Active Corrosion Protection of Mg–Al Layered Double Hydroxide for Magnesium Alloys: A Short Review
Abstract
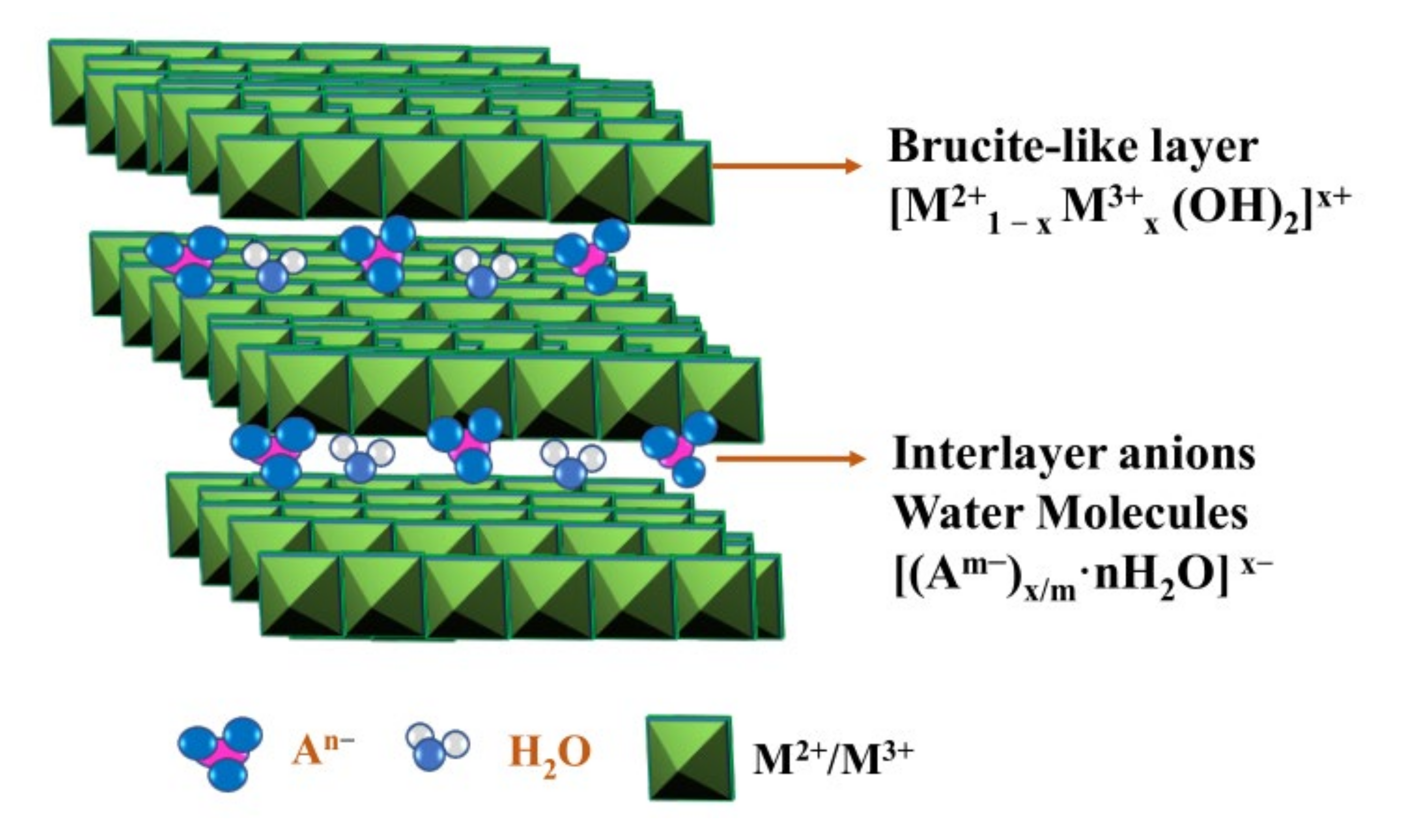
:1. Introduction
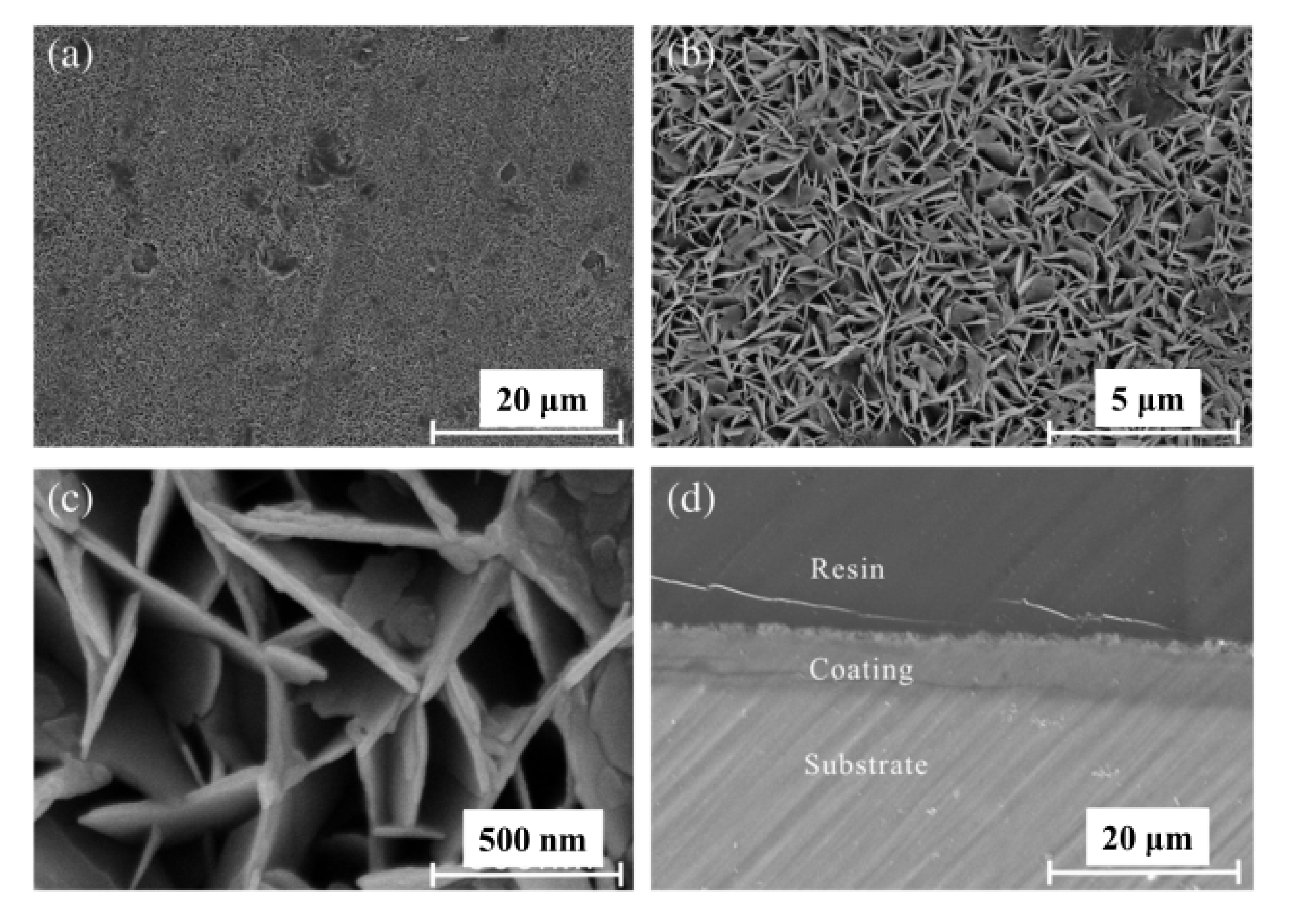
2. Synthesis of LDH
2.1. Co-Precipitation
2.2. In-Situ Growth Methods
2.2.1. One-Step In-Situ Growth Method
2.2.2. Two-Step In-Situ Growth Method
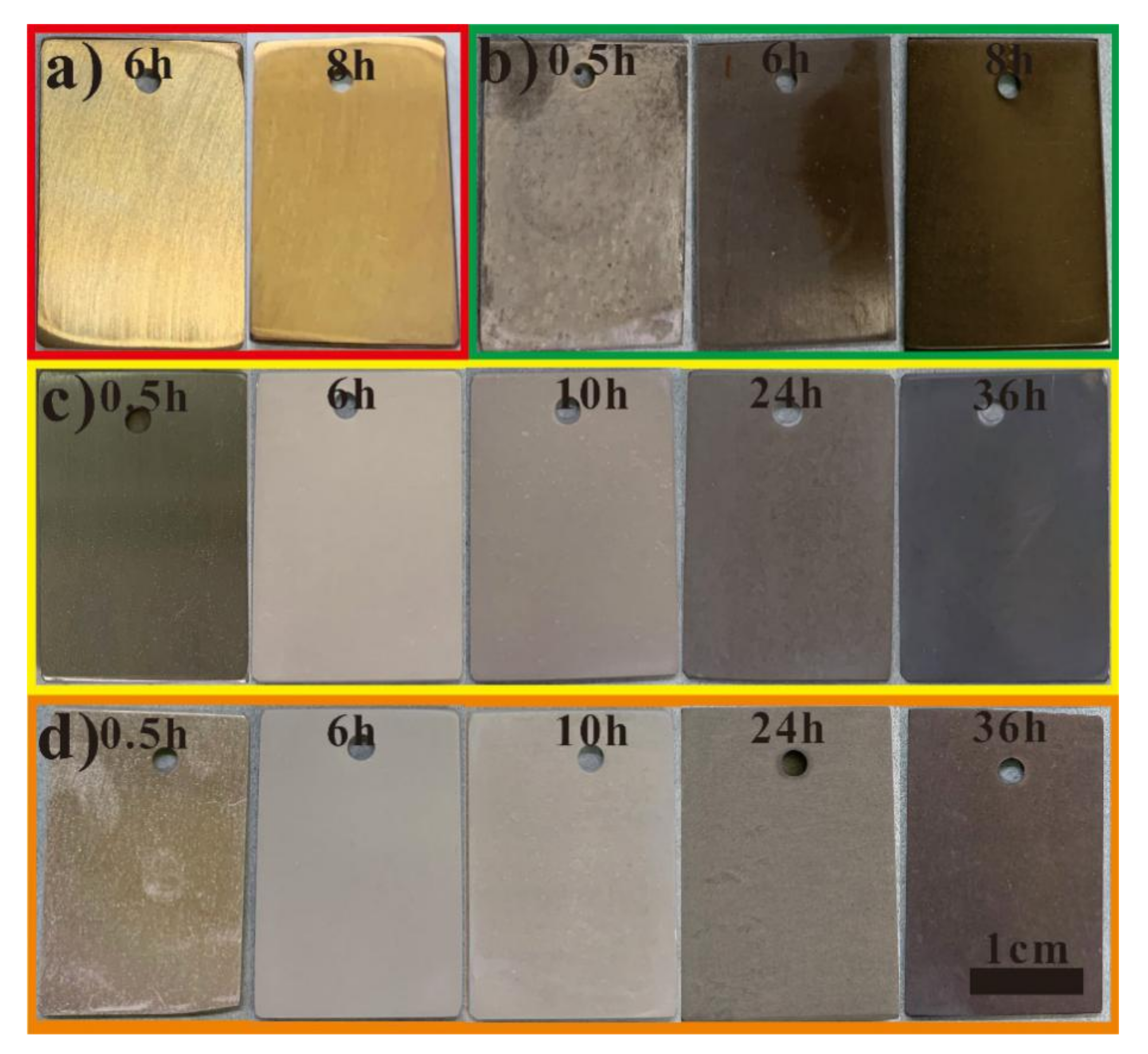
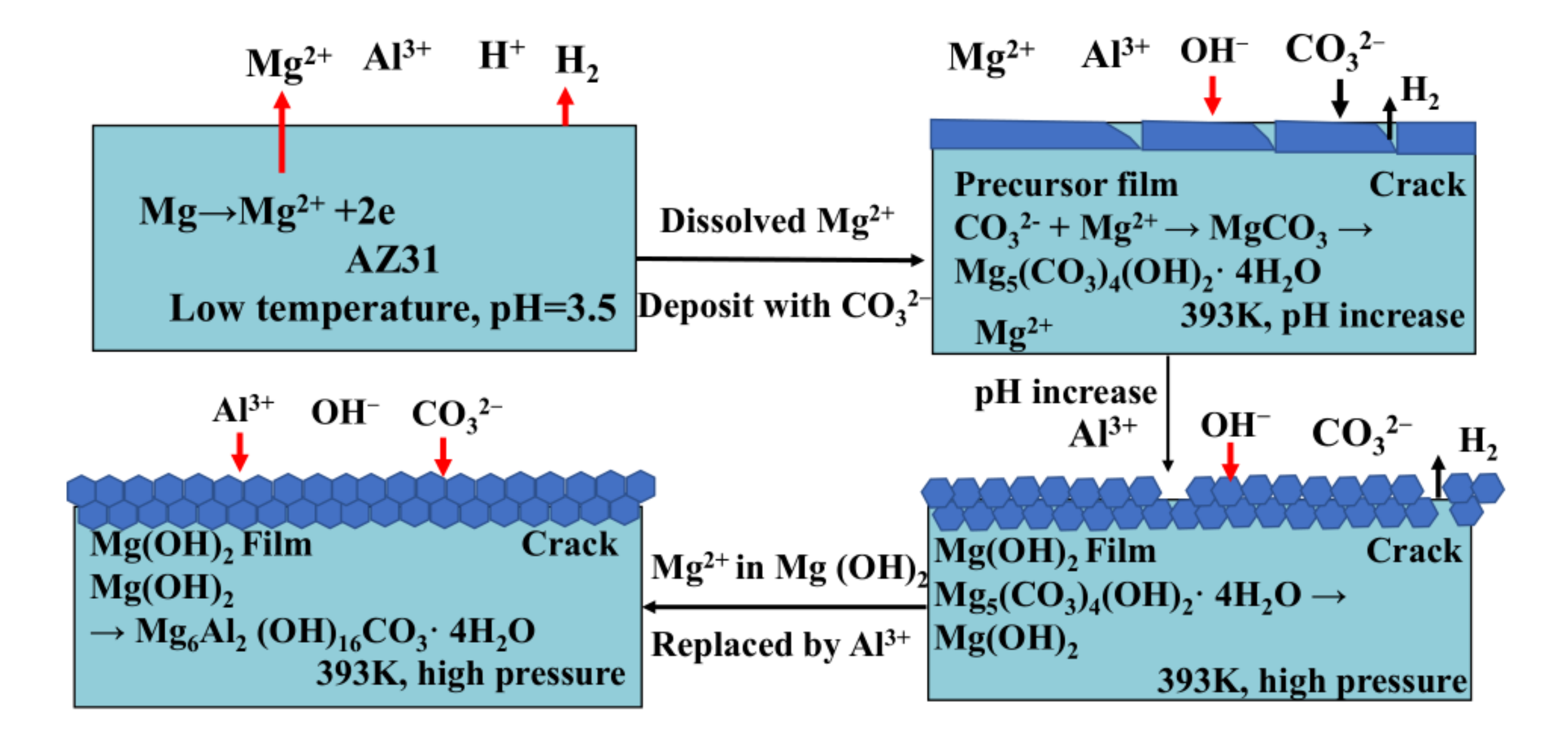
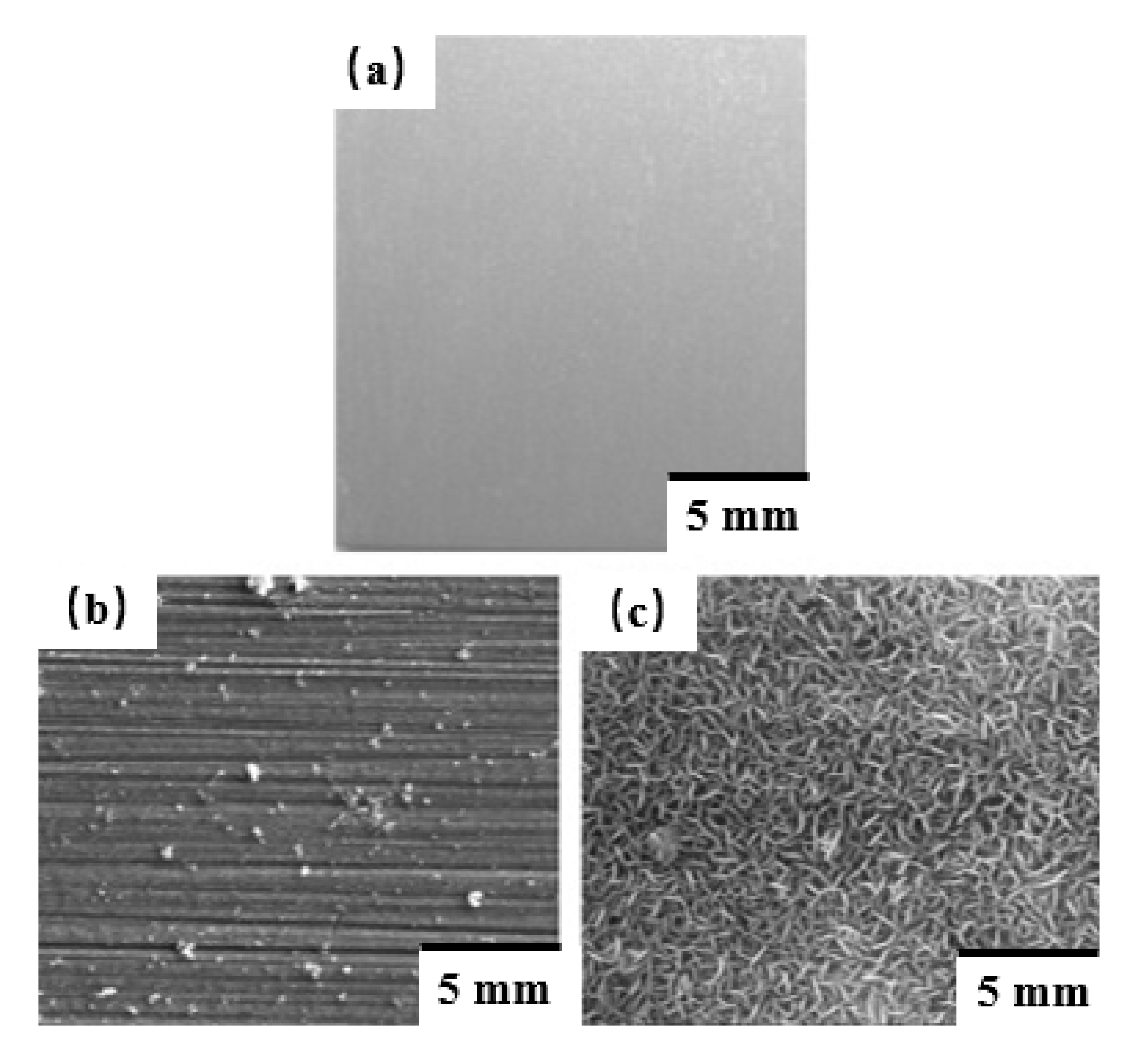
2.2.3. Hydrothermal Treatment
2.2.4. Urea Hydrolysis
2.2.5. Steam Coating
2.3. Electrochemical Deposition
2.4. Spinning Coating
2.5. Anion-Exchange
3. The Anti-Corrosion Mechanisms
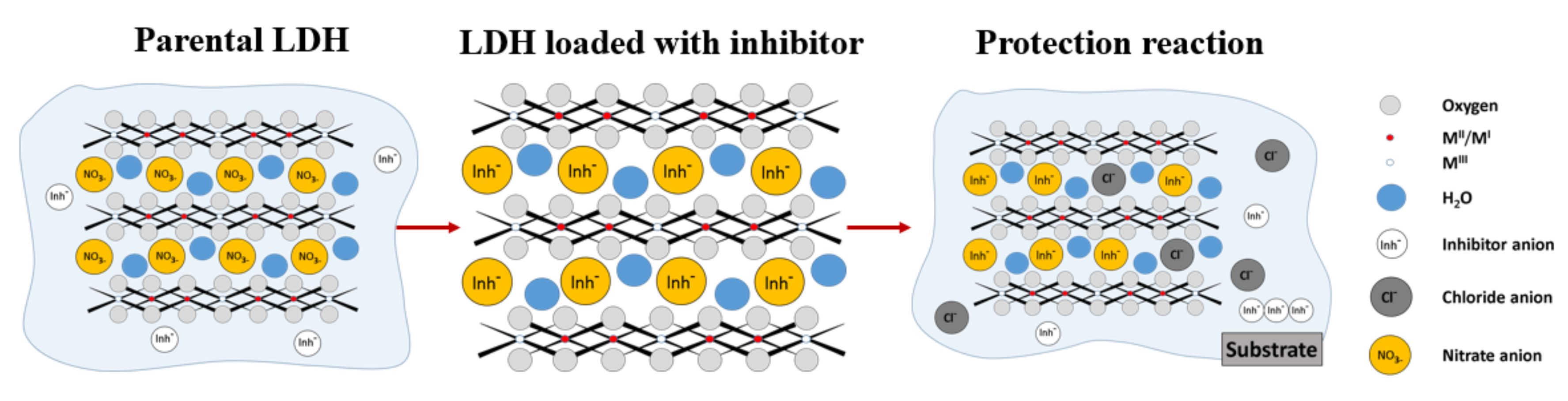
3.1. Interlayer Anion Exchange Mechanism
3.2. Composite Synergistic Corrosion Resistance Mechanisms
4. LDH Modified Coating on Magnesium Alloy
4.1. Anticorrosive Intercalated Structure
4.2. Super-Hydrophobic Modification
4.3. Biocompatible Coatings
5. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.-X.; Xie, Z.-H.; Fernandez, C.; Wu, L.; Cheng, D.; Jiang, X.-H.; Zhong, C.-J. Development of a thiophene derivative modified LDH coating for Mg alloy corrosion protection. Electrochim. Acta 2020, 330, 135186. [Google Scholar] [CrossRef]
- Yan, Y.; Qiu, Y.; Gharbi, O.; Birbilis, N.; Nakashima, P. Characterisation of Li in the surface film of a corrosion resistant Mg–Li(–Al–Y–Zr) alloy. Appl. Surf. Sci. 2019, 494, 1066–1071. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-Y.; Gao, L.; Fan, X.-L.; Zeng, R.-C.; Chen, D.-C.; Zhi, K.-Q. In vitro degradation and cytocompatibility of a low temperature in-situ grown self-healing Mg–Al LDH coating on MAO-coated magnesium alloy AZ31. Bioact. Mater. 2020, 5, 364–376. [Google Scholar] [CrossRef]
- Xu, D.; Wang, B.; Li, C.; Zu, T.; Han, E. Effect of icosahedral phase on the thermal stability and ageing response of a duplex structured Mg–Li alloy. Mater. Des. 2015, 69, 124–129. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.W.; Zhao, L.R.; Shi, X.D. Effects of polypropylene carbonate coating on the degradation and biocompatibility of degradable magnesium alloy AZ31. Proc. Est. Acad. Sci. 2019, 68, 13–21. [Google Scholar] [CrossRef]
- Chen, J.I.; Fang, L.; Wu, F.; Zeng, X.G.; Hu, J.; Zhang, S.F.; Jiang, B.; Luo, H.J. Comparison of corrosion resistance of MgAl-LDH and ZnAl-LDH films intercalated with organic anions ASP on AZ31 Mg alloys. Trans. Nonferrous Met. Soc. China 2020, 30, 2424–2434. [Google Scholar] [CrossRef]
