Abstract
The microcapsules were prepared by using melamine-formaldehyde resin as the wall material and aloin as the core material. The aloin was dissolved in ethanol and water to prepare microcapsules. The aloin powder, the aloin microcapsules prepared with ethanol as the solvent, and the aloin microcapsules prepared with water as the solvent were, respectively, added to the waterborne coating with different contents and coated on the surface of Tilia europaea. The effects of different modifiers and contents on the coating’s optical properties, mechanical properties, and antibacterial properties were explored. The results showed that the aloin microcapsules prepared with ethanol as the solvent had good morphology and comprehensive properties. When the content was 7.0%, the color difference of the waterborne coating was small, the adhesion was grade 3, the impact resistance was 12 kg·cm, and the antibacterial rate was 87.8%. In terms of antibacterial properties, the uncoated aloin powder, the coated aloin microcapsules prepared with ethanol as the solvent, and the aloin microcapsules prepared with water all have certain antibacterial properties and the antibacterial rates reached 99.2%, 97.3%, and 67.3%, respectively. This study provides a certain reference for developing antibacterial wood furniture coatings.
1. Introduction
As a natural polymer composite material, wood has a unique color, pattern, and texture [1]. Because of its high strength-to-weight ratio, convenient processing, environmental protection, natural degradation, and recyclability, it is widely used in the fields of wood products and furniture. However, wood has some problems, such as dry shrinkage and wet expansion, which will lead to dimensional instability. It is easy for wood to absorb moisture from the air, which provides a good environment for bacterial growth. At the same time, it is easy for the glucose produced in the process of wood degradation to cause damage to wood materials, such as decay, mildew, and mold [2,3]. These problems pose a new challenge to the antibacterial properties of wood furniture surface coatings. There are many kinds of coatings used in wood furniture. The traditional coatings include acid-curing coatings and polyurethane coatings. However, the acid-curing coating products contain free formaldehyde, which is harmful to the user’s body. Polyurethane coating has poor workability. Compared with these traditional coatings, the waterborne coating is more and more widely used because of its advantages of being green, energy-saving, and low formaldehyde. However, the mechanical properties of waterborne coatings need to be strengthened [4,5], and they do not have antibacterial properties, so they cannot protect the wood furniture substrate against bacteria.
Aloe is a perennial, evergreen, and fleshy herb belonging to aloe in Liliaceae. It is a green plant resource homologous to medicine and food. Its chemical composition is complex, including anthraquinone compounds, sugars, and amino acids. Aloin is an anthraquinone compound extracted from aloe, which has the physiological functions of diarrhea, depigmentation, tyrosinase inhibition, free radical scavenging, and antibacterial activity [6]. Aloin is also a natural organic compound, which has anti-inflammatory, bactericidal, antiviral, and other effects [7]. Aloin is the main antibacterial active component of aloe [8] and can inhibit the growth of several fungi and bacteria [9]. The crude extract of aloe is different from general antibiotics. It can not only effectively inhibit microbial proliferation but also effectively kill resistant bacteria induced by antibiotics [10]. The above research results show that aloin has antibacterial properties, but it is rarely used in wood surface waterborne coatings. However, the aloin core material is easy to decompose and unstable due to its high temperature, and its color is dark brown. When directly coated on the surface of wood furniture and wood products, it will obviously cover the wood texture and cannot reflect the beauty of the wood grain, which seriously limits the application on wood surfaces.
Microcapsules are a technology to wrap film-forming materials on the surface of another substance (liquid, gas, solid) through a series of physical or chemical methods to form small spherical particles with a “core-shell structure” so as to achieve some purpose [11,12,13]. Melamine-formaldehyde (MF) resin is a colorless transparent polymer. It has good interfacial compatibility with a waterborne coating when it is used as a wall material [14]. The preparation of the aloin microcapsules can not only improve the deep color defect of aloin but also realize the antibacterial property of furniture coatings, which has potential application value.
In this work, the microcapsules were prepared with melamine-formaldehyde resin as the wall material and the aloin powder as the core material. The aloin powder was dissolved in ethanol and water to prepare microcapsules, respectively. The microcapsules and the aloin powder were added to the waterborne coating with different contents and coated on the surface of Tilia europaea. The effects of the three different modifiers and different contents on the optical, mechanical, and antibacterial properties of the coating were explored so as to provide a certain reference basis for the development of antibacterial furniture coatings.
2. Experimental Materials and Methods
2.1. Experimental Materials
The experimental materials are shown in Table 1. The Dulux water-based coating consists of waterborne acrylic acid copolymer dispersion (the content is 90.0%), matting agent (the content is 2.0%), additives (the content is 2.0%), and water (the content is 6.0%). The specification of Tilia europaea boards is 100 × 50 × 5 mm3. Staphylococcus aureus AS1.89 (National strain collection and management center) is the second-generation standard strain (CMCC26003).

Table 1.
List of experimental materials.
