Enhancing Electrical Conductivity and Corrosion Resistance of CrN Coating by Pt Addition
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.Z.; Yan, F.Y.; Xue, Q.J. Friction and wear behavior of ultra-high molecular weight polyethylene sliding against GCr15 steel and electroless Ni-P alloy coating under the lubrication of seawater. Tribol. Lett. 2009, 35, 85–95. [Google Scholar] [CrossRef]
- Hannel, S.; Fouvry, S.; Kapsa, P.H.; Vinent, L. The fretting sliding transition as a criterion for electrical contact performance. Wear 2001, 249, 761–770. [Google Scholar] [CrossRef]
- Wang, J.J.; Pu, J.B.; Zhang, G.G.; Wang, L.P. Architecture of superthick diamond-like carbon films with excellent high temperature wear resistance. Tribol. Int. 2015, 81, 129–138. [Google Scholar] [CrossRef]
- Rezaee, S.; Arman, A.; Jurečka, S.; Korpi, A.G.; Mwema, F.; Luna, C.; Sobola, D.; Kulesza, S.; Shakoury, R.; Bramowicz, M.; et al. Effect of annealing on the micromorphology and corrosion properties of Ti/SS thin films. Superlattices Microstr. 2020, 146, 106681. [Google Scholar] [CrossRef]
