Validation of Antibacterial Systems for Sustainable Ceramic Tiles
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sciancalepore, C.; Manfredini, T.; Bondioli, F. Antibacterial and self-cleaning coatings for silicate ceramics: A review. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 92, 90–99. [Google Scholar]
- Seabra, M.P.; Grave, L.; Oliveira, C.; Alves, A.; Correia, A.; Labrincha, J.A. Porcelain stoneware tiles with antimicrobial action. Ceram. Int. 2014, 40, 6063–6070. [Google Scholar] [CrossRef]
- Berto, A.M. Ceramic tiles: Above and beyond traditional applications. J. Eur. Ceram. Soc. 2007, 27, 1607–1613. [Google Scholar] [CrossRef]
- Welch, K.; Sloan, G.; Ong, I.; Microban International. Antimicrobial Treatment for Ceramics and Sanitary Ware. World Ceram. Rev. 2017, 121, 104–108. [Google Scholar]
- Bianchi, C.L.; Cerrato, G.; Bresolin, B.M.; Djellabi, R.; Rtimi, S. Digitally printed AgNPs doped TiO2 on commercial porcelain-grès tiles: Synergistic effects and continuous photocatalytic antibacterial activity. Surfaces 2020, 3, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Baheiraeia, N.; Moztarzadeha, F.; Hedayati, M. Antibacterial Ag/SiO2 thin film on glazed ceramic tiles prepared by sol-gel method. In Proceedings of the 18th Iranian Conference of Biomedical Engineering (ICBME), Tehran, Iran, 14–16 December 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 105–108. [Google Scholar]
- Bondioli, F.; Manfredini, T. Le nanopolveri nella funzionalizzazione delle superfici ceramiche. Ceram. Inf. 2008, 468, 231–237. [Google Scholar]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnology 2017, 15, 65. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, F.; Olive, P.L.; Banuelos, A.; Orrantia, E.; Nino, N.; Sanchez, E.M.; Ruiz, F.; Bach, H.; Av-Gay, Y. Synthesis, characterization, and evaluation of antimicrobial and cytotoxic effect of silver and titanium nanoparticles. Nanomedicine 2010, 6, 681–688. [Google Scholar] [CrossRef]
- Simon-Deckers, A.; Loo, S.; Mayne-L’hermite, M.; Herlin-Boime, N.; Menguy, N.; Reynaud, C.; Gouget, B.; Carriere, M. Size-, composition- and shape-dependent toxicological impact of metal oxide nanoparticles and carbon nanotubes toward bacteria. Environ. Sci. Technol. 2009, 43, 8423–8429. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Hench, L.L.; Seal, S. The potential toxicity of nanomaterials—The role of surfaces. JOM 2006, 58, 77–82. [Google Scholar] [CrossRef]
- Choi, O.; Hu, Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef] [PubMed]
- Sohm, B.; Immel, F.; Bauda, P.; Pagnout, C. Insight into the primary mode of action of TiO2 nanoparticles on Escherichia coli in the dark. Proteomics 2015, 15, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryck, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Franzoni, E.; Bignozzi, M.C.; Rambaldi, E. TiO2 in the building sector. In Titanium Dioxide (TiO2) and Its Applications; Parrino, F., Palmisano, L., Dean, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 449–479. [Google Scholar]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Barmeh, A.; Nilforoushan, M.R.; Otroj, S. Wetting and photocatalytic properties of Ni-doped TiO2 coating on glazed ceramic tiles under visible light. Thin Solid Film. 2018, 666, 137–142. [Google Scholar] [CrossRef]
- Da Silva, A.L.; Dondi, M.; Raimondo, M.; Hotza, D. Photocatalytic ceramic tiles: Challenges and technological solutions. J. Eur. Ceram. Soc. 2018, 38, 1002–1017. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Epple, M.; Chernousova, S. Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chem. Int. 2013, 52, 1636–1653. [Google Scholar]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [Green Version]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial Silver in Medicinal and Consumer Applications: A Patent Review of the Past Decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef] [Green Version]
