Design of Icephobic Surfaces by Lowering Ice Adhesion Strength: A Mini Review
Abstract
:1. Introduction
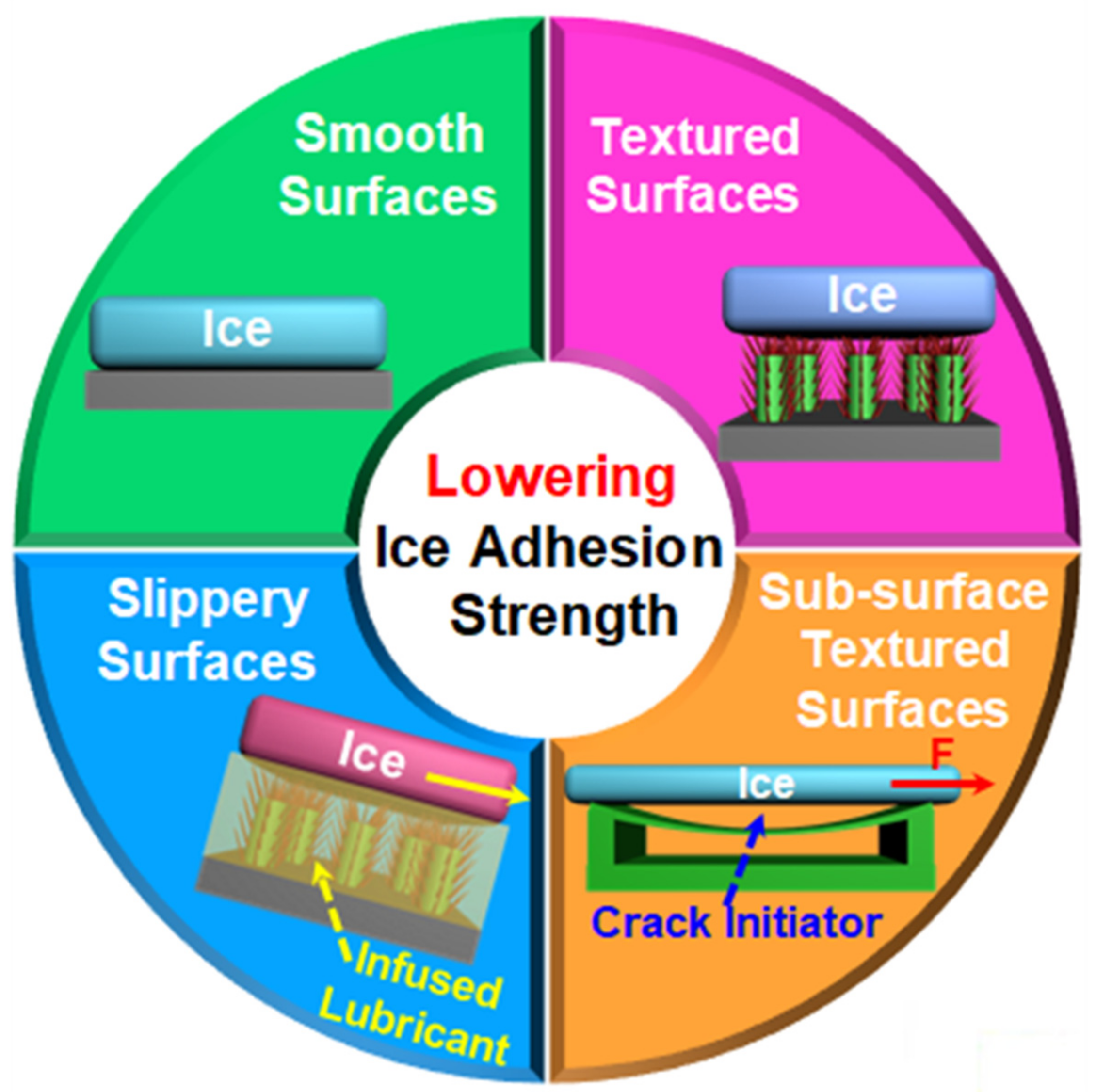
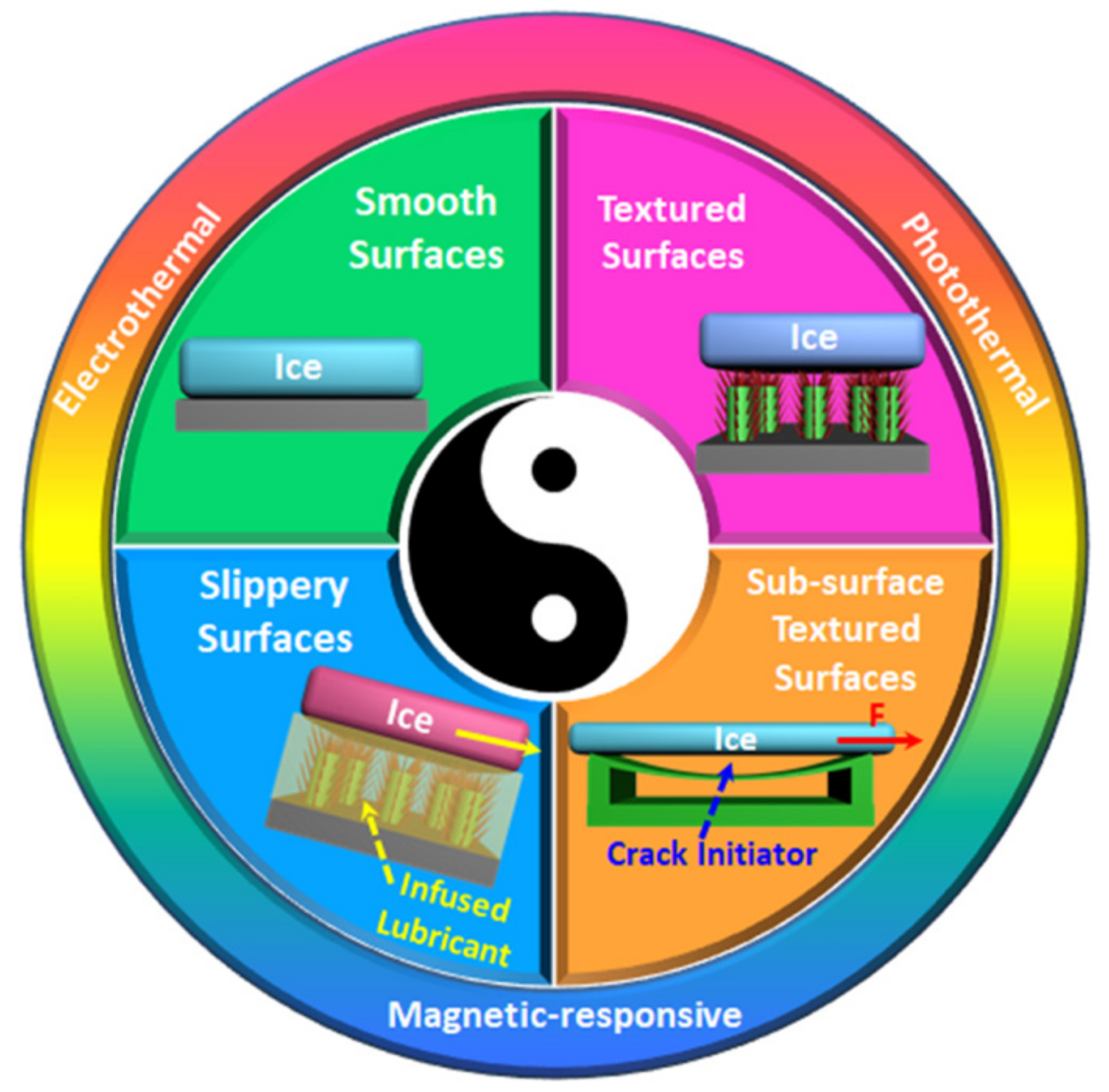
2. Strategies towards Designing of Icephobic Surfaces by Lowering Ice Adhesion Strength
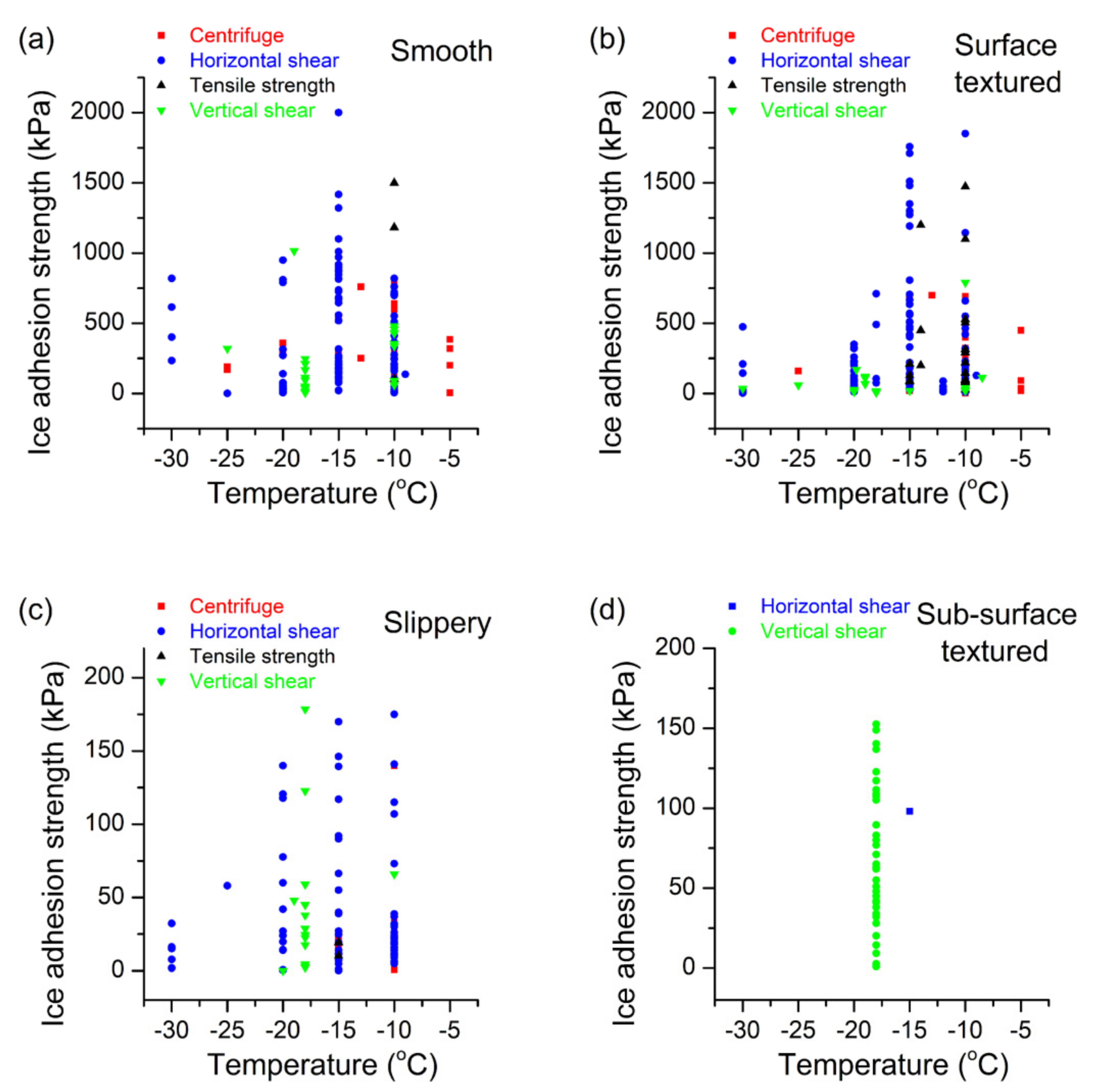
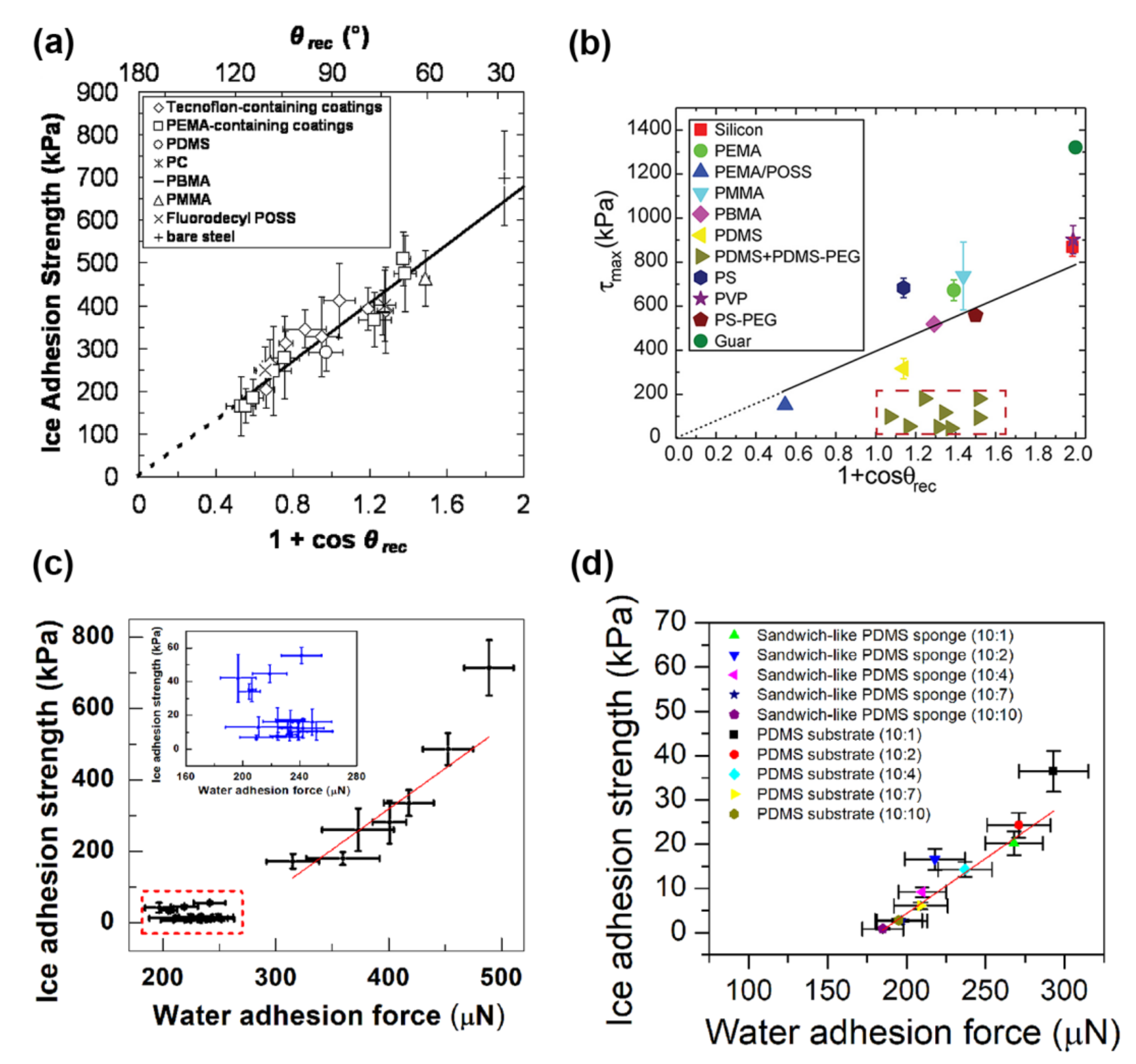
2.1. Smooth Surfaces
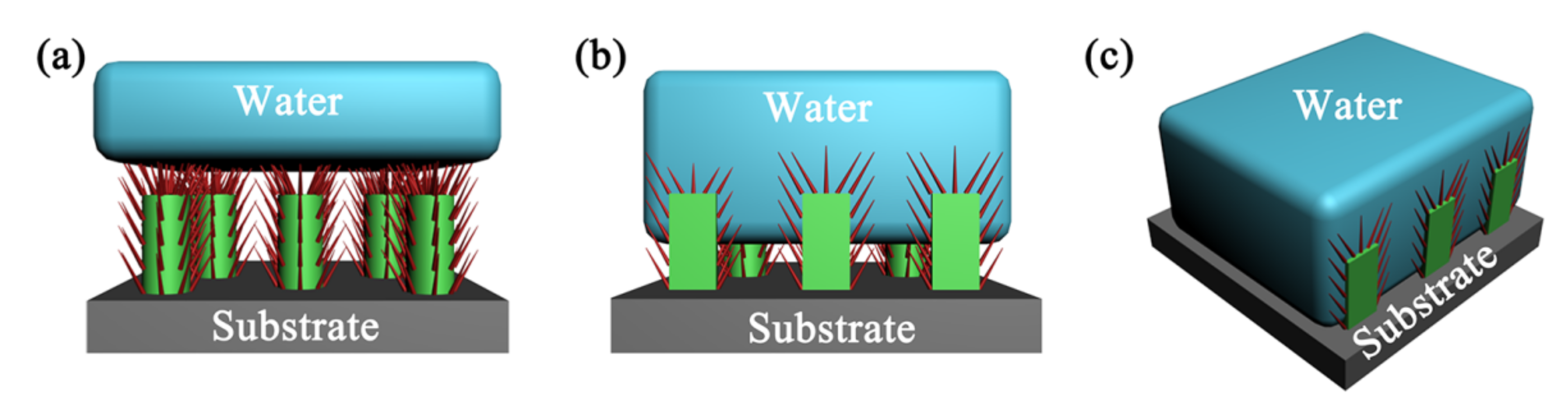
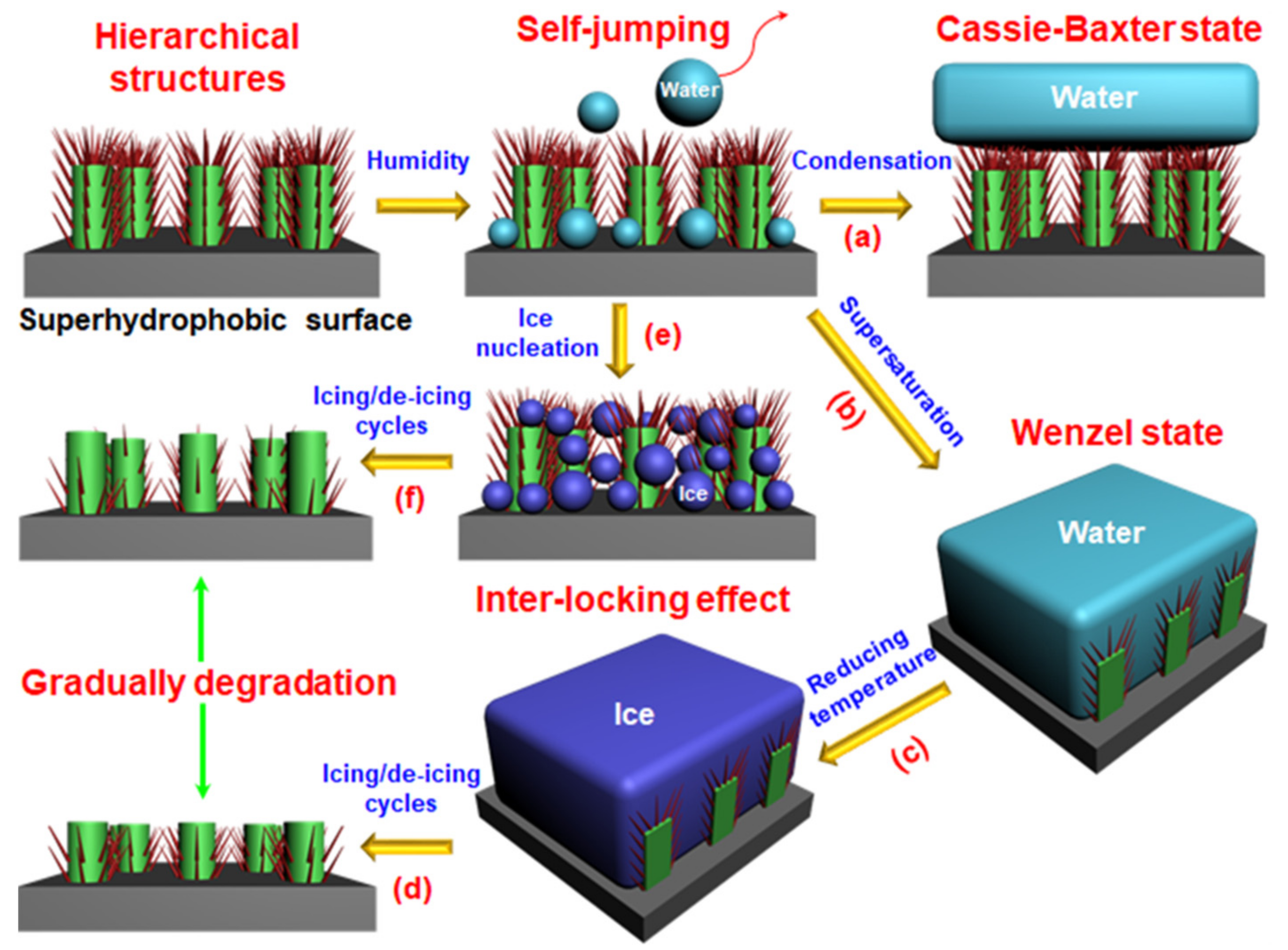
2.2. Textured Surfaces
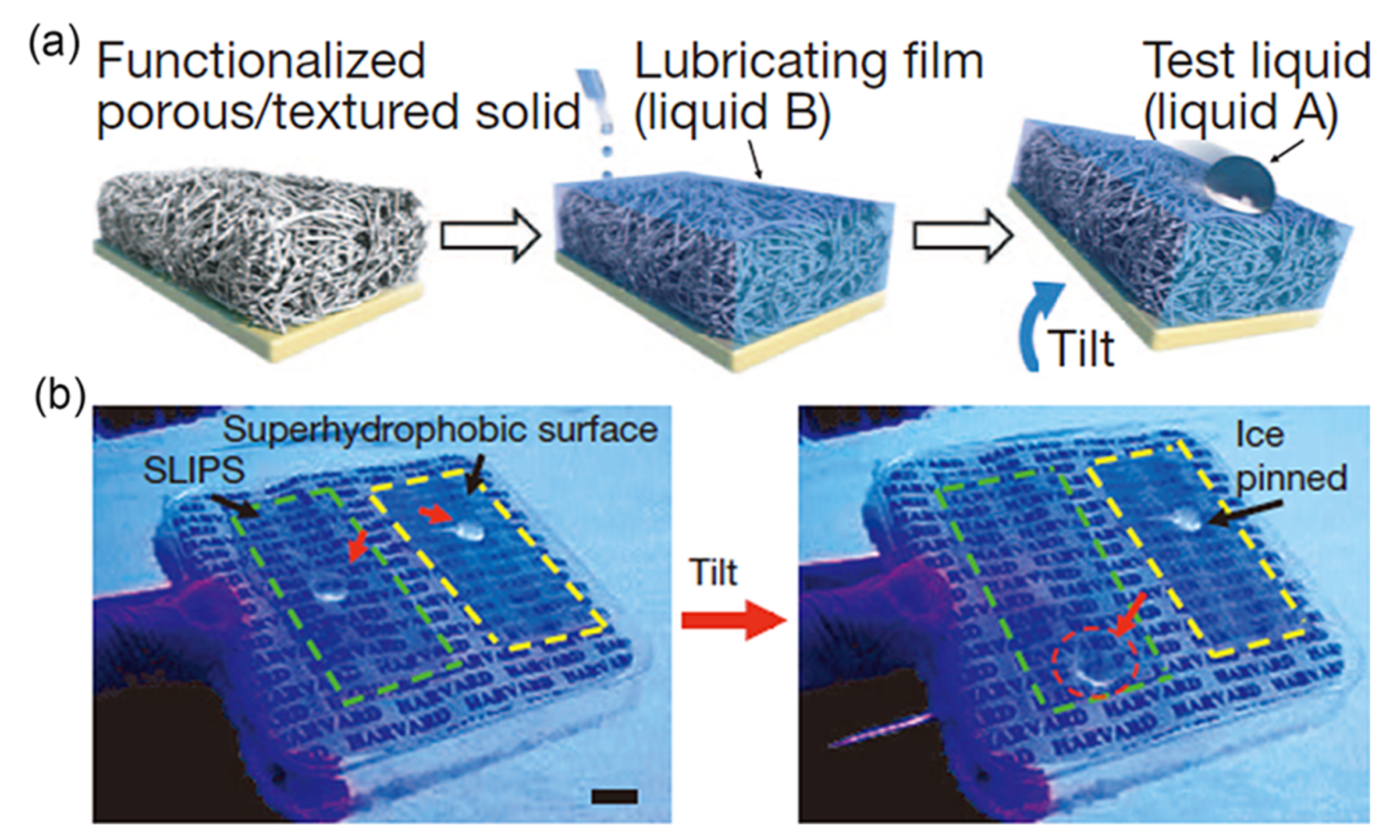
2.3. Slippery Surfaces
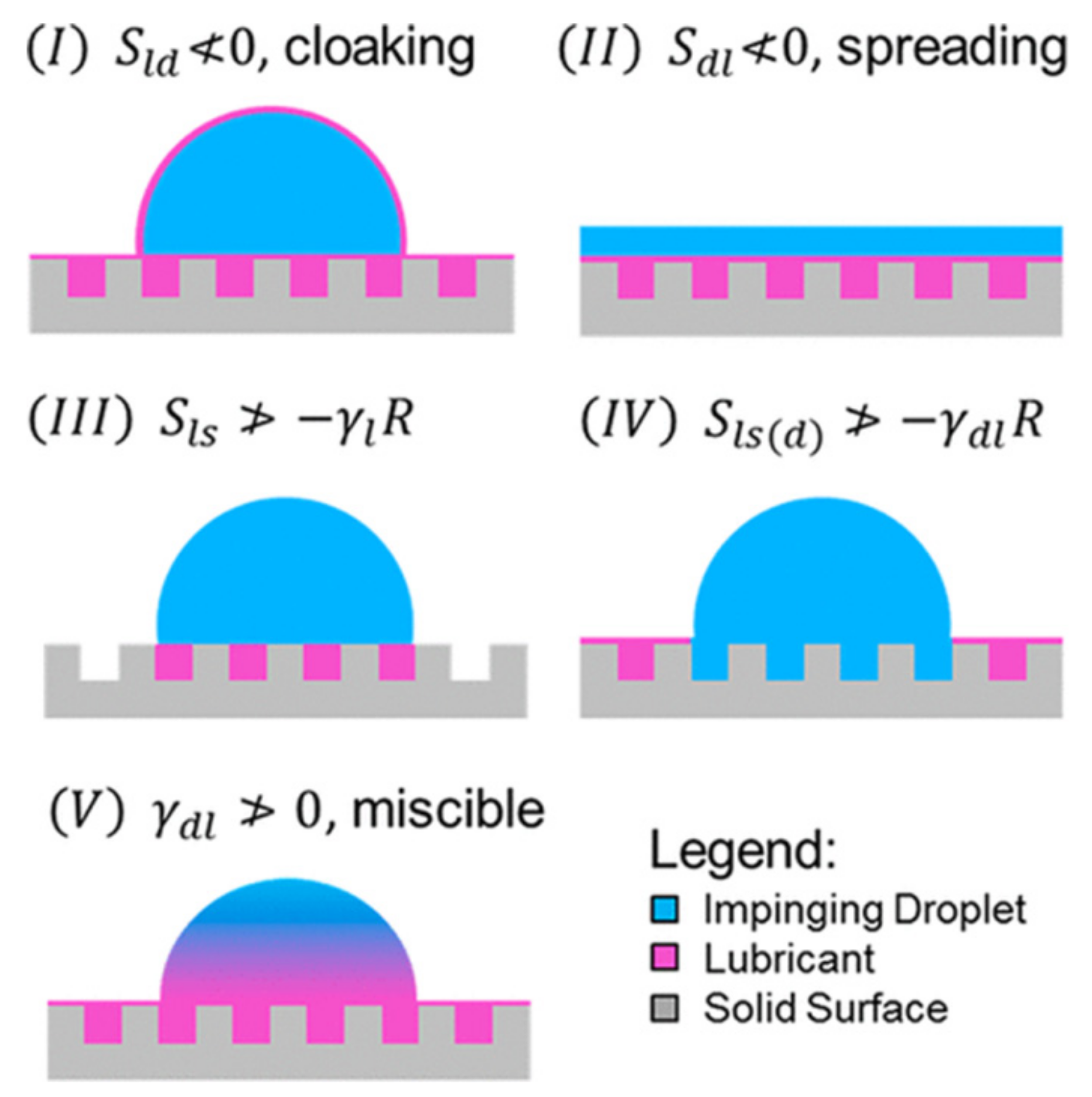
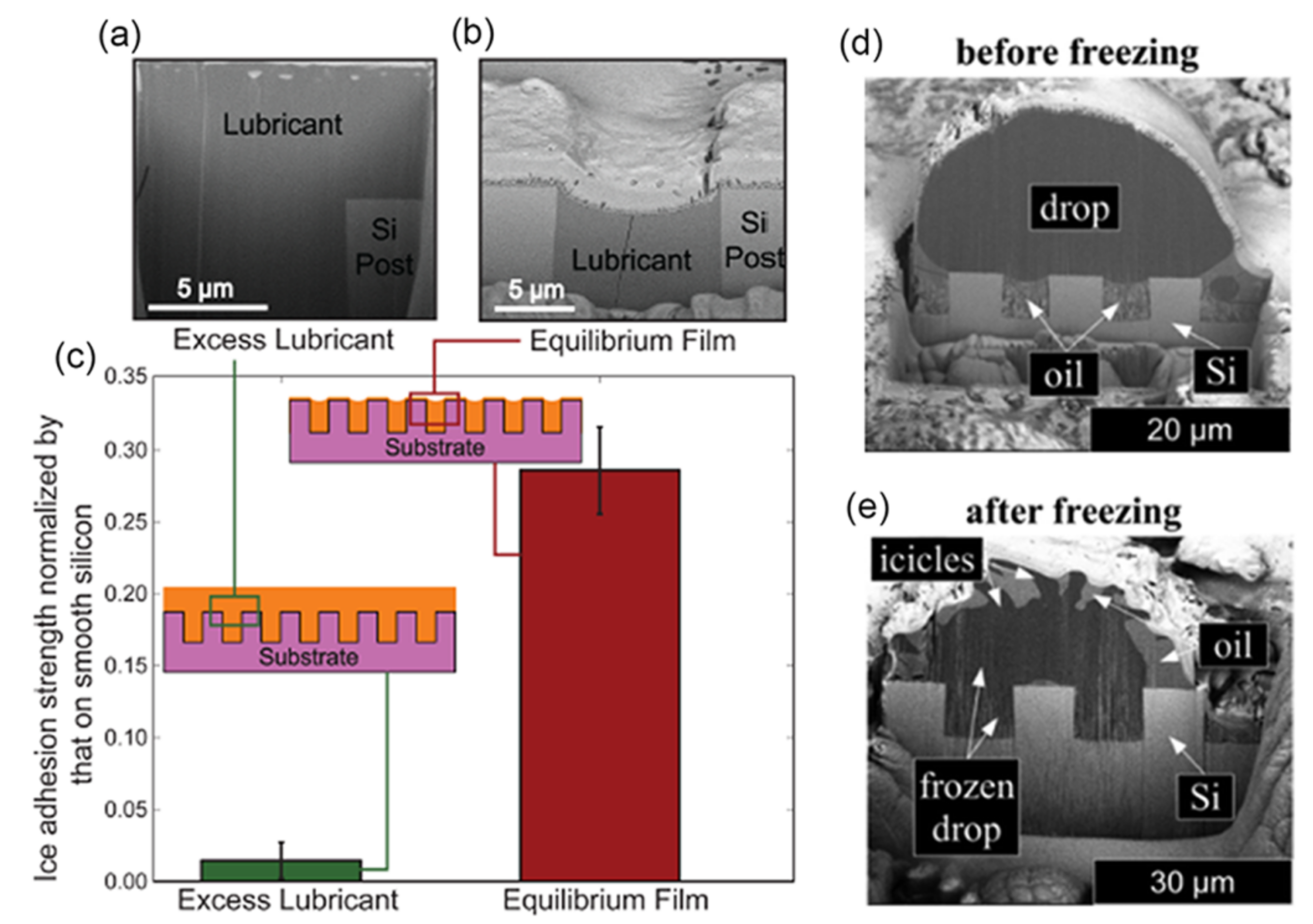
2.3.1. Organic Lubricating Surfaces
- (1)
- Sld ≥ 0, the lubricant spreads over the droplet; the droplet is cloaked,
- (2)
- Sdl ≥ 0, the impinging fluid spreads over the lubricant and forms a film instead of discrete droplets,
- (3)
- Sls ≤ −γlR, the lubricant does not infuse in the hierarchical structures of substrate in the presence of the surrounding vapor,
- (4)
- Sls(d) ≤ −γdlR, the lubricant does not infuse in the hierarchical structures of substrate in the presence of the impinging fluid,
- (5)
- γdl ≤ 0, the lubricant and the impinging fluid are miscible, where subscripts l, d, s refer to lubricant, impinging fluid and substrate, respectively, where Sxy represents the spreading parameter for x spreading over y, and R = (r − 1)(r − ϕ) is a roughness factor calculated using the roughness (r) and the solid fraction (ϕ), and is 0 for a flat substrate and 1 for a very rough substrate [221,222]. Thus, these five criteria need to be considered when a water droplet is on slippery surfaces at low temperature (i.e., before ice nucleation).
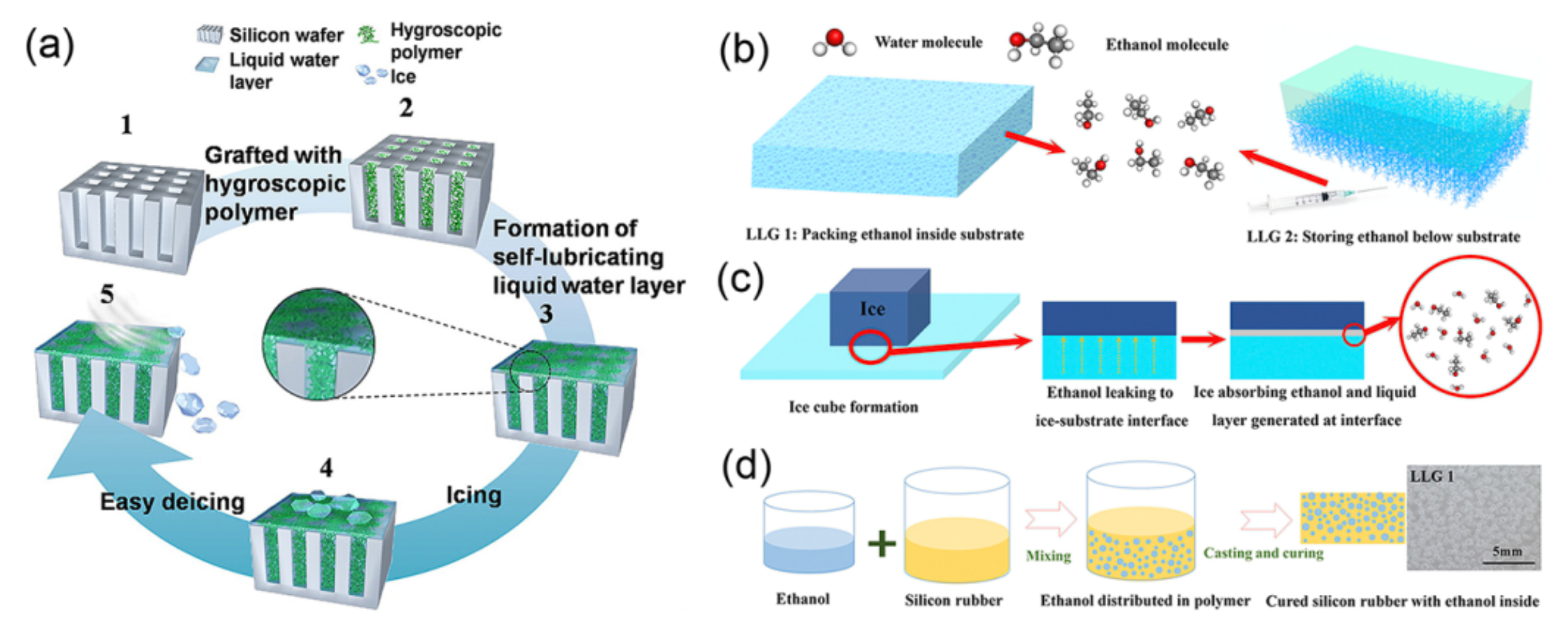
2.3.2. Aqueous Lubricating Surfaces
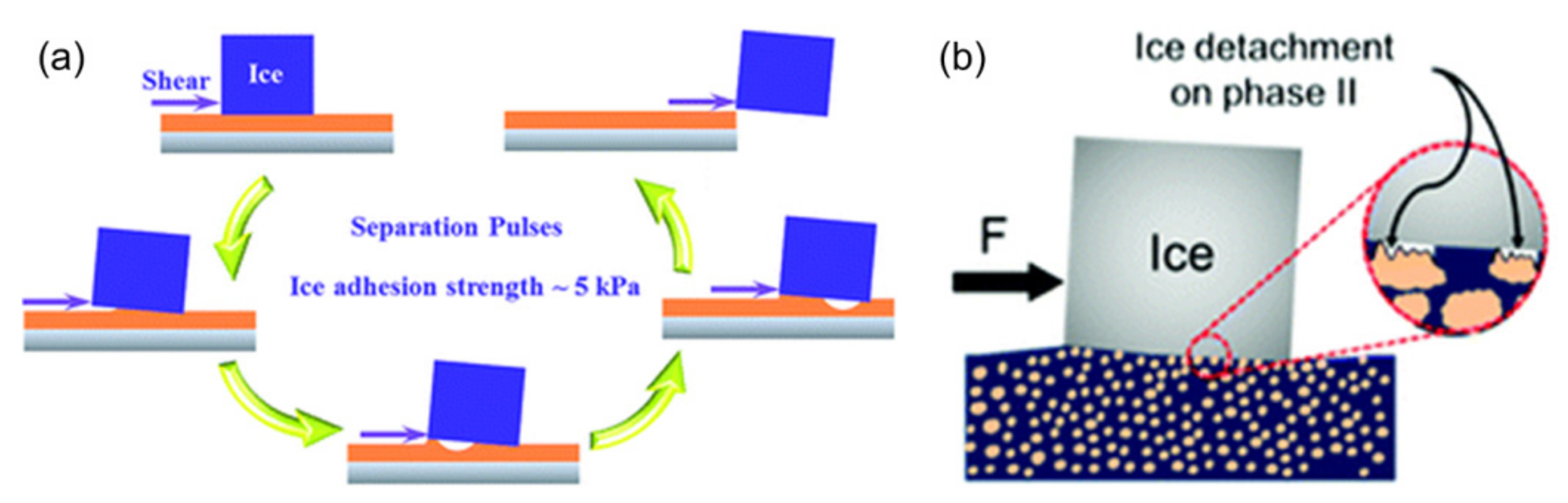
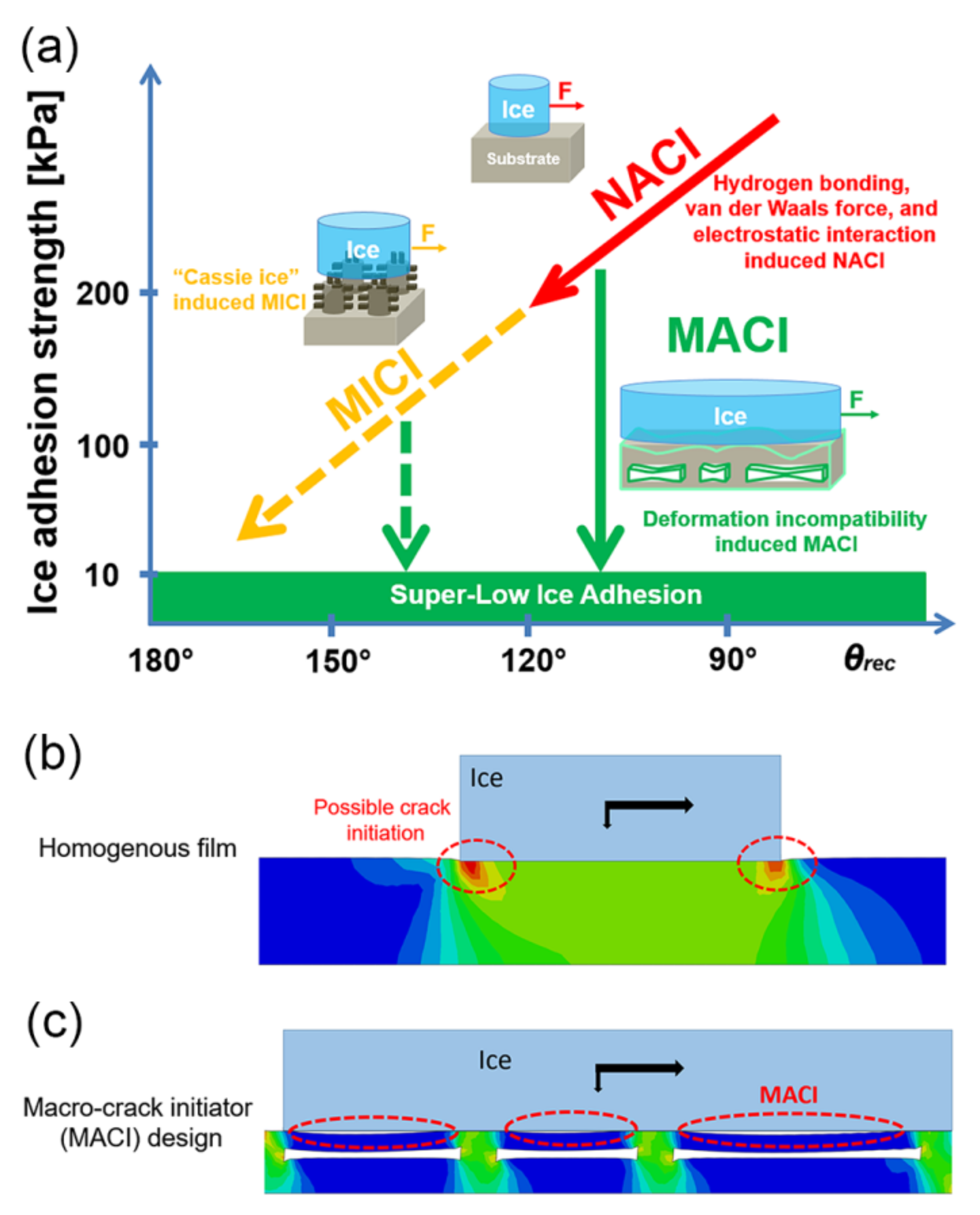
2.4. Sub-Surface Textured Surfaces
3. Synergy of Multiple Anti-Icing Strategies
4. Summary and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Meuler, A.J.; Smith, J.D.; Varanasi, K.K.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Relationships between water wettability and ice adhesion. ACS Appl. Mater. Interfaces 2010, 2, 3100–3110. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhuo, Y.; He, J.; Zhang, Z. Design and preparation of sandwich-like PDMS sponges with super-low ice adhesion. Soft Matter 2018, 14, 4846–4851. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.W.; Du, Y.J.; Alsaid, Y.; Wu, D.; Hua, M.T.; Yan, Y.C.; Yao, B.W.; Ma, Y.F.; Zhu, X.Y.; He, X.M. Superhydrophobic photothermal icephobic surfaces based on candle soot. Proc. Natl. Acad. Sci. USA 2020, 117, 11240–11246. [Google Scholar] [CrossRef]
- Hejazi, V.; Sobolev, K.; Nosonovsky, M. From superhydrophobicity to icephobicity: Forces and interaction analysis. Sci. Rep. 2013, 3, 2194. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Vågenes, E.T.; Delabahan, C.; He, J.; Zhang, Z. Room temperature characteristics of polymer-based low ice adhesion surfaces. Sci. Rep. 2017, 7, 42181. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.H.; Moevius, L.; Xu, X.P.; Qian, T.Z.; Yeomans, J.M.; Wang, Z.K. Pancake bouncing on superhydrophobic surfaces. Nat. Phys. 2014, 10, 515–519. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Mammen, L.; Butt, H.-J.; Vollmer, D. Candle soot as a template for a transparent robust superamphiphobic coating. Science 2012, 335, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Genzer, J.; Efimenko, K. Creating long-lived superhydrophobic polymer surfaces through mechanically assembled monolayers. Science 2000, 290, 2130–2133. [Google Scholar] [CrossRef] [Green Version]
- Wen, M.; Wang, L.; Zhang, M.; Jiang, L.; Zheng, Y. Antifogging and icing-delay properties of composite micro- and nanostructured surfaces. ACS Appl. Mater. Interfaces 2014, 6, 3963–3968. [Google Scholar] [CrossRef]
- Boreyko, J.B.; Collier, C.P. Delayed frost growth on jumping-drop superhydrophobic surfaces. ACS Nano 2013, 7, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zheng, Y.; Wen, M.; Song, C.; Lin, Y.; Jiang, L. Icephobic/anti-icing properties of micro/nanostructured surfaces. Adv. Mater. 2012, 24, 2642–2648. [Google Scholar] [CrossRef]
- Varanasi, K.K.; Deng, T.; Smith, J.D.; Hsu, M.; Bhate, N. Frost formation and ice adhesion on superhydrophobic surfaces. Appl. Phys. Lett. 2010, 97, 234102–234103. [Google Scholar] [CrossRef]
- He, Z.; Xiao, S.; Gao, H.; He, J.; Zhang, Z. Multiscale crack initiator promoted super-low ice adhesion surfaces. Soft Matter 2017, 13, 6562–6568. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Song, Y.; Jiang, L.; Wang, J. Bio-inspired strategies for anti-icing. ACS Nano 2014, 8, 3152–3169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, J.; Cheng, Y.; Yang, H.; Chen, Z.; Lai, Y. Bioinspired Surfaces with Superwettability for Anti-Icing and Ice-Phobic Application: Concept, Mechanism, and Design. Small 2017, 13, 1701867. [Google Scholar] [CrossRef] [PubMed]
- Sojoudi, H.; Wang, M.; Boscher, N.D.; McKinley, G.H.; Gleason, K.K. Durable and scalable icephobic surfaces: Similarities and distinctions from superhydrophobic surfaces. Soft Matter 2016, 12, 1938–1963. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhuo, Y.; He, Z.; Xiao, S.; He, J.; Zhang, Z. Dynamic Anti-Icing Surfaces (DAIS). Adv. Sci. 2021, 2101163. [Google Scholar] [CrossRef]
- Zhuo, Y.; Xiao, S.; Amirfazli, A.; He, J.; Zhang, Z. Polysiloxane as icephobic Materials—The past, present and the future. Chem. Eng. J. 2020, 405, 127088. [Google Scholar] [CrossRef]
- Zhuo, Y.; Chen, J.; Xiao, S.; Li, T.; Wang, F.; He, J.; Zhang, Z. Gels as emerging anti-icing materials: A mini review. Mater. Horizons 2021. [CrossRef]
- Esmeryan, K.D. From extremely water-repellent coatings to passive icing protection—Principles, limitations and lnnovative application aspects. Coatings 2020, 10, 66. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Shen, Z.; Qiu, H.; Chen, J.; Liang, L.; Wang, J. Research progress in aluminium-based anti-icing surfaces. J. Mater. Eng. 2021, 49, 41–50. [Google Scholar]
- Wang, G.; Guo, Z. Liquid infused surfaces with anti-icing properties. Nanoscale 2019, 11, 22615–22635. [Google Scholar] [CrossRef] [PubMed]
- Latthe, S.S.; Sutar, R.S.; Bhosale, A.K.; Nagappan, S.; Ha, C.-S.; Sadasivuni, K.K.; Liu, S.; Xing, R. Recent developments in air-trapped superhydrophobic and liquid-infused slippery surfaces for anti-icing application. Prog. Org. Coat. 2019, 137, 105373. [Google Scholar] [CrossRef]
- Golovin, K.; Kobaku, S.P.; Lee, D.H.; DiLoreto, E.T.; Mabry, J.M.; Tuteja, A. Designing durable icephobic surfaces. Sci. Adv. 2016, 2, e1501496. [Google Scholar] [CrossRef] [Green Version]
- Rønneberg, S.; He, J.; Zhang, Z. The need for standards in low ice adhesion surface research: A critical review. J. Adhes. Sci. Technol. 2019, 34, 1–29. [Google Scholar] [CrossRef]
- Rønneberg, S.; Laforte, C.; Volat, C.; He, J.; Zhang, Z. The effect of ice type on ice adhesion. AIP Adv. 2019, 9, 055304. [Google Scholar] [CrossRef] [Green Version]
- Brassard, J.-D.; Laforte, C.; Guérin, F.; Blackburn, C. Icephobicity: Definition and measurement regarding atmospheric icing. Adv. Polym. Sci. 2017, 284, 123–143. [Google Scholar]
- Golovin, K.; Dhyani, A.; Thouless, M.D.; Tuteja, A. Low–interfacial toughness materials for effective large-scale deicing. Science 2019, 364, 371–375. [Google Scholar] [CrossRef]
