RETRACTED: Advanced Binder-Free Electrode Based on CuCo2O4 Nanowires Coated with Polypyrrole Layer as a High-Performance Nonenzymatic Glucose Sensing Platform
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of CuCo2O4 NWs-pPy@CCE
2.2. Materials Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
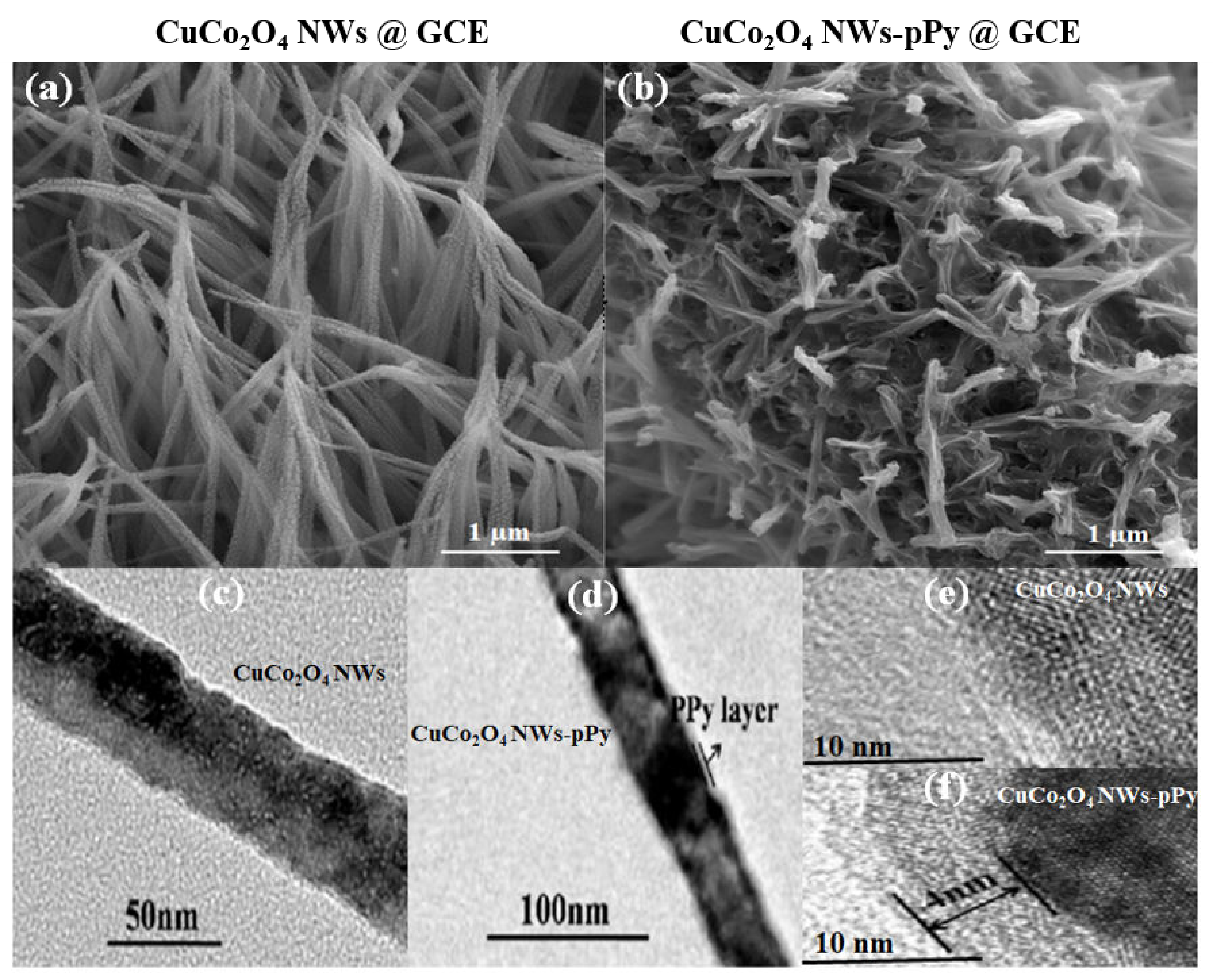
3.1. Materials Characterization
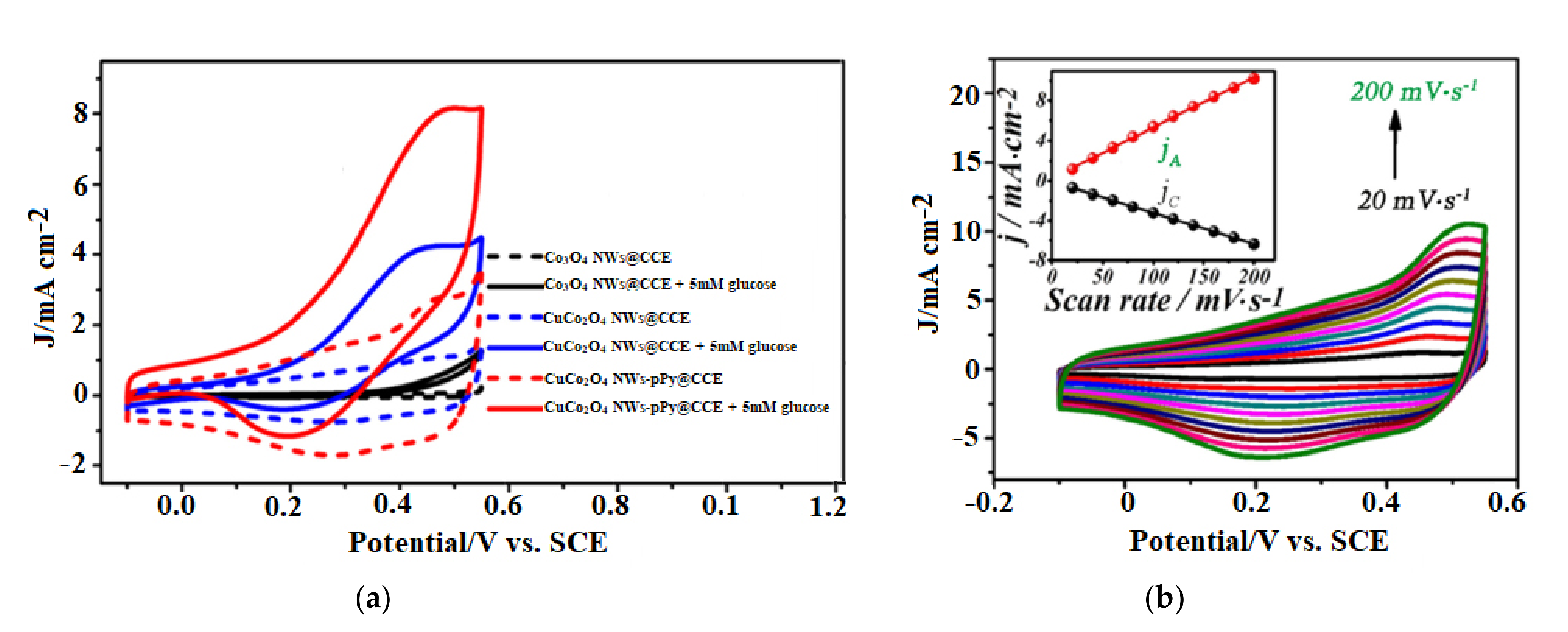
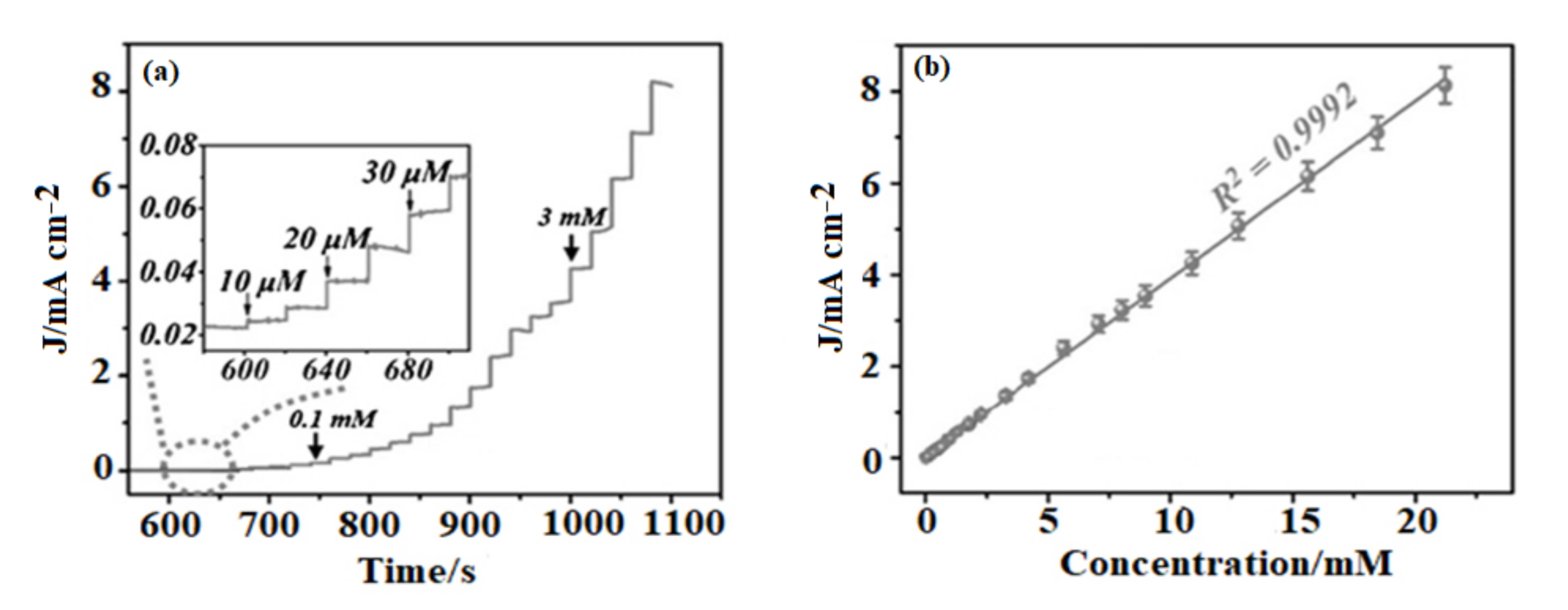
3.2. Sensor Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ni, J.; Zhuang, X.; Wahab, M.A. Review on The Prediction of Residual Stress in Welded Steel Components. Comput. Mater. Contin. 2020, 62, 495–523. [Google Scholar] [CrossRef]
- Kang, S.; Park, T. Detecting Outlier Behavior of Game Player Players using Multimodal Physiology Data. Intell. Autom. Soft Comput. 2019, 26, 205–214. [Google Scholar] [CrossRef]
- Kaur, S.; Joshi, V.K. Hybrid soft computing technique based trust evaluation protocol for wireless sensor networks. Intell. Autom. Soft Comput. 2020, 26, 217–226. [Google Scholar] [CrossRef]
- Sharma, M.; Pham, H.; Singh, V. Modeling and Analysis of Leftover Issues and Release Time Planning in Multi-Release Open Source Software Using Entropy Based Measure. Comput. Syst. Sci. Eng. 2019, 34, 33–46. [Google Scholar] [CrossRef]
- Vengadeswaran, S.; Balasundaram, S.R. Core—An optimal data placement strategy in hadoop for data intentitive applications based on cohesion relation. Comput. Syst. Sci. Eng. 2019, 34, 47–60. [Google Scholar] [CrossRef]
- Roshani, M.; Phan, G.T.; Ali, P.J.M.; Roshani, G.H.; Hanus, R.; Duong, T.; Corniani, E.; Nazemi, E.; Kalmoun, E.M. Evaluation of flow pattern recognition and void fraction measurement in two phase flow independent of oil pipeline’s scale layer thickness. Alex. Eng. J. 2021, 60, 1955–1966. [Google Scholar] [CrossRef]
- Roshani, M.; Phan, G.; Roshani, G.H.; Hanus, R.; Nazemi, B.; Corniani, E.; Nazemi, E. Combination of X-ray tube and GMDH neural network as a nondestructive and potential technique for measuring characteristics of gas-oil–water three phase flows. Measurement 2021, 168, 108427. [Google Scholar] [CrossRef]
- Roshani, M.; Phan, G.; Faraj, R.H.; Phan, N.-H.; Roshani, G.H.; Nazemi, B.; Corniani, E.; Nazemi, E. Proposing a gamma radiation based intelligent system for simultaneous analyzing and detecting type and amount of petroleum by-products. Nucl. Eng. Technol. 2021, 53, 1277–1283. [Google Scholar] [CrossRef]
- Roshani, M.; Sattari, M.A.; Ali, P.J.M.; Roshani, G.H.; Nazemi, B.; Corniani, E.; Nazemi, E. Application of GMDH neural network technique to improve measuring precision of a simplified photon attenuation based two-phase flowmeter. Flow Meas. Instrum. 2020, 75, 101804. [Google Scholar] [CrossRef]
