peng, B.; Su, G.; Cohan, B.E.; Major, T.C.; Meyerhoff, M.E. Measurement of Tear Glucose Levels with Amperometric Glucose Biosensor/Capillary Tube Configuration. Anal. Chem. 2011, 83, 8341–8346. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.C.; Overgaard, M.; Rasmussen, L.M. Plasma Proteomics to Identify Biomarkers—Application to Cardiovascular Diseases. Transl. Proteom. 2015, 7, 40–48. [Google Scholar] [CrossRef]
- Wolf, D.J.; Zitelli, J.A. Surgical Margins for Basal Cell Carcinoma. Arch. Dermatol. 1987, 123, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Siyal, S.H.; Javed, M.S.; Ahmad, A.; Sajjad, M.; Batool, S.; Khan, A.J.; Akramh, S.; Alothman, A.A.; Alshgarii, R.A.; Najam, T. Free-standing 3D Co3O4@ NF micro-flowers composed of porous ultra-long nanowires as an advanced cathode material for supercapacitor. Curr. Appl. Phys. 2021, 31, 221–227. [Google Scholar] [CrossRef]
- Kassa-Baghdouche, L. High-Sensitivity Spectroscopic Gas Sensor Using Optimized H1 Photonic Crystal Microcavities. J. Opt. Soc. Am. B 2020, 37, A277. [Google Scholar] [CrossRef]
