Optimization of Quantitative Analysis of Biofilm Cell from Pipe Materials
Abstract
:1. Introduction
2. Materials and Methods
- (1)
- HPC methods using A agar from BTL Sp. z o.o. Department of Enzymes and Peptons, catalog number P-0231 (composition: meat extract 2 g/L, yeast extract 2 g/L, glucose 10 g/L, peptone 5 g/L, sodium chloride 4 g/L, agar 15 g/L, and pH 7.2 ± 0.2), and R2A agar from Thermo Scientific with catalog number CM0906 (composition: yeast extract 0.5 g/L, starch 0.5 g/L, glucose 0.5 g/L, peptone 0.5 g/L, KH2PO4 0.3 g/L, MgSO4 0.024 g/L, sodium pyruvate 0.3 g/L, agar 15 g/L, and pH 7.2 ± 0.2 at 25 °C). To determine the number of bacteria present on agar A, the samples were incubated at 37 °C for 48 h (mesophilic bacteria) and at 22 °C for 72 h (psychrophilic bacteria). When using R2A agar, the incubation time was extended to 7 days. The results converted to CFU/mL/cm2 (taking into account the surface of the coupons of 2 cm2).
- (2)
- ATP value was analyzed using a luminometer (LuminUltra Photonmaster Luminometer, Fredericton, JCT, Canada), 100 uL of the sample was put in a sterile, polystyrene tube. For the determination of ATP(o)—the total number of microorganisms, 100 uL I chenged it. of enzyme (BacTiter-Glo Microbial Cell Enviability Assay) was injected into the sample by the automatic dispenser. For the determination of ATP(z)—the number of dead microorganisms, the test sample firstly was passed through a sterile MCE membrane filter and then the enzyme was delivered (this operation enabled the living organisms to remain on the surface of the filter so that it was possible to identify intracellular ATP(I) (ATP(I) = ATP(o)-ATP(z)). To enable proper dissolution and reaction of enzyme in the sample, the injection of enzyme was delayed by 30 s. A delay of 2 s was used between the injection of enzyme and the measuring time. The obtained values in RLU (Relative Light Units) were converted to RLU/mL/cm2 (taking into account the surface of 2 cm2).