- Karami, A.; Roshani, G.H.; Khazaei, A.; Nazemi, E.; Fallahi, M. Investigation of different sources in order to optimize the nuclear metering system of gas–oil–water annular flows. Neural Comput. Appl. 2018, 32, 3619–3631. [Google Scholar] [CrossRef]
- Shalaby, M.; Sakoury, M.M.A.; Harthi, S.M.; Alshalawi, F.M.; Alhajji, M.M.; Alshaikh, Z.H.; Aljaber, A.H. Vitamin D3 for Health and Muscle Functions of Athletes. Syst. Rev. Pharm. 2020, 11, 851–854. [Google Scholar] [CrossRef]
- Shalaby, M.N.; Saad, M.; Akar, S.; Reda, M.A.A.; Shalgham, A. The Role of Aerobic and Anaerobic Training Programs on CD34+ Stem Cells and Chosen Physiological Variables. J. Hum. Kinet. 2012, 35, 69–79. [Google Scholar] [CrossRef]
- Li, K.; Yang, W.; Li, K. A Hybrid Parallel Solving Algorithm on GPU for Quasi-Tridiagonal System of Linear Equations. IEEE Trans. Parallel Distrib. Syst. 2016, 27, 2795–2808. [Google Scholar] [CrossRef]
- Li, K.; Tang, X.; Veeravalli, B.; Li, K. Scheduling Precedence Constrained Stochastic Tasks on Heterogeneous Cluster Systems. IEEE Trans. Comput. 2015, 64, 191–204. [Google Scholar] [CrossRef]
- Yang, W.; Li, K.; Mo, Z.; Li, K. Performance Optimization Using Partitioned SpMV on GPUs and Multicore CPUs. IEEE Trans. Comput. 2015, 64, 2623–2636. [Google Scholar] [CrossRef]
- Mei, J.; Li, K.; Ouyang, A.; Li, K. A Profit Maximization Scheme with Guaranteed Quality of Service in Cloud Computing. IEEE Trans. Comput. 2015, 64, 3064–3078. [Google Scholar] [CrossRef]
- Li, K.; Yang, W.; Li, K. Performance Analysis and Optimization for SpMV on GPU Using Probabilistic Modeling. IEEE Trans. Parallel Distrib. Syst. 2015, 26, 196–205. [Google Scholar] [CrossRef]
- Li, K.; Ai, W.; Tang, Z.; Zhang, F.; Jiang, L.; Li, K.; Hwang, K. Hadoop Recognition of Biomedical Named Entity Using Conditional Random Fields. IEEE Trans. Parallel Distrib. Syst. 2015, 26, 3040–3051. [Google Scholar] [CrossRef]
- Xu, Y.; Li, K.; He, L.; Zhang, L.; Li, K. A Hybrid Chemical Reaction Optimization Scheme for Task Scheduling on Heterogeneous Computing Systems. IEEE Trans. Parallel Distrib. Syst. 2015, 26, 3208–3222. [Google Scholar] [CrossRef]
- Li, K.; Zheng, W.; Li, K. A Fast Algorithm with Less Operations for Length—N = q × 2 m DFTs. IEEE Trans. Signal Process 2015, 63, 673–683. [Google Scholar] [CrossRef]
- Li, K.; Tang, X.; Li, K. Energy-Efficient Stochastic Task Scheduling on Heterogeneous Computing Systems. IEEE Trans. Parallel Distrib. Syst. 2014, 25, 2867–2876. [Google Scholar] [CrossRef]
- Tang, X.; Li, K.; Zeng, Z. Bharadwaj Veeravalli: A Novel Security-Driven Scheduling Algorithm for Precedence-Constrained Tasks in Heterogeneous Distributed Systems. IEEE Trans. Comput. 2011, 60, 1017–1029. [Google Scholar]