- Makkonen, L. Ice adhesion —Theory, measurements and countermeasures. J. Adhes. Sci. Technol. 2012, 26, 413–445. [Google Scholar] [CrossRef]
- Andrews, E.H.; Majid, H.A.; Lockington, N.A. Adhesion of ice to a flexible substrate. J. Mater Sci. 1984, 19, 73–81. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, K.; Tao, C.; Zhao, Y.; Li, X.; Zhu, K.; Yuan, X. Strategies for anti-icing: Low surface energy or liquid-infused? RSC Adv. 2016, 6, 70251–70260. [Google Scholar] [CrossRef]
- Fu, Q.; Wu, X.; Kumar, D.; Ho, J.W.C.; Kanhere, P.D.; Srikanth, N.; Liu, E.; Wilson, P.; Chen, Z. Development of sol–gel icephobic coatings: Effect of surface roughness and surface energy. ACS Appl. Mater. Interfaces 2014, 6, 20685–20692. [Google Scholar] [CrossRef]
- Foroughi Mobarakeh, L.; Jafari, R.; Farzaneh, M. Robust icephobic, and anticorrosive plasma polymer coating. Cold Reg. Sci. Technol. 2018, 151, 89–93. [Google Scholar] [CrossRef]
- Foroughi Mobarakeh, L.; Jafari, R.; Farzaneh, M. The ice repellency of plasma polymerized hexamethyldisiloxane coating. Appl. Surf. Sci. 2013, 284, 459–463. [Google Scholar] [CrossRef]
- Golovin, K.; Tuteja, A. A predictive framework for the design and fabrication of icephobic polymers. Sci. Adv. 2017, 3, e1701617. [Google Scholar] [CrossRef] [Green Version]
- Dou, R.; Chen, J.; Zhang, Y.; Wang, X.; Cui, D.; Song, Y.; Jiang, L.; Wang, J. Anti-icing coating with an aqueous lubricating layer. ACS Appl. Mater. Interfaces 2014, 6, 6998–7003. [Google Scholar] [CrossRef]
- Cui, W.; Pakkanen, T.A. Fabrication of transparent icephobic surfaces with self-reparability: Effect of structuring and thickness of the lubricant-elastomer layer. Appl. Surf. Sci. 2019, 504, 144061. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Z.; Fan, Q.; Lv, J.; Wang, J. Anti-ice coating inspired by ice skating. Small 2014, 10, 4693–4699. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, J.; He, M.; Li, K.; Cui, D.; Zhang, Q.; Zeng, X.; Zhang, Y.; Wang, J.; Song, Y. Superhydrophobic surfaces cannot reduce ice adhesion. Appl. Phys. Lett. 2012, 101, 111603. [Google Scholar] [CrossRef]
- Balordi, M.; Santucci de Magistris, G.; Chemelli, C. A novel simple anti-ice aluminum coating: Synthesis and in-lab comparison with a superhydrophobic hierarchical surface. Coatings 2020, 10, 111. [Google Scholar] [CrossRef] [Green Version]
- Beemer, D.L.; Wang, W.; Kota, A.K. Durable gels with ultra-low adhesion to ice. J. Mater. Chem. A 2016, 4, 18253–18258. [Google Scholar] [CrossRef]
- Chen, G.; Liu, S.; Sun, Z.; Wen, S.; Feng, T.; Yue, Z. Intrinsic self-healing organogels based on dynamic polymer network with self-regulated secretion of liquid for anti-icing. Prog. Org. Coat. 2020, 144, 105641. [Google Scholar] [CrossRef]
- Barman, T.; Chen, H.; Liu, J.; Yang, G.; Zhao, W.; Peng, C.; Hou, X. Synthesis and characterization of styrene-based polyfluoroacrylate film for hydrophobic/icephobic applications. Thin Solid Films 2019, 687, 137462. [Google Scholar] [CrossRef]
- Barthwal, S.; Lee, B.; Lim, S.-H. Fabrication of robust and durable slippery anti-icing coating on textured superhydrophobic aluminum surfaces with infused silicone oil. Appl. Surf. Sci. 2019, 496, 143677. [Google Scholar] [CrossRef]
- Aguilar, D.; Zheng, S.; Huang, Y.; Zeng, X.; Zhang, Q.; Chen, Z. Solvent-free synthesis and hydrophobization of bio-based epoxy coatings for anti-icing and anticorrosion applications. ACS Sustain. Chem. Eng. 2019, 7, 19131–19141. [Google Scholar] [CrossRef]
- Arianpour, F.; Farzaneh, M.; Kulinich, S.A. Hydrophobic and ice-retarding properties of doped silicone rubber coatings. Appl. Surf. Sci. 2013, 265, 546–552. [Google Scholar] [CrossRef]
- Saleema, N.; Farzaneh, M.; Paynter, R.W.; Sarkar, D.K. Prevention of ice accretion on aluminum surfaces by enhancing their hydrophobic properties. J. Adhes. Sci. Technol. 2011, 25, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Arianpour, F.; Farzaneh, M.; Jafari, R. Hydrophobic and ice-phobic properties of self-assembled monolayers (SAMs) coatings on AA6061. Prog. Org. Coat. 2016, 93, 41–45. [Google Scholar] [CrossRef]
- Shen, Y.; Tao, H.; Chen, S.; Zhu, L.; Wang, T.; Tao, J. Icephobic/anti-icing potential of superhydrophobic Ti6Al4V surfaces with hierarchical textures. RSC Adv. 2015, 5, 1666–1672. [Google Scholar] [CrossRef]
- Susoff, M.; Siegmann, K.; Pfaffenroth, C.; Hirayama, M. Evaluation of icephobic coatings—Screening of different coatings and influence of roughness. Appl. Surf. Sci. 2013, 282, 870–879. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Wang, N.; Han, Z.; Sun, H.; Xiong, D. Robust superhydrophobic surface with wrinkle-like structures on AZ31 alloy that repels viscous oil and investigations of the anti-icing property. Colloid Surface. A 2020, 594, 124655. [Google Scholar] [CrossRef]
- Valentini, L.; Bittolo Bon, S.; Pugno, N.M.; Hernandez Santana, M.; Lopez-Manchado, M.A.; Giorgi, G. Synergistic icephobic behaviour of swollen nitrile butadiene rubber graphene and/or carbon nanotube composites. Compos. Part B-Eng. 2019, 166, 352–360. [Google Scholar] [CrossRef]
- Wang, C.; Fuller, T.; Zhang, W.; Wynne, K.J. Thickness dependence of ice removal stress for a polydimethylsiloxane nanocomposite: Sylgard. Langmuir 2014, 30, 12819–12826. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gupta, M.C.; Yeong, Y.H.; Wynne, K.J. Factors affecting the adhesion of ice to polymer substrates. J. Appl. Polym. Sci. 2017, 135, 45734. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhang, W.; Siva, A.; Tiea, D.; Wynne, K.J. Laboratory test for ice adhesion strength using commercial instrumentation. Langmuir 2014, 30, 540–547. [Google Scholar] [CrossRef]
- Wang, F.; Ding, W.; He, J.; Zhang, Z. Phase transition enabled durable anti-icing surfaces and its DIY design. Chem. Eng. J. 2019, 360, 243–249. [Google Scholar] [CrossRef]
- Wang, G.; Shen, Y.; Tao, J.; Luo, X.; Zhang, L.; Xia, Y. Fabrication of a superhydrophobic surface with a hierarchical nanoflake-micropit structure and its anti-icing properties. RSC Adv. 2017, 7, 9981–9988. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Memon, H.; Liu, J.; Yang, G.; Xu, F.; Hussain, T.; Scotchford, C.; Hou, X. Effect of surface adsorption on icing behaviour of metallic coating. Surf. Coat. Technol. 2019, 380, 125068. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Li, M.; Wang, Q.; Chen, Q. The icephobicity comparison of polysiloxane modified hydrophobic and superhydrophobic surfaces under condensing environments. Appl. Surf. Sci. 2016, 385, 472–480. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, J.; Wang, Q.; Chen, Q.; Ding, J. Verification of icephobic/anti-icing properties of a superhydrophobic surface. ACS Appl. Mater. Interfaces 2013, 5, 3370–3381. [Google Scholar] [CrossRef]
- Wu, X.; Chen, Z. A mechanically robust transparent coating for anti-icing and self-cleaning applications. J. Mater. Chem. A 2018, 6, 16043–16052. [Google Scholar] [CrossRef]