- Sheng, H.; Congqin, N.; Yue, Z.; Lei, C.; Kaili, L.; Jiang, C. Antibacterial Activity of Silicate Bioceramics. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2011, 26, 226–230. [Google Scholar]
- Kummala, R.; Brobbey, K.J.; Haapanen, J.; Mäkelä, J.M.; Gunell, M.; Eerola, E.; Huovinen, P.; Toivakka, M.; Saarinen, J.J. Antibacterial activity of silver and titania nanoparticles on glass surfaces. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 015012. [Google Scholar] [CrossRef]
- Petronella, F.; Truppi, A.; Striccioli, M.; Curri, M.L.; Comparelli, R. Photocatalytic Application of Ag/TiO2 Hybrid Nanoparticles. In Noble Metal-Metal Oxide Hybrid Nanoparticles, Fundamentals and Applications; Satyabrata Mohapatra, Phuong Nguyen-Tri, Tuan Anh Nguyen; Elsevier: Amsterdam, The Netherlands, 2019; pp. 373–394. [Google Scholar]
- Durango-Giraldo, G.; Cardona, A.; Zapata, J.F.; Santa, J.F.; Buitrago-Sierra, R. Titanium dioxide modified with silver by two methods for bactericidal applications. Helyon 2019, 5, e01608. [Google Scholar] [CrossRef] [Green Version]
- Djellabi, R.; Basilico, N.; Delbue, S.; D’Alessandro, S.; Parapini, S.; Cerrato, G.; Laurenti, E.; Falletta, E.; Bianchi, C.L. Oxidative inactivation of SARS-CoV-2 on Photoactive AgNPs@TiO2 Ceramic Tiles. Int. J. Mol. Sci. 2021, 22, 8836. [Google Scholar] [CrossRef]
- Confindustria Ceramica. Indagini Statistiche Sull’industria Italiana; Confindustria Ceramica: Sassuolo, Italy, 2020; pp. 8–35. [Google Scholar]
- Palmonari, C. Il Grès Porcellanato; Centro Ceramico: Bologna, Italy, 1989. [Google Scholar]
- Zannini, P. Treatments for ceramic surfaces. Ceram. World Rev. 2014, 107, 62–64. [Google Scholar]
- Dondi, M.; Garcia-Ten, J.; Rambaldi, E.; Zanelli, C.; Vicent-Cabedo, M. Resource efficiency versus market trends in the ceramic tile industry: Effect on the supply chain in Italy and Spain. Resour. Conserv. Recycl. 2021, 168, 105271. [Google Scholar] [CrossRef]
- Rambaldi, E.; Esposito, L.; Tucci, A.; Timellini, G. Recycling of polishing porcelain stoneware residues in ceramic tiles. J. Eur. Ceram. Soc. 2007, 27, 3509–3515. [Google Scholar] [CrossRef]
- Rambaldi, E.; Fazio, S.; Prete, F.; Bignozzi, M.C. Innovative ceramic tile mixes. Cfi/Ber. DKG 2016, 93, 57–60. [Google Scholar]
- Rambaldi, E. Pathway towards a high recycling content in traditional ceramics. Ceramics 2021, 4, 486–501. [Google Scholar] [CrossRef]
- International Organization for Standardization, ISO 22196: Measurement of antibacterial activity on plastics and other non-porous surfaces. International Organization for Standardization: Geneva, Switzerland. 2011. Available online: https://www.iso.org/standard/54431.html (accessed on 20 July 2020).
- International Organization for Standardization, ISO10545-14: Ceramic tiles-Part14: Determination of resistance to stains.International Organization for Standardization: Geneva, Switzerland. 2015. Available online: https://www.iso.org/standard/60972.html (accessed on 20 June 2021).
- Comité Euroéen de Notmalisation, EN 15802: Conservation of cultural property-test methods-Determinaation of static contact angle. Comité Euroéen de Notmalisation: Bruxelles, Belgium. 2010. Available online: https://standards.iteh.ai/catalog/standards/sist/c3a0a8e7-5437-499a-89e2-c4355aae5534/sist-en-15802-2010 (accessed on 20 June 2021).
