Shear Bond Strength of Ceramic Veneers to Zirconia–Calcium Silicate Cores
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of ZrO2-Based Cores
2.2. Plasma Treatment
2.3. Phase and Morphology
2.4. Contact Angle
2.5. Veneering Ceramic Binding to Zirconia
2.6. Shear Bond Strength
2.7. Fractured Surface Analysis
2.8. Statistical Analysis
3. Results
3.1. Phase Composition
3.2. Surface Morphology
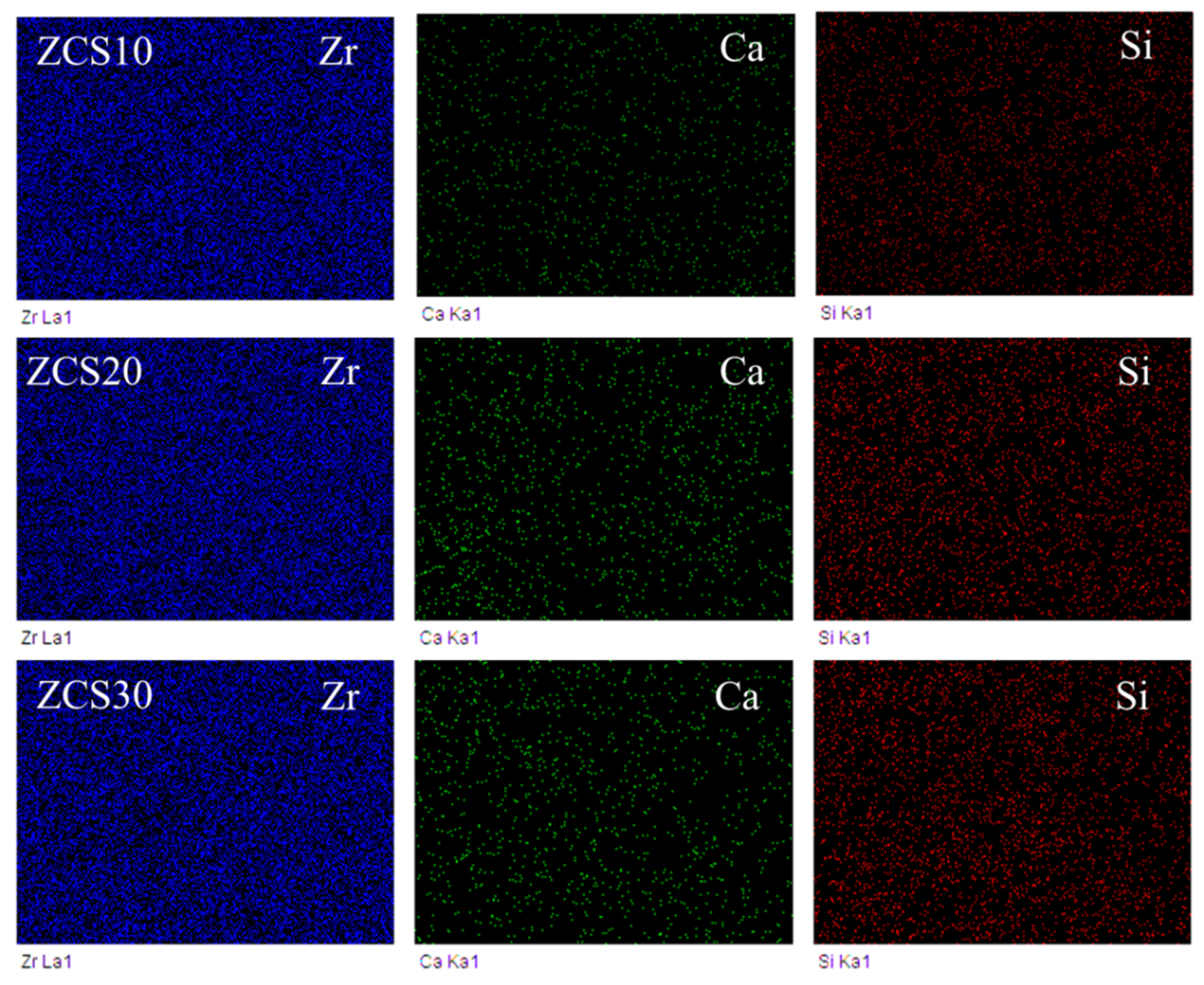
3.3. Elemental Mapping
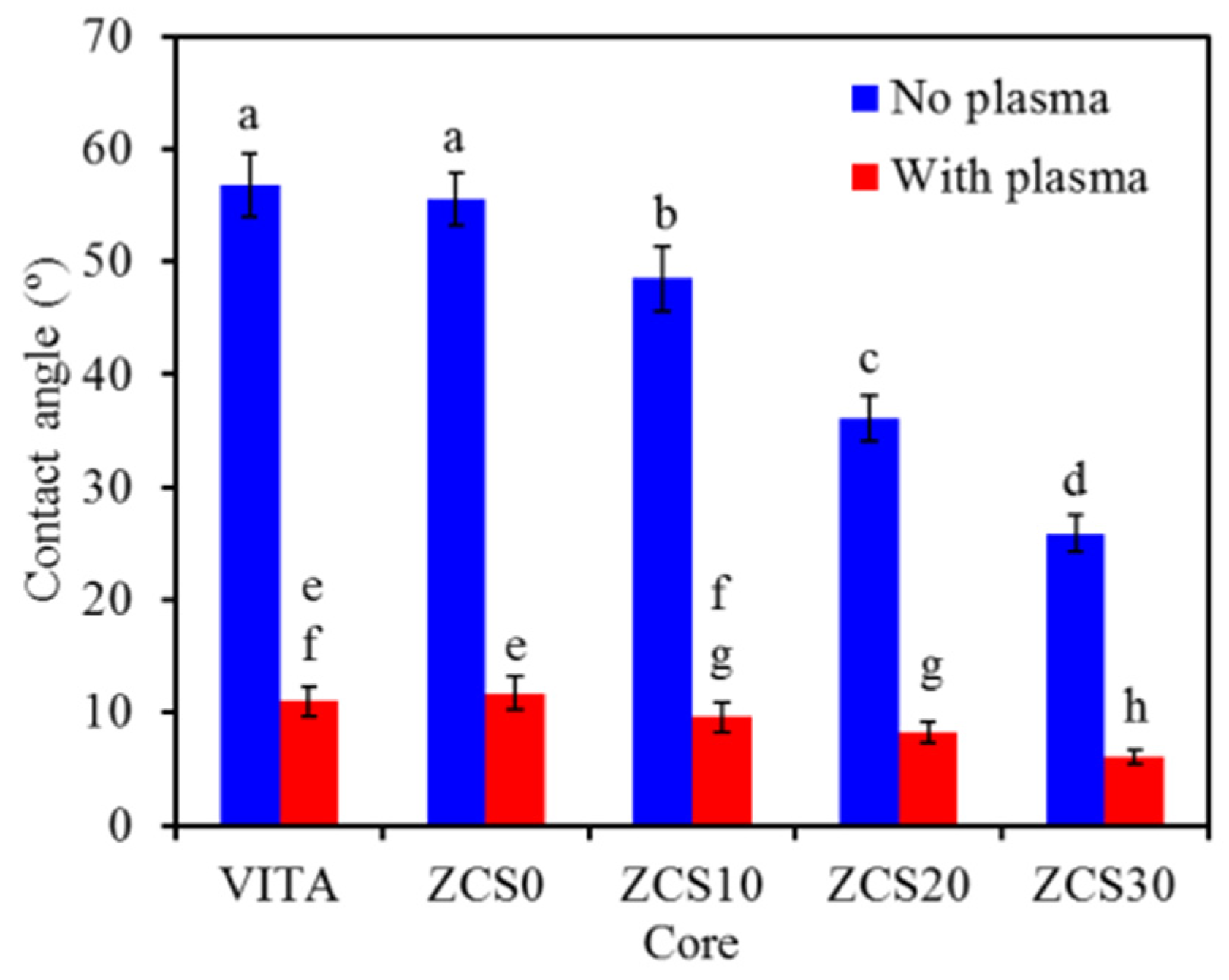
3.4. Contact Angle
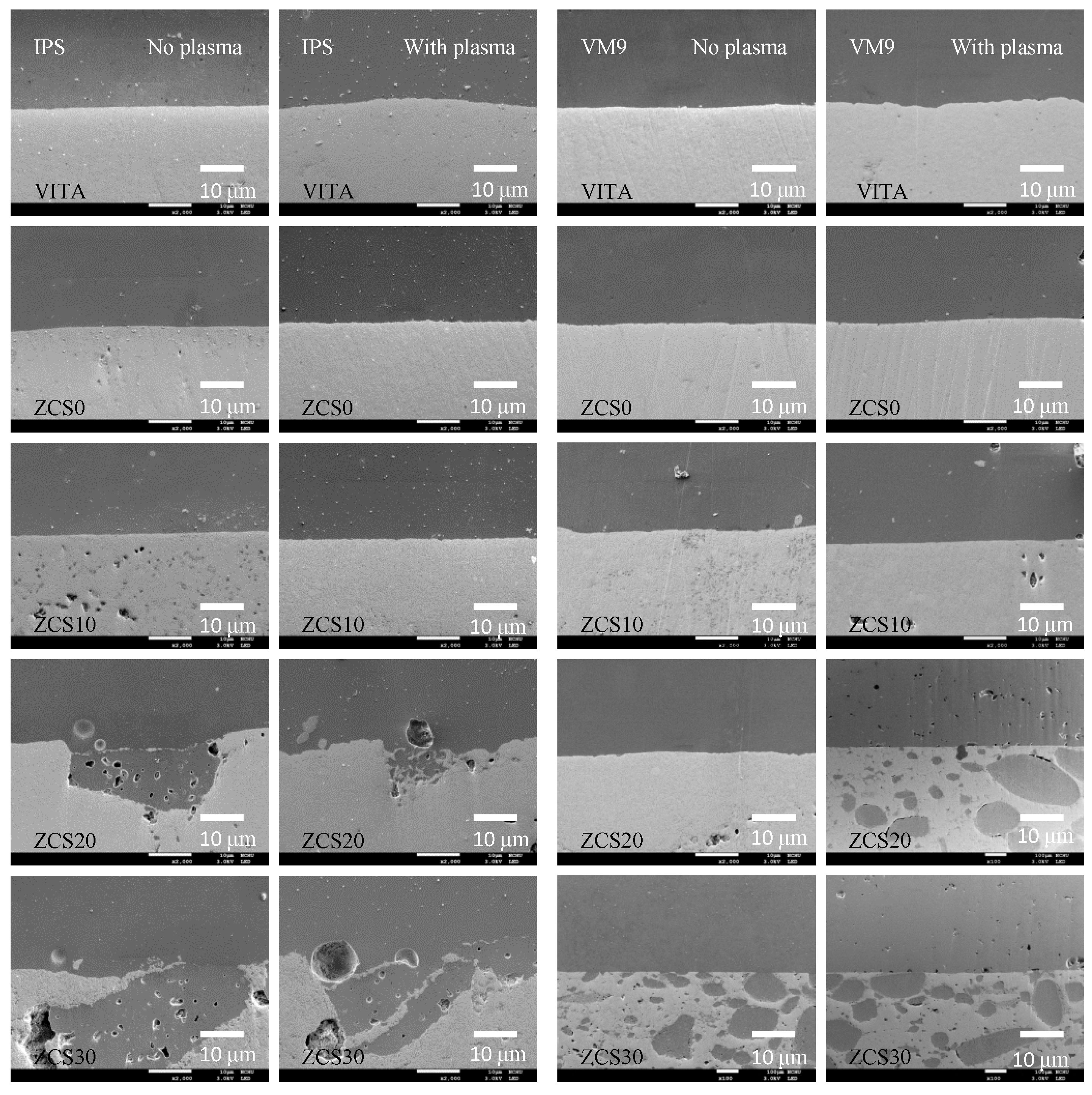
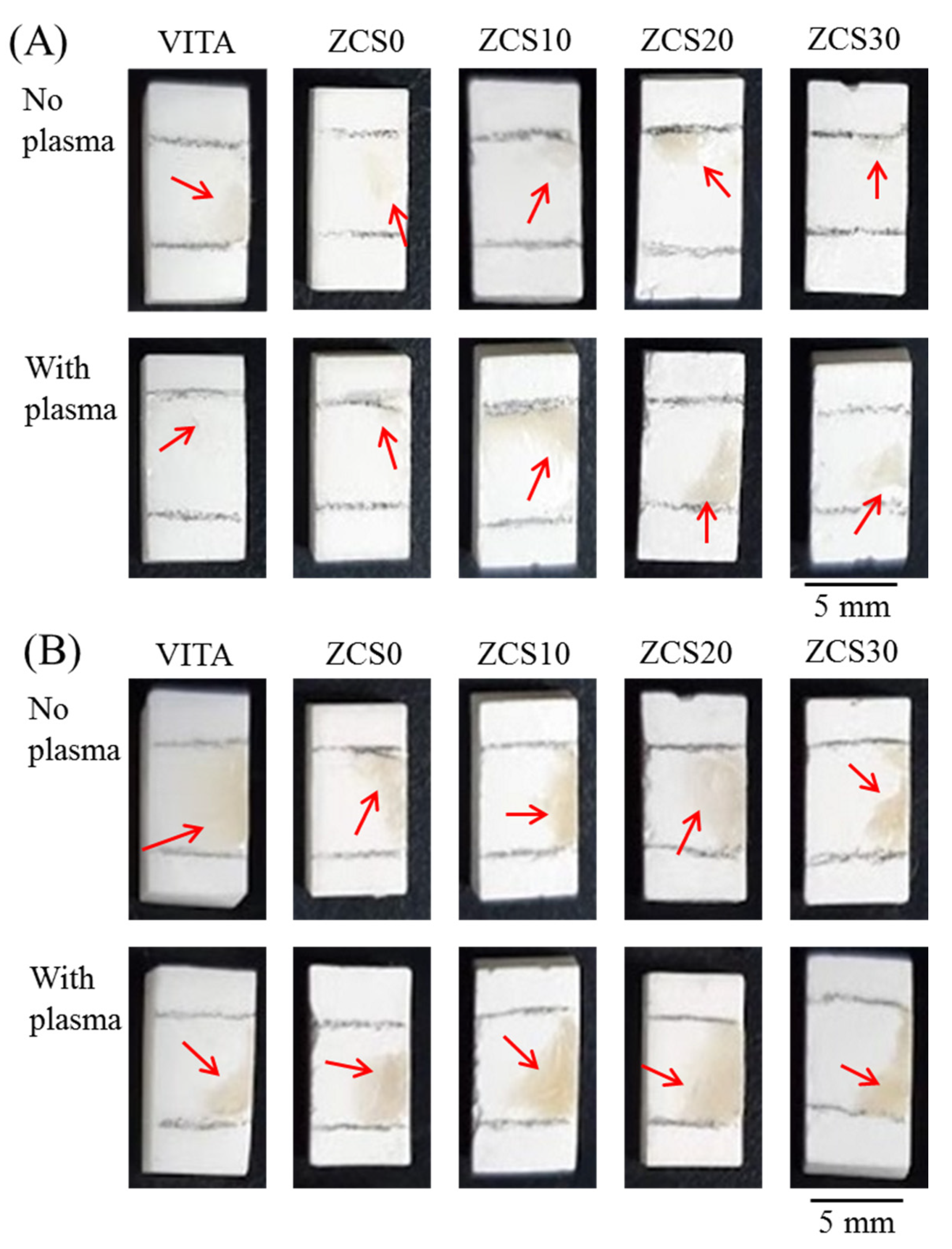
3.5. Interfacial Morphology
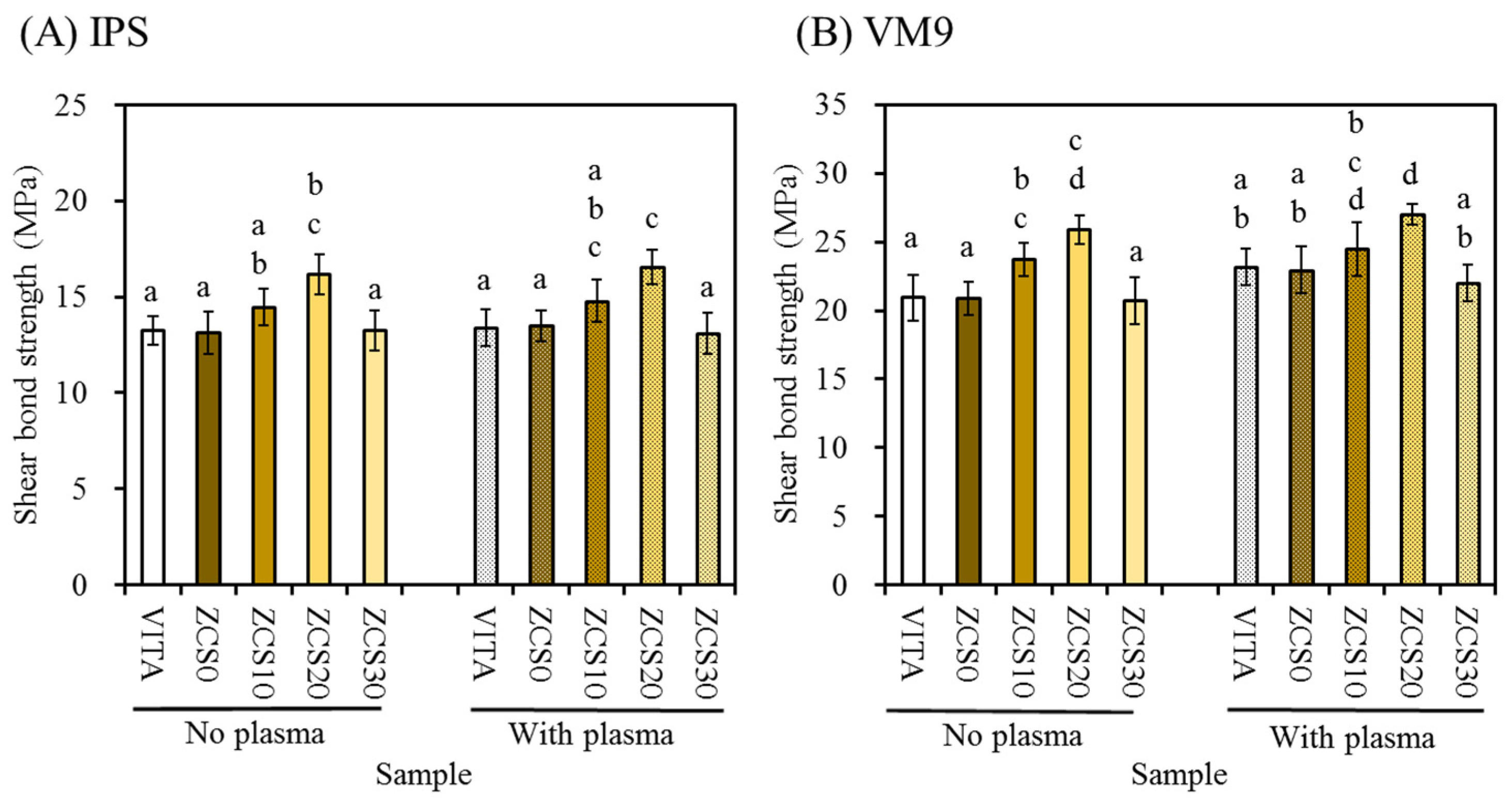
3.6. Shear Bond Strength
3.7. Fracture Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miyazaki, T.; Nakamura, T.; Matsumura, H.; Ban, S.; Kobayashi, T. Current status of zirconia restoration. J. Prosthodont. Res. 2013, 57, 236–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Ghany, G.O.S.A.; Sherief, A.H. Zirconia based ceramics, some clinical and biological aspects: Review. Future Dent. J. 2016, 2, 55–64. [Google Scholar] [CrossRef]