2.2. Preparation Method of Microcapsules with Aloin Powder as Core Material
In this experiment, two kinds of microcapsules were prepared: the aloin microcapsules prepared with ethanol as the solvent and the aloin microcapsules prepared with water as the solvent. The preparation process was as follows:
(1) Preparation of wall material: wall material prepolymer with melamine, formaldehyde solution, and distilled water were prepared in the mass ratio of 1:2:5. The 10.0 g of melamine powder and 20.0 g of formaldehyde solution were weighed. The melamine powder was poured into the formaldehyde solution, stirred until fully dissolved, and the 20.0 g of distilled water was added. A small amount of triethanolamine powder was absorbed with a dropper until the pH value was adjusted to 8.0–9.0. The diameter of the beaker was sealed with polyethylene film, and the beaker was put into a 70 °C constant temperature magnetic stirrer (Shanghai Lichen Technology Co., Ltd., Shanghai, China) at the rotational speed of 960 rpm. When the wall material was stirred to a transparent state, 30.0 g of distilled water was added. The rotational speed was adjusted to 1440 rpm, and the heating and stirring time was 30 min. Next, the solution was placed at room temperature and cooled naturally for 20 min. The obtained solution was melamine-formaldehyde resin wall material solution.
(2) Preparation of core material emulsion: when the aloin microcapsules with ethanol as the solvent were prepared, the 7.0 g of aloin powder was weighed and added to the 100.0 g of ethanol. It was stirred until it was completely dissolved and then centrifuged. Then, the supernatant was poured out. The undissolved aloin was taken out and dried in a dryer. The dried powder was weighed to 6.66 g. It was found that 0.34 g of aloin dissolved in ethanol. The 0.34 g of aloin was weighed and added to the 100.0 g of ethanol, stirred, and dissolved evenly. The 174 mL of distilled water was weighed in another beaker, the 1.76 g of sodium dodecylbenzene sulfonate was added to the water as an emulsifier and stirred at the speed of 640 rpm. Following this, the emulsifier solution was slowly poured into the core solution and fully mixed. The solution was put into a constant-temperature magnetic stirrer. The temperature was 70 °C, and the speed was 960 rpm. After being continuously stirred for 60 min, the solution was the core material emulsion prepared with ethanol as the solvent. When the aloin microcapsules with water as the solvent were prepared, the same method that was used to obtain the aloin powder was used, namely dissolved 6.67 g in 100.0 g water. The 6.67 g of aloin powder was added to the 100.0 g water, the other conditions remained unchanged, and the solution was the core material emulsion prepared with water as the solvent.
(3) Preparation of microcapsules: the melamine-formaldehyde resin wall solution was slowly added to the core material emulsion with a dropper under the rotational speed of 960 rpm. The citric acid monohydrate crystals were added until the pH value of the solution was adjusted to 2.5–3.0. The temperature was slowly raised to 70 °C. After being continuously stirred for 3 h, the resulting solution was aged at room temperature for 48 h. Then, the solution was filtered with a suction filter. The remained products were put into an oven to dry at 40 °C for 48 h. The light green powder obtained was the microcapsules.
2.3. Preparation of Coating
The Tilia europaea boards were pre-sanded with 600 mesh sandpaper. The aloin, the aloin microcapsules prepared with ethanol as the solvent, and the aloin microcapsules prepared with water as the solvent (modifier) were added to the waterborne coating according to the contents of 1.0%, 3.0%, 5.0%, 7.0%, 10.0%, 13.0%, and 15.0%, respectively. The total mass of the coating was 3.0 g, and the mass of the three kinds of modifiers was 0.03, 0.09, 0.15, 0.21, 0.30, 0.39, and 0.45 g, respectively. The modifiers were evenly mixed with the waterborne coating and then coated on the sample boards by SZQ tetrahedral fabricator. The samples 1–7 were coatings added with aloin. The samples 8–14 were coatings added with aloin microcapsules prepared with ethanol as the solvent. The samples 15–21 were coatings added with aloin microcapsules prepared with water as the solvent. They were dried at room temperature for 10 min and then were put into an oven at 40 °C for 20 min. The coatings were gently polished with 800 mesh sandpaper. After repeating the above process twice, the dry film thickness of the film was 60 μm. In addition, the waterborne coating without a modifier was used as the blank control sample.
2.4. Testing and Characterization
The morphologies of the microcapsules and waterborne coating film were characterized by a quanta-200 scanning electron microscope (SEM, FEI Company Hillsboro, OR, USA). The chemical components of the microcapsules were characterized by a VERTEX 80 V infrared spectrometer (Germany Bruker Co., Ltd., Karlsruhe, Germany).
According to the standard of GB/T 4893.6-2013 [15], the surface gloss of paint film was measured by HG268 intelligent gloss meter (Shenzhen 3nh Technology Co., Ltd., Shenzhen, China), and the gloss of the paint film was recorded at 20°, 60°, and 85° incident angles. According to the standard of GB/T 9286-1998 [16], the adhesion of the paint film was measured by a QFH-HG600 film gridding instrument (Dongguan Huaguo Precision Instrument Co., Ltd., Dongguan, China). The machine was used to cut the paint film, and the transparent tape was used to stick off the material falling off the paint film. The degree of adhesion of the coating was observed with a magnifying glass to judge the level of coating adhesion of each sample. There are adhesion grades: 0, 1, 2, 3, 4, and 5. The higher the grade, the worse the coating adhesion. According to the standard of GB/T 1732-1993 [17], the impact resistance of the coating was measured by a QCJ (Product model) paint film impactor tester (Shanghai Qigong Instrument Co., Ltd., Shanghai, China). The heavy hammer was controlled to fall on the boards, and the paint film was observed with a magnifying glass to record the test data. According to the standard of GB/T 11186.3-1989 [18], the color difference of the coating was tested by SEGT-J Portable Colorimeter (Guoti Precision Testing Instrument Co., Ltd., Shenyang, China). The L* represents brightness, the a* represents chromaticity changes from red to green, the b* represents chromaticity changes from yellow to blue, the h* represents hue, and the ∆E* represents color difference. The samples were placed flat on the table and tested by the colorimeter. The first set of data was L1*, a1*, b1*, h1*. The second set of data was L2*, a2*, b2*, h2*. The color difference of the coating was calculated by Formula (1). In the formula, ∆a* = a2* − a1*, ∆L* = L2* − L1*, ∆b* = b2* − b1*.