- Wu, X.; Silberschmidt, V.V.; Hu, Z.-T.; Chen, Z. When superhydrophobic coatings are icephobic: Role of surface topology. Surf. Coat. Technol. 2019, 358, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.H.; Tang, Y.X.; Silberschmidt, V.V.; Wilson, P.; Chen, Z. Mechanically robust transparent anti-icing coatings: Roles of dispersion status of titanate nanotubes. Adv. Mater. Interfaces 2018, 5, 1800773. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.H.; Zheng, S.L.; Bellido-Aguilar, D.A.; Silberschmidt, V.V.; Chen, Z. Transparent icephobic coatings using bio-based epoxy resin. Mater. Des. 2018, 140, 516–523. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, T.; Tenjimbayashi, M.; Manabe, K.; Moriya, T.; Nakamura, H.; Nakamura, T.; Matsubayashi, T.; Tsuge, Y.; Shiratori, S. Antifreeze liquid-Infused surface with high transparency, low ice adhesion strength, and antifrosting properties fabricated through a spray layer-by-layer method. Ind. Eng. Chem. Res. 2019, 58, 2225–2234. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, Z.; Tan, S.; Luo, Y.; Luo, Z. How micro-/nano-structure evolution influences dynamic wetting and natural deicing abilities of bionic lotus surfaces. Langmuir 2020, 36, 4005–4014. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, Y.; Wang, D.; Liu, Z.; Liu, Y.; Pei, X.; Yu, B.; Zhou, F. Integration of self-lubrication and near-infrared photothermogenesis for excellent anti-icing/deicing performance. Adv. Funct. Mater. 2015, 25, 4237–4245. [Google Scholar] [CrossRef]
- Coady, M.J.; Wood, M.; Wallace, G.Q.; Nielsen, K.E.; Kietzig, A.-M.; Lagugné-Labarthet, F.; Ragogna, P.J. Icephobic behavior of UV-cured polymer networks incorporated into slippery lubricant-infused porous surfaces: Improving SLIPS durability. ACS Appl. Mater. Interfaces 2018, 10, 2890–2896. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Q.; Cheng, T.; Zhan, X.; Chen, F. Polyols-infused slippery surfaces based on magnetic Fe3O4-functionalized polymer hybrids for enhanced multifunctional anti-icing and deicing properties. Langmuir 2018, 34, 4052–4058. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, C.; Tu, J. Robust slippery coating with superior corrosion resistance and anti-icing performance for AZ31B Mg alloy protection. ACS Appl. Mater. Interfaces 2017, 9, 11247–11257. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.; Tian, Y.; Wang, F.; Chen, Q.; Zhang, F.; Qian, H.; Ma, L. Facile one-step method to fabricate a slippery lubricant-infused surface (LIS) with self-replenishment properties for anti-icing applications. Coatings 2020, 10, 119. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.; Li, X.; Su, J.; Li, H.; Zhao, Y.; Yuan, X. Improvement of anti-icing properties of low surface energy coatings by introducing phase-change microcapsules. Polym. Eng. Sci. 2018, 58, 973–979. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, J.; Wang, Y.; Chen, Q.; Ding, J.; Wang, Q. Ice-phobic coatings based on silicon-oil-infused polydimethylsiloxane. ACS Appl. Mater. Interfaces 2013, 5, 4053–4062. [Google Scholar] [CrossRef]
- Zhuo, Y.; Wang, F.; Xiao, S.; He, J.; Zhang, Z. One-step fabrication of bioinspired lubricant-regenerable icephobic slippery liquid-infused porous surfaces. ACS Omega 2018, 3, 10139–10144. [Google Scholar] [CrossRef]
- Chen, D.; Gelenter, M.D.; Hong, M.; Cohen, R.E.; McKinley, G.H. Icephobic surfaces induced by interfacial nonfrozen water. ACS Appl. Mater. Interfaces 2017, 9, 4202–4214. [Google Scholar] [CrossRef]
- Chen, J.; Dou, R.; Cui, D.; Zhang, Q.; Zhang, Y.; Xu, F.; Zhou, X.; Wang, J.; Song, Y.; Jiang, L. Robust prototypical anti-icing coatings with a self-lubricating liquid water layer between ice and substrate. ACS Appl. Mater. Interfaces 2013, 5, 4026–4030. [Google Scholar] [CrossRef] [PubMed]
- Chernyy, S.; Järn, M.; Shimizu, K.; Swerin, A.; Pedersen, S.U.; Daasbjerg, K.; Makkonen, L.; Claesson, P.; Iruthayaraj, J. Superhydrophilic polyelectrolyte brush layers with imparted anti-icing properties: Effect of counter ions. ACS Appl. Mater. Interfaces 2014, 6, 6487–6496. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Xiao, S.; Håkonsen, V.; Li, T.; Wang, F.; He, J.; Zhang, Z. Ultrafast self-healing and highly transparent coating with mechanically durable icephobicity. Appl. Mater. Today 2020, 19, 100542. [Google Scholar] [CrossRef]
- Zou, M.; Beckford, S.; Wei, R.; Ellis, C.; Hatton, G.; Miller, M.A. Effects of surface roughness and energy on ice adhesion strength. Appl. Surf. Sci. 2011, 257, 3786–3792. [Google Scholar] [CrossRef]
- Zheng, S.; Li, C.; Fu, Q.; Xiang, T.; Hu, W.; Wang, J.; Ding, S.; Liu, P.; Chen, Z. Fabrication of a micro-nanostructured superhydrophobic aluminum surface with excellent corrosion resistance and anti-icing performance. RSC Adv. 2016, 6, 79389–79400. [Google Scholar] [CrossRef]
- Zhuo, Y.; Li, T.; Wang, F.; Håkonsen, V.; Xiao, S.; He, J.; Zhang, Z. Ultra-durable icephobic coating by molecular pulley. Soft Matter 2019, 15, 3607–3611. [Google Scholar] [CrossRef]
- Zheng, S.; Li, C.; Fu, Q.; Hu, W.; Xiang, T.; Wang, Q.; Du, M.; Liu, X.; Chen, Z. Development of stable superhydrophobic coatings on aluminum surface for corrosion-resistant, self-cleaning, and anti-icing applications. Mater. Des. 2016, 93, 261–270. [Google Scholar] [CrossRef]
- Zheng, S.; Bellido-Aguilar, D.A.; Wu, X.; Zhan, X.; Huang, Y.; Zeng, X.; Zhang, Q.; Chen, Z. Durable waterborne hydrophobic bio-epoxy coating with improved anti-icing and self-cleaning performance. ACS Sustain. Chem. Eng. 2018, 7, 641–649. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Z.; Sarma, J.; Dai, X. Passive removal of highly wetting liquids and ice on quasi-liquid surfaces. ACS Appl. Mater. Interfaces 2020, 12, 20084–20095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.-q.; Cai, J.-z.; Li, X.-h.; Li, H.; Zhao, Y.-h.; Yuan, X.-y. Balance of polyacrylate-fluorosilicone block copolymers as icephobic coatings. Chin. J. Polym. Sci. 2015, 33, 153–162. [Google Scholar] [CrossRef]
- Zhang, K.; Li, X.; Zhao, Y.; Zhu, K.; Li, Y.; Tao, C.; Yuan, X. UV-curable POSS-fluorinated methacrylate diblock copolymers for icephobic coatings. Prog. Org. Coat. 2016, 93, 87–96. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, B.; Jamil, M.I.; Cheng, D.-G.; Zhang, Q.; Zhan, X.; Chen, F. Highly stable amphiphilic organogel with exceptional anti-icing performance. ACS Appl. Mater. Interfaces 2019, 11, 12838–12845. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhao, Y.; Li, H.; Qi, H.; Li, B.; Yuan, X. Preparation and evaluation of hydrophobic surfaces of polyacrylate-polydimethylsiloxane copolymers for anti-icing. Prog. Org. Coat. 2013, 76, 1435–1444. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, M.H.; Wei, W.; Zhang, Y.F.; Gutowski, V.; Zhang, W.X.; Deng, P.Y. “Open-mouth” mesoporous hollow micro/nano coatings based on POSS/PDMS: Fabrication, mechanisms, and anti-icing performance. Part. Part. Syst. Char. 2018, 35, 1800323. [Google Scholar] [CrossRef]
- Yeong, Y.H.; Wang, C.; Wynne, K.J.; Gupta, M.C. Oil-infused superhydrophobic silicone material for low ice adhesion with long-term infusion stability. ACS Appl. Mater. Interfaces 2016, 8, 32050–32059. [Google Scholar] [CrossRef]
- Yeong, Y.H.; Gupta, M.C. Hot embossed micro-textured thin superhydrophobic Teflon FEP sheets for low ice adhesion. Surf. Coat. Technol. 2017, 313, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.H.; Zhao, X.; Ho, J.W.C.; Chen, Z. Design and durability study of environmental-friendly room-temperature processable icephobic coatings. Chem. Eng. J. 2019, 355, 901–909. [Google Scholar] [CrossRef]