- International Organizatio for Standardization, ISO 25178-2: Geometrical Product Specifications (GPS)—Surface Texture: Areal—Part 2: Terms, Definitions and Surface Texture Parameters. International Organization for Standardization: Geneva, Switzerland. 2012. Available online: https://www.iso.org/standard/42785.html (accessed on 20 March 2021).
- Intenational Organization for Standardization, ISO 4287: Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parameters; International Organization for Standardization: Geneva, Switzerland. 2009. Available online: https://www.iso.org/standard/10132.html (accessed on 20 March 2021).
- Rambaldi, E.; Lucchese, B.; Engels, M.; Bignozzi, M.C. Evaluation of durability and cleanability performances of protective treatments for lapped ceramic tiles—Part 2. Int. J. Appl. Ceram. Tech. 2018, 16, 625–637. [Google Scholar] [CrossRef] [Green Version]
- Donlan, R.M.; Costerton, J.W. Biofilm: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dondi, M.; Ercolani, G.; Guarini, G.; Melandri, C.; Raimondo, M.; Rocha e Almedra, E.; Tenorio Cavalcante, P.M. The role of surface microstructure on the resistance to stains of porcelain stoneware tiles. J. Eur. Ceram. Soc. 2005, 24, 357–365. [Google Scholar] [CrossRef] [Green Version]
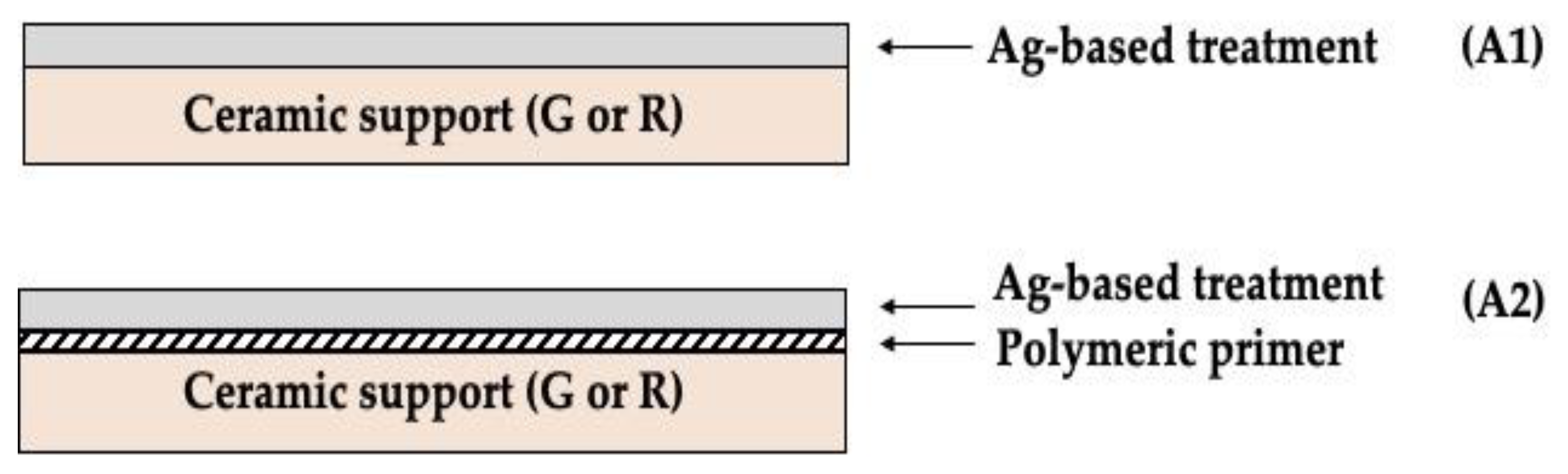

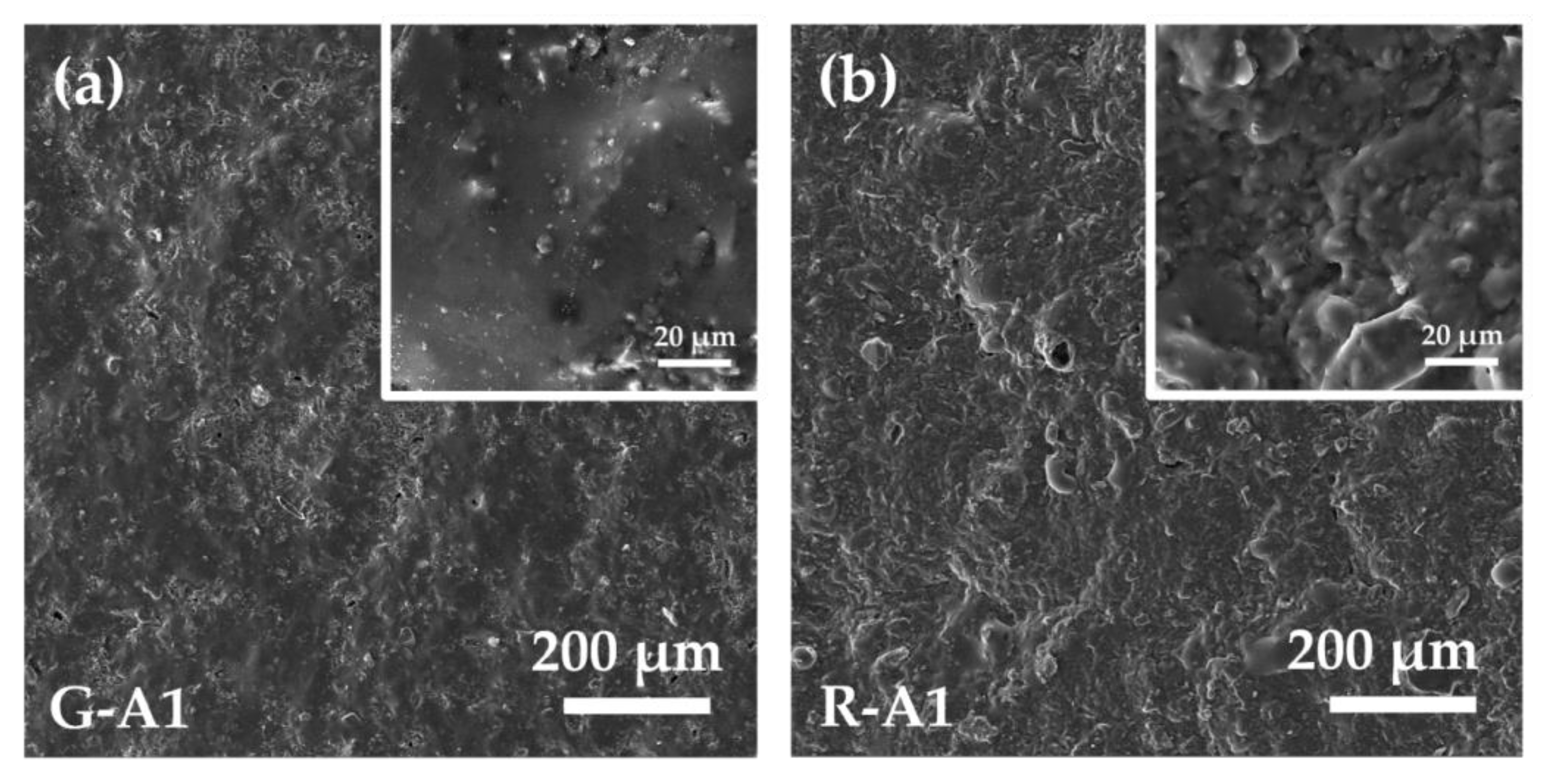


| Components | G (wt%) | R (wt%) |
|---|---|---|
| LOI | 2.95 | 4.45 |
| SiO2 | 79.0 | 75.0 |
| Al2O3 | 13.0 | 15.0 |
| TiO2 | 0.23 | 0.55 |
| Fe2O3 | 0.16 | 0.76 |
| CaO | 1.27 | 0.47 |
| MgO | 0.02 | 0.12 |
| K2O | 0.99 | 2.03 |
| Na2O | 1.77 | 0.79 |
| SO3 | 0.10 | 0.20 |
| ZrO2 | 0.40 | 0.07 |
| Components | G (mg/kg) | R (mg/kg) |
|---|---|---|
| CdO | 12 | 13 |
| Cr2O3 | 27 | 67 |
| CuO | 8 | 6 |
| NiO | <5 | <5 |
| MnO2 | 20 | 121 |
| ZnO | <5 | 111 |
| V2O5 | 39 | 74 |
| CoO | <5 | <5 |
| BaO | 103 | 282 |
| PbO | <20 | <20 |
| G-A1 | G-A2 | R-A1 | R-A2 | |
|---|---|---|---|---|