- (3)
- Flow cytometry (FC) analysis was performed using a Cy Flow Cube 8 cytometer (Sysmex Partec GmbH, Görlitz, Germany), 2 mL of the sample was put into a sterile, polystyrene tube and then 20 µL of fluorescent dye was added. The dye SYBRGreen was used for measurements of the total number of microorganisms, while dye propidium iodide enabled the number of dead organisms to be determined. Before analysis, the samples were subjected to 10 s vortexing and incubated at 37 °C for 10 min. The obtained values were converted to number of particles/mL/cm2 (taking into account the surface of 2 cm2).
3. Results
3.1. Detachment of the Biofilm
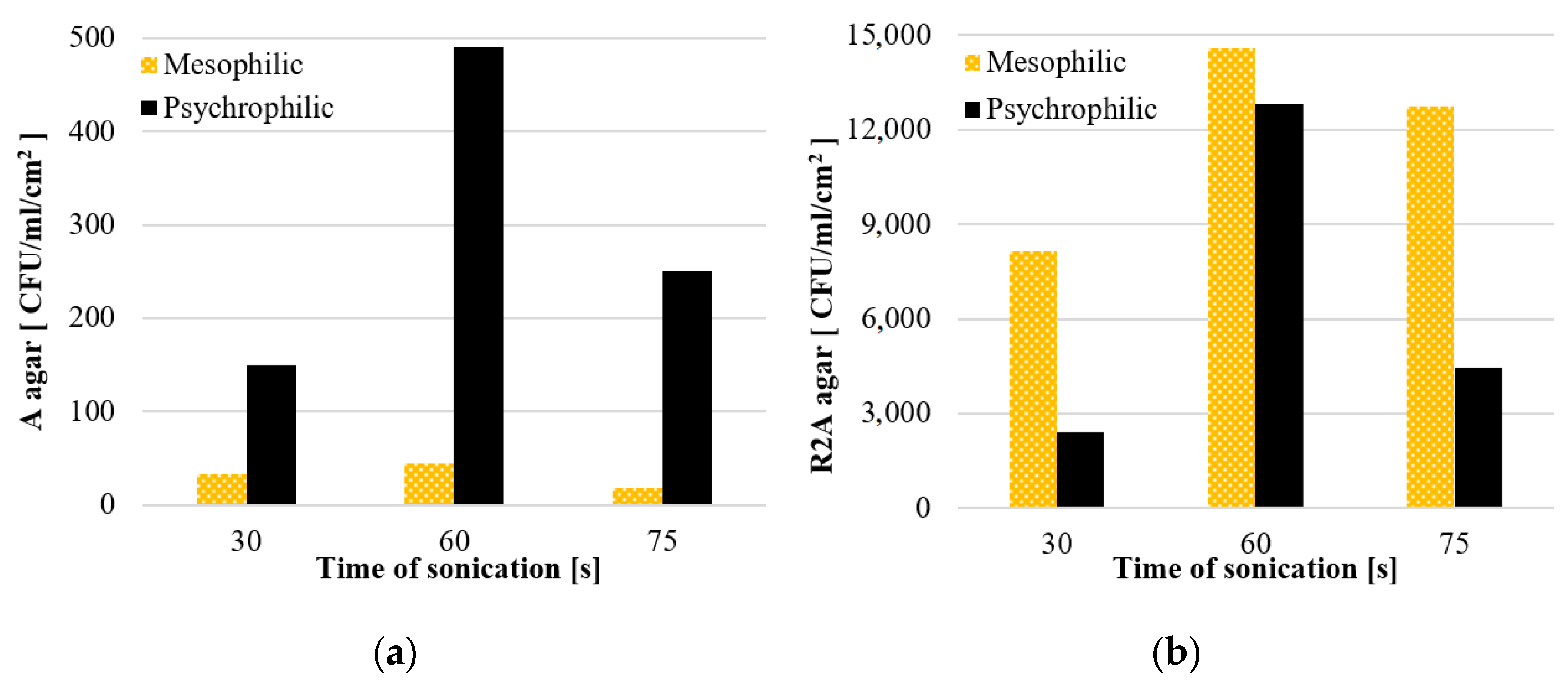
3.1.1. The Effect of Sonication Time on the Efficiency of Biofilm Detachment
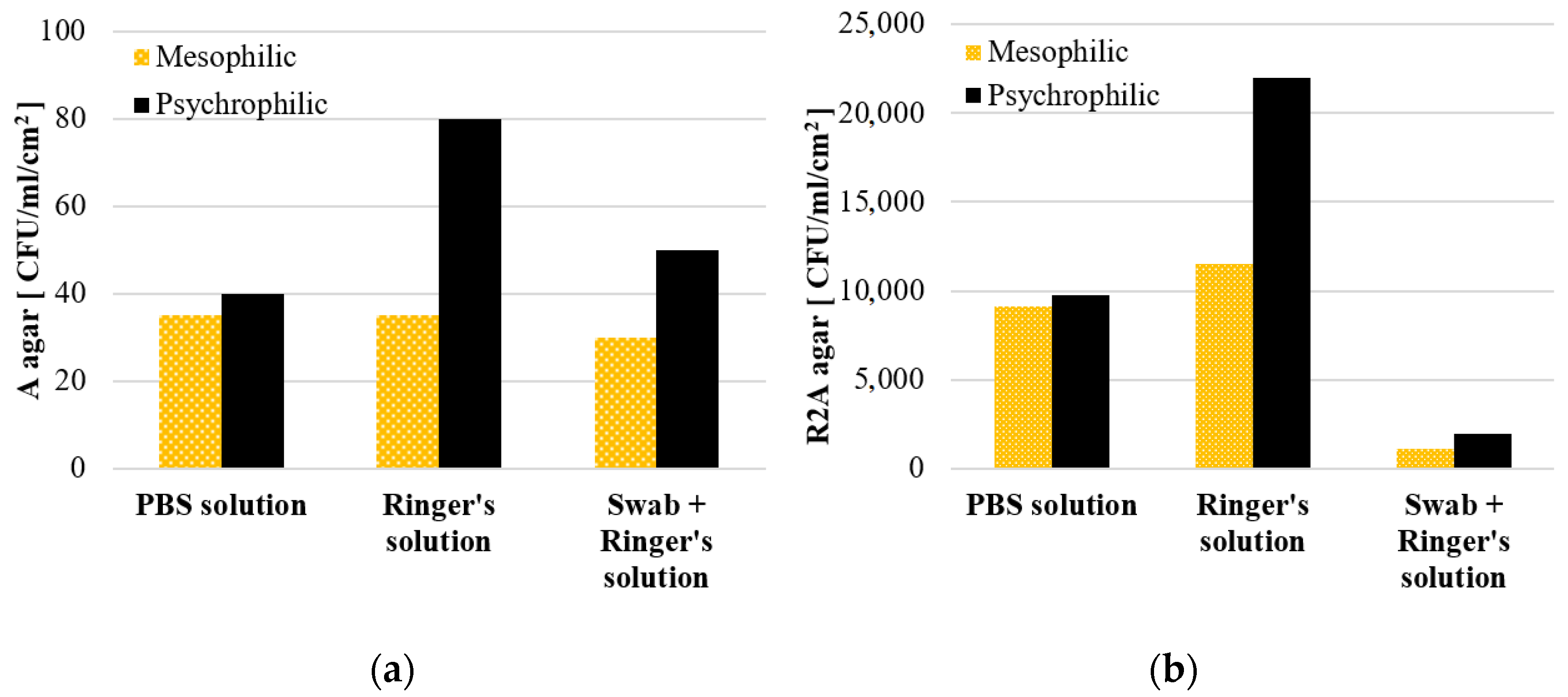
3.1.2. The Effect of Physiological Solutions on the Viability of the Biofilm Microorganisms
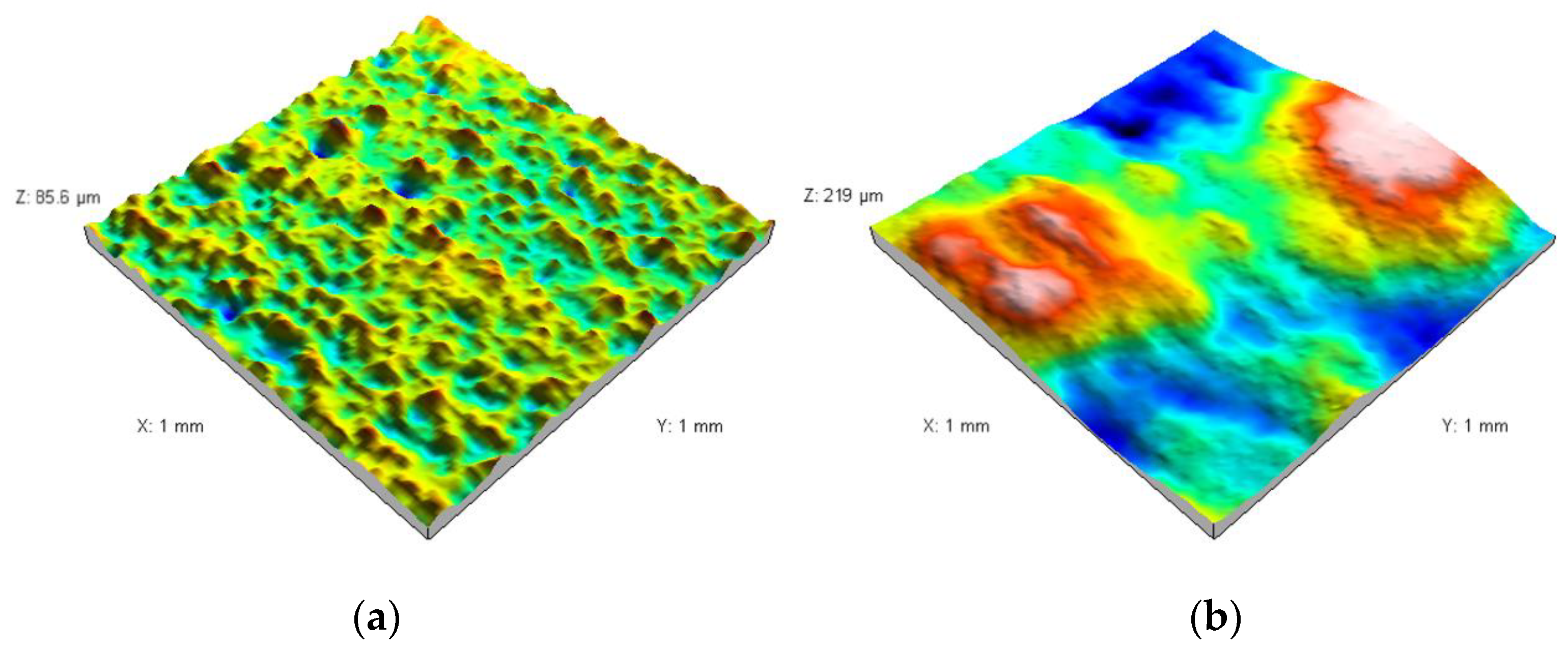
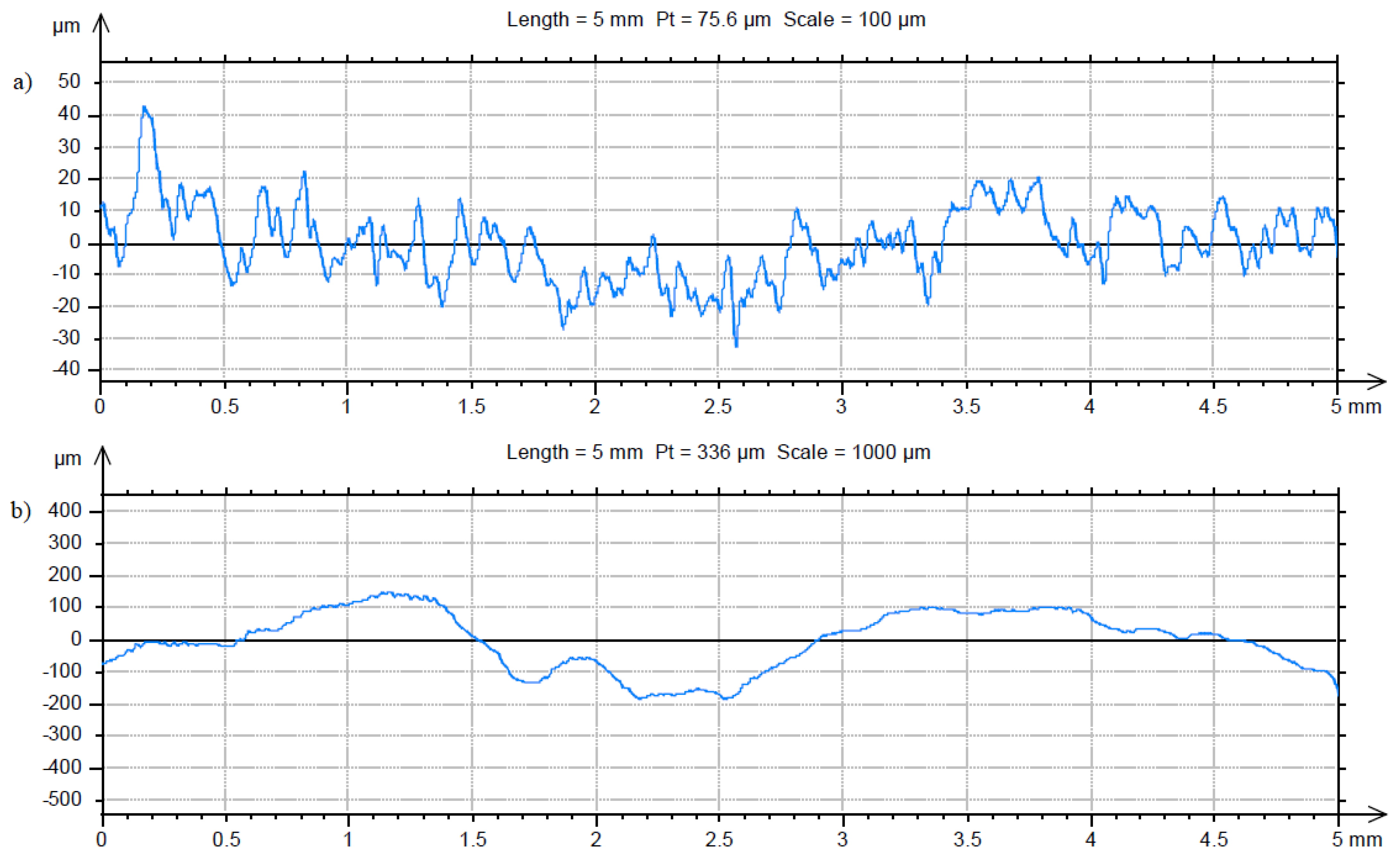
3.2. Characterization of the Sterile Coupon and Coupon with Biofilm
4. Discussion
5. Conclusions
- the most effective method of detaching bacterial cells from the surface of the coupons is a mechanical method using a sterile cotton swab, which is then subjected to ultrasound;
- the optimal time of exposure to ultrasound was 60 s (for the sonicator power of 28 W). At that time, the highest values obtained by the ATP bioluminescence assay, flow cytometry methods and the HPC method were recorded, while at the same time guaranteeing the least destruction of the bacterial cell);
- the use of buffered saline (Ringer’s solution) allowed to extend the viability of the microorganisms detached from the coupon surface compared to the PBS solution.