- Wu, Y.-l.; She, W.; Shi, D.; Jiang, T.; Hao, T.-h.; Liu, J.; Zhang, Q.-c.; You, J.; Li, R.Y. An extremely chemical and mechanically durable siloxane bearing copolymer coating with self-crosslinkable and anti-icing properties. Compos. Part B-Eng. 2020, 195, 108031. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, G.; Li, L.; Xu, C.; Lv, X.; Zhang, H.; Yao, W. Icephobic behaviors of superhydrophobic amorphous carbon nano-films synthesized from a flame process. J. Colloid Interface Sci. 2019, 552, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.F.; Wang, Y.M.; Zhang, Z.M.; Liu, Y.L. Application of anti-icing coating based on adsorption of functional substances by microporous sphere. Prog. Org. Coat. 2019, 137, 105320. [Google Scholar] [CrossRef]
- Wei, C.; Jin, B.; Zhang, Q.; Zhan, X.; Chen, F. Anti-icing performance of super-wetting surfaces from icing-resistance to ice-phobic aspects: Robust hydrophobic or slippery surfaces. J. Alloy Compd. 2018, 765, 721–730. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, X.; Chen, J.; He, Z.; Liu, J.; Li, Q.; Wang, J.; Jiang, L. Organogel as durable anti-icing coatings. Sci. China Mater. 2015, 58, 559–565. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Lv, T.; Wang, Q.; Chen, Q.; Ding, J. Influence of different chemical modifications on the icephobic properties of superhydrophobic surfaces in a condensate environment. J. Mater. Chem. A 2015, 3, 4967–4975. [Google Scholar] [CrossRef]
- Wang, N.; Tang, L.L.; Tong, W.; Xiong, D.S. Fabrication of robust and scalable superhydrophobic surfaces and investigation of their anti-icing properties. Mater. Des. 2018, 156, 320–328. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, S.; Zhuo, Y.; Ding, W.; He, J.; Zhang, Z. Liquid layer generators for excellent icephobicity at extremely low temperatures. Mater. Horizons 2019, 6, 2063–2072. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Tay, T.E.; Sun, Y.; Liang, W.; Yang, B. Low-voltage and -surface energy SWCNT/poly(dimethylsiloxane) (PDMS) nanocomposite film: Surface wettability for passive anti-icing and surface-skin heating for active deicing. Compos. Sci. Technol. 2019, 184, 107872. [Google Scholar] [CrossRef]
- Vercillo, V.; Tonnicchia, S.; Romano, J.M.; Garcia-Giron, A.; Aguilar-Morales, A.I.; Alamri, S.; Dimov, S.S.; Kunze, T.; Lasagni, A.F.; Bonaccurso, E. Design rules for laser-treated icephobic metallic surfaces for aeronautic applications. Adv. Funct. Mater. 2020, 30, 1910268. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Maghsoudi, K.; Jafari, R.; Momen, G. A comparative study of the icephobic and self-cleaning properties of teflon materials having different surface morphologies. J. Mater. Process. Tech. 2019, 276, 116415. [Google Scholar] [CrossRef]
- Urata, C.; Hönes, R.; Sato, T.; Kakiuchida, H.; Matsuo, Y.; Hozumi, A. Textured organogel films showing unusual thermoresponsive dewetting, icephobic, and optical properties. Adv. Mater. Interfaces 2019, 6, 1801358. [Google Scholar] [CrossRef]
- Upadhyay, V.; Galhenage, T.; Battocchi, D.; Webster, D. Amphiphilic icephobic coatings. Prog. Org. Coat. 2017, 112, 191–199. [Google Scholar] [CrossRef]
- Taş, M.; Memon, H.; Xu, F.; Ahmed, I.; Hou, X. Electrospun nanofibre membrane based transparent slippery liquid-infused porous surfaces with icephobic properties. Colloid Surface. A 2019, 585, 124177. [Google Scholar] [CrossRef]
- Tarquini, S.; Antonini, C.; Amirfazli, A.; Marengo, M.; Palacios, J. Investigation of ice shedding properties of superhydrophobic coatings on helicopter blades. Cold Reg. Sci. Technol. 2014, 100, 50–58. [Google Scholar] [CrossRef]
- Sojoudi, H.; McKinley, G.H.; Gleason, K.K. Linker-free grafting of fluorinated polymeric cross-linked network bilayers for durable reduction of ice adhesion. Mater. Horizons 2015, 2, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Sojoudi, H.; Arabnejad, H.; Raiyan, A.; Shirazi, S.A.; McKinley, G.H.; Gleason, K.K. Scalable and durable polymeric icephobic and hydrate-phobic coatings. Soft Matter 2018, 14, 3443–3454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivakumar, G.; Jackson, J.; Ceylan, H.; Sundararajan, S. Effect of plasticizer on the wear behavior and ice adhesion of elastomeric coatings. Wear 2019, 426–427, 212–218. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Y.; Tao, J.; Zhu, C.; Chen, H.; Wu, Z.; Xie, Y. Spraying fabrication of durable and transparent coatings for anti-icing application: Dynamic water repellency, icing delay, and ice adhesion. ACS Appl. Mater. Interfaces 2019, 11, 3590–3598. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Tao, J.; Tao, H.; Chen, S.; Pan, L.; Wang, T. Superhydrophobic Ti6Al4V surfaces with regular array patterns for anti-icing applications. RSC Adv. 2015, 5, 32813–32818. [Google Scholar] [CrossRef]
- Sandhu, A.; Walker, O.J.; Nistal, A.; Choy, K.L.; Clancy, A.J. Perfluoroalkane wax infused gels for effective, regenerating, anti-icing surfaces. Chem. Commun. 2019, 55, 3215–3218. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Mulroney, A.T.; Gupta, M.C. Anti-icing epoxy resin surface modified by spray coating of PTFE teflon particles for wind turbine blades. Mater. Today Commun. 2019, 22, 100770. [Google Scholar] [CrossRef]
- Nguyen, T.-B.; Park, S.; Lim, H. Effects of morphology parameters on anti-icing performance in superhydrophobic surfaces. Appl. Surf. Sci. 2018, 435, 585–591. [Google Scholar] [CrossRef]
- Nguyen, T.-B.; Park, S.; Jung, Y.; Lim, H. Effects of hydrophobicity and lubricant characteristics on anti-icing performance of slippery lubricant-infused porous surfaces. J. Ind. Eng. Chem. 2018, 69, 99–105. [Google Scholar] [CrossRef]
- Mulroney, A.T.; Kessler, E.D.; Combs, S.; Gupta, M.C. Low ice adhesion surfaces using microtextured hydrophobic tapes and their applications in refrigeration systems. Surf. Coat. Technol. 2018, 351, 108–114. [Google Scholar] [CrossRef]
- Ahn, J.; Jeon, J.; Heu, C.S.; Kim, D.R. Three-dimensionally programmed slippery wrinkles with high stretchability for tunable functionality of icephobicity and effective water harvesting. Adv. Mater. Interfaces 2018, 5, 1800980. [Google Scholar] [CrossRef]
- Arianpour, F.; Farhadi, S.; Farzaneh, M. Effect of heterogeneity on hydro/ice-phobic properties of alkylsilane/fluoro-alkylsilane-based coatings on Al substrates. J. Coat. Technol. Res. 2017, 14, 267–275. [Google Scholar] [CrossRef]
- Balordi, M.; Cammi, A.; de Magistris, G.S.; Chemelli, C. Role of micrometric roughness on anti-ice properties and durability of hierarchical super-hydrophobic aluminum surfaces. Surf. Coat. Technol. 2019, 374, 549–556. [Google Scholar] [CrossRef]
- Bleszynski, M.; Woll, R.; Middleton, J.; Kumosa, M. Effects of crosslinking, embedded TiO2 particles and extreme aging on PDMS icephobic barriers. Polym. Degrad. Stabil. 2019, 166, 272–282. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Zhevnenko, S.N.; Emel’yanenko, A.M.; Gol’dshtein, R.V.; Epifanov, V.P. Adhesive strength of the contact of ice with a superhydrophobic coating. Dokl. Chem. 2013, 448, 71–75. [Google Scholar] [CrossRef]
- Momen, G.; Jafari, R.; Farzaneh, M. Ice repellency behaviour of superhydrophobic surfaces: Effects of atmospheric icing conditions and surface roughness. Appl. Surf. Sci. 2015, 349, 211–218. [Google Scholar] [CrossRef]