- Shalaby, M.N.; Fadl, M.A. Relative Indicators and Predicative Ability of Some Biological Variables on Cardiac Neural Activity for Volleyball Players. Syst. Rev. Pharm. 2020, 11, 834–840. [Google Scholar] [CrossRef]
- Hedayati, S.A.A.; Khabbazi, M.; Harsij, M.; Hasan Gerami, M.; Ghafari Farsani, H. Investigation on destructive effect of water-born CuO nanoparticles on gill histopathology of rainbow trout, Oncorhynchus mykiss. Aquat. Physiol. Biotechnol. 2014, 2, 75–88. [Google Scholar]
- Khabbazi, M.; Harsij, M.; Hedayati, S.A.A.; Gholipoor, H.; Gerami, M.H.; Ghafari Farsani, H. Effect of CuO nanoparticles on some hematological indices of rainbow trout Oncorhynchus mykiss and their potential toxicity. Nanomed. J. 2015, 2, 67–73. [Google Scholar]
- Khabbazi, M.; Harsij, M.; Hedayati, S.A.A.; Gerami, M.H.; Ghafari, F.H. Effect of CuSo4 sub-lethal concentration on some hematological indices of Rainbow Trout Oncorhynchus mykiss. J. Aquat. Ecol. 2015, 4, 1–7. [Google Scholar]
- Ismail, U.U.; Shariff, M.H.M. Potential Therapeutic Effects of Olea Europaea (Olive) Fruit Oil as neuroprotective agent against neurotocixity induced opioid. J. Cell. Mol. Anesth. 2019, 3, 159. [Google Scholar]
- Othman, Z.; Khalep, H.R.H.; Abidin, A.Z.; Hassan, H.; Fattepur, S. The Anti-Angiogenic Properties of Morinda citrifolia. L (Mengkudu) Leaves Using Chicken Chorioallantoic Membrane (CAM) Assay. Pharmacogn. J. 2019, 11, 12–15. [Google Scholar] [CrossRef]
- Zharif, N.; Santosh, F.; Kiran, C.N.; Fadli, A.; Ibrahim, A.; Nizam, G. Synergistic effect of ethanolic extract of melastoma malabataricum leaves and antibiotics. Int. J. Med. Toxicol. Leg. Med. 2018, 21, 167. [Google Scholar] [CrossRef]
- Ngafwan, N.; Rasyid, H.; Abood, E.S.; Abdelbasset, W.K.; Al-Shawi, S.G.; Bokov, D.; Jalil, A.T. Study on novel fluorescent carbon nanomaterials in food analysis. Food Sci. Technol. 2021, 1, 1–6. [Google Scholar] [CrossRef]
- Hutapea, S.; Ghazi Al-Shawi, S.; Chen, T.C.; You, X.; Bokov, D.; Abdelbasset, W.K.; Suksatan, W. Study on food preservation materials based on nano-particle reagents. Food Sci. Technol. 2021, 1, 1–7. [Google Scholar] [CrossRef]
- King, H.; Aubert, E.R.; Herman, W.H. Global Burden of Diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care 1998, 21, 1414–1431. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-S.; Choi, S.-J.; Kim, S.-J.; Hakim, M.; Kim, I.-D. Rational Design of Highly Porous SnO2Nanotubes Functionalized with Biomimetic Nanocatalysts for Direct Observation of Simulated Diabetes. Adv. Funct. Mater. 2016, 26, 4740–4748. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, X.; Li, F.; Sangaiah, A.K.; Ding, X. Seam-Carved Image Tampering Detection Based on the Cooccurrence of Adjacent LBPs. Secur. Commun. Netw. 2020, 2020, 8830310. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, D.; Tang, Q.; Tang, S.; Yang, K. Local and nonlocal constraints for compressed sensing video and multi-view image recovery. Neurocomputing 2020, 406, 34–48. [Google Scholar] [CrossRef]