- Subasri, R.; Raju, K.R.C.S.; Reddy, D.S.; Jyothirmayi, A.; Ijeri, V.; Prakash, O.; Gaydos, S.P. Environmentally friendly Zn–Al layered double hydroxide (LDH)-based sol–gel corrosion protection coatings on AA 2024-T3. J. Coat. Technol. Res. 2019, 16, 1447–1463. [Google Scholar] [CrossRef]
- Jin, W.; Park, D.-H. Functional layered double hydroxide nanohybrids for biomedical imaging. Nanomaterials 2019, 9, 1404. [Google Scholar] [CrossRef] [Green Version]
- Mills, S.J.; Christy, A.; Schmitt, R.T. The creation of neotypes for hydrotalcite. Miner. Mag. 2016, 80, 1023–1029. [Google Scholar] [CrossRef]
- Liu, J.-C.; Qi, B.; Song, Y.-F. Engineering polyoxometalate-intercalated layered double hydroxides for catalytic applications. Dalton Trans. 2020, 49, 3934–3941. [Google Scholar] [CrossRef]
- Rezvani, Z.; Rad, F.A.; Khodam, F. Synthesis and characterization of Mg–Al-layered double hydroxides intercalated with cubane-1,4-dicarboxylate anions. Dalton Trans. 2015, 44, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Iyi, N.; Ebina, Y.; Sasaki, T. Water-swellable MgAl-LDH (layered double hydroxide) hybrids: Synthesis, characterization, and film preparation. Langmuir ACS J. Surf. Colloids 2008, 24, 5591–5598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Ren, Y.; Wang, H.; Chen, F. Double-doped LDH films on aluminum alloys for active protection. Mater. Lett. 2017, 192, 33–35. [Google Scholar] [CrossRef]
- Mohedano, M.; Serdechnova, M.; Starykevich, M.; Karpushenkov, S.; Bouali, A.; Ferreira, M.; Zheludkevich, M. Active protective PEO coatings on AA2024: Role of voltage on in-situ LDH growth. Mater. Des. 2017, 120, 36–46. [Google Scholar] [CrossRef]
- Wang, X.; Jing, C.; Chen, Y.X. Active corrosion protection of super-hydrophobic corrosion inhibitor intercalated Mg–Al layered double hydroxide coating on AZ31 magnesium alloy. J. Alloys Compd. 2021, 8, 291–300. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, S.; Zheng, D.; Wang, J.; Zhang, X.; Du, R.; Song, G.-L.; Lin, C. Multifunctional inhibition based on layered double hydroxides to comprehensively control corrosion of carbon steel in concrete. Corros. Sci. 2017, 126, 166–179. [Google Scholar] [CrossRef]
- Basu, D.; Das, A.; Stöckelhuber, K.W.; Wagenknecht, U.; Heinrich, G. Advances in layered double hydroxide (LDH)-based elastomer composites. Prog. Polym. Sci. 2014, 39, 594–626. [Google Scholar] [CrossRef]