- Yan, M.; Wei, C.K.; Lin, Y.Y.; Hu, S.W.; Ding, S.J. Impact behavior of three notched all-ceramic restorations after soaking in artificial saliva. Materials 2015, 8, 4479–4490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soon, G.; Pingguan-Murphy, B.; Lai, K.W.; Akbar, S.A. Review of zirconia-based bioceramic: Surface modification and cellular response. Ceram. Int. 2016, 42, 12543–12555. [Google Scholar] [CrossRef]
- Ding, S.J.; Chu, Y.H.; Wang, D.Y. Enhanced properties of novel zirconia-based osteo-implant systems. Appl. Mater. Today 2017, 9, 622–632. [Google Scholar] [CrossRef]
- Yan, M.; Csík, A.; Yang, C.C.; Luo, Y.; Fodor, T.; Ding, S.J. Synergistic reinforcement of surface modification on improving the bonding of veneering ceramics to zirconia. Ceram. Int. 2018, 44, 19665–19671. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.W.; Yang, C.C.; Yan, M. Bond strength of heat-pressed veneer ceramics to zirconia with various blasting conditions. J. Dent. Sci. 2018, 13, 301–310. [Google Scholar] [CrossRef]
- Farhan, F.A.; Sulaiman, E.; Kutty, M.G. Effect of new zirconia surface coatings on the surface properties and bonding strength of veneering zirconia substrate. Surf. Coat. Technol. 2018, 333, 247–258. [Google Scholar] [CrossRef]