- Zhou, S.; Qiu, J. Enhanced SSD with interactive multi-scale attention features for object detection. Multimed. Tools Appl. 2021, 80, 11539–11556. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, K.; Yang, K.; Luo, Y.-S. Congestion-Balanced and Welfare-Maximized Charging Strategies for Electric Vehicles. IEEE Trans. Parallel Distrib. Syst. 2020, 31, 2882–2895. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.; Ren, Y.; Alfarraj, O.; Wang, L. Blockchain Based Data Storage Mechanism in Cyber Physical System. J. Internet Technol. 2020, 21, 1681–1689. [Google Scholar]
- Song, Y.; Li, J.; Chen, X.; Zhang, D.; Tang, Q.; Yang, K. An efficient tensor completion method via truncated nuclear norm. J. Vis. Commun. Image Represent. 2020, 70, 102791. [Google Scholar] [CrossRef]
- Wang, J.; Wu, W.; Liao, Z.; Jung, Y.W.; Kim, J.U. An Enhanced PROMOT Algorithm with D2D and Robust for Mobile Edge Computing. J. Internet Technol. 2020, 21, 1437–1445. [Google Scholar]
- Zhang, D.; Wang, S.; Li, F.; Tian, S.; Wang, J.; Ding, X.; Gong, R. An Efficient ECG Denoising Method Based on Empirical Mode Decomposition, Sample Entropy, and Improved Threshold Function. Wirel. Commun. Mob. Comput. 2020, 2020, 8811962. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, K.; Song, Y.; Li, F.; Park, J.H. Waiting Time Minimized Charging and Discharging Strategy Based on Mobile Edge Computing Supported by Software-Defined Network. IEEE Internet Things J. 2020, 7, 6088–6101. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, K.; Xiang, L.; Luo, Y.; Xiong, B.; Tang, Q. A Self-Adaptive Regression-Based Multivariate Data Compression Scheme with Error Bound in Wireless Sensor Networks. Int. J. Distrib. Sens. Netw. 2013, 9, 913497. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Wang, J.; Yue, X.-G. Visual object tracking based on residual network and cascaded correlation filters. J. Ambient. Intell. Humaniz. Comput. 2021, 12, 8427–8440. [Google Scholar] [CrossRef]
- Gu, K.; Wang, Y.; Wen, S. Traceable Threshold Proxy Signature. J. Inf. Sci. Eng. 2017, 33, 63–79. [Google Scholar]
- Li, W.; Ding, Y.; Yang, Y.; Sherratt, R.S.; Park, J.H.; Wang, J. Parameterized algorithms of fundamental NP-hard problems: A survey. Human-Centric Comput. Inf. Sci. 2020, 10, 29. [Google Scholar] [CrossRef]
- Gu, K.; Yang, L.; Wang, Y.; Wen, S. Traceable Identity-Based Group Signature. RAIRO—Theor. Inform. Appl. 2016, 50, 193–226. [Google Scholar] [CrossRef]
- Yin, B.; Zhou, S.; Lin, Y.; Liu, Y.; Hu, Y. Efficient distributed skyline computation using dependency-based data partitioning. J. Syst. Softw. 2014, 93, 69–83. [Google Scholar] [CrossRef]