- Li, J.; Lin, K.; Luo, X.; Zhang, H.; Cheng, Y.F.; Li, X.; Liu, Y. Enhanced corrosion protection property of Li–Al layered double hydroxides (LDHs) film modified by 2-guanidinosuccinic acid with excellent self-repairing and self-antibacterial properties. Appl. Surf. Sci. 2019, 480, 384–394. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.G.; Zeng, R.C.; Li, S.Q.; Cui, H.Z.; Song, L.; Han, E.H. Corrosion resistance of Mg–Al-LDH coating on magnesi-um alloy AZ31. Surf. Coat. Technol. 2014, 258, 1152–1158. [Google Scholar] [CrossRef]
- Wu, W.; Yao, Q.S.; Li, Y.C. Biocorrosion resistance and biocompatibility of Mg–Al layered double hydroxide/poly-L-glutamic acid hybrid coating on magnesium alloy AZ31. Prog. Org. Coat. 2020, 147, 426–441. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Tabish, M.; Yasin, G.; Anjum, M.J.; Malik, M.U.; Zhao, J.; Yang, Q.; Manzoor, S.; Murtaza, H.; Khan, W.Q. Reviewing the current status of layered double hydroxide-based smart nanocontainers for corrosion inhibiting applications. J. Mater. Res. Technol. 2021, 10, 390–421. [Google Scholar] [CrossRef]
- Lin, J.; Uan, J. Formation of Mg,Al-hydrotalcite conversion coating on Mg alloy in aqueous HCO3−/CO32− and corresponding protection against corrosion by the coating. Corros. Sci. 2009, 51, 1181–1188. [Google Scholar] [CrossRef]
- Yang, C.; Yuan, J.; Guo, Y.; Luo, X. In situ nano-assembly of Mg/Al LDH embedded on phosphorylated cellulose microspheres for tetracycline hydrochloride removal. Cellulose 2020, 28, 301–316. [Google Scholar] [CrossRef]
- Hu, T.; Ouyang, Y.; Xie, Z.H.; Wu, L. One-pot scalable in situ growth of highly corrosion-resistant MgAl-LDH/MBT composite coating on magnesium alloy under mild conditions. J. Mater. Sci. Technol. 2021, 92, 225–235. [Google Scholar] [CrossRef]
- Anjum, M.J.; Zhao, J.; Asl, V.Z.; Yasin, G.; Wang, W.; Wei, S.; Zhao, Z.; Khan, W.Q. In-situ intercalation of 8-hydroxyquinoline in Mg–Al LDH coating to improve the corrosion resistance of AZ31. Corros. Sci. 2019, 157, 1–10. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.; Shan, D.; Han, E.-H. Influence of alloying elements and microstructure on the formation of hydrotalcite film on Mg alloys. Corros. Sci. 2015, 93, 90–99. [Google Scholar] [CrossRef]