- Zaher, A.M.; Hochstedler, J.L.; Rueggeberg, F.A.; Kee, E.L. Shear bond strength of zirconia-based ceramics veneered with 2 different techniques. J. Prosthet. Dent. 2017, 118, 221–227. [Google Scholar] [CrossRef]
- Song, K.H.; Im, Y.W.; Lee, J.H.; Lee, J.; Lee, H.H. Evaluation of mold-enclosed shear bond strength between zirconia core and porcelain veneer. Dent. Mater. J. 2018, 37, 783–788. [Google Scholar] [CrossRef] [Green Version]
- Elsaka, S.E. Influence of surface treatments on the surface properties of different zirconia cores and adhesion of zirconia-veneering ceramic systems. Dent. Mater. 2013, 29, e239–e251. [Google Scholar] [CrossRef]
- Liu, Y.C.; Hsieh, J.P.; Chen, Y.C.; Kang, L.L.; Hwang, C.S.; Chuang, S.F. Promoting porcelain–zirconia bonding using different atmospheric pressure gas plasmas. Dent. Mater. 2018, 34, 1188–1198. [Google Scholar] [CrossRef]
- Lung, C.Y.K.; Liu, D.; Matinlinna, J.P. Silica coating of zirconia by silicon nitride hydrolysis on adhesion promotion of resin to zirconia. Mater. Sci. Eng. C 2015, 46, 103–110. [Google Scholar] [CrossRef]