- Long, M.; Xiao, X. Outage performance of double-relay cooperative transmission network with energy harvesting. Phys. Commun. 2018, 29, 261–267. [Google Scholar] [CrossRef]
- Xu, Z.; Liang, W.; Li, K.-C.; Xu, J.; Jin, H. A blockchain-based Roadside Unit-assisted authentication and key agreement protocol for Internet of Vehicles. J. Parallel Distrib. Comput. 2021, 149, 29–39. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Li, J.; Hu, Y.; Luo, Y.; Wang, X. Woodland Labeling in Chenzhou, China, via Deep Learning Approach. Int. J. Comput. Intell. Syst. 2020, 13, 1393. [Google Scholar] [CrossRef]
- Qu, Y.; Xiong, N. RFH: A Resilient, Fault-Tolerant and High-Efficient Replication Algorithm for Distributed Cloud Storage. In Proceedings of the 2012 41st International Conference on Parallel Processing, Pittsburgh, PA, USA, 10–13 September 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 520–529. [Google Scholar]
- Fang, W.; Yao, X.; Zhao, X.; Yin, J.; Xiong, N. A Stochastic Control Approach to Maximize Profit on Service Provisioning for Mobile Cloudlet Platforms. IEEE Trans. Syst. Man Cybern. Syst. 2016, 48, 522–534. [Google Scholar] [CrossRef]
- Hahn, Y.-B.; Ahmad, R.; Tripathy, N. Chemical and biological sensors based on metal oxide nanostructures. Chem. Commun. 2012, 48, 10369–10385. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Ahmad, R.; Mousa, H.M.; Kim, I.-G.; Kim, J.I.; Neupane, M.P.; Park, C.H.; Kim, C.S. High-performance glucose biosensor based on chitosan-glucose oxidase immobilized polypyrrole/Nafion/functionalized multi-walled carbon nanotubes bio-nanohybrid film. J. Colloid Interface Sci. 2016, 482, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, J.; Kim, J.; Na, H.B.; Kim, B.; Shin, C.-H.; Kwak, J.H.; Dohnalkova, A.; Grate, J.W.; Hyeon, T.; et al. Simple Fabrication of a Highly Sensitive and Fast Glucose Biosensor Using Enzymes Immobilized in Mesocellular Carbon Foam. Adv. Mater. 2005, 17, 2828–2833. [Google Scholar] [CrossRef]
- Zarei, M.; Davarpanah, A.; Mokhtarian, N. and Farahbod, F. Integrated feasibility experimental investigation of hydrodynamic, geometrical and, operational characterization of methanol conversion to formaldehyde. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 42, 89–103. [Google Scholar] [CrossRef]
- Davarpanah, A. Feasible analysis of reusing flowback produced water in the operational performances of oil reservoirs. Environ. Sci. Pollut. Res. 2018, 25, 35387–35395. [Google Scholar] [CrossRef]