- Brassard, J.-D.; Laforte, J.-L.; Blackburn, C.; Perron, J.; Sarkar, D.K. Silicone based superhydrophobic coating efficient to reduce ice adhesion and accumulation on aluminum under offshore arctic conditions. Ocean Eng. 2017, 144, 135–141. [Google Scholar] [CrossRef]
- Chen, J.; Li, K.; Wu, S.; Liu, J.; Liu, K.; Fan, Q. Durable anti-icing coatings based on self-sustainable lubricating layer. ACS Omega 2017, 2, 2047–2054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, Z.M.; Jiao, W.C.; Huang, Y.F.; Ding, G.M.; Zhong, X.; Yan, M.L.; Zheng, Y.T.; Wang, R.G. FDTS-modified SiO2/rGO wrinkled films with a micro-nanoscale hierarchical structure and anti-icing/deicing properties under condensation condition. Adv. Mater. Interfaces 2020, 7, 1901446. [Google Scholar] [CrossRef]
- Coady, M.J.; Getangama, N.N.K.; Khalili, A.; Wood, M.; Nielsen, K.E.; de Bruyn, J.R.; Hutter, J.L.; Klassen, R.J.; Kietzig, A.M.; Ragogna, P.J. Highly cross-linked UV-cured siloxane copolymer networks as icephobic coatings. J. Polym. Sci. 2020, 58, 1022–1029. [Google Scholar] [CrossRef]
- Cui, W.; Jiang, Y.; Mielonen, K.; Pakkanen, T.A. The verification of icephobic performance on biomimetic superhydrophobic surfaces and the effect of wettability and surface energy. Appl. Surf. Sci. 2019, 466, 503–514. [Google Scholar] [CrossRef]
- Cui, W.; Pakkanen, T.A. Icephobic performance of one-step silicone-oil-infused slippery coatings: Effects of surface energy, oil and nanoparticle contents. J. Colloid Interface Sci. 2019, 558, 251–258. [Google Scholar] [CrossRef]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, Y.; Wei, W.; Yin, Y.; Liu, M.; Guo, H.; Zheng, C.; Deng, P. Liquid-infused micro-nanostructured MOF coatings (LIMNSMCs) with high anti-icing performance. ACS Appl. Mater. Interfaces 2019, 11, 47545–47552. [Google Scholar] [CrossRef]
- Gao, S.; Liu, B.; Peng, J.; Zhu, K.; Zhao, Y.; Li, X.; Yuan, X. Icephobic durability of branched PDMS slippage coatings co-cross-linked by functionalized POSS. ACS Appl. Mater. Interfaces 2019, 11, 4654–4666. [Google Scholar] [CrossRef]
- Ghalmi, Z.; Farzaneh, M. Experimental investigation to evaluate the effect of PTFE nanostructured roughness on ice adhesion strength. Cold Reg. Sci. Technol. 2015, 115, 42–47. [Google Scholar] [CrossRef]
- Gu, W.C.; Song, K.X.; Cheng, Z.; Wang, Q.L.; Wang, S.L.; Wang, X.K.; Yu, X.Q.; Zhang, Y.F. Water-based robust transparent superamphiphobic coatings for resistance to condensation, frosting, icing, and fouling. Adv. Mater. Interfaces 2020, 7, 1902201. [Google Scholar] [CrossRef]
- Guerin, F.; Laforte, C.; Farinas, M.I.; Perron, J. Analytical model based on experimental data of centrifuge ice adhesion tests with different substrates. Cold Reg. Sci. Technol. 2016, 121, 93–99. [Google Scholar] [CrossRef]
- Momen, G.; Farzaneh, M. Facile approach in the development of icephobic hierarchically textured coatings as corrosion barrier. Appl. Surf. Sci. 2014, 299, 41–46. [Google Scholar] [CrossRef]
- Menini, R.; Ghalmi, Z.; Farzaneh, M. Highly resistant icephobic coatings on aluminum alloys. Cold Reg. Sci. Technol. 2011, 65, 65–69. [Google Scholar] [CrossRef]
- He, Y.; Jiang, C.; Cao, X.; Chen, J.; Tian, W.; Yuan, W. Reducing ice adhesion by hierarchical micro-nano-pillars. Appl. Surf. Sci. 2014, 305, 589–595. [Google Scholar] [CrossRef]
- He, Z.; Wu, C.; Hua, M.; Wu, S.; Wu, D.; Zhu, X.; Wang, J.; He, X. Bioinspired multifunctional anti-icing hydrogel. Matter 2020, 2, 723–734. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Zhuo, Y.; Wang, F.; He, J.; Zhang, Z. Understanding the role of hollow sub-surface structures in reducing ice adhesion strength. Soft Matter 2019, 15, 2905–2910. [Google Scholar] [CrossRef]
- Hong, S.; Wang, R.; Huang, X.; Liu, H. Facile one-step fabrication of PHC/PDMS anti-icing coatings with mechanical properties and good durability. Prog. Org. Coat. 2019, 135, 263–269. [Google Scholar] [CrossRef]
- Idriss, H.; Guselnikova, O.; Postnikov, P.S.; Kolska, Z.; Haušild, P.; Čech, J.; Lyutakov, O.; Švorčík, V. Versatile and scalable icephobization of airspace composite by surface morphology and chemistry tuning. ACS Appl. Polym. Mater. 2020, 2, 977–986. [Google Scholar] [CrossRef]
- Irajizad, P.; Al-Bayati, A.; Eslami, B.; Shafquat, T.; Nazari, M.; Jafari, P.; Kashyap, V.; Masoudi, A.; Araya, D.; Ghasemi, H. Stress-localized durable icephobic surfaces. Mater. Horiz. 2019, 6, 758–766. [Google Scholar] [CrossRef]
- Jafari, R.; Menini, R.; Farzaneh, M. Superhydrophobic and icephobic surfaces prepared by RF-sputtered polytetrafluoroethylene coatings. Appl. Surf. Sci. 2010, 257, 1540–1543. [Google Scholar] [CrossRef] [Green Version]
- Jafari, R.; Momen, G.; Eslami, E. Fabrication of icephobic aluminium surfaces by atmospheric plasma jet polymerisation. Surf. Eng. 2018, 35, 450–455. [Google Scholar] [CrossRef]
- Jamil, M.I.; Zhan, X.; Chen, F.; Cheng, D.-G.; Zhang, Q. Durable and scalable candle soot icephobic coating with nucleation and fracture mechanism. ACS Appl. Mater. Interfaces 2019, 11, 31532–31542. [Google Scholar] [CrossRef]
- Jeon, J.; Jang, H.; Chang, J.; Lee, K.-S.; Kim, D.R. Fabrication of micro-patterned aluminum surfaces for low ice adhesion strength. Appl. Surf. Sci. 2018, 440, 643–650. [Google Scholar] [CrossRef]
- Jiang, G.; Chen, L.; Zhang, S.; Huang, H. Superhydrophobic SiC/CNTs coatings with photothermal deicing and passive anti-icing properties. ACS Appl. Mater. Interfaces 2018, 10, 36505–36511. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Shen, Y.; Luo, X.; Tao, J.; Xie, Y.; Chen, H.; Wu, Y. A combination structure of microblock and nanohair fabricated by chemical etching for excellent water repellency and icephobicity. Appl. Surf. Sci. 2018, 455, 883–890. [Google Scholar] [CrossRef]
- Juuti, P.; Haapanen, J.; Stenroos, C.; Niemelä-Anttonen, H.; Harra, J.; Koivuluoto, H.; Teisala, H.; Lahti, J.; Tuominen, M.; Kuusipalo, J.; et al. Achieving a slippery, liquid-infused porous surface with anti-icing properties by direct deposition of flame synthesized aerosol nanoparticles on a thermally fragile substrate. Appl. Phys. Lett. 2017, 110, 161603. [Google Scholar] [CrossRef]
- Kim, A.; Kim, S.; Huh, M.; Kim, H.; Lee, C. Superior anti-icing strategy by combined sustainable liquid repellence and electro/photo-responsive thermogenesis of oil/MWNT composite. J. Mater. Sci. Technol. 2020, 49, 106–116. [Google Scholar] [CrossRef]
- Kim, P.; Wong, T.-S.; Alvarenga, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. Liquid-infused nanostructured surfaces with extreme anti-ice and anti-frost performance. ACS Nano 2012, 6, 6569–6577. [Google Scholar] [CrossRef]
- Koivuluoto, H.; Hartikainen, E.; Niemelä-Anttonen, H. Thermally sprayed coatings: Novel surface engineering strategy towards icephobic solutions. Materials 2020, 13, 1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koivuluoto, H.; Stenroos, C.; Kylmälahti, M.; Apostol, M.; Kiilakoski, J.; Vuoristo, P. Anti-icing behavior of thermally sprayed polymer coatings. J. Therm. Spray Techn. 2017, 26, 150–160. [Google Scholar] [CrossRef]