- Campos, T.M.B.; Ramos, N.C.; Machado, J.P.B.; Bottino, M.A.; Souza, R.O.A.; Melo, R.M. A new silica-infiltrated Y-TZP obtained by the sol-gel method. J. Dent. 2016, 48, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Madani, A.; Nakhaei, M.; Karami, P.; Rajabzadeh, G.; Salehi, S.; Bagheri, H. Sol-gel dip coating of yttria-stabilized tetragonal zirconia dental ceramic by aluminosilicate nanocomposite as a novel technique to improve the bonding of veneering porcelain. Int J. Nanomed. 2016, 11, 3215–3223. [Google Scholar]
- Abdullah, A.O.; Hui, Y.; Pollington, S.; Muhammed, F.K.; Sun, X.; Liu, Y. Comparative effectiveness of multiple laser scanning and conventional techniques on zirconia shear bond strength. Coatings 2019, 9, 422. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Yang, C.C.; Chen, Y.H.; Ding, S.J. Oxygen plasma improved shear bond strength between zirconia and composite resin. Coatings 2020, 10, 635. [Google Scholar] [CrossRef]
- Juy, A.; Anglada, M. Surface phase transformation during grinding of Y-TZP. J. Am. Ceram. Soc. 2007, 90, 2618–2621. [Google Scholar] [CrossRef]
- Chintapalli, R.K.; Marro, F.G.; Jimenez-Pique, E.; Anglada, M. Phase transformation and subsurface damage in 3Y-TZP after sandblasting. Dent. Mater. 