- Valizadeh, K.; Davarpanah, A. Design and construction of a micro-photo bioreactor in order to dairy wastewater treatment by micro-algae: Parametric study. Energy Sources Part A Recover. Util. Environ. Eff. 2019, 42, 611–624. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Zhang, E.; Xie, Y.; Ci, S.; Jia, J.; Wen, Z. Porous Co3O4 hollow nanododecahedra for nonenzymatic glucose biosensor and biofuel cell. Biosens. Bioelectron. 2016, 81, 46–53. [Google Scholar] [CrossRef]
- Long, M.; Tan, L.; Liu, H.; He, Z.; Tang, A. Novel helical TiO2 nanotube arrays modified by Cu2O for enzyme-free glucose oxidation. Biosens. Bioelectron. 2014, 59, 243–250. [Google Scholar] [CrossRef]
- Syah, R.; Alizadeh, S.M.; Nasution, M.K.; Kashkouli, M.N.I.; Elveny, M. and Khan, A. Carbon dioxide-based enhanced oil recovery methods to evaluate tight oil reservoirs productivity: A laboratory perspective coupled with geo-sequestration feature. Energy Rep. 2021, 7, 4697–4704. [Google Scholar] [CrossRef]
- Zeng, G.; Li, W.; Ci, S.; Jia, J.; Wen, Z. Highly Dispersed NiO Nanoparticles Decorating graphene Nanosheets for Non-enzymatic Glucose Sensor and Biofuel Cell. Sci. Rep. 2016, 6, 36454. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.-X.; Wong, W.-T.; Liu, W.-R. Temperature effects on a nano-porous ZnCo2O4 anode with excellent capability for Li-ion batteries. RSC Adv. 2015, 5, 75838–75845. [Google Scholar] [CrossRef]
- Liu, L.; Hu, Z.; Sun, L.; Gao, G.; Liu, X. Controlled synthesis and enhanced electrochemical performance of Prussian blue analogue-derived hollow FeCo2O4 nanospheres as lithium-ion battery anodes. RSC Adv. 2015, 5, 36575–36581. [Google Scholar] [CrossRef]
- Shanmugavani, A.; Selvan, R.K. Improved electrochemical performances of CuCo2O4/CuO nanocomposites for asymmetric supercapacitors. Electrochim. Acta 2016, 188, 852–862. [Google Scholar] [CrossRef]
- Yang, J.; Ye, H.; Zhang, Z.; Zhao, F.; Zeng, B. Metal–organic framework derived hollow polyhedron CuCo2O4 functionalized porous graphene for sensitive glucose sensing. Sens. Actuators B Chem. 2017, 242, 728–735. [Google Scholar] [CrossRef]
- Luo, X.; Huang, M.; Bie, L.; He, D.; Zhang, Y.; Jiang, P. CuCo2O4 nanowire arrays supported on carbon cloth as an efficient 3D binder-free electrode for non-enzymatic glucose sensing. RSC Adv. 2017, 7, 23093–23101. [Google Scholar] [CrossRef]
- Liu, F.; Zhuang, Y.; Guo, M.; Chen, Y.; Tu, J.; Ding, L. 3D Copper Foam-Supported CuCo2O4 Nanosheet Arrays as Electrode for Enhanced Non-Enzymatic Glucose Sensing. Sensors 2018, 18, 1131. [Google Scholar] [CrossRef]
- Marimuthu, T.; Mohamad, S.; Alias, Y. Needle-like polypyrrole–NiO composite for non-enzymatic detection of glucose. Synth. Met. 2015, 207, 35–41. [Google Scholar] [CrossRef]