| Bacterial reduction | 99.999% | 99.999% | 99.71% | 99.999% |
| R-value | 5.880 ± 0.084 | 5.880 ± 0.084 | 2.538 ± 0.140 | 5.647 ± 0.008 |
| Sample | CHROME * | IODINE | OIL |
|---|---|---|---|
| G | 4 | 5 | 4 |
| G-A1 | 4 | 5 | 4 |
| G-A2 | 5 | 5 | 5 |
| R | 4 | 5 | 4 |
| R-A1 | 4 | 5 | 3 |
| R-A2 | 5 | 5 | 4 |
| G | G-A1 | G-A2 | R | R-A1 | R-A2 | |
|---|---|---|---|---|---|---|
| Contact angle (°) | 60 ± 5 | 63 ± 7 | 62 ± 6 | 69 ± 3 | 65 ± 7 | 69 ± 4 |
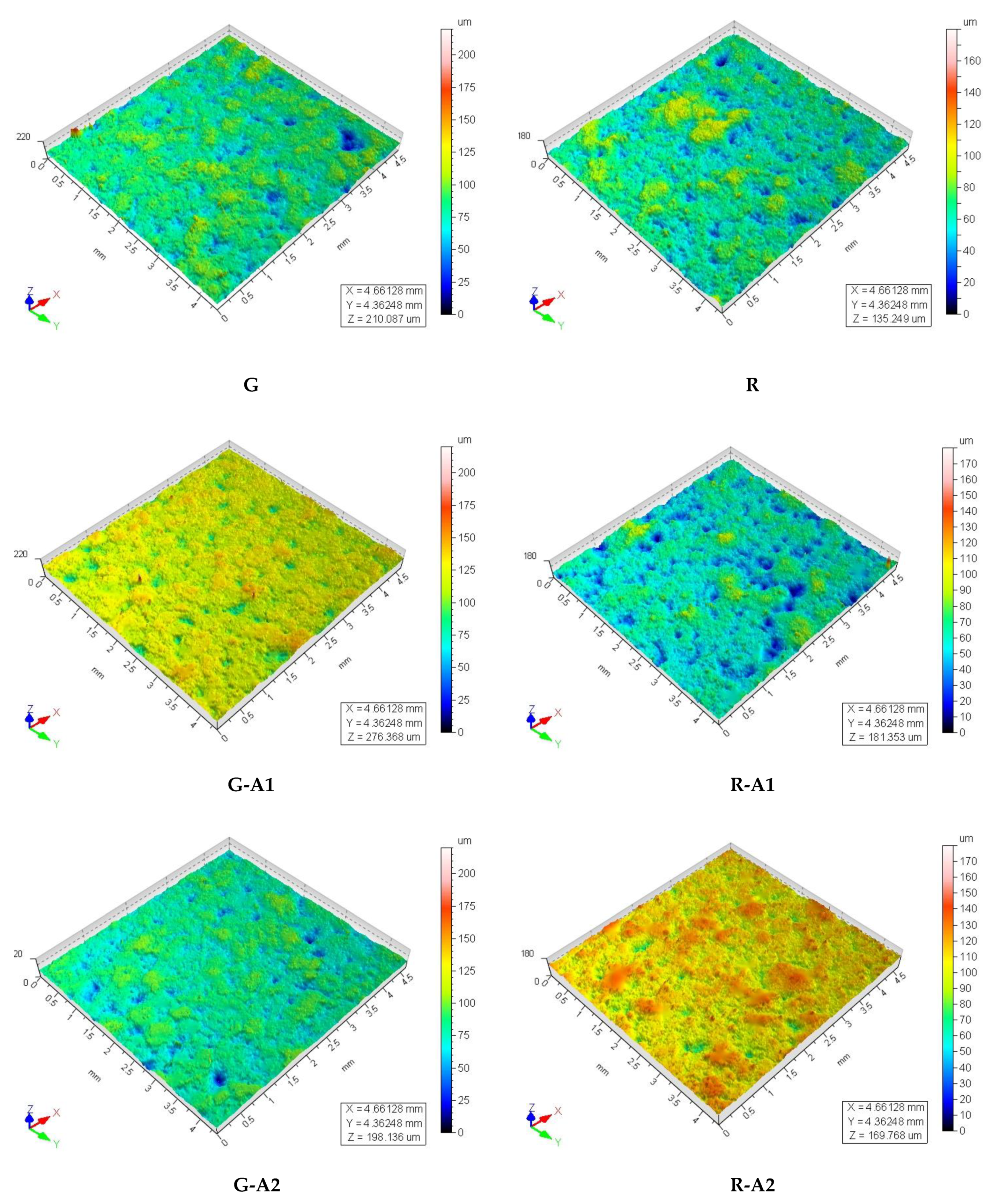
| Parameter | Description | G | G-A1 | GA2 | R | R-A1 | R-A2 |
|---|---|---|---|---|---|---|---|
| Ra (μm) | Average roughness | 4.8 ± 0.7 | 4.3 ± 0.6 | 4.6 ± 0.8 | 4.3 ± 0.8 | 4.4 ± 0.8 | 4.3 ± 0.6 |
| Rv (μm) | Maximum valley depht | 13 ± 3 | 12 ± 3 | 14 ± 4 | 12 ± 3 | 12 ± 3 | 12 ± 2 |
| Rp (μm) | Maximum peak height | 11 ± 2 | 10 ± 3 | 10 ± 2 | 10 ± 2 | 10 ± 2 | 12 ± 2 |