Staphylococcus aureus is frequently described as being responsible for a variety of human and animal diseases [19,20], which often parasitizes in the skin, nasal cavity, throat, gastrointestinal tract, and suppurative sores. It also exists in the air, sewage, and other environments. Its optimum growth temperature is 37 °C, and the cultivation process is easy to operate. Therefore, Staphylococcus aureus was selected as the microorganism for the antibacterial test. According to the standard of GB/T 21866-2008 [21], the antibacterial test method of the coating was as follows:
(1) Preparation of agar medium: the 33.0 g of agar medium powder was weighed and added to 1000 mL of distilled water. The beaker was heated in a water bath pot. After the solution was stirred and dissolved, the agar solution was poured into a triangular flask while it was hot and was put into a high-temperature and high-pressure sterilization pot at 121 °C for 30 min. Then it was poured into the sterilization culture dishes, and the injection was stopped at about one-third of the height of the plate. At this time, the cover was covered, and the agar medium was cooled and solidified at room temperature.
(2) Activation of bacteria: the inclined culture medium of Staphylococcus aureus was taken out from the refrigerator at 0–5 °C, and the inoculation ring was sterilized at high temperature on an alcohol lamp. The cooled inoculation ring was quickly extended into the inclined culture medium of Staphylococcus aureus, and a small number of bacteria were dipped. Then a horizontal line and continuous zigzag line were drawn on the agar medium. Next, it was placed at room temperature for 20 min, and the agar medium was placed upside down in a constant temperature and humidity incubator for 20 h at 36–38 °C. This was to reduce water loss and avoid increasing the salt concentration; otherwise, the osmotic pressure would change, which was not conducive to the growth of bacteria. Following the activation of bacteria, the culture was completed when there was dense golden bacterial material in the scribed part of the agar medium.
(3) Preparation of suspension: the bacterial culture medium was nutrient broth (NB). The culture concentration suitable for Staphylococcus aureus is 1.0%. The preparation method was to add 2.0 g of nutrient broth powder to 198.0 g of distilled water and then stir to dissolve. The pH value was adjusted to 7.0–7.2 with 0.1 mol/L HCl solution. The nutrient broth was sub-packed and put into a high-temperature and high-pressure sterilization pot and sterilized at 121 °C for 30 min. The inoculation ring was put on the alcohol lamp for high-temperature sterilization, and the fresh bacteria with 1–2 rings scraped from the culture medium were added to the nutrient broth. Then the suspension containing the bacteria was prepared.
(4) Inoculation and culture of samples: first, the polyethylene films were prepared, the size was (40 ± 2) × (40 ± 2) mm2, and the thickness was (0.05–0.10) mm. The films were soaked in 70% ethanol solution for 10 min, then washed with 0.85% normal saline and dried naturally. A total of 0.4–0.5 mL of the above suspension was taken with a pipette and dropped onto the surface of the blank control sample and the samples 1–21, respectively. Next, the polyethylene films were clamped by sterilization tweezers to cover the samples. It should be noted that the suspension shall not overflow from the edge of the polyethylene covering film. If the suspension overflows, the area of the polyethylene film can be increased, or the volume of the suspension can be reduced, but the volume shall not be less than 0.1 mL. The films were gently pressed to spread the suspension around. We were sure to lay it flat without bubbles so that the suspension can contact the samples evenly. The samples were placed at 36–38 °C and cultured for 24 h under the relative humidity of RH > 90%.
(5) Elution and culture of bacteria: after 24 h of culture, the samples were taken out. A normal saline solution with a concentration of 0.85% was prepared, which was used as the eluent. A total of 10 mL of the eluent was added to each sample. The polyethylene films and the surface of the samples were washed with the eluent repeatedly. The eluent-containing bacteria were mixed with the agar medium liquid that had been melted and cooled to about 45 °C. Then it was shaken fully and injected into the sterilized culture dishes. When the agar was solidified, the agar plate containing bacteria was made. After being cultured at 36–37 °C for 24 h, colonies can be formed.
(6) Count of live bacteria: the corresponding number of colonies was recorded by the colony counter. Colony count was expressed in colony-forming units (CFU). According to the standard of HG/T 3950-2007 [22], the culture dishes to be tested were put into the instrument. They were observed with a magnifying glass, and all colonies at the bottom of the agar medium were counted one by one and marked with color with a probe pen. If there was no omission in the counting process, the counting was completed, and the number on the display screen was the colony number of the agar medium. The number of viable bacteria determined above multiplied by 1000 was the actual value of recovered viable bacteria after 24 h of culturing of each sample, as shown in Formula (2). In the formula, R* represents the bacterial resistance rate. B* represents the average number of bacteria recovered after 24 h of the blank control sample, expressed in (CFU/piece). C* represents the average number of bacteria recovered after 24 h of antibacterial coating sample, expressed in (CFU/piece).