- Koshio, K.; Waku, T.; Hagiwara, Y. Ice-phobic glass-substrate surfaces coated with polypeptides inspired by antifreeze protein. Int. J. Refrig. 2020, 114, 201–209. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farhadi, S.; Nose, K.; Du, X.W. Superhydrophobic surfaces: Are they really ice-repellent? Langmuir 2011, 27, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kulinich, S.A.; Farzaneh, M. How wetting hysteresis influences ice adhesion strength on superhydrophobic surfaces. Langmuir 2009, 25, 8854–8856. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farzaneh, M. Ice adhesion on super-hydrophobic surfaces. Appl. Surf. Sci. 2009, 255, 8153–8157. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farzaneh, M. On ice-releasing properties of rough hydrophobic coatings. Cold Reg. Sci. Technol. 2011, 65, 60–64. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Honda, M.; Zhu, A.L.; Rozhin, A.G.; Du, X.W. The icephobic performance of alkyl-grafted aluminum surfaces. Soft Matter 2015, 11, 856–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Li, X.; Luo, C.; Zhao, Y.; Yuan, X. Icephobicity of polydimethylsiloxane-b-poly(fluorinated acrylate). Thin Solid Films 2014, 573, 67–73. [Google Scholar] [CrossRef]
- Li, T.; Zhuo, Y.; Håkonsen, V.; Rønneberg, S.; He, J.; Zhang, Z. Epidermal gland inspired self-repairing slippery lubricant-infused porous coatings with durable low ice adhesion. Coatings 2019, 9, 602. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, Y.; Ren, L.; Zhu, K.; Zhao, Y.; Yuan, X. Self-crosslinking coatings of fluorinated polysiloxanes with enhanced icephobicity. Thin Solid Films 2017, 639, 113–122. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Sofia Moita, A.; Zhang, C.; Wang, S.; Liu, Y. Fabrication of bio-inspired non-fluorinated superhydrophobic surfaces with anti-icing property and its wettability transformation analysis. Appl. Surf. Sci. 2019, 505, 144386. [Google Scholar] [CrossRef]
- Li, X.; Zhang, K.; Zhao, Y.; Zhu, K.; Yuan, X. Formation of icephobic film from POSS-containing fluorosilicone multi-block methacrylate copolymers. Prog. Org. Coat. 2015, 89, 150–159. [Google Scholar] [CrossRef]
- Li, X.; Zhang, K.; Zhao, Y.; Zhu, K.; Yuan, X. Enhancement of icephobic properties based on UV-curable fluorosilicone copolymer films. RSC Adv. 2015, 5, 90578–90587. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Li, H.; Yuan, X. Preparation and icephobic properties of polymethyltrifluoropropylsiloxane–polyacrylate block copolymers. Appl. Surf. Sci. 2014, 316, 222–231. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Zhan, B.; Li, S.; Han, Z.; Liu, Y. A novel icephobic strategy: The fabrication of biomimetic coupling micropatterns of superwetting surface. Adv. Mater. Interfaces 2019, 6, 1900864. [Google Scholar] [CrossRef]
- Li, Y.; Luo, C.; Li, X.; Zhang, K.; Zhao, Y.; Zhu, K.; Yuan, X. Submicron/nano-structured icephobic surfaces made from fluorinated polymethylsiloxane and octavinyl-POSS. Appl. Surf. Sci. 2016, 360, 113–120. [Google Scholar] [CrossRef]
- Ling, E.J.Y.; Uong, V.; Renault-Crispo, J.-S.; Kietzig, A.-M.; Servio, P. Reducing ice adhesion on nonsmooth metallic surfaces: Wettability and topography effects. ACS Appl. Mater. Interfaces 2016, 8, 8789–8800. [Google Scholar] [CrossRef]
- Liu, F.; Pan, Q. Facile fabrication of robust ice-phobic polyurethane sponges. Adv. Mater. Interfaces 2015, 2, 1500219. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Z.; Pan, Q. Intelligent icephobic surface towards self-deicing capability. ACS Sustain. Chem. Eng. 2019, 8, 792–799. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, Y.; Jiang, Z.; Youdong, J.; Liang, W. Anti-frosting/anti-icing property of nano-ZnO superhydrophobic surface on Al alloy prepared by radio frequency magnetron sputtering. Mater. Res. Express 2020, 7, 026401. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Janjua, Z.; Roe, M.; Xu, F.; Turnbull, B.; Choi, K.-S.; Hou, X. Super-hydrophobic/icephobic coatings based on silica nanoparticles modified by self-assembled monolayers. Nanomaterials 2016, 6, 232. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Mazzola, L.; Memon, H.; Barman, T.; Turnbull, B.; Mingione, G.; Choi, K.-S.; Hou, X. Development and evaluation of poly(dimethylsiloxane) based composite coatings for icephobic applications. Surf. Coat. Technol. 2018, 349, 980–985. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Ru, Y.; Fang, R.; Gu, Z.; Jiang, L. Reversibly thermosecreting organogels with switchable lubrication and anti-icing performance. Angew. Chem. Int. Ed. 2020, 59, 11876–11880. [Google Scholar]
- Liu, Q.; Yang, Y.; Huang, M.; Zhou, Y.; Liu, Y.; Liang, X. Durability of a lubricant-infused electrospray silicon rubber surface as an anti-icing coating. Appl. Surf. Sci. 2015, 346, 68–76. [Google Scholar] [CrossRef]
- Liu, W.; Chen, H.; Shen, Y.; Wu, Z. Facilely fabricating superhydrophobic resin-based coatings with lower water freezing temperature and ice adhesion for anti-icing application. J. Bionic Eng. 2019, 16, 794–805. [Google Scholar] [CrossRef]
- Liu, X.; Chen, H.; Zhao, Z.; Yan, Y.; Zhang, D. Slippery liquid-infused porous electric heating coating for anti-icing and de-icing applications. Surf. Coat. Technol. 2019, 374, 889–896. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, L.; Wang, W.; Kota, A.K.; Hu, H. An experimental study on soft PDMS materials for aircraft icing mitigation. Appl. Surf. Sci. 2018, 447, 599–609. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Chen, J.; Gu, H.; Liu, J.; Wang, R.; Zhang, B.; Zhang, H.; Zhang, Q. Design and preparation of bioinspired slippery liquid-infused porous surfaces with anti-icing performance via delayed phase inversion process. Colloid Surface. A 2020, 588, 124384. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Jarrell, R.; Nair, S.S.; Wynne, K.J.; Di, D. Icephobic, Pt-cured, polydimethylsiloxane nanocomposite coatings. ACS Appl. Mater. Interfaces 2020, 12, 11180–11189. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Hu, H.; Hu, H.; Samanta, A.; Wang, Q.; Ding, H. An experimental study to characterize a surface treated with a novel laser surface texturing technique: Water repellency and reduced ice adhesion. Surf. Coat. Technol. 2019, 374, 634–644. [Google Scholar] [CrossRef]
- Memon, H.; Liu, J.; De Focatiis, D.S.A.; Choi, K.S.; Hou, X. Intrinsic dependence of ice adhesion strength on surface roughness. Surf. Coat. Technol. 2020, 385, 125382. [Google Scholar] [CrossRef]
- He, Z.; Zhuo, Y.; Wang, F.; He, J.; Zhang, Z. Design and preparation of icephobic PDMS-based coatings by introducing an aqueous lubricating layer and macro-crack initiators at the ice-substrate interface. Prog. Org. Coat. 2020, 147, 105737. [Google Scholar] [CrossRef]
- Yao, H.; Gao, H. Gibson-soil-like materials achieve flaw-tolerant adhesion. J. Comput. Theor. Nanos. 2010, 7, 1299–1305. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Hejazi, V. Why superhydrophobic surfaces are not always icephobic. ACS Nano 2012, 6, 8488–8491. [Google Scholar] [CrossRef]
- Viswanathan, K.; Sundaram, N.K.; Chandrasekar, S. Stick-slip at soft adhesive interfaces mediated by slow frictional waves. Soft Matter 2016, 12, 5265–5275. [Google Scholar] [CrossRef]
- Chaudhury, M.K.; Kim, K.H. Shear-induced adhesive failure of a rigid slabin contact with a thin confined film. Eur. Phys. J. E 2007, 23, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, M.K.; Chakrabarti, A.; Ghatak, A. Adhesion-induced instabilities and pattern formation in thin films of elastomers and gels. Eur. Phys. J. E 2015, 38, 82. [Google Scholar] [CrossRef] [Green Version]