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashbolt, N.J. Microbial Contamination of Drinking water and human health from community water systems. Curr. Environ. Health Rep. 2015, 2, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manuel, C.M.; Nunes, O.C.; Melo, L.F. Unsteady state flow and stagnation in distribution systems affect the biological stability of drinking water. Biofouling 2009, 26, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Wingender, J.; Flemming, H.-C. Biofilms in drinking water and their role as reservoir for pathogens. Int. J. Hyg. Environ. Health 2011, 214, 417–423. [Google Scholar] [CrossRef]
- Gomes, I.B.; Simões, M.; Simões, L.C. An overview on the reactors to study drinking water biofilms. Water Res. 2014, 62, 63–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proctor, C.R.; Hammes, F. Drinking water microbiology—From measurement to management. Curr. Opin. Biotechnol. 2015, 33, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, Z.; Liu, K.; Zhang, Y.; Peng, H.; Hu, B.; Ren, H.; Zhou, X.; Qiu, S.; He, X.; et al. Effect of flushing on the detachment of biofilms attached to the walls of metal pipes in water distribution systems. J. Zhejiang Univ.-Sci. A 2017, 18, 313–328. [Google Scholar] [CrossRef]
- Tsvetanova, Z.G.; Hoekstra, E.J. A study on assessment of biomass production potential of pipe materials in contact with drinking water. Water Supply 2019, 9, 423–429. [Google Scholar] [CrossRef]
- Pietrucha-Urbanik, K.; Tchórzewska-Cieślak, B.; Papciak, D.; Skrzypczak, I. Analysis of chemical stability of tap water in terms of required level of technological safety. Arch. Environ. Prot. 2017, 43, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Papciak, D.; Tchórzewska-Cieslak, B.; Pietrucha-Urbanik, K.; Pietrzyk, A. Analysis of the biological stability of tap water on the basis of risk analysis and parameters limiting the secondary growth of microorganisms in water distribution. Desalin. Water Treat. 2018, 117, 1–8. [Google Scholar] [CrossRef]
- Waller, S.A.; Packman, A.I.; Hausner, M. Comparison of biofilm cell quantification methods for drinking water distribution systems. J. Microbiol. Methods 2018, 144, 8–21. [Google Scholar] [CrossRef]