- Richetta, M.; Medaglia, P.; Mattoccia, A.; Varone, A.; Pizzoferrato, R. Layered double hydroxides: Tailoring interlamellar nanospace for a vast field of applications. J. Mater. Sci. Eng. 2017, 6, 2122–2169. [Google Scholar]
- Yao, Q.S.; Li, Z.C.; Qiu, Z.M. Corrosion resistance of Mg(OH)2 /Mg–Al-layered double hydroxide coatings on magnesium alloy AZ31: Influence of hydrolysis degree of silane. Rare Met. 2019, 38, 76–84. [Google Scholar] [CrossRef]
- Li, C.-F.; Wang, M.-J.; Ho, W.-H.; Li, H.-N.; Yen, S.-K. Effects of electrolytic MgO coating parameters on corrosion resistance of AZ91D magnesium alloy. J. Electrochem. Soc. 2011, 158, C11–C16. [Google Scholar] [CrossRef]
- Liao, L.; Zhao, N.; Xia, Z. Hydrothermal synthesis of Mg–Al layered double hydroxides (LDHs) from natural brucite and Al(OH)3. Mater. Res. Bull. 2012, 47, 3897–3901. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, F.; Fang, L.; Guan, T.; Hu, J.; Zhang, S. A comparative study and optimization of corrosion resistance of ZnAl layered double hydroxides films intercalated with different anions on AZ31 Mg alloys. Surf. Coat. Technol. 2019, 358, 594–603. [Google Scholar] [CrossRef]
- Bouali, A.; Serdechnova, M.; Blawert, C.; Tedim, J.; Ferreira, M.; Zheludkevich, M. Layered double hydroxides (LDHs) as functional materials for the corrosion protection of aluminum alloys: A review. Appl. Mater. Today 2020, 21, 100857. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, L.; Zhang, Y.; Long, S.; Jie, X. Corrosion behavior of Mg–Al LDH film in-situ assembled with graphene on Mg alloy pre-sprayed Al layer. J. Alloy. Compd. 2020, 834, 155107. [Google Scholar] [CrossRef]
- Hibino, T.; Ohya, H. Synthesis of crystalline layered double hydroxides: Precipitation by using urea hydrolysis and subsequent hydrothermal reactions in aqueous solutions. Appl. Clay Sci. 2009, 45, 123–132. [Google Scholar] [CrossRef]
- Zeng, R.-C.; Liu, Z.-G.; Zhang, F.; Li, S.-Q.; He, Q.-K.; Cui, H.-Z.; Han, E.-H. Corrosion resistance of in-situ Mg–Al hydrotalcite conversion film on AZ31 magnesium alloy by one-step formation. Trans. Nonferrous Met. Soc. China 2015, 25, 1917–1925. [Google Scholar] [CrossRef]
- Radulescu, A.; Fetters, L.J.; Richter, D. Polymer-driven wax crystal control using partially crystalline polymeric materials. Adv. Polym. Sci. 2007, 210, 1–100. [Google Scholar]
- Ishizaki, T.; Chiba, S.; Watanabe, K.; Suzuki, H. Corrosion resistance of Mg–Al layered double hydroxide container-containing magnesium hydroxide films formed directly on magnesium alloy by chemical-free steam coating. J. Mater. Chem. A 2013, 1, 8968–8977. [Google Scholar] [CrossRef]