- Kendall, K. The adhesion and surface energy of elastic solids. J. Phys. D Appl. Phys. 1971, 4, 1186–1195. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, R.K. Fundamentals of Polymer Engineering, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Morelle, X.P.; Illeperuma, W.R.; Tian, K.; Bai, R.; Suo, Z.; Vlassak, J.J. Highly stretchable and tough hydrogels below water freezing temperature. Adv. Mater. 2018, 30, 1801541. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Xiao, S.; Håkonsen, V.; He, J.; Zhang, Z. Anti-icing ionogel surfaces: Inhibiting ice nucleation, growth, and adhesion. ACS Mater. Lett. 2020, 2, 616–623. [Google Scholar] [CrossRef]
- He, Z.; He, J.; Zhang, Z. Selective growth of metallic nanostructures on microstructured copper substrate in solution. CrystEngComm 2015, 17, 7262–7269. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Yang, Y.L.; Pan, J.F.; Long, H.; Huang, L.S.; Zhang, X.K. Compare study between icephobicity and superhydrophobicity. Phys. B Condens. Matter 2019, 556, 118–130. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Z.; He, J. CuO/Cu based superhydrophobic and self-cleaning surfaces. Scr. Mater. 2016, 118, 60–64. [Google Scholar] [CrossRef]
- Cheng, Y.-T.; Rodak, D.E. Is the lotus leaf superhydrophobic? Appl. Phys. Lett. 2005, 86, 144101. [Google Scholar] [CrossRef]
- Papadopoulos, P.; Mammen, L.; Deng, X.; Vollmer, D.; Butt, H.-J. How superhydrophobicity breaks down. Proc. Natl. Acad. Sci. USA 2013, 110, 3254–3258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Yang, X.; Li, Y.; Lu, Y.; Zhu, D. Robust micro-nanostructured superhydrophobic surfaces for long-term dropwise condensation. Nano Lett. 2021. [Google Scholar] [CrossRef]
- Ezazi, M.; Shrestha, B.; Klein, N.; Lee, D.H.; Seo, S.; Kwon, G. Self-healable superomniphobic surfaces for corrosion protection. ACS Appl. Mater. Interfaces 2019, 11, 30240–30246. [Google Scholar] [CrossRef]
- Zhang, H. Concrete. In Building Materials in Civil Engineering; Woodhead Publishing: Beijing, China, 2011; pp. 81–423. [Google Scholar]
- Cao, L.; Jones, A.K.; Sikka, V.K.; Wu, J.; Gao, D. Anti-icing superhydrophobic coatings. Langmuir 2009, 25, 12444–12448. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Ivanov, V.K.; Pashinin, A.S. Durable icephobic coating for stainless steel. ACS Appl. Mater. Interfaces 2013, 5, 2549–2554. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.B.; Emelyanenko, A.M.; Emelyanenko, K.A.; Modin, E.B. Modus operandi of protective and anti-icing mechanisms underlying the design of longstanding outdoor icephobic coatings. ACS Nano 2019, 13, 4335–4346. [Google Scholar] [CrossRef]
- Wang, L.; Gong, Q.; Zhan, S.; Jiang, L.; Zheng, Y. Robust anti-icing performance of a flexible superhydrophobic surface. Adv. Mater. 2016, 28, 7729–7735. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, T.; Schutzius, T.M.; Poulikakos, D. Imparting icephobicity with substrate flexibility. Langmuir 2017, 33, 6708–6718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Yu, W.; Zhang, Y.; Woo, J.-Y.; Chen, Y.; Wang, B.; Yun, Y.; Liu, G.; Lee, J.K.; Wang, L. A novel flexible micro-ratchet/ZnO nano-rods surface with rapid recovery icephobic performance. J. Ind. Eng. Chem. 2018, 62, 52–57. [Google Scholar] [CrossRef]
- Wong, T.-S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef]
- Han, L.; Zhao, X.; Kidalla, J.E. An investigation into the anti-icing properties of fabrics used for the outer layer of firefighter clothing. Text. Res. J. 2018, 89, 1500–1511. [Google Scholar] [CrossRef]
- Wang, N.; Xiong, D.; Lu, Y.; Pan, S.; Wang, K.; Deng, Y.; Shi, Y. Design and fabrication of the lyophobic slippery surface and its application in anti-icing. J. Phys. Chem. C 2016, 120, 11054–11059. [Google Scholar] [CrossRef] [Green Version]
- Stamatopoulos, C.; Hemrle, J.; Wang, D.; Poulikakos, D. Exceptional anti-icing performance of self-impregnating slippery surfaces. ACS Appl. Mater. Interfaces 2017, 9, 10233–10242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Xiong, D.; Pan, S.; Wang, K.; Shi, Y.; Deng, Y. Robust superhydrophobic coating and the anti-icing properties of its lubricants-infused-composite surface under condensing condition. New J. Chem. 2017, 41, 1846–1853. [Google Scholar] [CrossRef]
- Sun, J.; Wang, C.; Song, J.; Huang, L.; Sun, Y.; Liu, Z.; Zhao, C.; Li, Y. Multi-functional application of oil-infused slippery Al surface: From anti-icing to corrosion resistance. J. Mater. Sci 2018, 53, 16099–16109. [Google Scholar] [CrossRef]
- Niemelä-Anttonen, H.; Koivuluoto, H.; Tuominen, M.; Teisala, H.; Juuti, P.; Haapanen, J.; Harra, J.; Stenroos, C.; Lahti, J.; Kuusipalo, J.; et al. Icephobicity of slippery liquid infused porous surfaces under multiple freeze-thaw and ice accretion-detachment cycles. Adv. Mater. Interfaces 2018, 5, 1800828. [Google Scholar] [CrossRef]
- Metya, A.K.; Singh, J.K. Ice adhesion mechanism on lubricant-impregnated surfaces using molecular dynamics simulations. Mol. Simulat. 2018, 45, 1–9. [Google Scholar] [CrossRef]
- Alcaire, M.; Lopez-Santos, C.; Aparicio, F.J.; Sanchez-Valencia, J.R.; Obrero, J.M.; Saghi, Z.; Rico, V.J.; De la Fuente, X.; Gonzalez-Elipe, A.R.; Barranco, A.; et al. 3D organic nanofabrics: Plasma-assisted synthesis and anti-freezing behavior of superhydrophobic and lubricant-infused slippery surfaces. Langmuir 2019, 35, 16876–16885. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wen, G.; Guo, Z. Superhydrophobic and slippery cotton fabrics with robust nanolayers for stable wettability, anti-fouling and anti-icing properties. New J. Chem. 2019, 43, 16656–16663. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, C.; Li, J.; Liu, Y.; Zheng, Y. Anti-icing properties of bioinspired liquid-infused double-layer surface with internal wetting transport ability. Adv. Mater. Interfaces 2019, 6, 1900244. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Lu, C.; Liu, Y.; Feng, S.; Liu, Y. Robust slippery liquid-infused porous network surfaces for enhanced anti-/de-icing performance. ACS Appl. Mater. Interfaces 2020, 12, 25471–25477. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.J.; Song, Y.; Lu, Z.; Antao, D.S.; Wang, E.N. Design of lubricant infused surfaces. ACS Appl. Mater. Interfaces 2017, 9, 42383–42392. [Google Scholar] [CrossRef]
- Peppou-Chapman, S.; Hong, J.K.; Waterhouse, A.; Neto, C. Life and death of liquid-infused surfaces: A review on the choice, analysis and fate of the infused liquid layer. Chem. Soc. Rev. 2020, 49, 3688–3715. [Google Scholar] [CrossRef]
- Subramanyam, S.B.; Rykaczewski, K.; Varanasi, K.K. Ice adhesion on lubricant-impregnated textured surfaces. Langmuir 2013, 29, 13414–13418. [Google Scholar] [CrossRef]
- Rykaczewski, K.; Anand, S.; Subramanyam, S.B.; Varanasi, K.K. Mechanism of frost formation on lubricant-impregnated surfaces. Langmuir 2013, 29, 5230–5238. [Google Scholar] [CrossRef]
- Pang, H.; Zhou, S.; Gu, G.; Wu, L. Long-term hydrophobicity and ice adhesion strength of latex paints containing silicone oil microcapsules. J. Adhes. Sci. Technol. 2012, 27, 46–57. [Google Scholar] [CrossRef]
- Tao, C.; Li, X.; Liu, B.; Zhang, K.; Zhao, Y.; Zhu, K.; Yuan, X. Highly icephobic properties on slippery surfaces formed from polysiloxane and fluorinated POSS. Prog. Org. Coat. 2017, 103, 48–59. [Google Scholar] [CrossRef]