- Rahman, A.M.A.E. Synthesis and annealing effects on the properties of nanostructured Ti-Al-V-N coatings deposited by plasma enhanced magnetron sputtering. Mater. Chem. Phys. 2015, 149, 179–187. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, T.F.; Lee, H.B.R.; Yang, J.H.; Choi, W.C.; Han, B.; Kim, K.H.; Kwon, S.H. Improved corrosion resistance and mechanical properties of CrN hard coatings with an atomic layer deposited Al2O3 interlayer. ACS Appl. Mater. Inter. 2015, 7, 26716–26725. [Google Scholar] [CrossRef]
- Madaoui, N.; Saoula, N.; Zaid, B.; Saidi, D.; Ahmed, A.S. Structural, mechanical and electrochemical comparison of TiN and TiCN coatings on XC48 steel substrates in NaCl 3.5% water solution. Appl. Surf. Sci. 2014, 312, 134–138. [Google Scholar] [CrossRef]
- Ruden, A.; Restrepo Parra, E.; Paladines, A.U.; Sequeda, F. Corrosion resistance of CrN thin films produced by dc magnetron sputtering. Appl. Surf. Sci. 2013, 270, 150–156. [Google Scholar] [CrossRef]
- Martinez, E.; Sanjinés, R.; Karimi, A.; Esteve, J.; Lévy, F. Mechanical properties of nanocomposite and multilayered Cr-Si-N sputtered thin films. Surf. Coat. Technol. 2004, 180, 570–574. [Google Scholar] [CrossRef]
- Gilewicz, A.; Warcholinski, B. Tribological properties of CrCN/CrN multilayer coatings. Tribol. Int. 2014, 80, 34–40. [Google Scholar] [CrossRef]
- Xu, X.; Sun, J.F.; Xu, Z.B.; Li, Z.J.; Su, F.H. Microstructure, electrochemical and tribocorrosion behaviors of CrCN nanocomposite coating with various carbon content. Surf. Coat. Technol. 2021, 411, 126997. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhang, J.W.; Zhou, S.G.; Wang, Y.C.; Wang, C.T.; Wang, Y.X.; Sui, Y.F.; Lan, J.B.; Xue, Q. Improvement in the tribocorrosion performance of CrCN coating by multilayered design for marine protective application. Appl. Surf. Sci. 2020, 528, 147061. [Google Scholar] [CrossRef]
- Lamastra, F.R.; Leonardi, F.; Montanari, R.; Casadei, F.; Valente, T.; Gusmano, G. X-ray residual stress analysis on CrN/Cr/CrN multilayer PVD coatings deposited on different steel substrates. Surf. Coat. Technol. 2006, 200, 6172–6175. [Google Scholar] [CrossRef]