3. Results and Discussion
3.1. Analysis of Microcapsules Prepared with Different Solvent
The macro morphology of the aloin powder and the aloin microcapsules is shown in Figure 1. Figure 1A shows that the aloin powder itself is dark and brown. Figure 1B shows that the aloin microcapsules prepared with ethanol as the solvent have the lightest color. Figure 1C shows the aloin microcapsules prepared with water as the solvent has a slightly lighter color. The micromorphology of microcapsules is shown in Figure 2. Figure 2A shows that when the aloin powder is not coated, the surface is very rough, oval, large, and uneven. The diameter of the aloin powder is about 60–80 μm. Figure 2B shows that the aloin microcapsules prepared with ethanol as the solvent are well coated, the microcapsules are round and dispersed, the wall materials of melamine-formaldehyde resin are less precipitated and agglomerated, and the particle size is uniform. The diameter of the microcapsules is about 5 μm. Figure 2C shows that the aloin microcapsules prepared with water as the solvent are relatively poorly coated, with a large amount of precipitation and agglomeration, and the particle size is uneven, about 2–80 μm. This may be because the solubility of aloin in water is much higher than that in ethanol, resulting in excessive content of core materials and poor coating effect of the microcapsules. Through the comparison between the aloin microcapsules prepared with different solvents and the morphology comparison between microcapsules and the aloin powder, it can be seen that the aloin microcapsules prepared with ethanol as the solvent have better morphological characteristics, smooth surface, uniform particle size, and round dispersion, followed by the aloin microcapsules prepared with water as the solvent. It can be seen from the morphology that the surface of the aloin powder is very rough, the particle size is large and not round. Compared with the morphology after coating, it is not suitable for direct application in coatings. The coating of the aloin microcapsules can increase the beauty of its surface morphology, so it can be better applied to the surface of painted furniture.
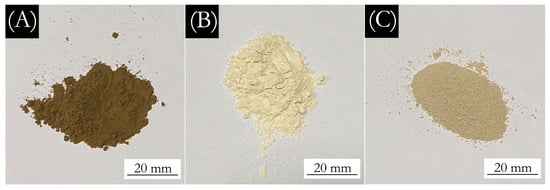
Figure 1.
The macroscopic topography: (A) the aloin powder, (B) the aloin microcapsules prepared with ethanol as the solvent, and (C) the aloin microcapsules prepared with water as the solvent.

Figure 2.
The microtopography: (A) the aloin, (B) the aloin microcapsules prepared with ethanol as the solvent, and (C) the aloin microcapsules prepared with water as the solvent.
Figure 3 is the infrared spectrum of the aloin powder, the aloin microcapsules prepared with ethanol as the solvent, and the aloin microcapsules prepared with water as the solvent. The characteristic absorption peak of C-H is at 2950 cm−1. The absorption peaks at 1614 cm−1 and 1432 cm−1 are the vibration absorption peaks of the aloin aromatic ring skeleton. The stretching vibration peak of C=O in the aloin molecule is at 1633 cm−1. At 1148 cm−1, it is the stretching vibration of C-O in the aloin molecule. The vibration mode of O-H in aloin molecule is mainly in-plane bending vibration (δOH), the vibration peaks at 592 and 862 cm−1 can refer to the in-plane bending vibration considered as O-H [23,24,25]. The corresponding peaks also appeared in the infrared spectra of the two aloin microcapsules, indicating the existence of the aloin core material in the microcapsules and that the components were not damaged. The peak values of 3370, 1570, and 1344 cm−1 are the vibration absorption peaks of N-H, C=N, and C-N bonds in melamine-formaldehyde resin [26], which indicates that the wall material melamine-formaldehyde resin has successfully coated the aloin core material.
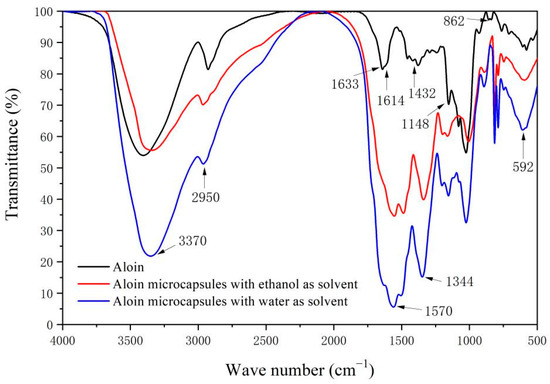
Figure 3.
Infrared spectrum of the aloin, the aloin microcapsules prepared with ethanol as the solvent, and the aloin microcapsules prepared with water as the solvent.
3.2. Effects of Different Microcapsules on Optical and Mechanical Properties of Coating
The test results of sample color differences are shown in Table 2 and Figure 4. In the sample, the color difference of No. 1–2, No. 8–12, and No. 15–16 is small. The color difference value is inversely proportional to the amount of modifier added. The more modifier added, the greater the color difference of the sample, which may be caused by the color of the aloin powder and the microcapsules themselves. By comparing the color difference results of the coating with three different modifiers, it was concluded that the overall color difference of the coating added with the aloin microcapsules prepared with ethanol as the solvent was the smallest and the performance was obviously the best, followed by the aloin microcapsules prepared with water as the solvent. The overall color difference of the coating added with the aloin powder was the largest, and the performance was poor. This is because the color of the aloin powder becomes lighter after being coated, so the overall color difference of the two aloin microcapsules is small. However, with the increase of the addition amount, the concentration of the three aloin modifiers increases, which will lead to uneven distribution in the coating, so the color difference of the coating becomes larger.