- Papi, M.A.P.; Caetano, F.R.; Bergamini, M.F.; Marcolino-Junior, L.H. Facile synthesis of a silver nanoparticles/polypyrrole nanocomposite for non-enzymatic glucose determination. Mater. Sci. Eng. C 2017, 75, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Shanmugavalli, V.; Vishista, K. An Investigation on the Supercapacitive Performance of CuCo2O4/Polyaniline, a Nanocomposite of Spinel Structured Transition Binary Metal Oxide and Conducting Polymer, with a Special Focus on Bonding and Electron Density Distribution Through MEM. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1448–1462. [Google Scholar] [CrossRef]
- Fu, Y.; Manthiram, A. Core-shell structured sulfur-polypyrrole composite cathodes for lithium-sulfur batteries. RSC Adv. 2012, 2, 5927–5929. [Google Scholar] [CrossRef]
- Han, F.; Li, D.; Li, W.-C.; Lei, C.; Sun, Q.; Lu, A.-H. Nanoengineered Polypyrrole-Coated Fe2O3@C Multifunctional Composites with an Improved Cycle Stability as Lithium-Ion Anodes. Adv. Funct. Mater. 2013, 23, 1692–1700. [Google Scholar] [CrossRef]
- Xu, C.; Sun, J.; Gao, L. Synthesis of novel hierarchical graphene/polypyrrole nanosheet composites and their superior electrochemical performance. J. Mater. Chem. 2011, 21, 11253–11258. [Google Scholar] [CrossRef]
- Dong, X.-C.; Xu, H.; Wang, X.-W.; Huang, Y.-X.; Chan-Park, M.B.; Zhang, H.; Wang, L.-H.; Huang, W.; Chen, P. 3D Graphene–Cobalt Oxide Electrode for High-Performance Supercapacitor and Enzymeless Glucose Detection. ACS Nano 2012, 6, 3206–3213. [Google Scholar] [CrossRef]
- Yu, Z.; Li, H.; Zhang, X.; Liu, N.; Tan, W.; Zhang, X.; Zhang, L. Facile synthesis of NiCo2O4@Polyaniline core–shell nanocomposite for sensitive determination of glucose. Biosens. Bioelectron. 2016, 75, 161–165. [Google Scholar] [CrossRef]
- Yang, J.; Cho, M.; Lee, Y. Synthesis of hierarchical NiCo2O4 hollow nanorods via sacrificial-template accelerate hydrolysis for electrochemical glucose oxidation. Biosens. Bioelectron. 2016, 75, 15–22. [Google Scholar] [CrossRef]
- Wu, M.; Meng, S.; Wang, Q.; Si, W.; Huang, W.; Dong, X. Nickel–Cobalt Oxide Decorated Three-Dimensional Graphene as an Enzyme Mimic for Glucose and Calcium Detection. ACS Appl. Mater. Interfaces 2015, 7, 21089–21094. [Google Scholar] [CrossRef]
- Xue, B.; Li, K.; Feng, L.; Lu, J.; Zhang, L. Graphene wrapped porous Co3O4/NiCo2O4 double-shelled nanocages with enhanced electrocatalytic performance for glucose sensor. Electrochim. Acta 2017, 239, 36–44. [Google Scholar] [CrossRef]
- Ji, S.; Yang, Z.; Zhang, C.; Miao, Y.-E.; Tjiu, W.W.; Pan, J.; Liu, T. Nonenzymatic sensor for glucose based on a glassy carbon electrode modified with Ni(OH)2 nanoparticles grown on a film of molybdenum sulfide. Microchim. Acta 2013, 180, 1127–1134. [Google Scholar] [CrossRef]