- Kassa-Baghdouche, L.; Cassan, E. Mid-Infrared Gas Sensor Based on High-Q/V Point-Defect Photonic Crystal Nanocavities. Opt. Quantum Electron. 2020, 52, 260. [Google Scholar] [CrossRef]
- Ahmad, A.; Jini, D.; Aravind, M.; Parvathiraja, C.; Ali, R.; Kiyani, M.Z.; Alothman, A. A novel study on synthesis of egg shell based activated carbon for degradation of methylene blue via photocatalysis. Arab. J. Chem. 2020, 13, 8717–8722. [Google Scholar] [CrossRef]
- Shvalov, A.N.; Soini, J.T.; Chernyshev, A.V.; Tarasov, P.A.; Soini, E.; Maltsev, V.P. Light-Scattering Properties of Individual Erythro-Cytes. Appl. Opt. 1999, 38, 230–235. [Google Scholar] [CrossRef]
- Mroczka, J.; Wysoczanski, D.; Onofri, F. Optical Parameters and Scattering Properties of Red Blood Cells. Opt. Appl. 2002, 32, 691–700. [Google Scholar]
- Xu, X.; Wang, R.K.; Elder, J.B.; Tuchin, V. Effect of Dextran-Induced Changes in Refractive Index and Aggregation on Optical Properties of Whole Blood. Phys. Med. Biol. 2003, 48, 1205–1221. [Google Scholar] [CrossRef] [PubMed]
- Cailleau, R.; Young, R.; Olivé, M.; Reeves, W.J., Jr. Breast Tumor Cell Lines from Pleural Effusions 2. J. Natl. Cancer Inst. 1974, 53, 661–674. [Google Scholar] [CrossRef]
