Microstructure and Oxidation Behavior of Nb-Si-Based Alloys for Ultrahigh Temperature Applications: A Comprehensive Review
Abstract
:1. Introduction
2. Nb-Si-Based Alloys
3. Modified Nb-Si-Based Multi-Element Alloys
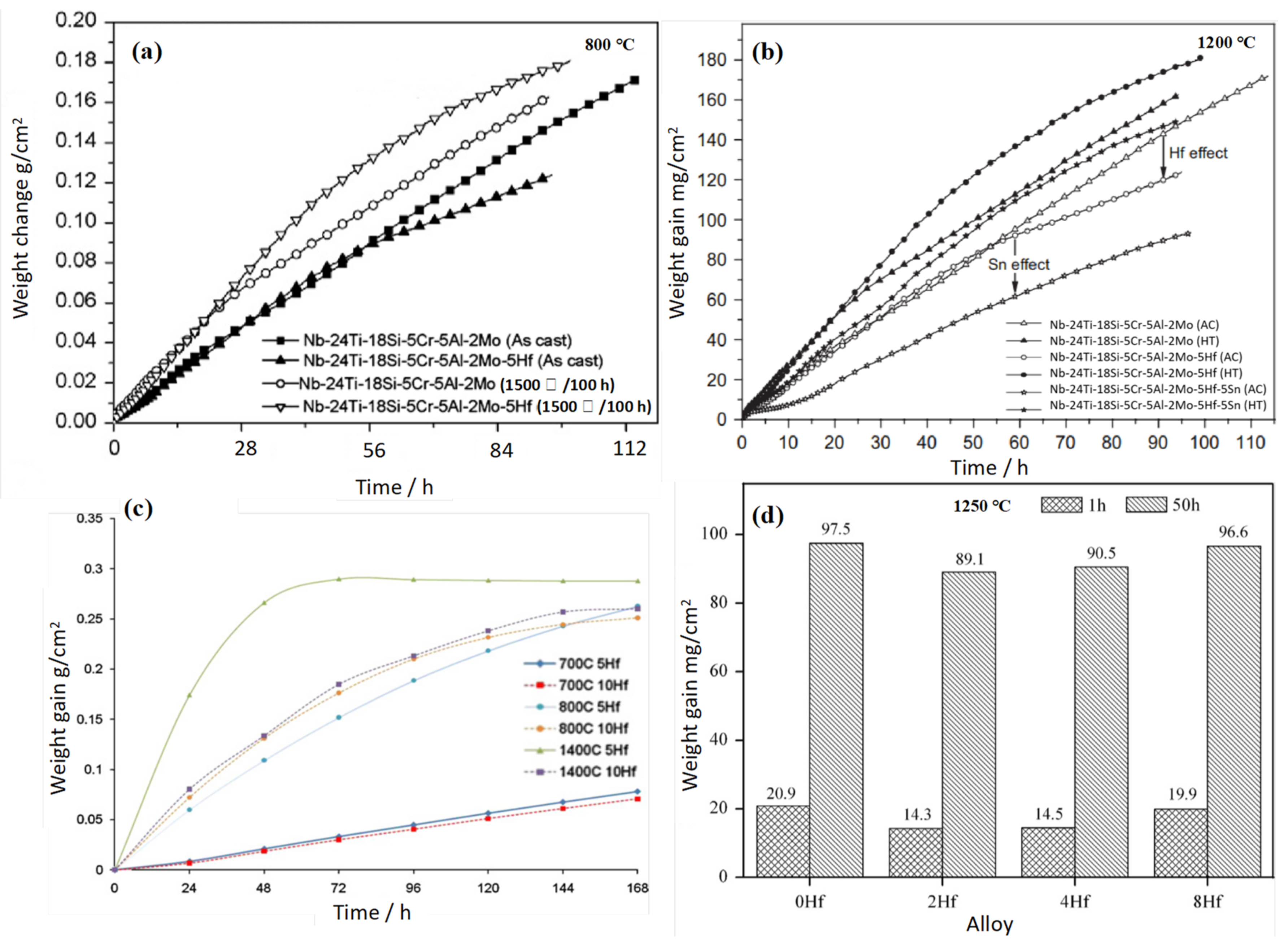
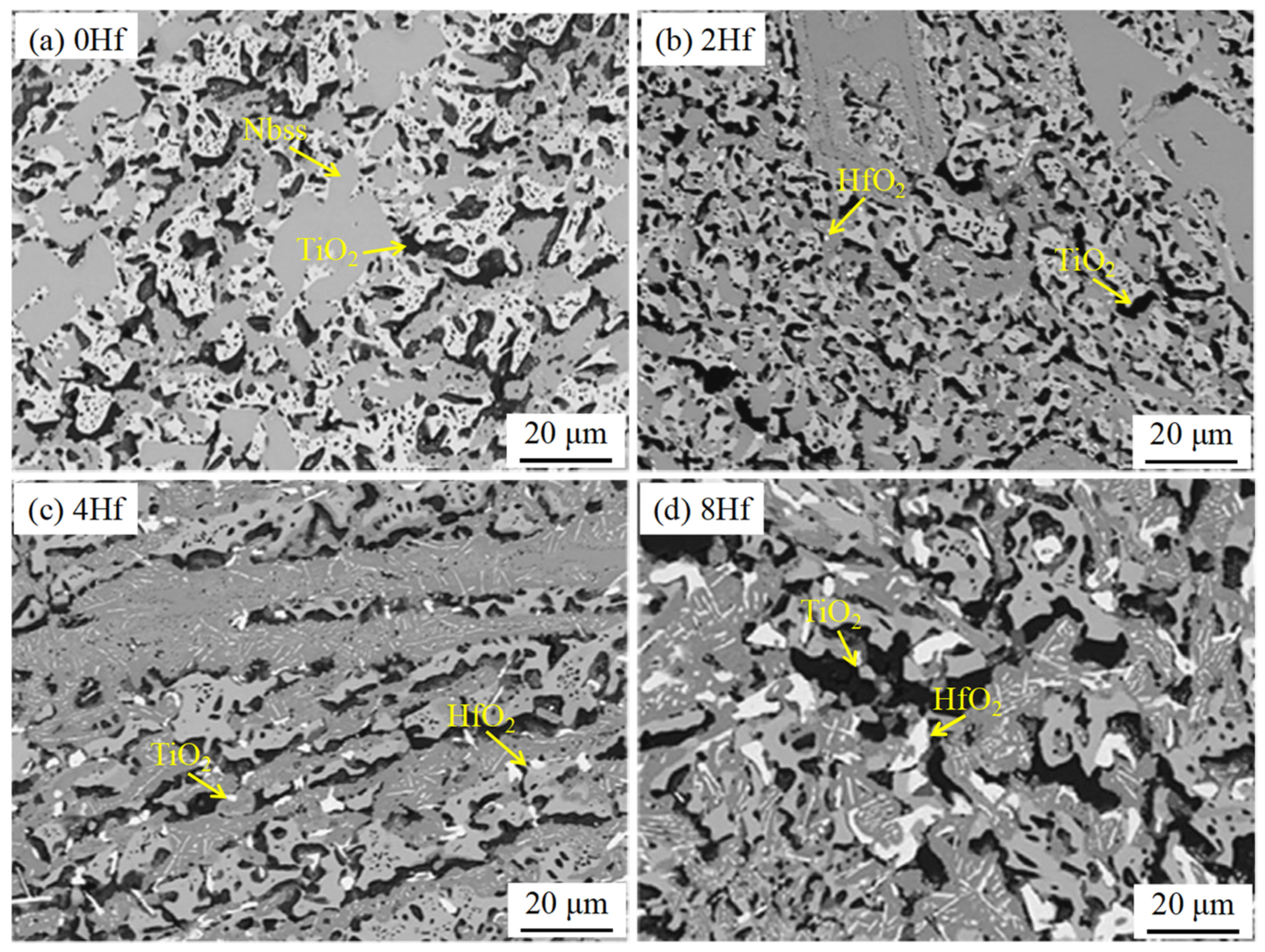
3.1. Nb-Si-Based Alloys Modified with Hf Elements
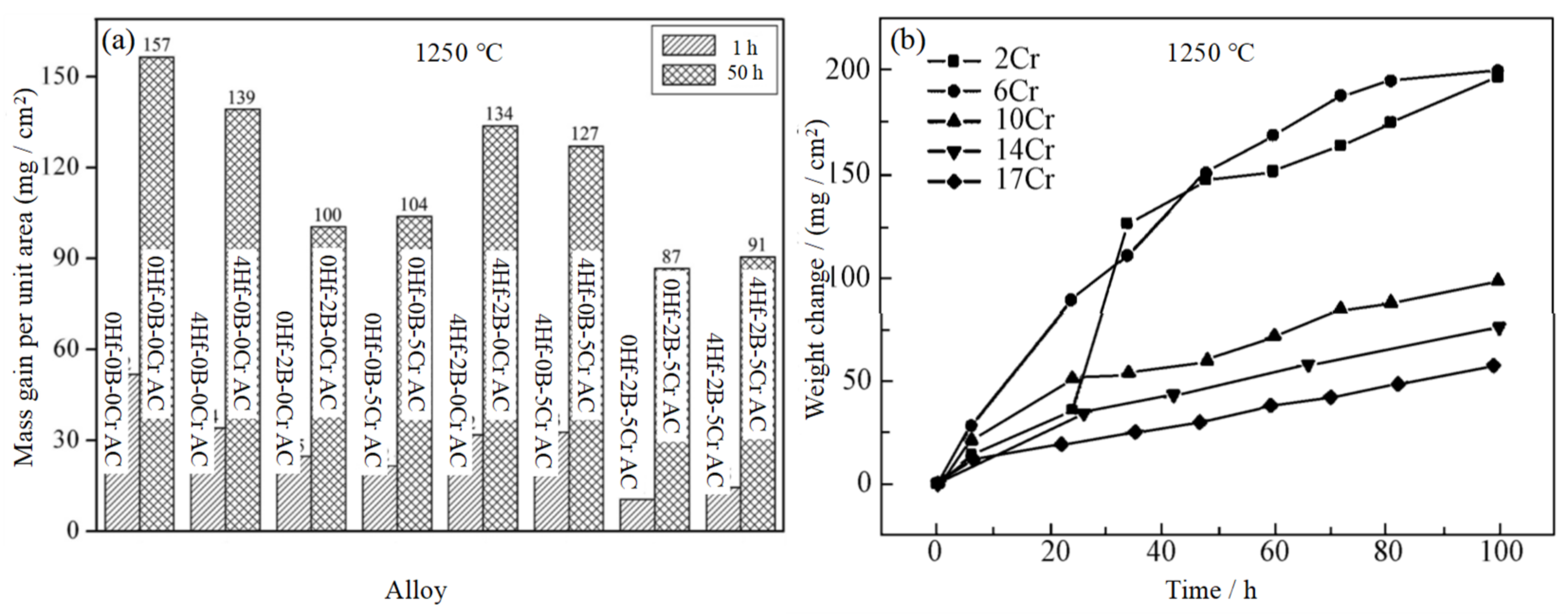
3.2. Nb-Si-Based Alloys Modified with Cr Elements
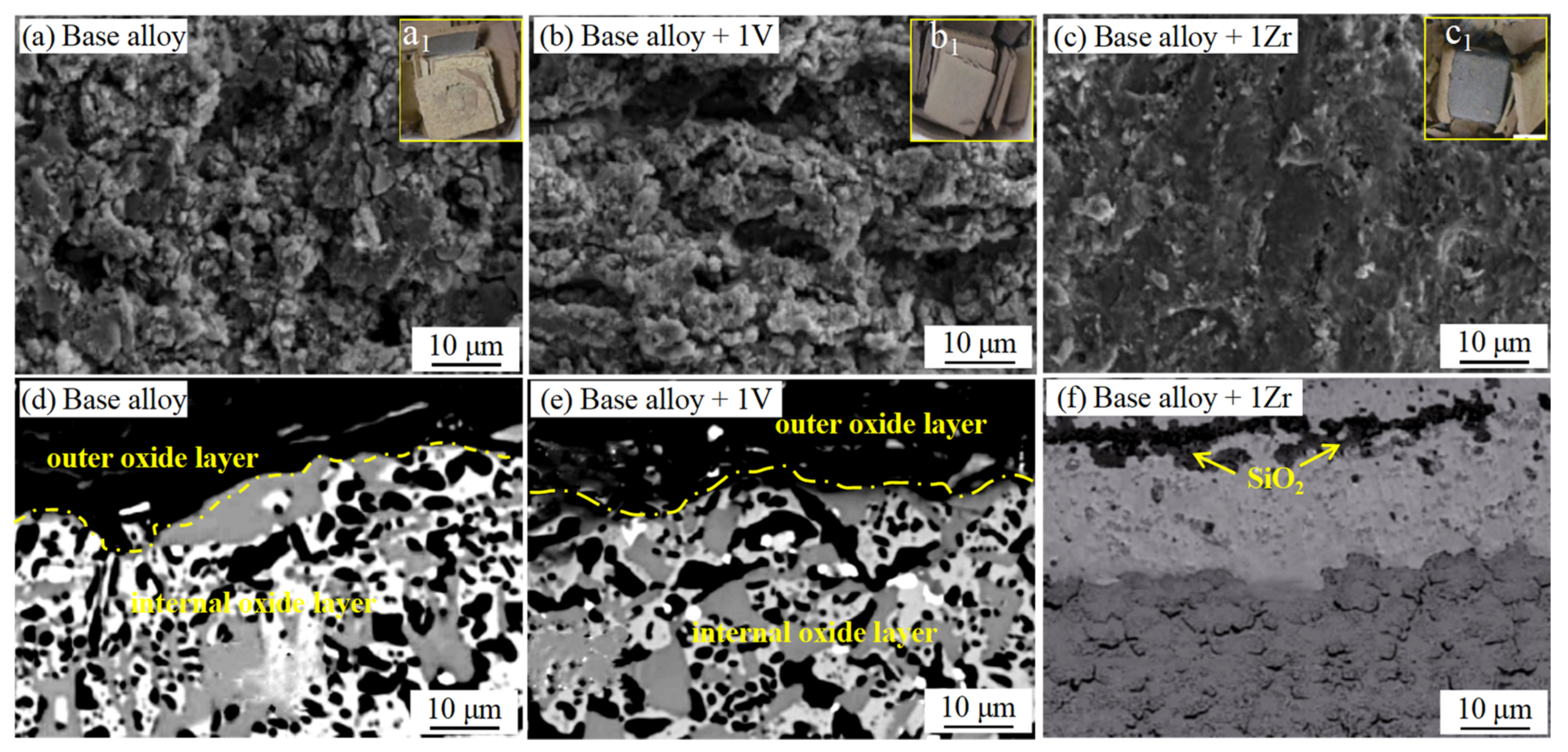
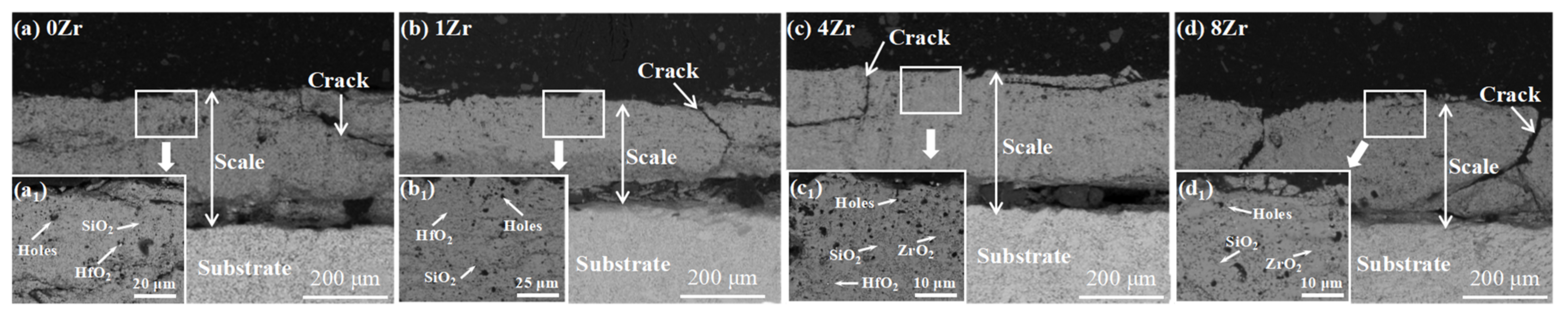
3.3. Nb-Si-Based Alloys Modified with Zr Elements
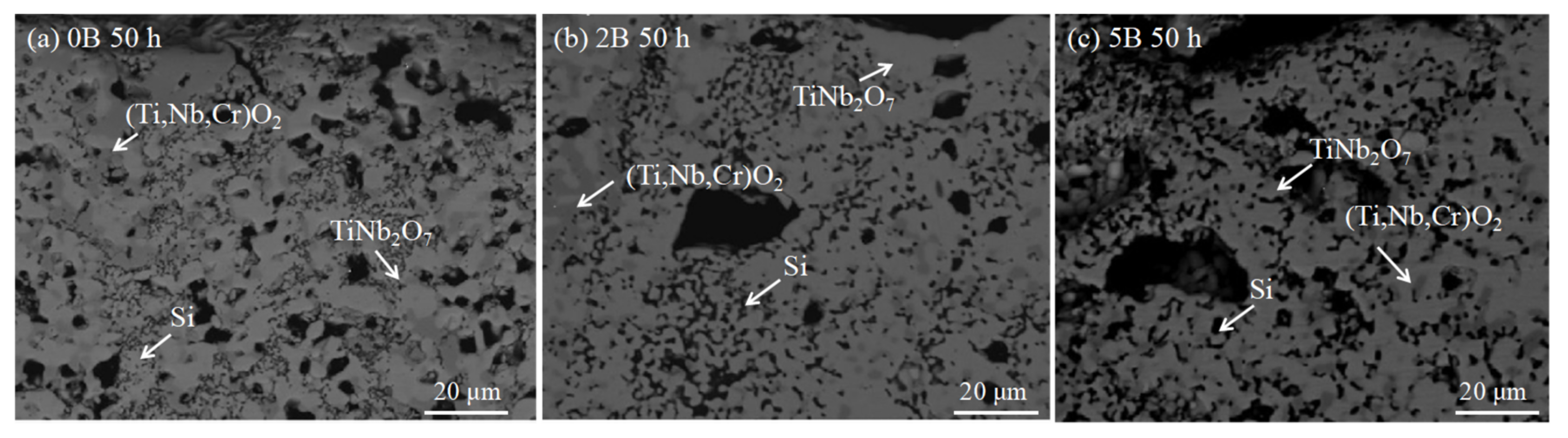
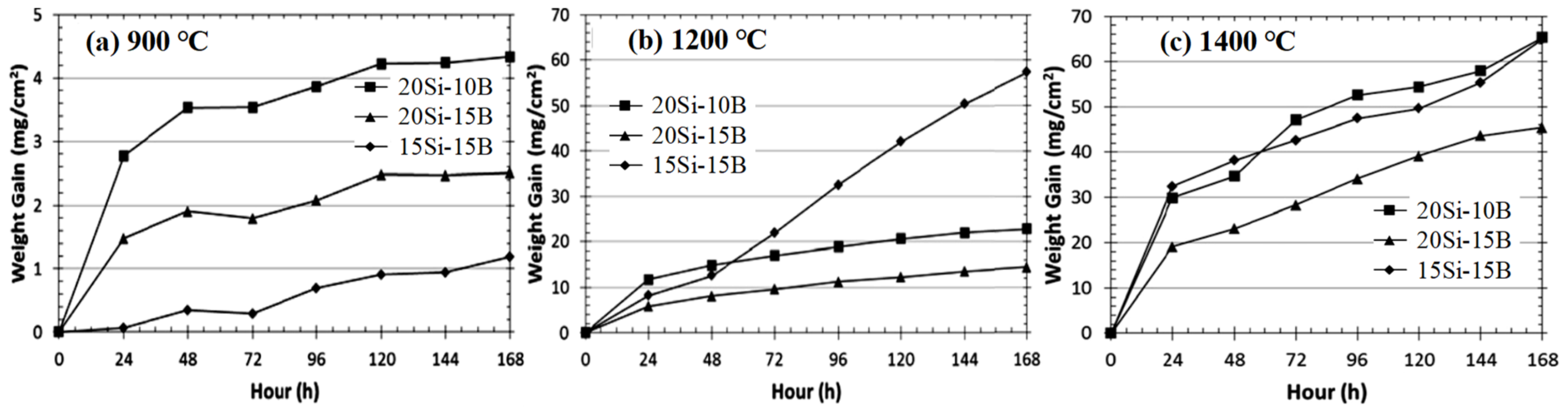
3.4. Nb-Si-Based Alloys Modified with B Elements
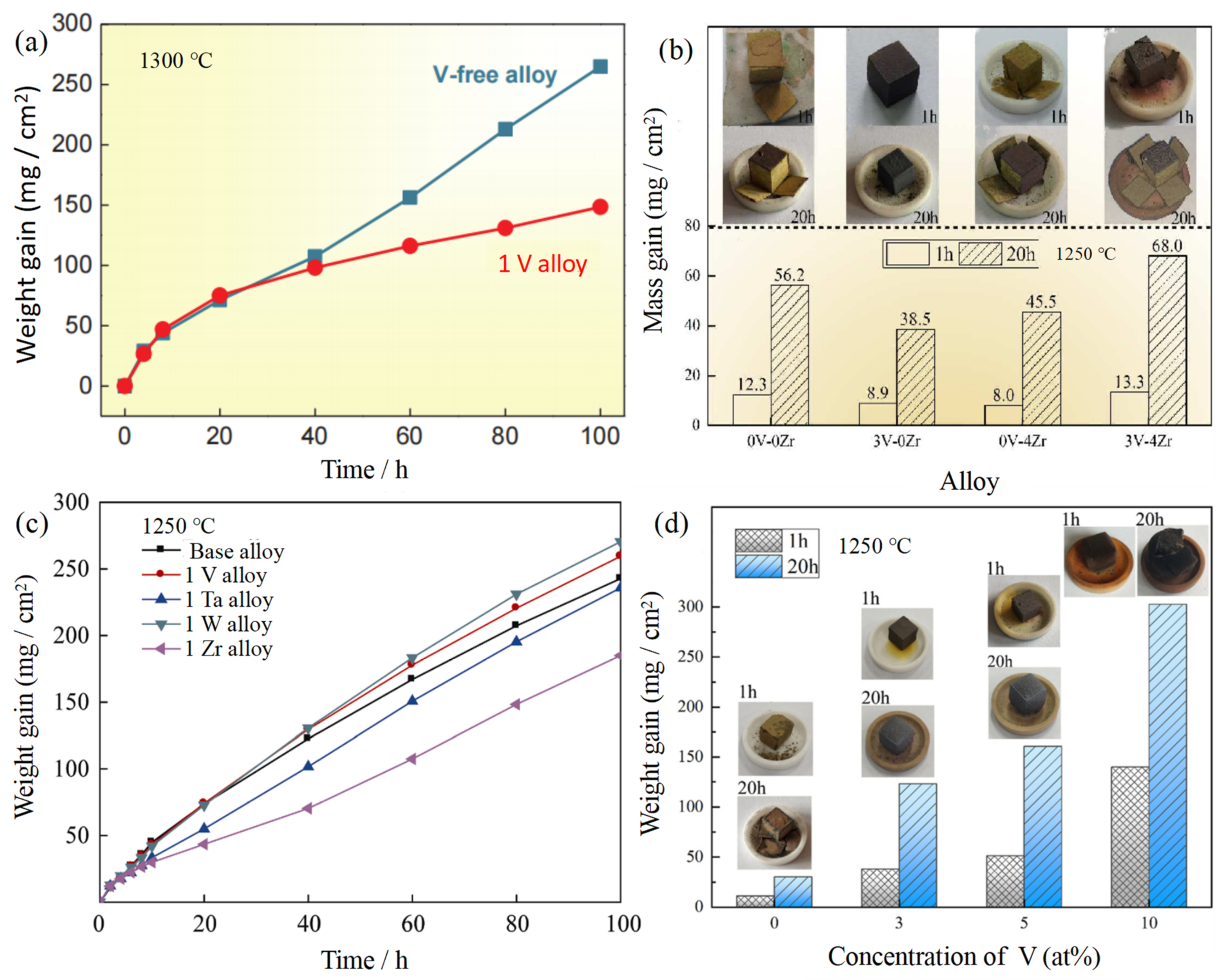
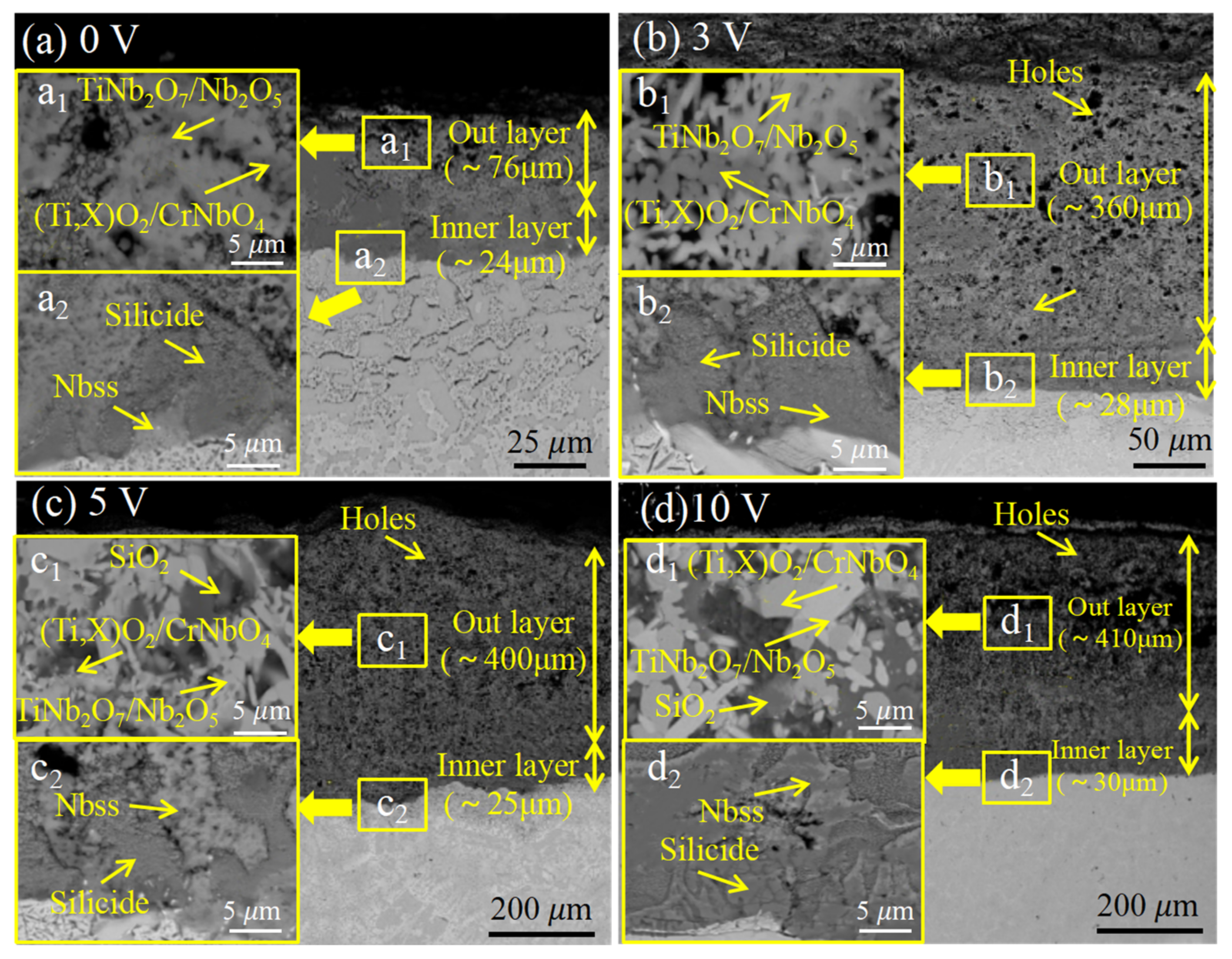
3.5. Nb-Si-Based Alloys Modified with V Elements
3.6. Nb-Si-Based Alloys Modified with Other Elements
4. Mechanisms of Oxidation and Failure
5. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsakiropoulos, P. Alloys for application at ultra-high temperatures: Nb-silicide in situ composites challenges, breakthroughs and opportunities. Prog. Mater. Sci. 2022, 123, 100714. [Google Scholar] [CrossRef]
- Cheng, J.C.; Yi, S.H.; Park, J.S. Oxidation behaviors of Nb-Si-B ternary alloys at 1100 °C under ambient atmosphere. Intermetalics 2012, 23, 12–19. [Google Scholar] [CrossRef]
- Murakamia, T.; Sasakia, S.; Ichikawaa, K.; Kitahara, A. Oxidation resistance of powder compacts of the Nb-Si-Cr system and Nb3Si5Al2 substrate compacts prepared by spark plasma sintering. Intermetallics 2001, 9, 629–635. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Kamil, M.P.; Ko, Y.G. Synergistic influence of inorganic oxides (ZrO2 and SiO2) with N2H4 to protect composite coatings obtained via plasma electrolyte oxidation on Mg alloy. Phys. Chem. Chem. Phys. 2017, 19, 2372–2382. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.F.; Tsakiropoulos, P. Study of the effects of Ge addition on the microstructure of Nb-18Si in situ composites. Intermetallics 2010, 18, 1072–1078. [Google Scholar]
- Sala, K.; MitraK, R. Microstructural evolution and mechanical properties of as-cast and annealed Nb-Si-Mo based hypoeutectic alloys with quaternary additions of Ti or Fe. Mater. Sci. Eng. A 2021, 802, 140663. [Google Scholar] [CrossRef]
- Maji, P.; Mitra, R.; Ray, K. Effect of Cr on the evolution of microstructures in as-cast ternary niobium-silicide-based composites. Intermetallics 2017, 85, 34–47. [Google Scholar] [CrossRef]