2013, 29, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Elias, A.B.; Simao, R.A.; Prado, M.; Cesar, P.F.; Dos Santos, G.B.; da Silva, E.M. Effect of different times of nonthermal argon plasma treatment on the microtensile bond strength of self-adhesive resin cement to yttria-stabilized tetragonal zirconia polycrystal ceramic. J. Prosthet. Dent. 2019, 121, 485–491. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, C.J.; Cho, L.R.; Huh, Y.H. Evaluation of the ceramic liner bonding effect between zirconia and lithium disilicate. J. Prosthet. Dent. 2018, 120, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.J.; Chu, Y.H.; Chen, P.T. Mechanical biocompatibility, osteogenic activity and antibacterial efficacy of calcium silicate-zirconia biocomposites. ACS Omega 2021, 6, 7106–7118. [Google Scholar] [CrossRef]
- Huang, Y.R.; Wu, I.T.; Chen, C.C.; Ding, S.J. In vitro comparisons of microscale and nanoscale calcium silicate particles. J. Mater. Chem. B 2020, 8, 6034–6047. [Google Scholar] [CrossRef]
- Shuai, C.; Feng, P.; Yang, B.; Cao, Y.; Min, A.; Peng, S. Effect of nano-zirconia on the mechanical and biological properties of calcium silicate scaffolds. Int. J. Appl. Ceram. Technol. 2015, 12, 1148–1156. [Google Scholar] [CrossRef]