Table 2.
Color difference measurement data of coating surface.
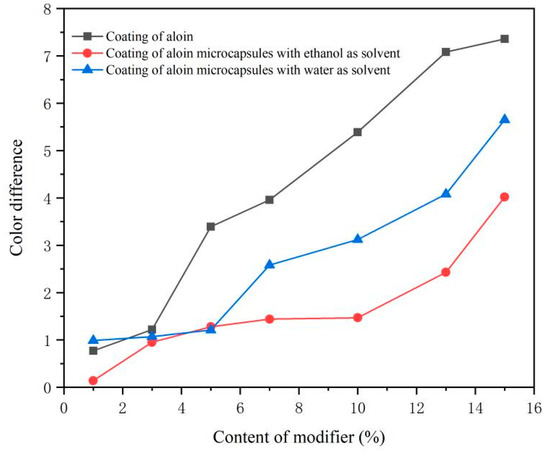
Figure 4.
Color difference of coating surface with different content of modifier.
The gloss test results of the coating at an incident angle of 60° are shown in Figure 5. It can be seen from the data that the gloss of the coating is inversely proportional to the amount of modifier added. The more modifier added, the lower the gloss of the coating. This may be caused by the uneven coating surface and the enhancement of diffuse reflection after the addition of the aloin powder and the microcapsules. The gloss of the coating added with aloin powder only was higher than that of the two groups with microcapsules. The gloss of the coating with microcapsules prepared with ethanol as the solvent was the second, and the gloss of the coating with microcapsules prepared with water as the solvent was the lowest. This may be that the gloss of the aloin powder was better, resulting in a higher gloss of the coating than that of the coating with the aloin microcapsules.
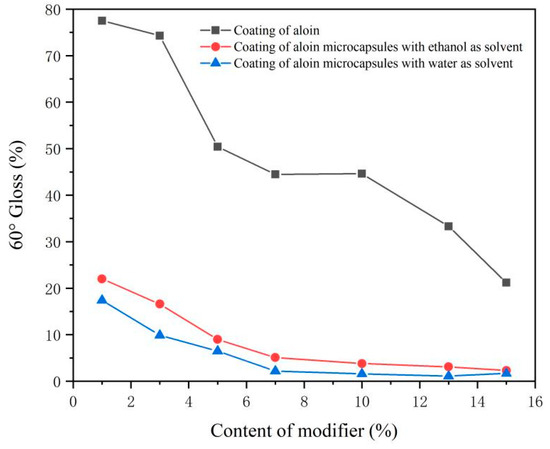
Figure 5.
Surface gloss of coating with different content of modifier.
The adhesion test results of the coating are shown in Figure 6. The adhesion performance of the coating is inversely proportional to the content of the modifier. The overall adhesion grade of the coating with the microcapsules prepared with ethanol as the solvent is low, and the adhesion performance was the best. The microcapsules prepared with water as the solvent was the second. The coating added with the aloin powder has a high overall adhesion grade and poor adhesion performance. The higher the content of the aloin modifiers, the worse the adhesion performance of the coating. This may be because when there are more aloin powder and microcapsules in the coating, the binder content of the coating decreases, resulting in the decrease of adhesion performance to Tilia europaea substrate.
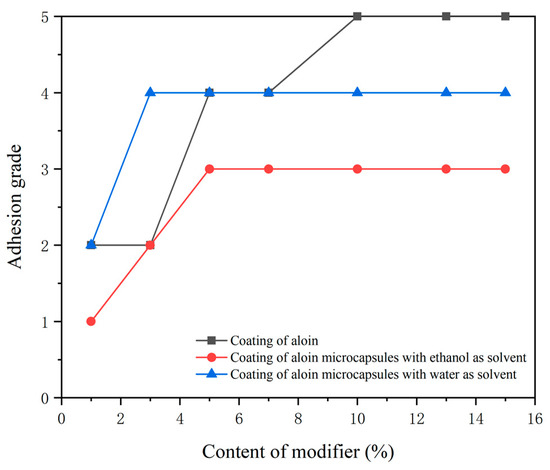
Figure 6.
Adhesion of coatings with different content of modifiers.
The test results of the impact strength of the coating surface are shown in Figure 7. The impact resistance of the coating is directly proportional to the content of the modifier. The higher the content of the modifier, the stronger the impact resistance of the coating. This shows that the aloin powder and the aloin microcapsules can increase the impact resistance of the coating surface. The impact strength of the coating added with the microcapsules prepared with water as the solvent is the highest. The impact strength of the coating added with the microcapsules prepared with ethanol as the solvent is the second. The impact strength of the coating added with the aloin powder is poor. This may be because aloin modifiers enhance the mechanical chain force of the coating, so the impact strength of the coating increases with the increase of content.