- Zoubi, W.A.; Ko, Y.G. Chemical stability of synergistic inorganic materials for enhancing electrochemical performance. Compos. Sci. Technol. 2020, 199, 108383. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Cui, K.; Fu, T.; Gao, J.; Hussain, S.; AlGarni, T.S. Pyrometallurgical recovery of zinc and valuable metals from electric arc furnace dust—A review. J. Clean. Prod. 2021, 298, 126788. [Google Scholar] [CrossRef]
- Liu, W.; Ren, X.Y.; Li, N.; Gao, C.; Xiong, H.P. Rapid directionally solidified microstructure characteristic and fracture be-haviour of laser melting deposited Nb-Si-Ti alloy. Prog. Nat. Sci. Mater. Int. 2021, 31, 113–120. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, W.; Sha, J. Microstructural evolution and mechanical properties of Nb-Si-Cr ternary alloys with a tri-phase Nb/Nb5Si3/Cr2Nb microstructure fabricated by spark plasma sintering. Prog. Nat. Sci. 2018, 28, 626–634. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Z.; He, J.; Su, H.; Jia, L.; Zhang, J.; Liu, L.; Zhang, H. Surface microstructure modification of hypereutectic Nb-Si based alloys to improve oxidation resistance without damaging fracture toughness. Mater. Charact. 2020, 159, 110051. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Kamil, M.P.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent advances in hybrid organic-inorganic materials with spatial architecture for state-of-the-art applications. Prog. Mater. Sci. 2020, 112, 100663. [Google Scholar] [CrossRef]
- Shen, F.Q.; Yu, L.H.; Fu, T.; Zhang, Y.Y.; Wang, H.; Cui, K.K.; Wang, J.; Hussain, S.; Akhtar, N. Effect of the Al, Cr and B elements on the mechanical properties and oxidation resistance of Nb-Si based alloys: A review. Appl. Phys. A Mater. Sci. Process. 2021, 127, 852. [Google Scholar] [CrossRef]
- Jiang, W.; Shao, W.; Sha, J.; Zhou, C. Experimental studies and modeling for the transition from internal to external oxidation of three-phase Nb-Si-Cr alloys. Prog. Nat. Sci. 2018, 28, 740–748. [Google Scholar] [CrossRef]
- Ma, R.; Guo, X. Effects of Mo and Zr composite additions on the microstructure, mechanical properties and oxidation resistance of multi-elemental Nb-Si based ultrahigh temperature alloys. J. Alloys Compd. 2021, 870, 159437. [Google Scholar] [CrossRef]
- He, J.-H.; Guo, X.-P.; Qiao, Y.-Q. Oxidation and hot corrosion behaviors of Nb−Si based ultrahigh temperature alloys at 900 °C. Trans. Nonferrous Met. Soc. China 2021, 31, 207–221. [Google Scholar] [CrossRef]
- Yu, L.H.; Shen, F.Q.; Fu, T.; Zhang, Y.Y.; Cui, K.K.; Wang, J.; Zhang, X. Microstructure and oxidation behavior of metal-modified mo-si-b alloys: A review. Coatings 2021, 11, 1256. [Google Scholar] [CrossRef]