- Dong, Y.-D.; Zhang, H.; Zhong, G.-J.; Yao, G.; Lai, B. Cellulose/carbon Composites and their Applications in Water Treatment—A Review. Chem. Eng. J. 2021, 405, 126980. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Knibbe, W.-J.; Feng, C.; Liu, W.; Medema, G.; van der Meer, W. Potential impacts of changing supply-water quality on drinking water distribution: A review. Water Res. 2017, 116, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Lin, Q.; Li, C.; He, G.; Deng, Y. Impacts of pre-oxidation on the formation of disinfection byproducts from algal organic matter in subsequent chlor(am)ination: A review. Sci. Total Environ. 2021, 754, 141955. [Google Scholar] [CrossRef] [PubMed]
- Lehtola, M.J.; Miettinen, I.T.; Keinänen, M.M.; Kekki, T.K.; Laine, O.; Hirvonen, A.; Vartiainen, P.J.; Martikainen, P.J. Microbiology, chemistry and biofilm development in a pilot drinking water distribution system with copper and plastic pipes. Water Res. 2004, 38, 3769–3779. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Kim, D.; Lee, T. Microbial diversity in biofilms on water distribution pipes of different materials. Water Sci. Technol. 2010, 61, 163–171. [Google Scholar] [CrossRef]
- Zacheus, O.M.; Iivanainen, E.K.; Nissinen, T.K.; Lehtola, M.J.; Martikainen, P.J. Bacterial biofilm formation on polyvinyl chloride, polyethylene and stainless steel exposed to ozonated water. Water Res. 2000, 34, 63–70. [Google Scholar] [CrossRef]
- Liu, G.; Bakker, G.L.; Li, S.; Vreeburg, J.H.G.; Verberk, J.Q.J.C.; Medema, G.J.; Liu, W.T.; Van Dijk, J.C. Pyrosequencing Reveals Bacterial Communities in Unchlorinated Drinking Water Distri-bution System: An Integral Study of Bulk Water, Suspended Solids, Loose Deposits, and Pipe Wall Biofilm. Environ. Sci. Technol. 2014, 48, 5467–5476. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C. Biofouling in water systems—Cases, causes and counter-measures. Appl. Microbiol. Biotechnol. 