- Chen, C.C.; Lai, M.H.; Wang, W.C.; Ding, S.J. Properties of anti-washout-type calcium silicate bone cements containing gelatin. J. Mater. Sci. Mater. Med. 2010, 21, 1057–1068. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Kolb, J.F.; Holub, M.; Uhrlandt, D.; Šimek, M.; Ostrikov, K.; Hamaguchi, S.; Cvelbar, U.; Černák, M.; Locke, B.; et al. The future for plasma science and technology. Plasma. Process. Polym. 2019, 16, e1800118. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.C.; Wei, C.K.; Ho, C.C.; Ding, S.J. Enhanced hydrophilicity and biocompatibility of dental zirconia ceramics by oxygen plasma treatment. Materials 2015, 8, 684–699. [Google Scholar] [CrossRef] [Green Version]
- Park, C.; Park, S.W.; Yun, K.D.; Ji, M.K.; Kim, S.; Yang, Y.P.; Lim, H.P. Effect of plasma treatment and its post process duration on shear bonding strength and antibacterial effect of dental zirconia. Materials 2018, 11, 2233. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.C.; Ding, S.J.; Lin, T.H.; Yan, M. Mechanical and optical properties evaluation of rapid sintered dental zirconia. Ceram. Int. 2020, 46, 26668–26674. [Google Scholar] [CrossRef]