- Yeong, Y.H.; Milionis, A.; Loth, E.; Sokhey, J. Self-lubricating icephobic elastomer coating (SLIC) for ultralow ice adhesion with enhanced durability. Cold Reg. Sci. Technol. 2018, 148, 29–37. [Google Scholar] [CrossRef]
- Xiao, S.; He, J.; Zhang, Z. Nanoscale deicing by molecular dynamics simulation. Nanoscale 2016, 8, 14625–14632. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R. Why is ice slippery? Phys. Today 2005, 58, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Ozbay, S.; Yuceel, C.; Erbil, H.Y. Improved icephobic properties on surfaces with a hydrophilic lubricating liquid. ACS Appl. Mater. Interfaces 2015, 7, 22067–22077. [Google Scholar] [CrossRef]
- Zhao, T.Y.; Jones, P.R.; Patankar, N.A. Thermodynamics of sustaining liquid water within rough icephobic surfaces to achieve ultra-low ice adhesion. Sci. Rep. 2019, 9, 258. [Google Scholar] [CrossRef]
- Fletcher, N.H. Surface structure of water and ice. Phil. Mag. 1968, 18, 1287–1300. [Google Scholar] [CrossRef]
- Döppenschmidt, A.; Kappl, M.; Butt, H.-J. Surface properties of ice studied by atomic force microscopy. J. Phys. Chem. B 1998, 102, 7813–7819. [Google Scholar] [CrossRef]
- Wettlaufer, J.S. Impurity effects in the premelting of ice. Phys. Rev. Lett. 1999, 82, 2516–2519. [Google Scholar] [CrossRef] [Green Version]
- Ikeda-Fukazawa, T.; Kawamura, K. Molecular-dynamics studies of surface of ice Ih. J. Chem. Phys. 2004, 120, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Menini, R.; Farzaneh, M. Advanced icephobic coatings. J. Adhes. Sci. Technol. 2011, 25, 971–992. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Tao, C.; Ren, L.; Zhao, Y.; Bai, S.; Yuan, X. Amphiphilic antifogging/anti-icing coatings containing POSS-PDMAEMA-b-PSBMA. ACS Appl. Mater. Interfaces 2017, 9, 22959–22969. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, J.S.; Pavía-Sanders, A.; Russell, J.D.; Wooley, K.L. Dynamic anti-icing coatings: Complex, amphiphilic hyperbranched fluoropolymer poly(ethylene glycol) cross-linked networks with an integrated liquid crystalline comonomer. Chem. Mater. 2016, 28, 5471–5479. [Google Scholar] [CrossRef]
- Koshio, K.; Arai, K.; Waku, T.; Wilson, P.W.; Hagiwara, Y. Suppression of droplets freezing on glass surfaces on which antifreeze polypeptides are adhered by a silane coupling agent. PLoS ONE 2018, 13, e0204686. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; He, Z.; Lv, J.; Jin, Y.; Wu, S.; Liu, G.; Zhou, F.; Wang, J. Ion-specific ice propagation behavior on polyelectrolyte brush surfaces. RSC Adv. 2017, 7, 840–844. [Google Scholar] [CrossRef] [Green Version]
- Ezzat, M.; Huang, C.-J. Zwitterionic polymer brush coatings with excellent anti-fog and anti-frost properties. RSC Adv. 2016, 6, 61695–61702. [Google Scholar] [CrossRef]
- Tao, C.; Bai, S.; Li, X.; Li, C.; Ren, L.; Zhao, Y.; Yuan, X. Formation of zwitterionic coatings with an aqueous lubricating layer for antifogging/anti-icing applications. Prog. Org. Coat. 2018, 115, 56–64. [Google Scholar] [CrossRef]
- Lee, H.; Alcaraz, M.L.; Rubner, M.F.; Cohen, R.E. Zwitter-wettability and antifogging coatings with frost-resisting capabilities. ACS Nano 2013, 7, 2172–2185. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhang, G.; Zhong, Z.; Huang, Y.; Su, Z. Superhydrophilic anti-icing coatings based on polyzwitterion brushes. Langmuir 2019, 35, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ibáñez Ibáñez, P.F.; Håkonsen, V.; Wu, J.; Xu, K.; Zhuo, Y.; Luo, S.; He, J.; Zhang, Z. Self-deicing electrolyte hydrogel surfaces with Pa-level ice adhesion and durable anti-freezing/frost performance. ACS Appl. Mater. Interfaces 2020, 12, 35572–35578. [Google Scholar] [CrossRef]
- Xu, X.; Jerca, V.V.; Hoogenboom, R. Bio-inspired hydrogels as multi-task anti-icing hydrogel coatings. Chem 2020, 6, 808–831. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.K.; Cong, Q.; Li, Y.; Jin, J.F.; Choy, K.L. Utilizing swelling force to decrease the ice adhesion strength. Cold Reg. Sci. Technol. 2018, 146, 122–126. [Google Scholar] [CrossRef]
- Chen, T.; Jin, J.; Qi, Y.; Tian, W.; Cong, Q.; Choy, K.-L. Disturbing stability of interface by adopting phase-change temperature gradient to reduce ice adhesion strength. Cold Reg. Sci. Technol. 2019, 158, 69–75. [Google Scholar] [CrossRef]
- Irajizad, P.; Hasnain, M.; Farokhnia, N.; Sajadi, S.M.; Ghasemi, H. Magnetic slippery extreme icephobic surfaces. Nat. Commun. 2016, 7, 13395. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-H.; Seong, M.; Kwak, M.K.; Ko, H.; Kang, M.; Park, H.W.; Kang, S.M.; Jeong, H.E. Tunable multimodal drop bouncing dynamics and anti-icing performance of a magnetically responsive hair array. ACS Nano 2018, 12, 10693–10702. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Zhao, F.; Wu, D.; Huang, Y.; Zhang, D.; Zhang, Z.; Fu, W.; Li, X.; Fan, Y. Plasmon-mediated photothermal and superhydrophobic TiN-PTFE film for anti-icing/deicing applications. Compos. Sci. Technol. 2019, 181, 107696. [Google Scholar] [CrossRef]
- Wu, D.; Ma, L.; Zhang, F.; Qian, H.; Minhas, B.; Yang, Y.; Han, X.; Zhang, D. Durable deicing lubricant-infused surface with photothermally switchable hydrophobic/slippery property. Mater. Des. 2020, 185, 108236. [Google Scholar] [CrossRef]
- Jamil, M.I.; Wang, Q.; Ali, A.; Hussain, M.; Aziz, T.; Zhan, X.; Zhang, Q. Slippery photothermal trap for outstanding deicing surfaces. J. Bionic Eng. 2021, 18, 548–558. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Liu, Y.; Xu, R.; Liu, S.; Zhou, F. Robust photothermal coating strategy for efficient ice removal. ACS Appl. Mater. Interfaces 2020, 12, 46981–46990. [Google Scholar] [CrossRef]
- Hao, T.; Zhu, Z.; Yang, H.; He, Z.; Wang, J. All-day anti-icing/deicing film based on combined photo-electro-thermal conversion. ACS Appl. Mater. Interfaces 2021, 13, 44948–44955. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yao, T.; Li, Z.; Wei, W.; Xie, Q.; Duan, W.; Han, H. A superhydrophobic/electrothermal synergistically anti-icing strategy based on graphene composite. Compos. Sci. Technol. 2020, 198, 108307. [Google Scholar] [CrossRef]
- Harper, A.; Liu, G. Thickness of the ice-shedding lubricant layer in equilibrium with an underlying crosslinked polymer film. ACS Appl. Polym. Mater. 2020, 2, 1369–1377. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Y.; Liu, X.; Zhao, Z.; Chen, J.; Jing, X.; Chen, H. Temperature self-regulating electrothermal pseudo-slippery surface for anti-icing. Chem. Eng. J. 2021, 422, 130110. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Zhuo, Y.; Zhang, Z.; He, J. Design of Icephobic Surfaces by Lowering Ice Adhesion Strength: A Mini Review. Coatings 2021, 11, 1343. https://doi.org/10.3390/coatings11111343
He Z, Zhuo Y, Zhang Z, He J. Design of Icephobic Surfaces by Lowering Ice Adhesion Strength: A Mini Review. Coatings. 2021; 11(11):1343. https://doi.org/10.3390/coatings11111343
Chicago/Turabian StyleHe, Zhiwei, Yizhi Zhuo, Zhiliang Zhang, and Jianying He. 2021. "Design of Icephobic Surfaces by Lowering Ice Adhesion Strength: A Mini Review" Coatings 11, no. 11: 1343. https://doi.org/10.3390/coatings11111343
APA StyleHe, Z., Zhuo, Y., Zhang, Z., & He, J. (2021). Design of Icephobic Surfaces by Lowering Ice Adhesion Strength: A Mini Review. Coatings, 11(11), 1343. https://doi.org/10.3390/coatings11111343