2002, 59, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Edwards, M.A.; Falkinham, J.O.; Pruden, A. Probiotic approach to pathogen control in premise plumbing systems? A Review. Environ.-Ment. Sci. Technol. 2013, 47, 10117–10128. [Google Scholar] [CrossRef]
- Vital, M.; Hammes, F.; Egli, T. Competition of Escherichia coli O157 with a drinking water bacterial community at low nutrient concentrations. Water Res. 2012, 46, 6279–6290. [Google Scholar] [CrossRef]
- Chan, S.; Pullerits, K.; Keucken, A.; Persson, K.M.; Paul, C.J.; Rådström, P. Bacterial release from pipe biofilm in a full-scale drinking water distribution system. Biofilms Microbiomes 2019, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Xu, J.; Junzhi, Q.; Xiong, G.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.M.; Ashbolt, N.J. Do Free-living amoebae in treated drinking water systems present an emerging health risk? Environ. Sci. Technol. 2011, 45, 860–869. [Google Scholar] [CrossRef]
- Christensen, S.C.B.; Nissen, E.; Arvin, E.; Albrechtsen, H.J. Distribution of asellus aquaticus and microinvertebrates in a non-chlorinated drinking water supply system—Effects of pipe material and sedimentation. Water Res. 2011, 45, 3215–3224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prest, E.I.; Hammes, F.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Biological stability of drinking water: Controlling factors, methods, and challenges. Front. Microbiol. 2016, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Douterelo, I.; Husband, S.; Loza, V.; Boxall, J. Dynamics of biofilm regrowth in drinking water distribution systems. Appl. Environ. Microbiol. 2016, 82, 4155–4168. [Google Scholar] [CrossRef] [Green Version]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Williams, R.C.; Lo Giudice, A. Analysis of galectin-3 levels as a source of coronary heart disease risk during periodontitis. J. Periodontal Res. 2021, 56, 597–605. [Google Scholar] [CrossRef]