- Wu, I.T.; Kao, P.F.; Huang, Y.R.; Ding, S.J. In vitro and in vivo osteogenesis of gelatin-modified calcium silicate cement with washout resistance. Mater. Sci. Eng. C 2020, 117, 111297. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, M.; Verné, E.; Appendino, P.; Moisescu, C.; Krajewski, A.; Ravaglioli, A.; Piancastelli, A. Coatings on zirconia for medical applications. Biomaterials 2000, 21, 765–773. [Google Scholar] [CrossRef]
- Abdalla, M.M.; Lung, C.Y.K.; Tsoi, J.K.H.; Matinlinna, J.P. Dental resin-zirconia bonding promotion using high-silica PVD coating with high ionization sputtering processing. Coatings 2019, 9, 182. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Sun, T.; Zhang, Y.; Zhang, Y.; Jiang, D.; Shao, L. The effect of graded glass–zirconia structure on the bond between core and veneer in layered zirconia restorations. J. Mech. Behav. Biomed. Mater. 2015, 46, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.X.; Chen, X.P.; Song, X.F.; Yin, L. Responses of pre-crystallized and crystallized zirconia-containing lithium silicate glass ceramics to diamond machining. Ceram. Int. 2020, 46, 1924–1933. [Google Scholar] [CrossRef]


| Core or Veneer | Composition | Manufacturer | Lot No. |
|---|---|---|---|
| Core | |||
| In-Ceram YZ | ZrO2-Y2O3-HfO2 | VITA Zahnfabrik | 30030 |
| ZSC0 | ZrO2 powder | Tosoh | Z305653B |