| Spd (1/mm2) | Density of peaks | 9.0 | 4.8 | 7.5 | 17.6 | 8.4 | 10.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Torre, V.; Rambaldi, E.; Masi, G.; Nici, S.; Ghezzi, D.; Cappelletti, M.; Bignozzi, M.C. Validation of Antibacterial Systems for Sustainable Ceramic Tiles. Coatings 2021, 11, 1409. https://doi.org/10.3390/coatings11111409
La Torre V, Rambaldi E, Masi G, Nici S, Ghezzi D, Cappelletti M, Bignozzi MC. Validation of Antibacterial Systems for Sustainable Ceramic Tiles. Coatings. 2021; 11(11):1409. https://doi.org/10.3390/coatings11111409
Chicago/Turabian StyleLa Torre, Valeria, Elisa Rambaldi, Giulia Masi, Silvia Nici, Daniele Ghezzi, Martina Cappelletti, and Maria Chiara Bignozzi. 2021. "Validation of Antibacterial Systems for Sustainable Ceramic Tiles" Coatings 11, no. 11: 1409. https://doi.org/10.3390/coatings11111409
APA StyleLa Torre, V., Rambaldi, E., Masi, G., Nici, S., Ghezzi, D., Cappelletti, M., & Bignozzi, M. C. (2021). Validation of Antibacterial Systems for Sustainable Ceramic Tiles. Coatings, 11(11), 1409. https://doi.org/10.3390/coatings11111409