- Yaroslavsky, A.N.; Patel, R.; Salomatina, E.; Li, C.; Lin, C.; Al-Arashi, M.; Neel, V. High-Contrast Mapping of Basal Cell Carcinomas. Opt. Lett. 2012, 37, 644–646. [Google Scholar] [CrossRef]
- Gharsallah, Z.; Najjar, M.; Suthar, B.; Janyani, V. High Sensitivity and Ultra-Compact Optical Biosensor for Detection of UREA Concentration. Opt. Quantum Electron. 2018, 50, 249. [Google Scholar] [CrossRef]
- Kashif, M.; Jafaar, E.; Sahari, S.K.; Low, F.W.; Hoa, N.D.; Ahmad, A.; Abbas, A.; Ngaini, Z.; Shafa, Z.; Qurashi, A. Organic sensitization of graphene oxide and reduced graphene oxide thin films for photovoltaic applications. Int. J. Energy Res. 2021, 45, 9657–9666. [Google Scholar] [CrossRef]
- Arafa, S.; Bouchemat, M.; Bouchemat, T.; Benmerkhi, A.; Hocini, A. Infiltrated Photonic Crystal Cavity as a Highly Sensitive Platform for Glucose Concentration Detection. Opt. Commun. 2017, 384, 93–100. [Google Scholar] [CrossRef]
- Arunkumar, R.; Suaganya, T.; Robinson, S. Design and Analysis of 2D Photonic Crystal Based Biosensor to Detect Different Blood Components. Photon. Sens. 2018, 9, 69–77. [Google Scholar] [CrossRef]