- Greczynski, G.; Lu, J.; Petrov, I.; Greene, J.E.; Hultman, L. Nitrogen-doped bcc-Cr films: Combining ceramic hardness with metallic toughness and conductivity. Scr. Mater. 2016, 122, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.Y.; Li, H.X.; Ji, L.; Liu, X.H.; Chen, J.M.; Wen, L.S. Insight into Al existing form and its role on microstructure and properties of Cr1-xAlxN films. Surf. Interface Anal. 2016, 48, 26–33. [Google Scholar] [CrossRef]
- Ibrahim, K.; Rahman, M.M.; Taha, H.; Mohammadpour, E.; Zhou, Z.F.; Yin, C.Y.; Nikoloski, A.; Jiang, Z.T. Structural, morphological, and optical characterizations of Mo, CrN and Mo:CrN sputtered coatings for potential solar selective applications. Appl. Surf. Sci. 2018, 440, 1001–1010. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.Y.; Zhang, X.H.; Wu, X.J.; Fang, F.; Jiang, J.Q. Comparison study on structure of Si and Cu doping CrN films by reactive sputtering. Appl. Surf. Sci. 2011, 257, 5595–5600. [Google Scholar] [CrossRef]
- Qi, D.L.; Lei, H.; Wang, T.G.; Pei, Z.; Gong, J.; Sun, C. Mechanical, microstructural and tribological properties of reactive magnetron sputtered Cr-Mo-N films. J. Mater. Sci. Technol. 2015, 31, 55–64. [Google Scholar] [CrossRef]
- Kong, J.Z.; Hou, T.J.; Wang, Q.Z.; Yin, L.; Zhou, F.; Zhou, Z.F.; Li, K.Y. Influence of titanium or aluminum doping on the electrochemical properties of CrN coatings in artificial seawater. Surf. Coat. Technol. 2016, 307, 118–124. [Google Scholar] [CrossRef]
- Lin, J.; Mishra, B.; Moore, J.J.; Sproul, W.D. Microstructure, mechanical and tribological properties of Cr1−xAlxN films deposited by pulsed-closed field unbalanced magnetron sputtering (P-CFUBMS). Surf. Coat. Technol. 2006, 201, 4329–4334. [Google Scholar] [CrossRef]
- Huang, X.F.; Xie, Z.W.; Li, K.S.; Chen, Q.; Gong, F.; Chen, Y.J.; Hu, S.Y.; Chen, Y.; Han, B.; Wu, D.; et al. Gong, Microstructure, wear and oxidation resistance of CrWN glass molding coatings synthesized by plasma enhanced magnetron sputtering. Vacuum 2020, 174, 12–23. [Google Scholar] [CrossRef]