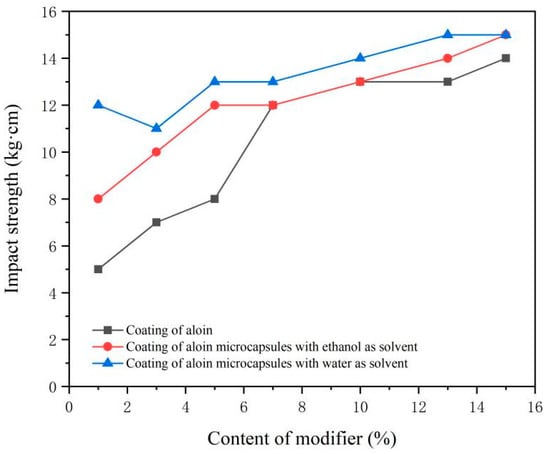
Figure 7.
Impact strength of coatings with different content of modifiers.
3.3. Microstructure Analysis of Coating
According to the analysis of the above test results, when the addition amount of the aloin powder, the aloin microcapsules prepared with ethanol as the solvent, and the aloin microcapsules prepared with water as the solvent, is 7.0%, the coating has a small color difference, good gloss, high adhesion, strong impact resistance, and the best comprehensive performance. Therefore, three samples with a content of 7.0%, i.e., No. 4, No. 11, No. 18, and the blank control sample of waterborne coating, are selected for further analysis through a scanning electron microscope and infrared spectrum analysis so as to investigate the reasons affecting the performance of the coating.
The micromorphology of the coating is shown in Figure 8. Figure 8A is the waterborne coating template. It is the blank control group, without any modifier, with a very smooth surface and few particles bulge. Figure 8B shows a small number of large particles can be seen on the surface of the coating with 7.0% aloin powder, and the aloin powder is evenly dispersed in the coating. Figure 8C shows the particles on the surface of the coating with 7.0% aloin microcapsules prepared with ethanol as the solvent are evenly dispersed, slightly agglomerated, and the particle size is about 6 μm. Figure 8D shows that the coating of 7.0% aloin microcapsules prepared with water as the solvent has an extremely rough surface, a large number of particles, and is unevenly dispersed on the coating surface with obvious agglomeration. Through the comparison between the coating surface with three different modifiers and the blank sample, it can be seen that the coating surface with 7.0% aloin powder as the modifier is smooth and basically free of particles, resulting in a high gloss of the paint film. The coating surface of 7.0% aloin microcapsules is prepared with ethanol as the solvent is smooth and the microcapsules are evenly dispersed, which is beneficial to reduce the color difference of the coating. The coating surface of 7.0% aloin microcapsules prepared with water as the solvent has serious agglomeration, cannot be well mixed with a water-based coating, and cannot be evenly dispersed on the coating surface, resulting in a large color difference and the lowest gloss of paint film, which is not suitable for applications in the coating. Comprehensively considered, the coating of 7.0% aloin microcapsules prepared with ethanol as the solvent has good comprehensive performance.

Figure 8.
SEM of coating: (A) waterborne coating, (B) coating with 7.0% aloin, (C) coating of 7.0% aloin microcapsules prepared with ethanol as the solvent, and (D) coating of 7.0% aloin microcapsules prepared with water as the solvent.
Figure 9 is the infrared spectrum of waterborne coating, coating added with 7.0% aloin, coating added with 7.0% aloin microcapsules prepared with ethanol as the solvent, and coating added with 7.0% aloin microcapsules prepared with water as the solvent. The peak at 2950 cm−1 is the characteristic absorption peak of C-H. The absorption peak at 1432 cm−1 is the vibration absorption peak of the aloin aromatic ring skeleton. The peak at 1633 cm−1 is the stretching vibration peak of C=O in the aloin molecule, which is a unique peak in the coating added with three modifiers, but there is no such peak in the waterborne coating. The peak at 1148 cm−1 represents the vibration of the aloin molecule and C-O in aqueous acrylic acid, and the peak at 862 cm−1 represents the vibration of the aloin molecule and O-H in aqueous acrylic acid. In the coatings with three modifiers, the two peaks are significantly enhanced due to the addition of aloin, which proves the existence of aloin in the coatings. The peak at 1570 cm−1 is the vibration absorption peak of C=N in melamine-formaldehyde resin, which is a unique peak in the coating added with three modifiers, but not in the waterborne coating. The absorption peak at 1726 cm−1 represents the characteristic peak of C=O in waterborne acrylic paint. Infrared spectra showed that there was no chemical reaction between the aloin powder, the aloin microcapsules prepared with ethanol as the solvent, and the aloin microcapsules prepared with water as the solvent and waterborne acrylic coating.
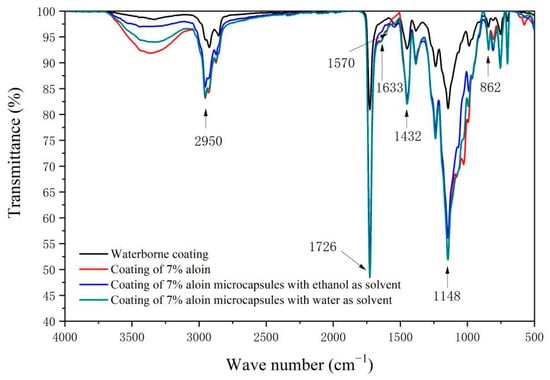
Figure 9.