- Almpanis, E.; Papanikolaou, N. Dielectric Nanopatterned Surfaces for Subwavelength Light Localization and Sensing Applications. Microelectron. Eng. 2016, 159, 60–63. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, T.; Wan, R.; Xu, Y.; Zhao, C.; Guo, S. Numerical Investigation of Narrowband Infrared Absorber and Sensor Based on Dielectric-Metal Metasurface. Opt. Express 2018, 26, 10179–10187. [Google Scholar] [CrossRef] [PubMed]
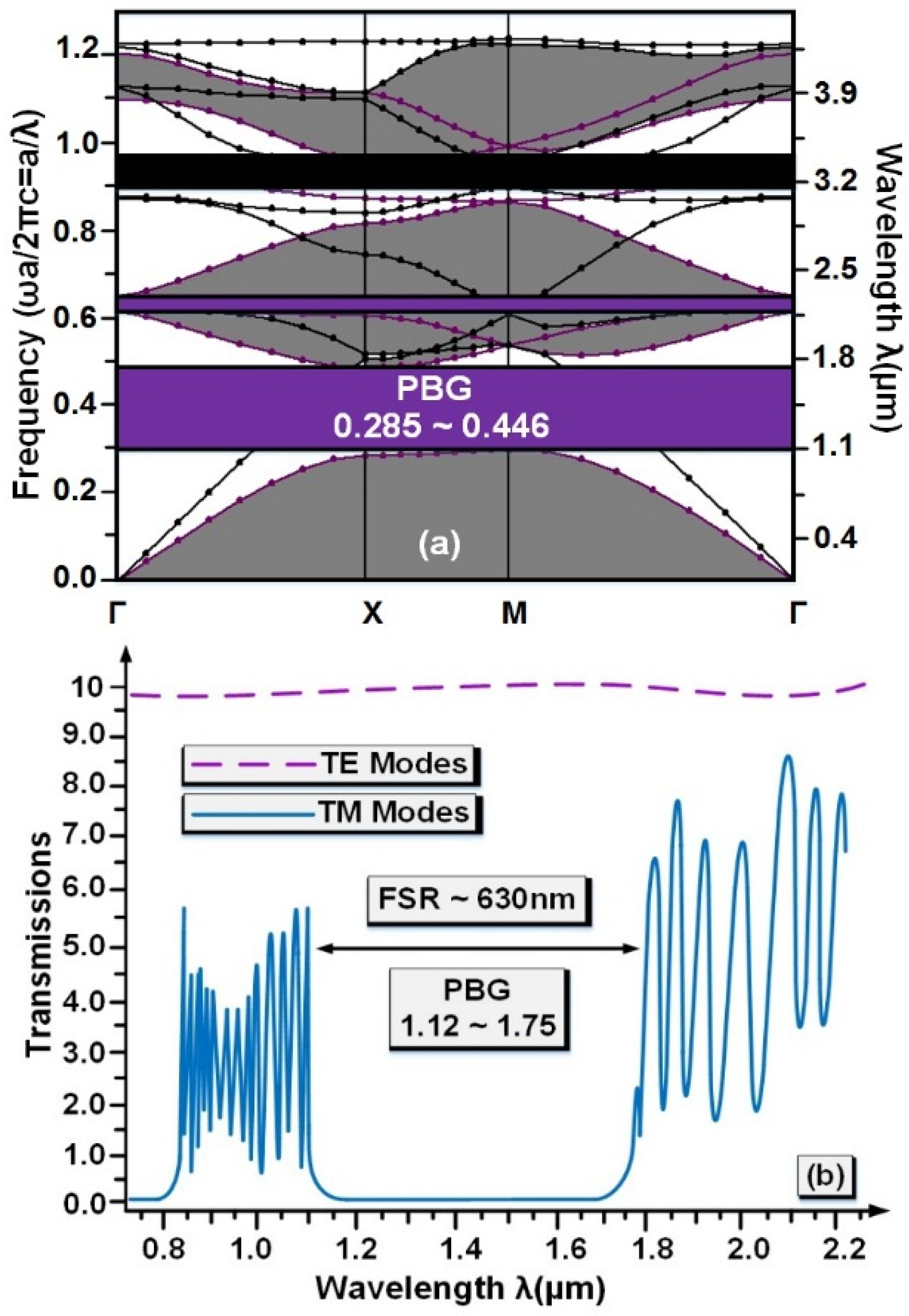

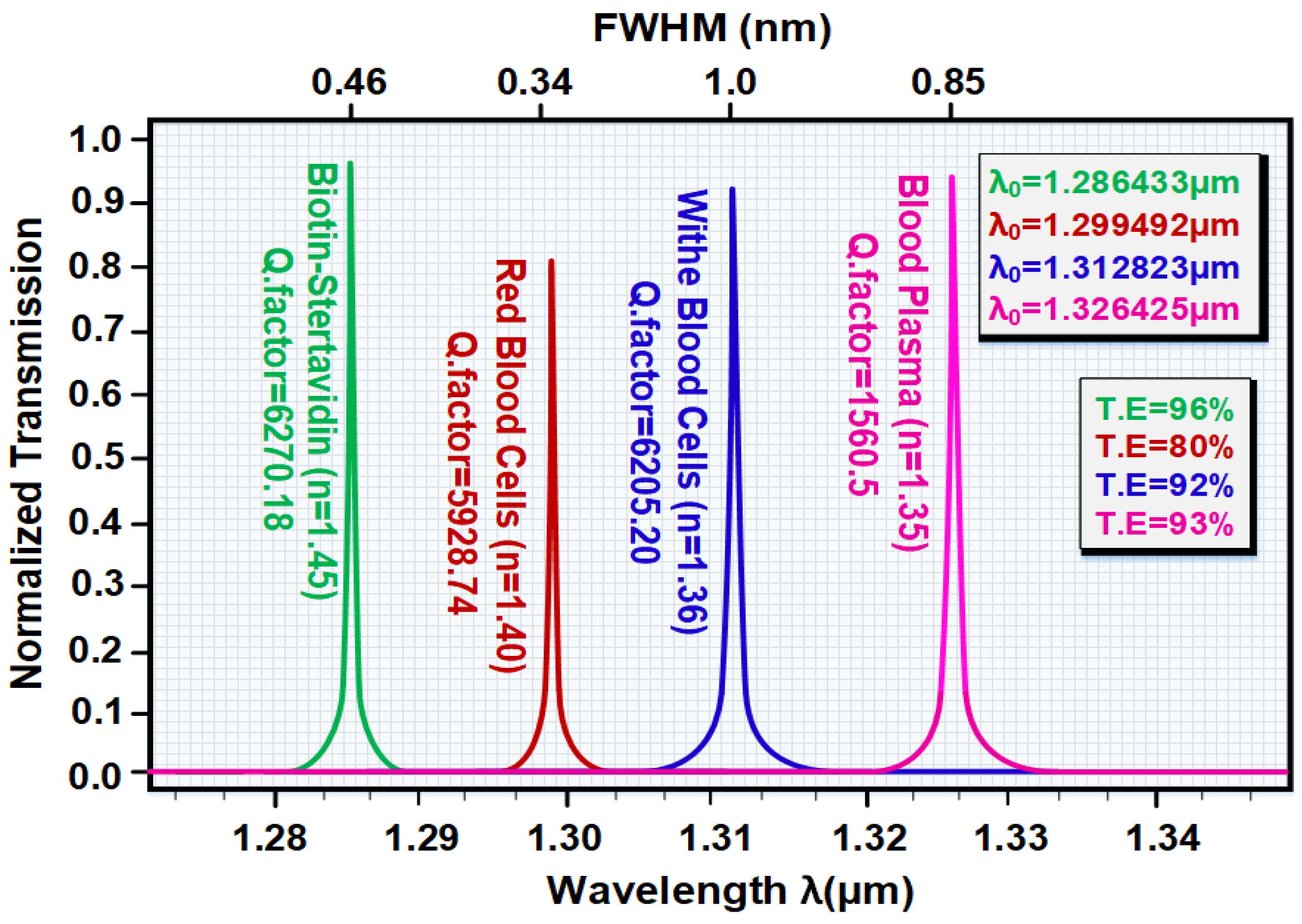

| Analytic | RI | λ0 (µm) | Δλ (nm) | Q.f | TE (%) | S |
|---|---|---|---|---|---|---|
| plasma | 1.35 | 1.326425 | 0.85 | 1560.5 | 99 | Ref |
| WBC | 1.36 | 1.312823 | 1 | 1312.82 | 80 | 1360.2 |
| RBC | 1.41 | 1.299492 | 0.34 | 3822.03 | 92 | Ref |
| Biotin | 1.45 | 1.286433 | 0.46 | 2796.59 | 93 | 326.47 |
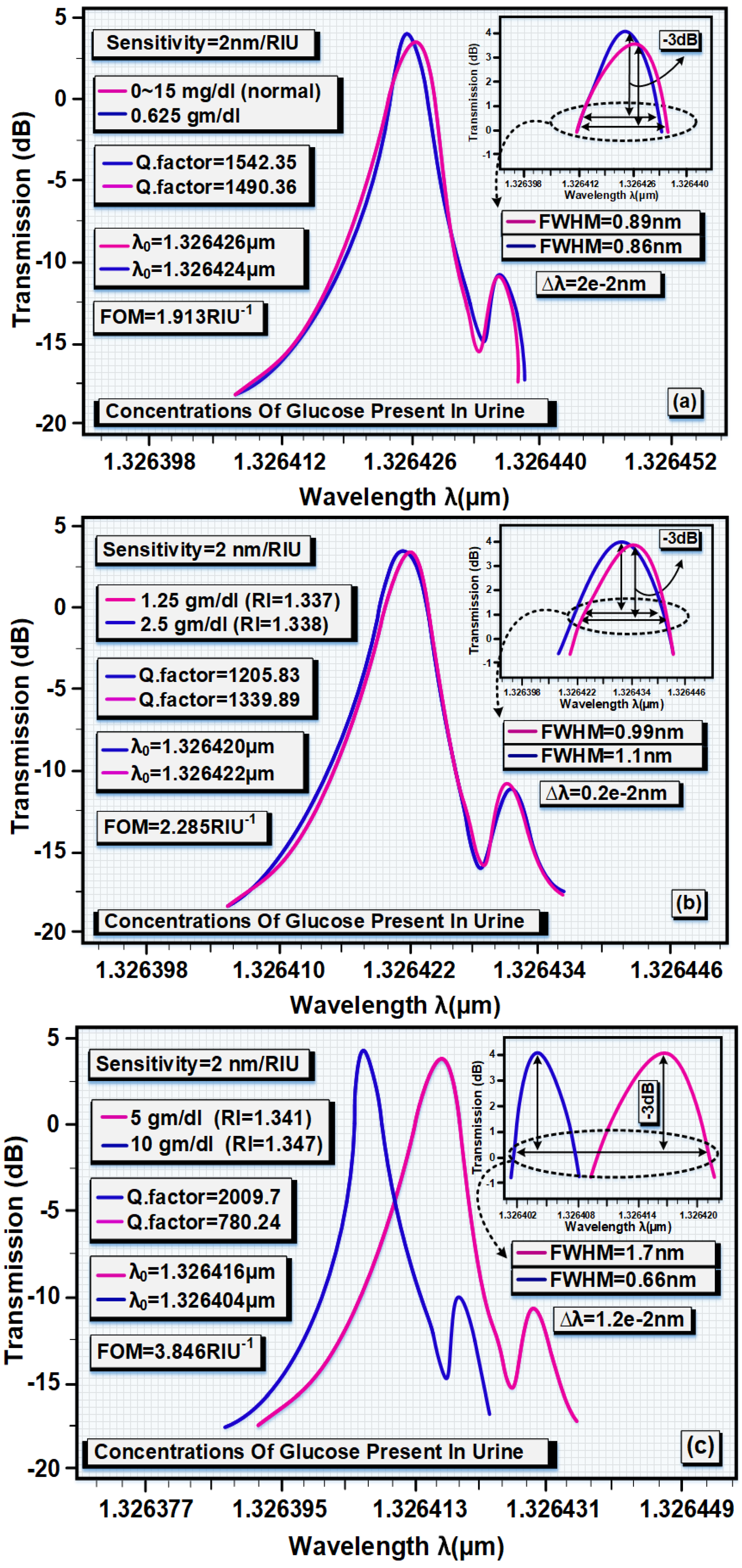
| Urine Sample with Glucose Concentration | Refractive Index |
|---|---|
| Normal (0~15 mg/dL) | 1.335 |
| 0.625 gm/dL | 1.336 |
| 1.25 gm/dL | 1.337 |
| 2.5 gm/dL | 1.338 |
| 5 gm/dL | 1.341 |
| 10 gm/dL | 1.347 |
| Glucose | RI | λ0 (µm) | Δλ (nm) | Q.f | TE (%) | S |