- Zhang, G.P.; Sun, J.; F, Q.G. Effect of mullite on the microstructure and oxidation behavior of thermalsprayed MoSi2 coating at 1500 °C. Ceram. Int. 2020, 46, 10058–10066. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H.Q.; Lei, H.; Li, H.Q.; Gong, J.; Sun, C. Influence of different coating structures on the oxidation resistance of MoSi2 coatings. Ceram. Int. 2020, 46, 5993–5997. [Google Scholar] [CrossRef]
- Fu, T.; Cui, K.; Zhang, Y.; Wang, J.; Zhang, X.; Shen, F.; Yu, L.; Mao, H. Microstructure and oxidation behavior of anti-oxidation coatings on Mo-based alloys through hapc process: A review. Coatings. 2021, 11, 883. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yu, L.H.; Fu, T.; Wang, J.; Shen, F.Q.; Cui, K.K. Microstructure evolution and growth mechanism of Si-MoSi2 composite coatings on TZM (Mo-0.5Ti-0.1Zr-0.02 C) alloy. J. Alloys Compd. 2022, 894, 162403. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, X.; Yu, Y.; Mei, F.; Zou, L.; Gao, J. Positive modification on the mechanical, tribological and oxidation properties of AlCrNbSiN coatings by regulating the Nb/Si-doping ratio. Ceram. Int. 2021, 47, 31603–31616. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Cui, K.K.; Gao, Q.J.; Hussain, S.; Lv, Y. Investigation of morphology and texture pr1operties of WSi2 coatings on W substrate based on contact-mode AFM and EBSD. Surf. Coat. Technol. 2020, 396, 125966. [Google Scholar] [CrossRef]
- Fu, T.; Cui, K.; Zhang, Y.; Wang, J.; Shen, F.; Yu, L.; Qie, J.; Zhang, X. Oxidation protection of tungsten alloys for nuclear fusion applications: A comprehensive review. J. Alloys Compd. 2021, 884, 161057. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, X. High temperature deformation behavior of an optimized Nb–Si based ultrahigh temperature alloy. Scr. Mater. 2016, 116, 16–20. [Google Scholar] [CrossRef]
- Cui, K.K.; Fu, T.; Zhang, Y.Y.; Wang, J.; Mao, H.B.; Tan, T.B. Microstructure and mechanical properties of CaAl12O19 rein-forced Al2O3-Cr2O3 composites. J. Eur. Ceram. Soc. 2021, 41, 7935–7945. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, T.; Cui, K.; Shen, F.; Wang, J.; Yu, L.; Mao, H. Evolution of surface morphology, roughness and texture of tungsten disilicide coatings on tungsten substrate. Vacuum 2021, 191, 110297. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Ni, W.J.; Li, Y.G. Effect of siliconizing temperature on microstructure and phase constitution of Mo-MoSi2 functionally graded materials. Ceram. Int. 2018, 44, 11166–11171. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Fu, T.; Wang, J.; Cui, K.; Shen, F. Rare earth elements enhanced the oxidation resistance of Mo-Si-based alloys for high temperature application: A review. Coatings 2021, 11, 1144. [Google Scholar] [CrossRef]