- Kamiyama, N.; Panomsuwan, G.; Yamamoto, E.; Sudare, T.; Saito, N.; Ishizaki, T. Effect of treatment time in the Mg(OH)2/Mg–Al LDH composite film formed on Mg alloy AZ31 by steam coating on the corrosion resistance. Surf. Coat. Technol. 2016, 286, 172–177. [Google Scholar] [CrossRef]
- Yan, L.; Zhou, M.; Pang, X.; Gao, K. One-step in situ synthesis of reduced graphene oxide/Zn–Al layered double hydroxide film for enhanced corrosion protection of magnesium alloys. Langmuir 2019, 35, 6312–6320. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ding, X.; Zheng, Z.; Ma, Y.; Atrens, A.; Chen, X.; Xie, Z.; Sun, D.; Pan, F. Fabrication and characterization of an actively protective Mg–Al LDHs/Al2O3 composite coating on magnesium alloy AZ31. Appl. Surf. Sci. 2019, 487, 558–568. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, D.; Kang, Z. One-Step Electrodeposition Process To Fabricate corrosion-resistant superhydrophobic surface on magnesium alloy. ACS Appl. Mater. Interfaces 2015, 7, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; Wang, K.; Duan, X.; Zhang, Y.; Cai, H.; Wang, Z.-H. Effect of hydrothermal treatment time on microstructure and corrosion behavior of micro-arc oxidation/layered double hydroxide composite coatings on LA103Z Mg–Li Alloy in 3.5 wt.% NaCl solution. J. Mater. Eng. Perform. 2020, 29, 4032–4039. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, M.; Xu, S.; Zhao, L.; Zhang, B. Fabrication of oriented layered double hydroxide films by spin coating and their use in corrosion protection. Chem. Eng. J. 2008, 141, 362–367. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kang, J.-K.; Lee, S.-C.; Kim, S.-B. Immobilization of layered double hydroxide in poly(vinylidene fluoride)/poly(vinyl alcohol) polymer matrices to synthesize bead-type adsorbents for phosphate removal from natural water. Appl. Clay Sci. 2019, 170, 1–12. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.; Shan, D.; Han, E.H. Study of the corrosion mechanism of the in situ grown Mg–Al–CO32− hydrotalcite film on AZ31 alloy. Corros. Sci. 2012, 65, 268–277. [Google Scholar] [CrossRef]
- Kaseem, M.; Ramachandraiah, K.; Hossain, S.; Dikici, B. A Review on LDH-smart functionalization of anodic films of Mg alloys. Nanomaterials 2021, 11, 536. [Google Scholar] [CrossRef]