| ZCS10 | 10 wt % CaSi-90 wt % ZrO2 powder | ||
| ZCS20 | 20 wt % CaSi-80 wt % ZrO2 powder | ||
| ZCS30 | 30 wt % CaSi-70 wt % ZrO2 powder | ||
| Veneer | |||
| IPS e.max Ceram | SiO2-LiO2-Na2O-K2O-Al2O3-CaO-P2O5-F | Ivoclar Vivadent AG | V45249 |
| VM9 | SiO2-Al2O3-K2O-Na2O-B2O | VITA Zahnfabrik | 11040 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, T.-Y.; Yang, C.-C.; Chen, Y.-H.; Yan, M.; Ding, S.-J. Shear Bond Strength of Ceramic Veneers to Zirconia–Calcium Silicate Cores. Coatings 2021, 11, 1326. https://doi.org/10.3390/coatings11111326
Chiang T-Y, Yang C-C, Chen Y-H, Yan M, Ding S-J. Shear Bond Strength of Ceramic Veneers to Zirconia–Calcium Silicate Cores. Coatings. 2021; 11(11):1326. https://doi.org/10.3390/coatings11111326
Chicago/Turabian StyleChiang, Ting-Yi, Chun-Chuan Yang, Yi-Hsuan Chen, Min Yan, and Shinn-Jyh Ding. 2021. "Shear Bond Strength of Ceramic Veneers to Zirconia–Calcium Silicate Cores" Coatings 11, no. 11: 1326. https://doi.org/10.3390/coatings11111326
APA StyleChiang, T.-Y., Yang, C.-C., Chen, Y.-H., Yan, M., & Ding, S.-J. (2021). Shear Bond Strength of Ceramic Veneers to Zirconia–Calcium Silicate Cores. Coatings, 11(11), 1326. https://doi.org/10.3390/coatings11111326