- Jiang, W.; Li, M.; Sha, J.; Zhou, C. Microstructure and oxidation resistance of composition gradients Nb-Si based alloy thin film. Mater. Des. 2020, 192, 108687. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, T.; Cui, K.; Zhang, Y.; Shen, F.; Wang, J.; Yu, L.; Mao, H. The protection, challenge, and prospect of anti-oxidation coating on the surface of niobium alloy. Coatings 2021, 11, 742. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Qie, J.M.; Cui, K.K.; Fu, T.; Fan, X.L.; Wang, J.; Zhang, X. Effect of hot dip silicon-plating temperature on mi-crostructure characteristics of silicide coating on tungsten substrate. Ceram. Int. 2020, 46, 5223–5228. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, K.; Fu, T.; Wang, J.; Qie, J.; Zhang, X. Synthesis WSi2 coating on W substrate by HDS method with various deposition times. Appl. Surf. Sci. 2020, 511, 145551. [Google Scholar] [CrossRef]
- Pu, D.; Pan, Y. Influence of high pressure on the structure, hardness and brittle-to-ductile transition of NbSi2 ceramics. Ceram. Int. 2021, 47, 2311–2318. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhao, J.; Li, J.H.; Lei, J.; Cheng, X.K. Effect of hot-dip siliconizing time on phase composition and microstructure of Mo-MoSi2 high temperature structural materials. Ceram. Int. 2019, 45, 5588–5593. [Google Scholar] [CrossRef]
- Cui, K.K.; Zhang, Y.Y.; Fu, T.; Wang, J.; Zhang, X. Toughening mechanism of mullite substrate composites: A review. Coatings 2020, 10, 672. [Google Scholar] [CrossRef]
- Geng, J.; Tsakiropoulos, P.; Shao, G.S. Oxidation of Nb-Si-Cr-Al in situ composites with Mo, Ti and Hf additions. Mater. Sci. Eng. A 2006, 441, 26–38. [Google Scholar] [CrossRef]
- Geng, J.; Tsakiropoulos, P. A study of the microstructures and oxidation of Nb-Si-Cr-Al-Mo in situ composites alloyed with Ti, Hf and Sn. Intermetallics 2007, 15, 382–395. [Google Scholar] [CrossRef]
- Vazquez, A.; Varma, S.K. High-temperature oxidation behavior of Nb-Si-Cr alloys with Hf additions. J. Alloys Compd. 2011, 509, 7027–7033. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, X. Alloying effects on the microstructure and properties of Nb–Si based ultrahigh temperature alloys. Intermetallics 2016, 70, 33–44. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, X.P. Microstructure, mechanical properties and oxidation resistance of Nb silicide based ultrahigh temperature alloys with Hf addition. Mater. Sci. Eng. A 2015, 645, 88–98. [Google Scholar] [CrossRef]
- Cui, K.K.; Zhang, Y.Y.; Fu, T.; Hussain, S.; AlGarni, T.S.; Wang, J.; Zhang, X.; Ali, S. Effects of Cr2O3 content on microstructure and mechanical properties of Al2O3 substrate composites. Coatings 2021, 11, 234. [Google Scholar] [CrossRef]