- Rajput, S.S.; Gangopadhyay, S.; Cavaleiro, A.; Al-Rjoub, A.; Kumar, C.S.; Fernandes, F. Influence of Ag additions on the structure, mechanical properties and oxidation behaviour of CrAlNAg coatings deposited by sputtering. Surf. Coat. Technol. 2018, 333, 167–180. [Google Scholar] [CrossRef]
- Hong, C.F.; Huan, Y.X.; Zhang, P.S.; Zhang, K.; Dai, P.Q. Effect of silver content on the microstructure, thermal stability and mechanical properties of CrNx/Ag nanocomposite films. Ceram. Int. 2021, 47, 25324–25336. [Google Scholar] [CrossRef]
- Heo, S.J.; Kim, S.W.; Yeo, I.W.; Park, S.J.; Oh, Y.S. Effect of bias voltage on microstructure and phase evolution of Cr-Mo-N coatings by an arc bonded sputter system. Ceram. Int. 2016, 42, 5231–5237. [Google Scholar] [CrossRef]
- Thornton, J.A.; Lamb, J.L. Thermal stability studies of sputter-deposited multilayer selective absorber coatings. Thin Solid Films 1982, 96, 175–183. [Google Scholar] [CrossRef]
- Bagcivan, N.; Bobzin, K.; Theiss, S. (Cr1-xAlx)N: A comparison of direct current, middle frequency pulsed and high power pulsed magnetron sputtering for injection molding components. Thin Solid Films 2013, 528, 180–186. [Google Scholar] [CrossRef]
- Pan, P.J.; Drnovsek, A.; Kovac, J.; Gselman, P.; Boncina, T.; Paskvale, S.; Cekada, M.; Merl, D.K.; Man, P.J. Oxidation resistance of CrN/(Cr,V)N hard coatings deposited by DC magnetron sputtering. Thin Solid Films 2015, 591, 323–329. [Google Scholar] [CrossRef]
- Beliardouh, N.E.; Bouzid, K.; Nouveau, C.; Tlili, B.; Walock, M.J. Tribological and electrochemical performances of Cr/CrN and Cr/CrN/CrAlN multilayer coatings deposited by RF magnetron sputtering. Tribol. Int. 2015, 82, 443–452. [Google Scholar] [CrossRef] [Green Version]
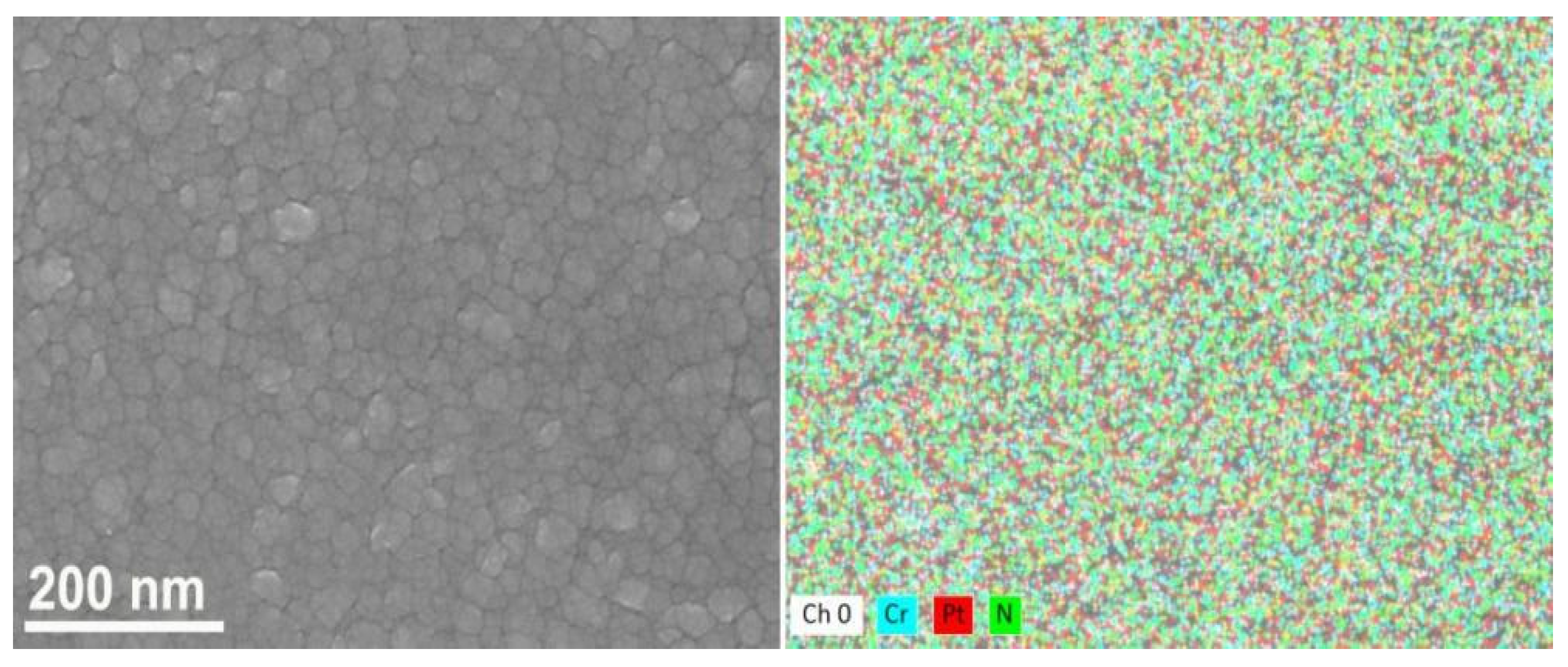
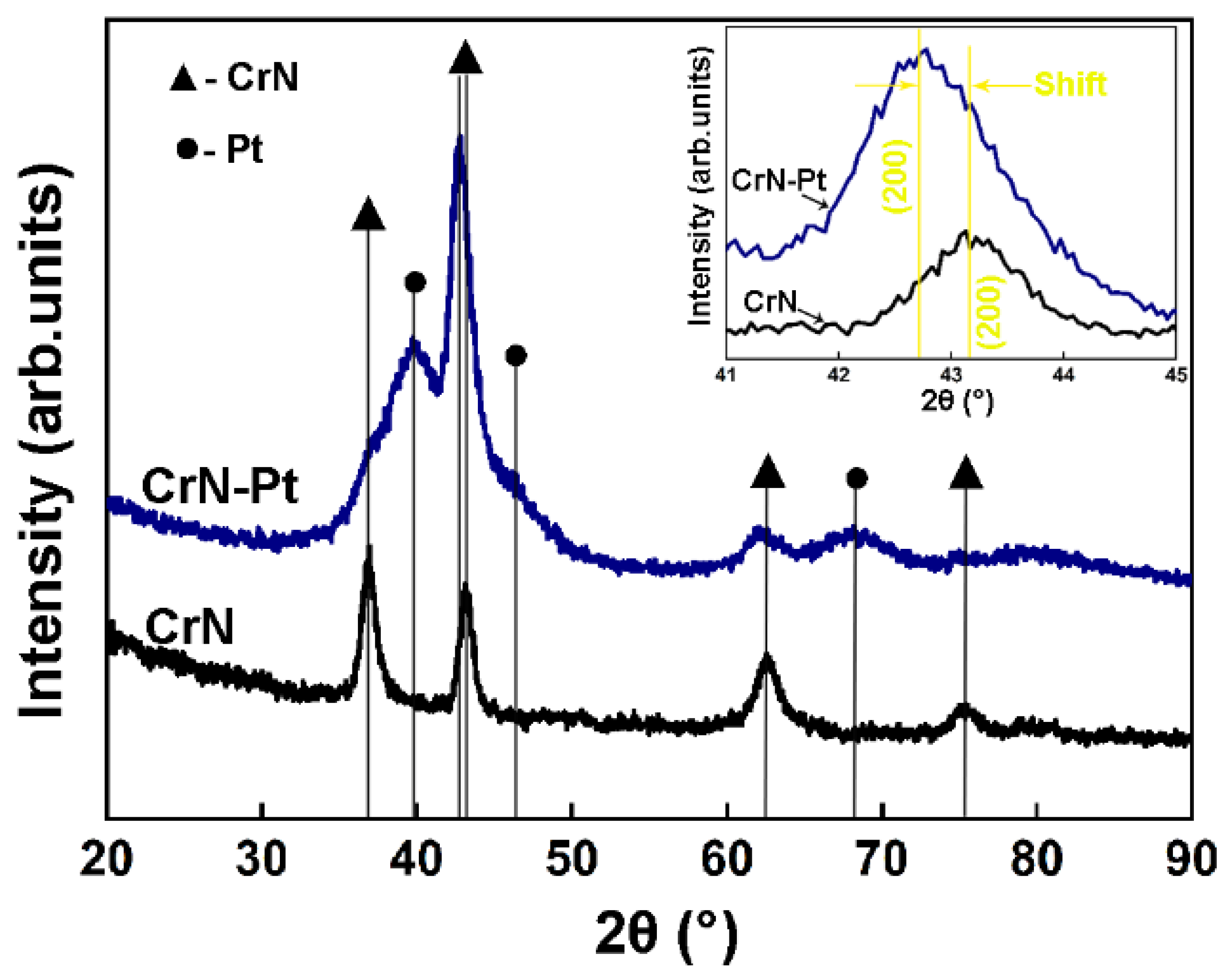
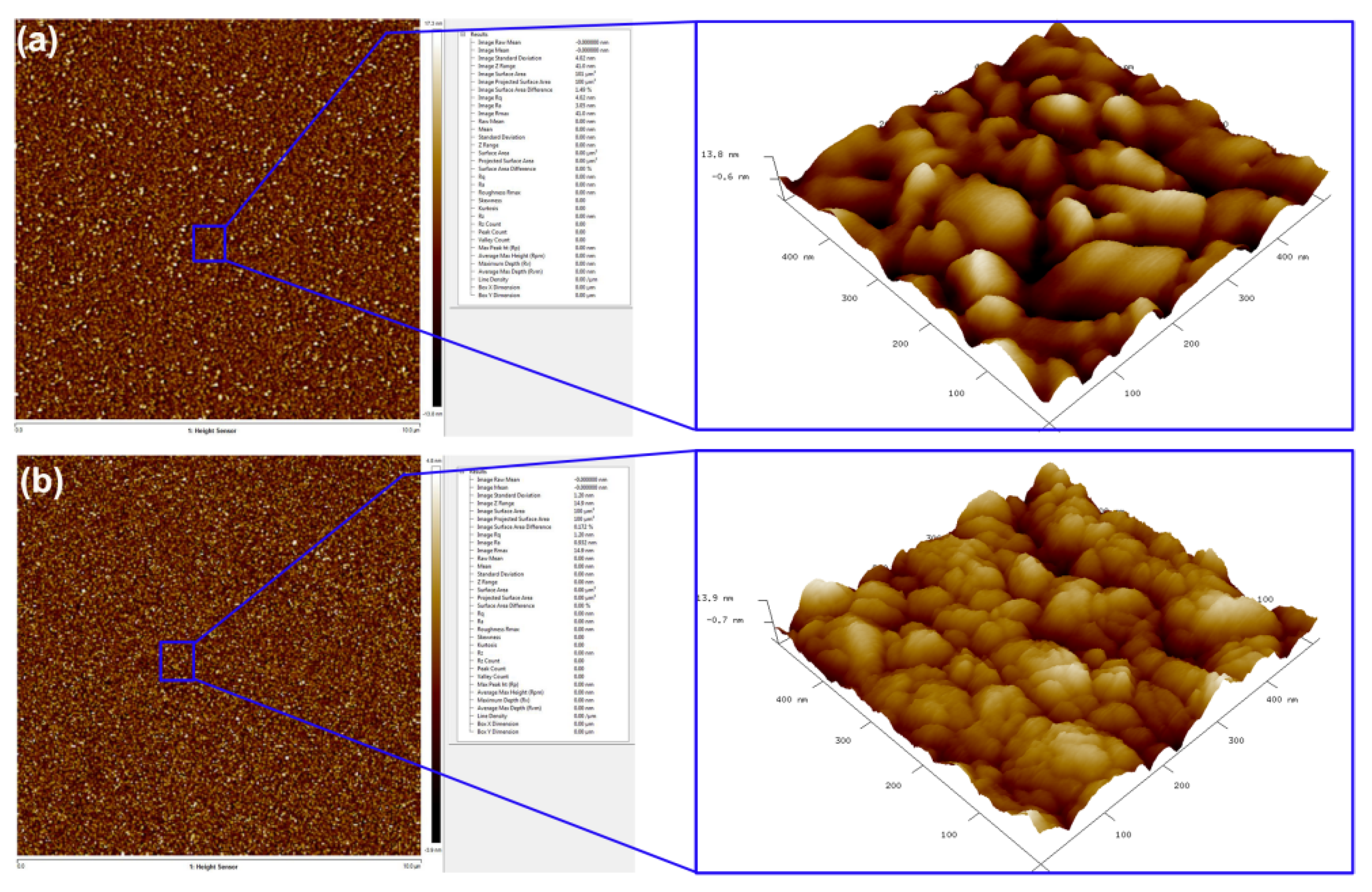
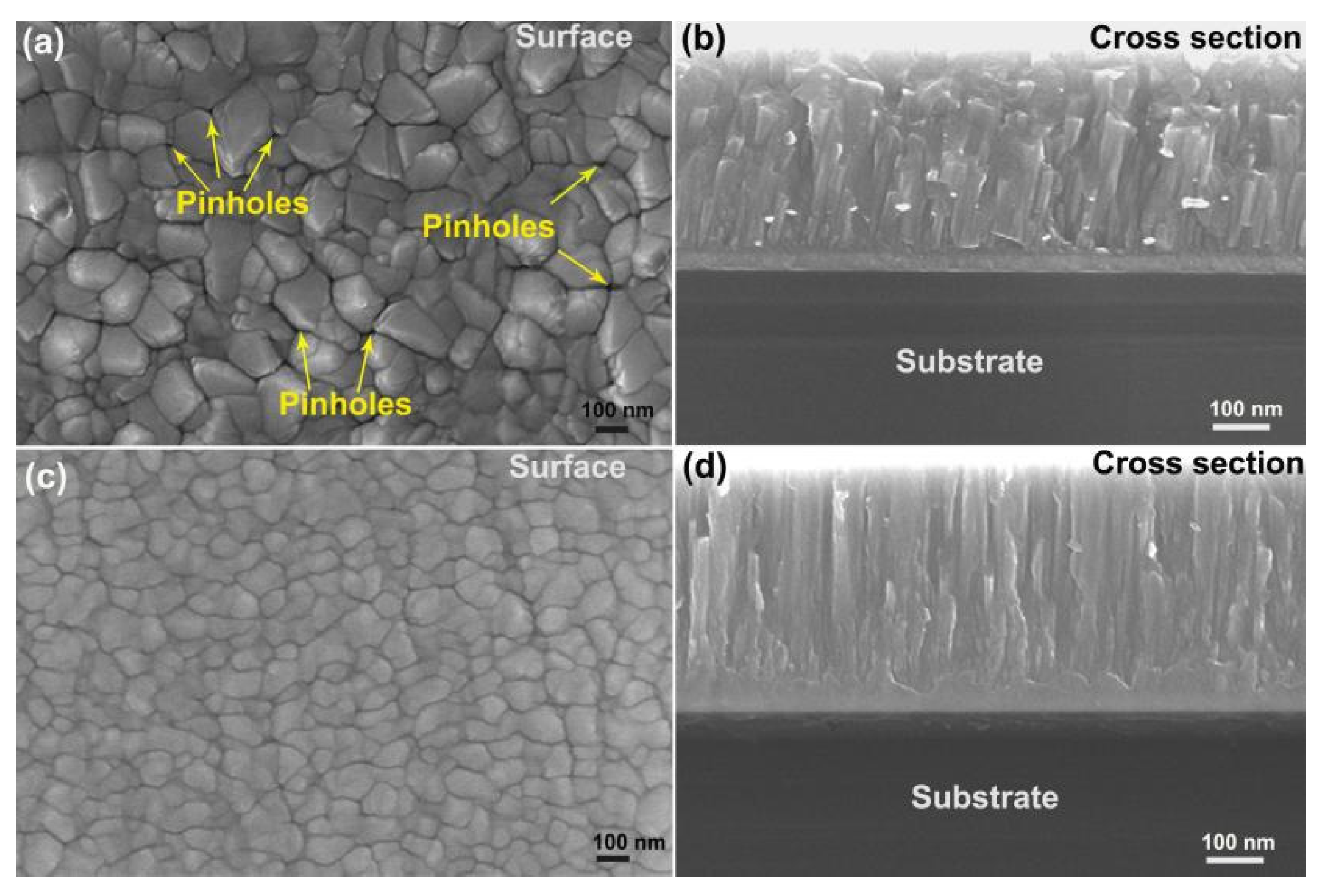
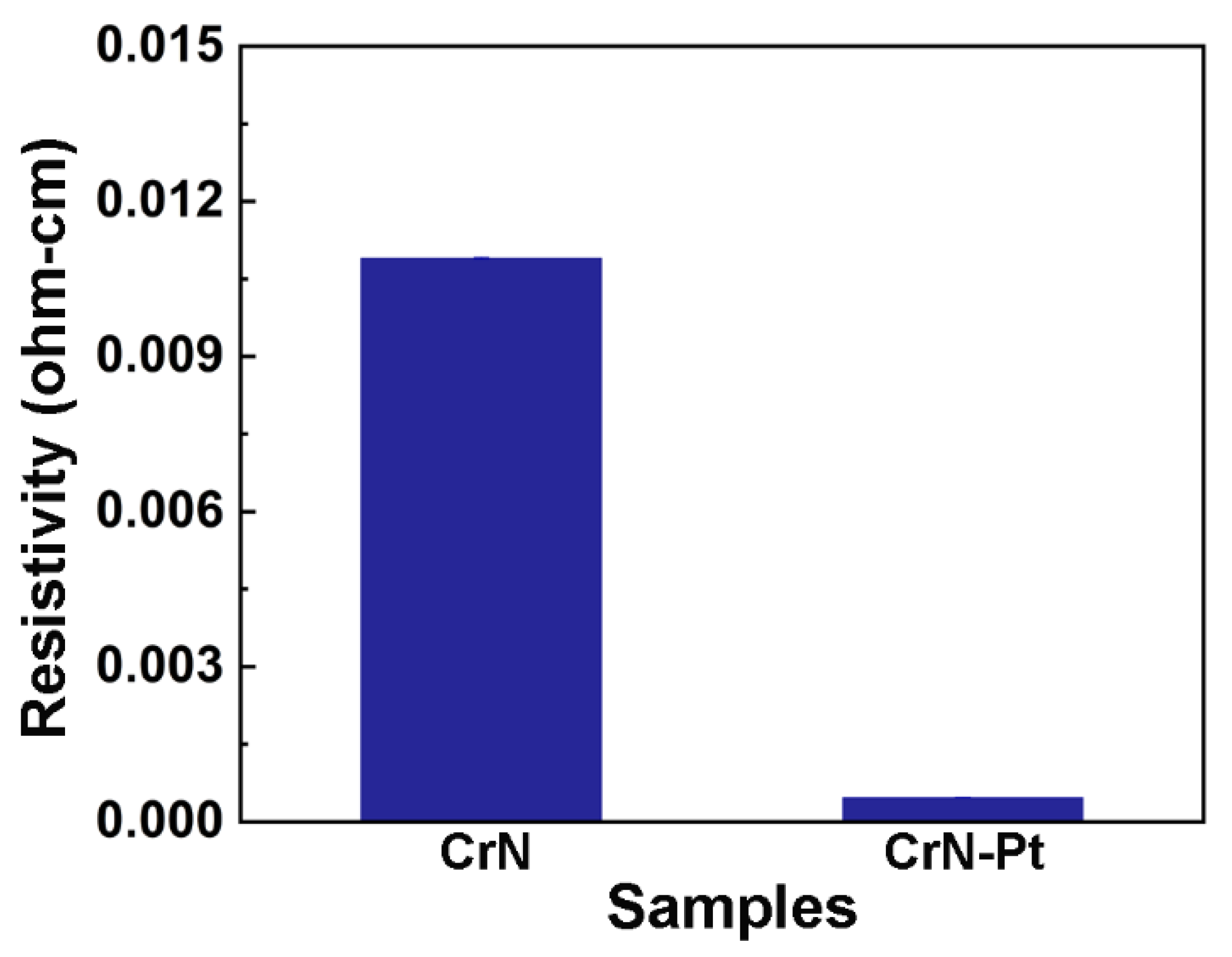
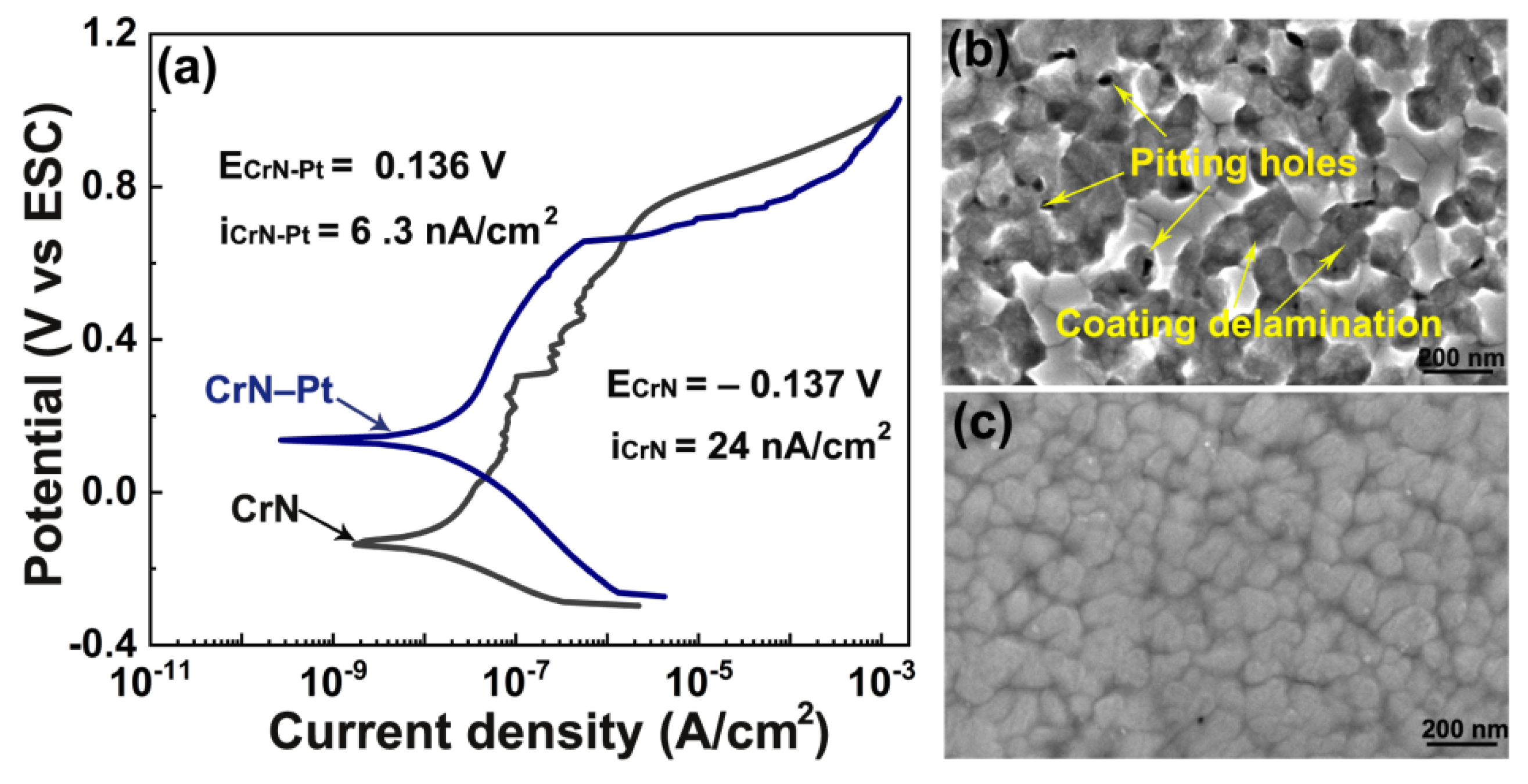
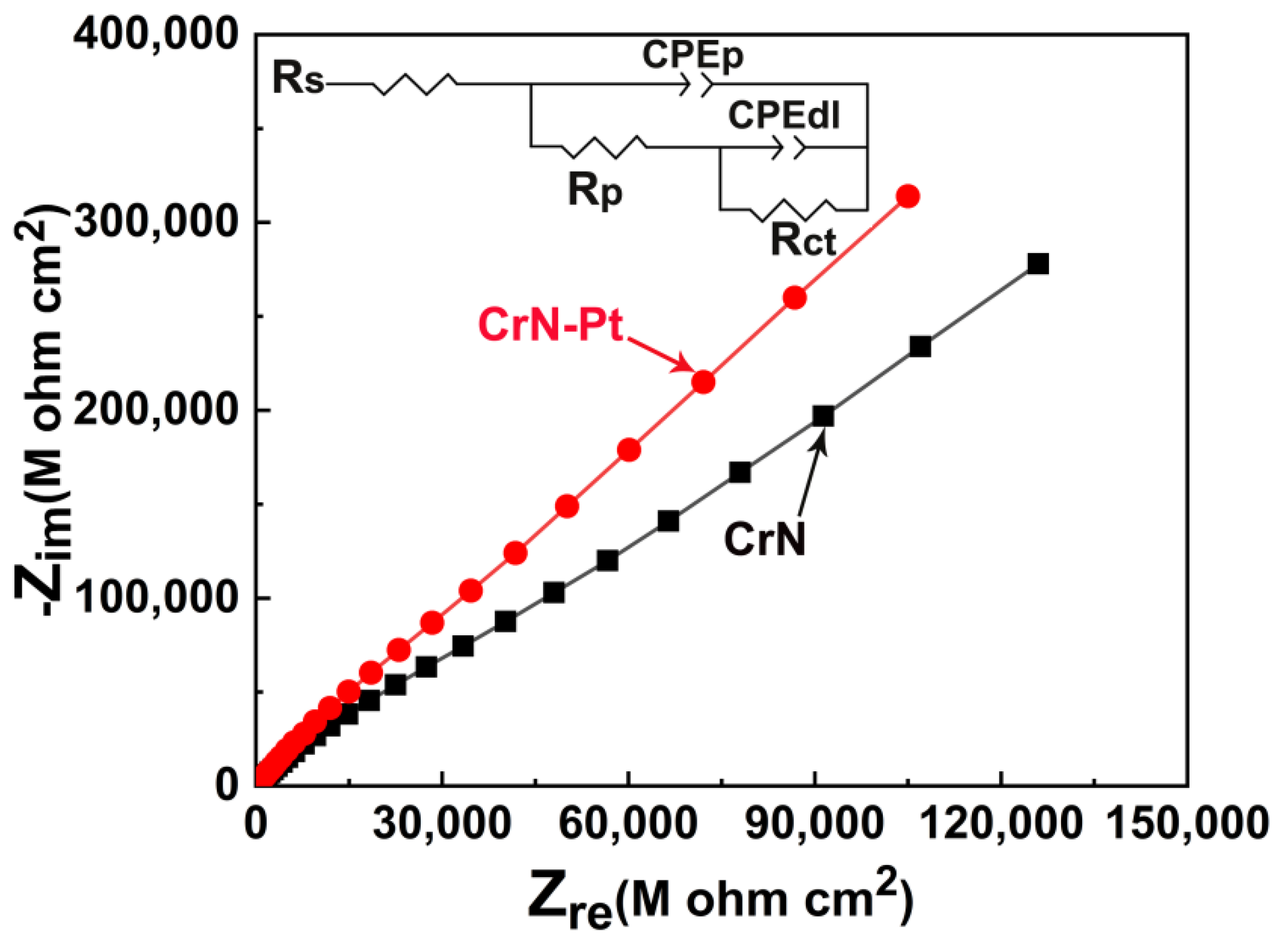