|---|---|---|---|---|---|---|
| 0~15 mg/dL | 1.335 | 1.326426 | 0.89 | 1490.36 | 91 | Ref. |
| 0.625 gm/dL | 1.336 | 1.326424 | 0.86 | 1542.35 | 98 | 2 |
| 1.25 gm/dL | 1.337 | 1.326422 | 0.99 | 1339.89 | 84 | Ref. |
| 2.5 gm/dL | 1.338 | 1.32642 | 1.1 | 1205.83 | 83 | 2 |
| 5 gm/dL | 1.341 | 1.326416 | 1.7 | 780.24 | 81 | Ref. |
| 10 gm/dL | 1.347 | 1.326404 | 0.66 | 2009.7 | 100 | 2 |
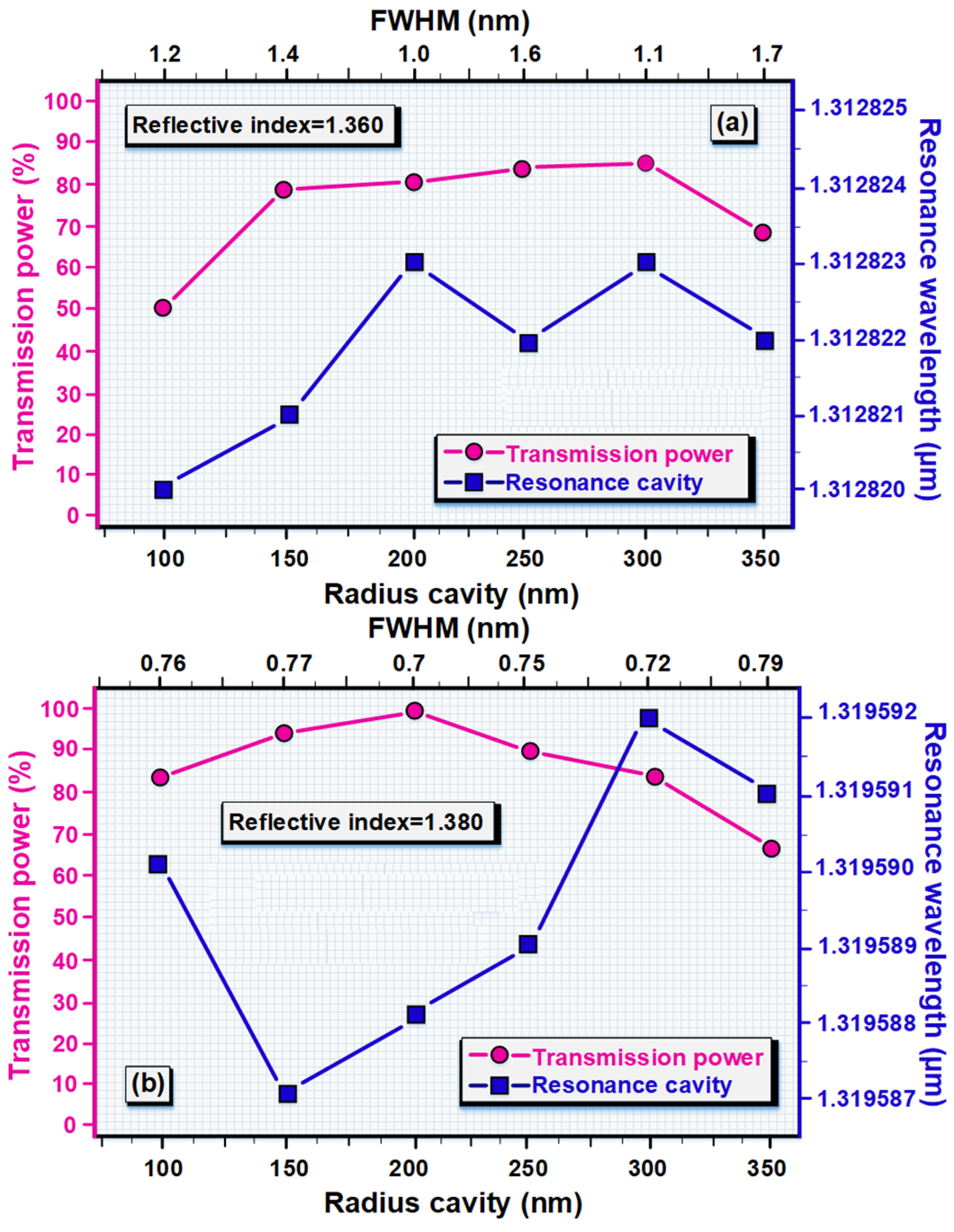
| Ref | Detection Sample | Q.f | FOM | TE | S nm/RIU |
|---|---|---|---|---|---|
| Ref. [45] | Blood and Tears fluid | 1082 | - | - | 6.5764 |
| Ref. [52] | Glucose | - | - | 86 | 422 |
| Ref. [53] | Glucose | 1.11 × 105 | 1117 | 92 | 462 |
| Ref. [54] | Blood | 262 | - | 100 | - |
| Ref. [55] | - | - | 88 | 98 | 263 |
| Ref. [56] | - | 1264 | 84 | 90 | 840 |
| The present study | Urine, Tears fluid, Blood | 3822.03 | 1320.23 | 100 | 1360.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalil, A.T.; Ashfaq, S.; Bokov, D.O.; Alanazi, A.M.; Hachem, K.; Suksatan, W.; Sillanpää, M. RETRACTED: High-Sensitivity Biosensor Based on Glass Resonance PhC Cavities for Detection of Blood Component and Glucose Concentration in Human Urine. Coatings 2021, 11, 1555. https://doi.org/10.3390/coatings11121555