- Pan, Y. Structural Prediction and overall performances of CrSi2 disilicides: DFT investigations. ACS Sustain. Chem. Eng. 2020, 8, 11024–11030. [Google Scholar] [CrossRef]
- Chan, K.S. Cyclic oxidation response of multiphase niobium-based alloys. Met. Mater. Trans. A 2004, 35, 589–597. [Google Scholar] [CrossRef]
- Wang, L.G.; Jia, L.N.; Cui, R.J.; Zheng, L.L.; Zhang, H. Microstructure, mechanical properties and oxidation resistance of Nb-22Ti-14Si-2Hf-2Al-xCr alloys. Chin. J. Aeronaut. 2012, 25, 292–296. [Google Scholar] [CrossRef] [Green Version]
- Zelenitsas, K.; Tsakiropoulos, P. Study of the role of Al and Cr additions in the microstructureof Nb-Ti-Si in situ composites. Intermetallics 2005, 13, 1079–1095. [Google Scholar] [CrossRef]
- Zelenitsas, K.; Tsakiropoulos, P. Effect of Al, Cr and Ta additions on the oxidation behaviour of Nb-Ti-Si in situ composites at 800 °C. Mater. Sci. Eng. A 2006, 416, 269–280. [Google Scholar] [CrossRef]
- Esparza, N.; Rangel, V.; Gutierrez, A.; Arellano, B.; Varma, S.K. A comparison of the effect of Cr and Al additions on the oxidation behaviour of alloys from the Nb–Cr–Si system. Mater. High Temp. 2016, 33, 105–114. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, X. Effects of Cr and Hf additions on the microstructure and properties of Nb silicide based ultrahigh temperature alloys. Mater. Sci. Eng. A 2015, 638, 121–131. [Google Scholar] [CrossRef]
- Pan, Y.; Pu, D.; Yu, E. Structural, electronic, mechanical and thermodynamic properties of Cr–Si binary silicides from first-principles investigations. Vacuum 2021, 185, 110024. [Google Scholar] [CrossRef]
- Su, L.; Jia, L.; Jiang, K.; Zhang, H. The oxidation behavior of high Cr and Al containing Nb-Si-Ti-Hf-Al-Cr alloys at 1200 and 1250 °C. Int. J. Refract. Met. Hard Mater. 2017, 69, 131–137. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Wang, S.F.; Bor, H.Y.; Hsu, Y.F. Effects of Zr addition on the microstructure and mechanical behavior of a fine-grained nickel-based superalloy at elevated temperatures. Mater. Sci. Eng. A 2014, 607, 294–301. [Google Scholar] [CrossRef]
- Luo, C.; Lu, N.; Zhu, C.L.; Li, H.Z.; Liu, X.Q. Effect of trace zirconium addition on high temperature mechanical properties of casting TiAl alloy. Foundry 2012, 61, 754–757. [Google Scholar]
- Swadźba, R.; Swadźba, L.; Wiedermann, J.; Hetmańczyk, M.; Witala, B. Characterization of alumina scales grown on a 2nd generation single crystal Ni superalloy during isothermal oxidation at 1050, 1100 and 1150 °C. Oxid. Met. 2014, 82, 195–208. [Google Scholar] [CrossRef]