- Li, P.; Lv, F.; Xu, Z.; Qi, G.; Zhang, Y. Functions of surfactants in the one-step synthesis of surfactant-intercalated LDHs. J. Mater. Sci. 2013, 48, 5437–5446. [Google Scholar] [CrossRef]
- Hang, T.T.X.; Truc, T.A.; Duong, N.T.; Pébère, N.; Olivier, M.-G. Layered double hydroxides as containers of inhibitors in organic coatings for corrosion protection of carbon steel. Prog. Org. Coat. 2012, 74, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Fang, L.; Wu, F.; Xie, J.; Hu, J.; Jiang, B.; Luo, H. Corrosion resistance of a self-healing rose-like MgAl-LDH coating intercalated with aspartic acid on AZ31 Mg alloy. Prog. Org. Coat. 2019, 136, 105234. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Zhang, S.; Yuan, B.; Zheng, Z.; Pan, F. A Novel approach to fabricate protective layered double hydroxide films on the surface of anodized Mg–Al alloy. Adv. Mater. Interfaces 2017, 4, 4. [Google Scholar] [CrossRef]
- Allada, R.K.; Navrotsky, A.; Berbeco, H.T.; Casey, W.H. Thermochemistry and aqueous solubilities of hydrotalcite-like solids. Science 2002, 296, 721–723. [Google Scholar] [CrossRef]
- Yu, D.; Wen, S.; Yang, J.; Wang, J.; Chen, Y.; Luo, J.; Wu, Y. RGO modified ZnAl-LDH as epoxy nanostructure filler: A novel synthetic approach to anticorrosive waterborne coating. Surface Coat. Technol. 2017, 326, 207–215. [Google Scholar] [CrossRef]
- Hayatdavoudi, H.; Rahsepar, M. Smart inhibition action of layered double hydroxide nanocontainers in zinc-rich epoxy coating for active corrosion protection of carbon steel substrate. J. Alloy. Compd. 2017, 711, 560–567. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Yao, W.; Zhong, Z.; Chen, Y.; Wu, J.; Pan, F. One-step in situ synthesis of graphene oxide/MgAl-layered double hydroxide coating on a micro-arc oxidation coating for enhanced corrosion protection of magnesium alloys. Surf. Coat. Technol. 2021, 413, 127083. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Ma, Y.L. Active corrosion protection by a smart coating based on a MgAl-layered double hydroxide on a cerium-modified plasma electrolytic oxidation coating on Mg alloy AZ31. Corros. Sci. 2018, 139, 370–382. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Song, Y.; Shan, D.; Han, E.-H. Modifications of the hydrotalcite film on AZ31 Mg alloy by phytic acid: The effects on morphology, composition and corrosion resistance. Corros. Sci. 2013, 74, 130–138. [Google Scholar] [CrossRef]