Jalil AT, Ashfaq S, Bokov DO, Alanazi AM, Hachem K, Suksatan W, Sillanpää M. RETRACTED: High-Sensitivity Biosensor Based on Glass Resonance PhC Cavities for Detection of Blood Component and Glucose Concentration in Human Urine. Coatings. 2021; 11(12):1555. https://doi.org/10.3390/coatings11121555
Chicago/Turabian StyleJalil, Abduladheem Turki, Shameen Ashfaq, Dmitry Olegovich Bokov, Amer M. Alanazi, Kadda Hachem, Wanich Suksatan, and Mika Sillanpää. 2021. "RETRACTED: High-Sensitivity Biosensor Based on Glass Resonance PhC Cavities for Detection of Blood Component and Glucose Concentration in Human Urine" Coatings 11, no. 12: 1555. https://doi.org/10.3390/coatings11121555
APA StyleJalil, A. T., Ashfaq, S., Bokov, D. O., Alanazi, A. M., Hachem, K., Suksatan, W., & Sillanpää, M. (2021). RETRACTED: High-Sensitivity Biosensor Based on Glass Resonance PhC Cavities for Detection of Blood Component and Glucose Concentration in Human Urine. Coatings, 11(12), 1555. https://doi.org/10.3390/coatings11121555