- Hong, S.; Hwang, G.; Han, W.; Lee, K.; Kang, S. Effect of zirconium addition on cyclic oxidation behavior of platinum-modified aluminide coating on nickel-based superalloy. Intermetallics 2010, 18, 864–870. [Google Scholar] [CrossRef]
- Zhang, S.-N.; Jia, L.-N.; Guo, Y.; Kong, B.; Zhang, F.-X.; Zhang, H. High-temperature oxidation behavior of Nb–Si-based alloy with separate vanadium, tantalum, tungsten and zirconium addition. Rare Met. 2021, 40, 607–615. [Google Scholar] [CrossRef]
- Zhang, S.; Jia, L.; Guo, Y.; Kong, B.; Zhou, C.; Zhang, H. Improvement in the oxidation resistance of Nb-Si-Ti based alloys containing zirconium. Corros. Sci. 2020, 163, 108294. [Google Scholar] [CrossRef]
- Qiao, Y.; Guo, X.; Zeng, Y. Study of the effects of Zr addition on the microstructure and properties of Nb-Ti-Si based ultrahigh temperature alloys. Intermetallics 2017, 88, 19–27. [Google Scholar] [CrossRef]
- Ma, R.; Guo, X. Composite alloying effects of V and Zr on the microstructures and properties of multi-elemental Nb–Si based ultrahigh temperature alloys. Mater. Sci. Eng. A 2021, 813, 141175. [Google Scholar] [CrossRef]
- Behrani, V.; Thom, A.J.; Kramer, M.J.; Akinc, M. Microstructure and oxidation behavior of Nb-Mo-Si-B alloys. Intermetallics 2006, 14, 24–32. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Cui, K.K.; Fu, T.; Wang, J.; Shen, F.Q.; Zhang, X.; Yu, L.H. Formation of MoSi2 and Si/MoSi2 coatings on TZM (Mo-0.5Ti-0.1Zr-0.02C) alloy by hot dip silicon-plating method. Ceram. Int. 2021, 47, 23053–23065. [Google Scholar] [CrossRef]
- Wu, M.L.; Jiang, L.W.; Qu, S.Y.; Guo, F.W.; Li, M.; Kang, Y.W.; Han, Y.F. Effect of trace Ce and B additions on the microstructure of Nb-3Si-22Ti alloys. Prog. Nat. Sci. Mater. Int. 2017, 27, 362–368. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, Y.G.; Bai, C.G. Microstructure and oxidation behavior of Si-MoSi2 functionally graded coating on Mo substrate. Ceram. Int. 2017, 43, 6250–6256. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, X. Effects of B addition on the microstructure and properties of Nb silicide based ultrahigh temperature alloys. Intermetallics 2015, 57, 83–92. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, X.; Guo, B. Effect of B and Ti on the directionally solidified microstructure of the Nb–Si alloys. Int. J. Refract. Met. Hard Mater. 2015, 51, 243–249. [Google Scholar] [CrossRef]
- Thomas, K.S.; Varma, S.K. Oxidation response of three Nb-Cr-Mo-Si-B alloys in air. Corros. Sci. 2015, 99, 145–153. [Google Scholar] [CrossRef]
- Su, L.F.; Jia, L.N.; Weng, J.F.; Hong, Z.; Zhou, C.G.; Zhang, H. Improvement in the oxidation resistance of Nb-Ti-Si-Cr-Al-Hf alloys containing alloyed Ge and B. Corros. Sci. 2014, 88, 460–465. [Google Scholar] [CrossRef]