- Goh, K.-H.; Lim, T.-T. Influences of co-existing species on the sorption of toxic oxyanions from aqueous solution by nanocrystalline Mg/Al layered double hydroxide. J. Hazard. Mater. 2010, 180, 401–408. [Google Scholar] [CrossRef]
- Xu, J.; Tan, Q.; Mei, Y. Corrosion protection of steel by Mg–Al layered double hydroxides in simulated concrete pore solution: Effect of SO42-. Corros. Sci. 2020, 163, 163. [Google Scholar] [CrossRef]
- Mashtalyar, D.; Nadaraia, K.; Imshinetskiy, I.; Belov, E.; Filonina, V.; Suchkov, S.; Sinebryukhov, S.; Gnedenkov, S. Composite coatings formed on Ti by PEO and fluoropolymer treatment. Appl. Surf. Sci. 2021, 536, 147976. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, L.; Zheludkevich, M.L.; Chen, Y.; Serdechnova, M.; Yao, W.; Blawert, C.; Atrens, A.; Pan, F. MgAl-V2O74- LDHs/(PEI/MXene)10 composite film for magnesium alloy corrosion protection. J. Mater. Sci. Technol. 2021, 91, 28–39. [Google Scholar] [CrossRef]
- Kameda, T.; Uchida, H.; Kumagai, S.; Saito, Y.; Mizushina, K.; Itou, I.; Han, T.; Yoshioka, T. Regeneration of car-bonate-intercalated Mg–Al layered double hydroxides (CO3·Mg–Al LDHs) by CO2-induced desorption of anions (X) from X·Mg–Al LDH (X = Cl, SO4, or NO3): A kinetic study. Chem. Eng. Res. Des. 2021, 165, 207–213. [Google Scholar] [CrossRef]
- Wu, L.; Wu, J.; Zhang, Z.; Zhang, C.; Zhang, Y.; Tang, A.; Li, L.; Zhang, G.; Zheng, Z.; Atrens, A.; et al. Corrosion resistance of fatty acid and fluoroalkylsilane-modified hydrophobic Mg–Al LDH films on anodized magnesium alloy. Appl. Surf. Sci. 2019, 487, 569–580. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, G.; Peng, X.; Li, L.; Feng, H.; Gao, B.; Huo, K.; Chu, P.K. Mitigation of corrosion on magnesium alloy by predesigned surface corrosion. Sci. Rep. 2015, 5, 17399. [Google Scholar] [CrossRef] [Green Version]
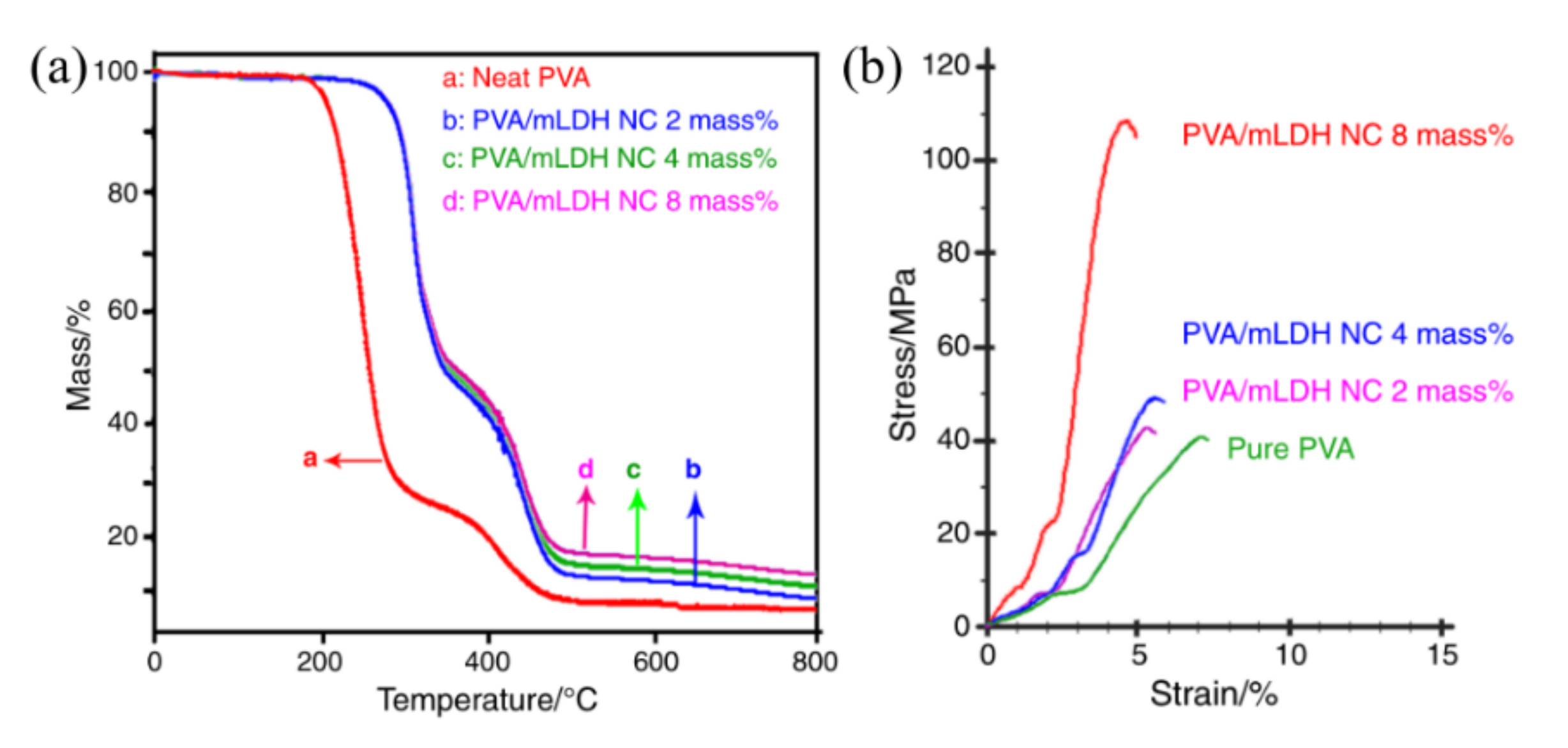
- Huang, S.; Cen, X.; Zhu, H.; Yang, Z.; Yang, Y.; Tjiu, W.W.; Liu, T. Facile preparation of poly(vinyl alcohol) nanocomposites with pristine layered double hydroxides. Mater. Chem. Phys. 2011, 130, 890–896. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M.; Hatami, M. Novel nanocomposites of poly(vinyl alcohol) and Mg–Al layered double hydroxide intercalated with diacid N-tetrabromophthaloyl-aspartic. J. Therm. Anal. Calorim. 2015, 120, 1293–1302. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, T.; Tang, J.; Liu, X.; Shen, Y. Enhanced antitumor effect of poly(L-glutamic acid)-rose bengal conjugate nanoparticle. J. Control. Release 2017, 259, e122–e123. [Google Scholar] [CrossRef]