Infrared spectra of waterborne coating, coating with 7.0% aloin, coating with 7.0% aloin microcapsules prepared with ethanol as the solvent, and coating with 7.0% aloin microcapsules prepared with water as the solvent.
3.4. Effects of Different Microcapsules on Antibacterial Property
The antibacterial rate data of the coatings are shown in Table 3 and Figure 10. The results show that the antibacterial rate of each sample coating is directly proportional to the content of modifiers. The higher the content of modifiers, the stronger the antibacterial property of the coating. This shows that the aloin powder and the two aloin microcapsules all have antibacterial properties. Among them, the aloin powder has the best antibacterial effect, up to 99.2%. This may be because the aloin powder was not coated and can directly play an antibacterial role, so the antibacterial effect is better than the two coated aloin microcapsules. However, the uncoated aloin powder has a negative influence on the color and roughness of the coating. The antibacterial effect of the aloin microcapsules prepared with ethanol as the solvent is the second, up to 97.3%. Compared with aloin powder, its antibacterial ability only has a small gap. The aloin microcapsules prepared with water as the solvent present the lowest antibacterial property, only 67.3%. This may be because the aloin microcapsules prepared with water as the solvent were relatively poorly coated; therefore, there are some impurities in the prepared microcapsule powder, which affect the antibacterial effect. The research results [27,28] proved that aloe has antibacterial and bactericidal effects, and anthraquinone compounds are the main antibacterial substances in aloe. The aloin, as an anthraquinone compound, can inhibit the growth of Staphylococcus aureus, remove harmful metabolites released during bacterial infection, and kill the endotoxin left by bacteria.

Table 3.
Actual recovery of viable bacteria and antibacterial rate of each sample.
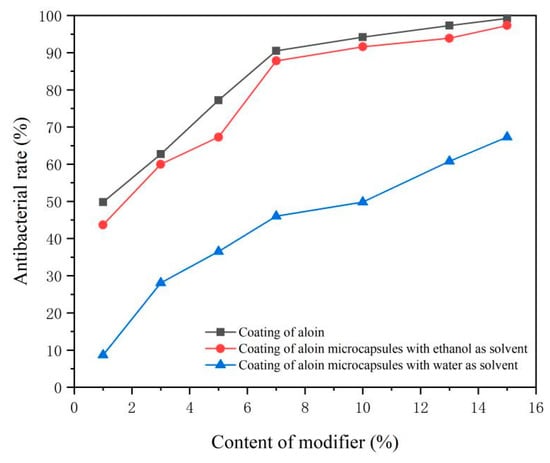
Figure 10.
Antibacterial rate of the coating surface with different content of modifiers.
4. Conclusions
In this work, the microcapsules were prepared with melamine-formaldehyde resin as the wall material and aloin powder as the core material. The aloin powder was dissolved in ethanol and water, respectively. The aloin powder, the aloin microcapsules prepared with ethanol, and the aloin microcapsules prepared with water were added to the waterborne coating with different contents, respectively. The effects of different modifiers and different contents on the color difference, gloss, adhesion, impact resistance, and antibacterial property of Staphylococcus aureus of the coating were tested. It was determined that the comprehensive performance of coating added with the aloin microcapsules prepared with ethanol as the solvent was the best. When the content of the aloin microcapsules with ethanol as the solvent was 7.0%, the color difference of the coating was small, the adhesion was grade 3, the impact resistance was 12 kg·cm, and the antibacterial rate was 87.8%. From the perspective of antibacterial property, the three modifiers added to waterborne coatings all have certain antibacterial properties. The results provide a technical reference for the application of the aloin in antibacterial coating for wood furniture.
Author Contributions
Conceptualization, methodology, validation, resources, data management, and supervision, X.Y.; formal analysis, investigation, and writing—review and editing, N.H. All authors have read and agreed to the published version of the manuscript.