- Guo, Y.L.; Jia, L.N.; Kong, B.; Zhang, H.R.; Zhang, H. Simultaneous improvement in fracture toughness and oxidation re-sistance of Nb-Si based alloys by vanadium addition. Mater. Sci. Eng. A 2017, 701, 149–157. [Google Scholar] [CrossRef]
- Kang, Y.W.; Qu, S.Y.; Song, J.X.; Huang, Q.; Han, Y.F. Microstructure and mechanical properties of Nb-Ti-Si-Al-Hf-xCr-yV multi-element in situ composite. Mater. Sci. Eng. A 2012, 534, 323–328. [Google Scholar] [CrossRef]
- Zheng, J.S.; Hou, X.M.; Wang, X.B.; Meng, Y.; Zheng, X.; Zheng, L. Isothermal oxidation mechanism of Nb-Ti-V-Al-Zr alloy at 700–1200 °C Diffusion and interface reaction. Corros. Sci. 2015, 96, 186–195. [Google Scholar] [CrossRef]
- Gang, F.; Klinski-Wetzel, K.V.; Wagner, J.N.; Heilmaieret, M. Influence of vanadium on the oxidation resistance of the intermetallic phase Nb5Si3. Oxid. Met. 2015, 83, 119–132. [Google Scholar] [CrossRef]
- Xu, C.; Gao, W. Pilling-Bedworth ratio for oxidation of alloys. Mater. Res. Innov. 2000, 3, 231–235. [Google Scholar] [CrossRef]
- Dasary, R.M.; Varma, S.K. Short-term oxidation response of Nb-15Re-15Si-10Cr-20Mo alloy. J. Mater. Res. Technol. 2014, 3, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Ma, R.; Guo, X. Effects of V addition on the microstructure and properties of multi-elemental Nb-Si based ultrahigh temperature alloys. J. Alloys Compd. 2020, 845, 156254. [Google Scholar] [CrossRef]
- Murayama, Y.; Hanada, S. High temperature strength, fracture toughness and oxidation resistance of Nb-Si-Al-Ti multi-phase alloys. Sci. Technol. Adv. Mater. 2002, 3, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, K.; Balachandran, G.; Mitra, R.; Ray, K. Effect of Mo on microstructure and mechanical behaviour of as-cast Nbss–Nb5Si3 in situ composites. Intermetallics 2006, 14, 1452–1460. [Google Scholar] [CrossRef]
- Ma, R.; Guo, X. Influence of molybdenum contents on the microstructure, mechanical properties and oxidation behavior of multi-elemental Nb–Si based ultrahigh temperature alloys. Intermetallics 2021, 129, 107053. [Google Scholar] [CrossRef]
- Guo, Y.; Jia, L.; Zhang, H.; Zhang, F.; Zhang, H. Enhancing the oxidation resistance of Nb-Si based alloys by yttrium addition. Intermetallics 2018, 101, 165–172. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, L.; Sun, G.; Zhang, F.; Ye, C.; Zhang, H. Synchronous improvement in room-temperature fracture toughness and high-temperature oxidation resistance of Nb Si based alloys with Erbium addition. Int. J. Refract. Met. Hard Mater. 2021, 94, 105359. [Google Scholar] [CrossRef]