| Coating | Cr at.% | N at.% | Pt at.% | O at.% |
|---|---|---|---|---|
| CrN | 53.48 | 45.16 | - | 1.36 |
| CrN–Pt | 45.97 | 39.26 | 13.33 | 1.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Wang, Y.; Xiang, L.; Song, G.; Xie, Z. Enhancing Electrical Conductivity and Corrosion Resistance of CrN Coating by Pt Addition. Coatings 2021, 11, 1479. https://doi.org/10.3390/coatings11121479
Wu H, Wang Y, Xiang L, Song G, Xie Z. Enhancing Electrical Conductivity and Corrosion Resistance of CrN Coating by Pt Addition. Coatings. 2021; 11(12):1479. https://doi.org/10.3390/coatings11121479
Chicago/Turabian StyleWu, Hulin, Yihe Wang, Lin Xiang, Guanlin Song, and Zhiwen Xie. 2021. "Enhancing Electrical Conductivity and Corrosion Resistance of CrN Coating by Pt Addition" Coatings 11, no. 12: 1479. https://doi.org/10.3390/coatings11121479
APA StyleWu, H., Wang, Y., Xiang, L., Song, G., & Xie, Z. (2021). Enhancing Electrical Conductivity and Corrosion Resistance of CrN Coating by Pt Addition. Coatings, 11(12), 1479. https://doi.org/10.3390/coatings11121479