- Laipan, M.; Xiang, L.; Yu, J.; Martin, B.R.; Zhu, R.; Zhu, J.; He, H.; Clearfield, A.; Sun, L. Layered intercalation compounds: Mechanisms, new methodologies, and advanced applications. Prog. Mater. Sci. 2020, 109, 100631. [Google Scholar] [CrossRef]
- Kaseem, M.; Ko, Y.G. A novel hybrid composite composed of albumin, WO3, and LDHs film for smart corrosion protection of Mg alloy. Compos. Part. B Eng. 2021, 204, 108490. [Google Scholar] [CrossRef]
- Peng, F.; Wang, D.; Zhang, D.; Yan, B.; Cao, H.; Qiao, Y.; Liu, X. PEO/Mg–Zn–Al LDH Composite Coating on Mg Alloy as a Zn/Mg Ion-Release Platform with Multifunctions: Enhanced Corrosion Resistance, Osteogenic, and Antibacterial Activities. ACS Biomater. Sci. Eng. 2018, 4, 4112–4121. [Google Scholar] [CrossRef] [PubMed]


| Sample | Electrolyte | Ecorr (V/SCE) | Icorr (μAcm−2) |
|---|---|---|---|
| CO3·Mg–Al LDH | 3.5 wt.% NaCl | −0.805 | 1.13 × 10−7 |
| Cl·Mg–Al LDH | 3.5 wt.% NaCl | −1.300 | 2.52 × 10−7 |
| NO3·Mg–Al LDH | 3.5 wt.% NaCl | −1.357 | 5.580 × 10−7 |
| V2O7·Mg–Al LDH | 3.5 wt.% NaCl | −0.92 | 1.30 × 10−8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, M.; Li, R.; Feng, X.; Pang, X.; Rao, J.; Cong, D.; Yin, C.; Zhang, Y. Active Corrosion Protection of Mg–Al Layered Double Hydroxide for Magnesium Alloys: A Short Review. Coatings 2021, 11, 1316. https://doi.org/10.3390/coatings11111316
Zhang X, Zhang M, Li R, Feng X, Pang X, Rao J, Cong D, Yin C, Zhang Y. Active Corrosion Protection of Mg–Al Layered Double Hydroxide for Magnesium Alloys: A Short Review. Coatings. 2021; 11(11):1316. https://doi.org/10.3390/coatings11111316
Chicago/Turabian StyleZhang, Xinfang, Min Zhang, Ruihong Li, Xiaoyan Feng, Xue Pang, Jinsong Rao, Dalong Cong, Changqing Yin, and Yuxin Zhang. 2021. "Active Corrosion Protection of Mg–Al Layered Double Hydroxide for Magnesium Alloys: A Short Review" Coatings 11, no. 11: 1316. https://doi.org/10.3390/coatings11111316
APA StyleZhang, X., Zhang, M., Li, R., Feng, X., Pang, X., Rao, J., Cong, D., Yin, C., & Zhang, Y. (2021). Active Corrosion Protection of Mg–Al Layered Double Hydroxide for Magnesium Alloys: A Short Review. Coatings, 11(11), 1316. https://doi.org/10.3390/coatings11111316