- Liu, A.Q.; Sun, L.; Li, S.S.; Han, Y.F. Effect of cerium on micmstiuctms and high temperature oxidation resistance of an Nb-Si system in-situ composite. J. Rare Earth 2007, 25, 474–479. [Google Scholar] [CrossRef]
- Guo, Y.; Jia, L.; Kong, B.; Zhang, F.; Liu, J.; Zhang, H. Improvement in the oxidation resistance of Nb-Si based alloy by selective laser melting. Corros. Sci. 2017, 127, 260–269. [Google Scholar] [CrossRef]
- Guo, Y.L.; Jia, L.N.; Sun, S.B.; Kong, B.; Liu, J.H.; Zhang, H. Rapid fabrication of Nb-Si based alloy by selective laser melting: Microstructure, hardness and initial oxidation behavior. Mater. Des. 2016, 109, 37–46. [Google Scholar] [CrossRef]
- Mathieu, S.; Knittel, S.; Berthod, P.; Vilasi, M. On the oxidation mechanism of niobium-base in situ composites. Corros. Sci. 2012, 60, 181–192. [Google Scholar] [CrossRef]
- Alvarez, D.; Varma, S.K. Characterization of microstructures and oxidation behavior of Nb-20Si-20Cr-5Al alloy. Corros. Sci. 2011, 53, 2161–2167. [Google Scholar] [CrossRef]






| Alloy Composition | Melting Point (°C) | Density (g/cm3) | Operating Temperature (°C) | Reference |
|---|---|---|---|---|
| Ni-based superalloy | 1350–1400 | 8.1–8.5 | 1150 | [1,5,17,19] |
| Nb-based superalloy | >1700 | 6.6–7.2 | 1200–1400 | [11,16,22,25] |
| Alloy Composition (at.%) | Oxidation Conditions (°C and h) | Weight Gain (mg/cm2) | Room Temperature Fracture Toughness (MPa·m1/2) | Reference |
|---|---|---|---|---|
| Nb-22Ti-16Si-3Cr-3Al-2B-8Hf | 1250/50 | 96.6 | 14.1 | [42] |
| Nb-22Ti-14Si-2Al-2Hf-17Cr | 1250/100 | 60 | 8.7 | [46] |
| Nb-22Ti-15Si-5Cr-3Hf-3Al-8Zr | 1250/20 | 46.3 | 15.01 | [59] |
| Nb-22Ti-16Si-5Cr-4Hf-3Al-5B | 1250/50 | 67.6 | 11.0 | [65] |
| Nb-15Si-24Ti-4Cr-2Al-2Hf-1V | 1300/100 | 148.5 | 12.98 | [69] |
| Nb-22Ti-15Si-5Cr-5Mo-4Zr-3Al-2Hf-10V | 1250/20 | 302.8 | 6.42 | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, F.; Zhang, Y.; Yu, L.; Fu, T.; Wang, J.; Wang, H.; Cui, K. Microstructure and Oxidation Behavior of Nb-Si-Based Alloys for Ultrahigh Temperature Applications: A Comprehensive Review. Coatings 2021, 11, 1373. https://doi.org/10.3390/coatings11111373
Shen F, Zhang Y, Yu L, Fu T, Wang J, Wang H, Cui K. Microstructure and Oxidation Behavior of Nb-Si-Based Alloys for Ultrahigh Temperature Applications: A Comprehensive Review. Coatings. 2021; 11(11):1373. https://doi.org/10.3390/coatings11111373
Chicago/Turabian StyleShen, Fuqiang, Yingyi Zhang, Laihao Yu, Tao Fu, Jie Wang, Hong Wang, and Kunkun Cui. 2021. "Microstructure and Oxidation Behavior of Nb-Si-Based Alloys for Ultrahigh Temperature Applications: A Comprehensive Review" Coatings 11, no. 11: 1373. https://doi.org/10.3390/coatings11111373