Funding
This project was partly supported by the Natural Science Foundation of Jiangsu Province (Grant No. BK20201386).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Yan, X.X.; Zhao, W.T.; Wang, L. Preparation and performance of thermochromic and self-repairing dual function paint film with lac resin microcapsules and fluorane microcapsules. Polymers 2021, 13, 3109. [Google Scholar] [CrossRef] [PubMed]
- Nan, F.; Liu, C.B.; Pu, J.B. Anticorrosive performance of waterborne epoxy coatings containing attapulgite/graphene nanocomposites. Surf. Topogr. Metrol. 2019, 7, 024002. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Gao, D.; Xu, W. Influence of the bottom color modification and material color modification process on the perfor-mance of modified Poplar. Coatings 2021, 11, 660. [Google Scholar] [CrossRef]
- Zigon, J.; Kovac, J.; Petric, M. The influence of mechanical, physical and chemical pre-treatment processes of wood surface on the relationships of wood with a waterborne opaque coating. Prog. Org. Coat. 2022, 162, 106574. [Google Scholar] [CrossRef]
- Zhao, Z.N.; Niu, Y.T.; Chen, F.Y. Development and finishing technology of waterborne UV lacquer-coated wooden flooring. Bioresources 2021, 16, 1101–1114. [Google Scholar] [CrossRef]
- Mahajan, K.; Kumar, S.; Naqvi, Z.; Mungure, T.E.; Bekhit, A.E.A. Functionalization of carrageenan based edible film using Aloe vera for improved lipid oxidative and microbial stability of frozen dairy products. Food Biosci. 2021, 43, 101336. [Google Scholar] [CrossRef]
- Lone, M.A.; Malviya, D.; Mishra, P.; Dubey, A.; Saxena, R.C. Antiinflammatory and antimicrobial activity of anthraquinone isolated from Aloe vera (Liliaceae). ASIAN J. Chem. 2009, 21, 1807–1811. [Google Scholar]
- Tian, B.; Hua, Y.J.; Ma, X.Q.; Wang, G.L. Relationship between antibacterial activity of aloe and its anthaquinone compounds. Zhongguo Zhong Yao Za Zhi 2003, 28, 1034–1037. [Google Scholar]
- Oumer, A.; Bisrat, D.; Mazumder, A.; Asres, K. A new antimicrobial anthrone from the leaf latex of aloe trichosantha. Nat. Prod. Commun. 2014, 9, 949–952. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, T.; Dweck, A.C. Aloe vera leaf gel: A review update. J. Ethnopharmacol. 1999, 68, 3–37. [Google Scholar] [CrossRef]
- Yan, X.X.; Zhao, W.T.; Qian, X.Y. Effect of water-based emulsion core microcapsules on aging resistance and self-repairing properties of water-based coatings on Linden. Appl. Sci. 2021, 11, 4662. [Google Scholar] [CrossRef]
- Yan, X.X.; Wang, L.; Qian, X.Y. Effect of microcapsules with different core-wall ratios on properties of waterborne primer coating for European Linden. Coatings 2020, 10, 826. [Google Scholar] [CrossRef]
- Yan, X.X.; Peng, W.W.; Qian, X.Y. Effect of water-based acrylic acid microcapsules on the properties of paint film for furniture surface. Appl. Sci. 2021, 11, 7586. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, C.; Du, Z.; Zou, W. Fabrication of shape-stabilized phase change materials with melamine foam/multi-walled carbon nanotubes composite as container. Compos. Interfaces 2020, 28, 255–271. [Google Scholar] [CrossRef]
- GB/T 4893.4-2013. Test of Surface Coatings of Furniture; Standardization Administration of the People’s Republic of China: Beijing, China, 2013. [Google Scholar]
- GB/T 9286-1998. Paint and Varnishes-Cross Cut Test for Films; Standardization Administration of the People’s Republic of China: Beijing, China, 1998. [Google Scholar]
- GB/T 1732-1993. Determination of Impact Resistance of Film; Standardization Administration of the People’s Republic of China: Beijing, China, 1993. [Google Scholar]
- GB/T 11186.3-1989. Methods for Measuring the Colour of Paint Films. Part Ⅲ: Calculation of Colour Differences; Standardization Administration of the People’s Republic of China: Beijing, China, 1990. [Google Scholar]
- Jamali, H.; Paydar, M.; Radmehr, B.; Ismail, S.; Dadrasnia, A. Prevalence and antimicrobial resistance of Staphylococcus aureus isolated from raw milk and dairy products. Food Control 2015, 54, 383–388. [Google Scholar] [CrossRef]
- Gao, J.; Ferreri, M.; Yu, F.Q.; Liu, X.Q.; Chen, L.B.; Su, J.L.; Han, B. Molecular types and antibiotic resistance of Staphylococcus aureus isolates from bovine mastitis in a single herd in China. Equine Vet. J. 2011, 192, 550–552. [Google Scholar] [CrossRef]
- GB/T 21866-2008. Test Method and Effect for Antibacterial Capability of Paints Film; Standardization Administration of the People’s Republic of China: Beijing, China, 2008. [Google Scholar]
- HG/T 3950-2007. Antibacterial Coatings; National Development and Reform Commission of the People’s Republic of China: Beijing, China, 2007. [Google Scholar]
- Xie, Y.F.; Huan, N.; Cao, Y.Y.; Wang, H.Y.; Zhong, Y.; Yao, W.R.; Qian, H. Structural characterization and spectroscopic analysis of the aloin. Spectrosc. Spect. Anal. 2014, 34, 385–388. [Google Scholar]
- ElSohly, M.A.; Gul, W.; Murphy, T.P. Analysis of the anthraquinones aloe-emodin and aloin by gas chromatography/mass spectrometry. Int. Immunopharmacol. 2004, 4, 1739–1744. [Google Scholar] [CrossRef]
- Wang, P.G.; Zhou, W.L.; Wamer, W.G.; Krynitsky, A.J.; Rader, J.I. Simultaneous determination of aloin A and aloe emodin in products containing aloe vera by ultra-performance liquid chromatography with tandem mass spectrometry. Anal. Methods 2012, 4, 3612–3619. [Google Scholar] [CrossRef]
- Chang, Y.J.; Yan, X.X. Preparation and self-repairing properties of MF-coated shellac water-based microcapsules. Coatings 2020, 10, 778. [Google Scholar] [CrossRef]
- Donkor, A.M.; Donkor, M.N.; Kuubabongnaa, N. Evaluation of anti-infective potencies of formulated aloin A ointment and aloin A isolated from Aloe barbadensis Miller. BMC Chem. 2020, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Coopoosamy, R.M.; Magwa, M.L. Antibacterial activity of aloe emodin and aloin A isolated from Aloe excelsa. Afr. J. Biotechnol. 2006, 5, 1092–1094. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).