- Mu, Y.; Jia, D.; He, Y.; Miao, Y.; Wu, H.-L. Nano nickel oxide modified non-enzymatic glucose sensors with enhanced sensitivity through an electrochemical process strategy at high potential. Biosens. Bioelectron. 2011, 26, 2948–2952. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-M.; Lin, Z.-J.; Chen, D.; Jia, T.-T.; Cai, Z.-M.; Wang, X.-R.; Chen, X.; Chen, G.-N.; Oyama, M. Nonenzymatic amperometric sensing of glucose by using palladium nanoparticles supported on functional carbon nanotubes. Biosens. Bioelectron. 2010, 25, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, A. Parametric Study of Polymer-Nanoparticles-Assisted Injectivity Performance for Axisymmetric Two-Phase Flow in EOR Processes. Nanomaterials 2020, 10, 1818. [Google Scholar] [CrossRef]

| Electrode | Sensitivity (μA μM−1 cm−2) | Linear Range (mM) | Potential (V) | Response Time (s) | Ref. |
|---|---|---|---|---|---|
| NiCo2O4@Polyaniline | 4.5 | 0.015–4.7 | 0.5 | 5 | [78] |
| NiCo2O4 nanorod | 1.68 | 0.0003–1 | 0.6 | 2 | [79] |
| NiCo2O4/3D graphene | 2.5 | 0.005–0.59 | 0.5 | - | [80] |
| Co3O4/NiCo2O4/graphene | 0.304 | 0.01–3.52 | 0.55 | - | [81] |
| Ni(OH)2/MoSx | - | 0.01–1.3 | 0.6 | 2 | [82] |
| CuCo2O4 NWs-pPy | 4.41 | 0.01–21.3 | 0.45 | 1 | Our work |
| Sample | Commercial Analyzer (mM) | Our Designed Sensor (mM) | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| 1 | 5.31 | 5.42 | 3.2 | 102.7 |
| 2 | 3.71 | 3.63 | 2.3 | 97.8 |
| 3 | 7.11 | 7.23 | 4.1 | 101.7 |
| 4 | 4.76 | 4.63 | 2.7 | 97.2 |
| 5 | 10.6 | 10.4 | 3.1 | 98.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatshan, M.R.; Aslam, S.; Bokov, D.; Ibrahim, A.J.; Mustafa, Y.F.; Davarpanah, A.; Elveny, M.; Ali, S. RETRACTED: Advanced Binder-Free Electrode Based on CuCo2O4 Nanowires Coated with Polypyrrole Layer as a High-Performance Nonenzymatic Glucose Sensing Platform. Coatings 2021, 11, 1462. https://doi.org/10.3390/coatings11121462
Hatshan MR, Aslam S, Bokov D, Ibrahim AJ, Mustafa YF, Davarpanah A, Elveny M, Ali S. RETRACTED: Advanced Binder-Free Electrode Based on CuCo2O4 Nanowires Coated with Polypyrrole Layer as a High-Performance Nonenzymatic Glucose Sensing Platform. Coatings. 2021; 11(12):1462. https://doi.org/10.3390/coatings11121462
Chicago/Turabian StyleHatshan, Mohammad Rafe, Sadia Aslam, Dmitry Bokov, Ahmed Jaber Ibrahim, Yasser Fakri Mustafa, Afshin Davarpanah, Marischa Elveny, and Shafaqat Ali. 2021. "RETRACTED: Advanced Binder-Free Electrode Based on CuCo2O4 Nanowires Coated with Polypyrrole Layer as a High-Performance Nonenzymatic Glucose Sensing Platform" Coatings 11, no. 12: 1462. https://doi.org/10.3390/coatings11121462
APA StyleHatshan, M. R., Aslam, S., Bokov, D., Ibrahim, A. J., Mustafa, Y. F., Davarpanah, A., Elveny, M., & Ali, S. (2021). RETRACTED: Advanced Binder-Free Electrode Based on CuCo2O4 Nanowires Coated with Polypyrrole Layer as a High-Performance Nonenzymatic Glucose Sensing Platform. Coatings, 11(12), 1462. https://doi.org/10.3390/coatings11121462