- Isola, G.; Lo Giudice, A.; Polizzi, A.; Alibrandi, A.; Murabito, P.; Indelicato, F. Identification of the different salivary Interleukin-6 profiles in patients with periodontitis: A cross-sectional study. Arch. Oral Biol. 2021, 122, 104997. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Williams, R.C.; Leonardi, R. Independent impact of periodontitis and cardiovascular disease on elevated soluble urokinase-type plasminogen activator receptor (suPAR) levels. J. Periodontol. 2021, 92, 896–906. [Google Scholar] [CrossRef]
- Masters, E.A.; Trombetta, R.P.; de Mesy Bentley, K.L.; Boyce, B.F.; Gill, A.L.; Gill, S.R.; Nishitani, K.; Ishikawa, M.; Morita, Y.; Ito, H.; et al. Evolving concepts in bone infection: Redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy”. Bone Res. 2019, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Akyıldız, İ.; Take, G.; Uygur, K.; Kızıl, Y.; Aydil, U. Bacterial biofilm formation in the middle-ear mucosa of chronic otitis media patients. Indian J. Otolaryngol. Head Neck Surg. 2013, 65, 557–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, W.; Yang, F.; Bick, A.; Oron, G.; Herzberg, M. Extracellular polymeric substances (EPS) in a hybrid growth membrane bioreactor (HG-MBR): Viscoelastic and adherence characteristics. Environ. Sci. Technol. 2010, 44, 8636–8643. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Shao, Y.; Zhang, Y.; Wang, R.; Zhu, D.Z.; Chen, H.; Liu, J. Optimization of ultrasonic parameters for effective detachment of biofilm cells in an actual drinking water distribution system. J. Zhejiang Univ.-Sci. A 2020, 21, 167–178. [Google Scholar] [CrossRef]
- Pan, R.; Zhang, K.; Cen, C.; Zhou, X.; Xu, J.; Wu, J.; Wu, X. Characteristics of biostability of drinking water in aged pipes after water source switching: ATP evaluation, biofilms niches and microbial community transition. Environ. Pollut. 2021, 271, 116293. [Google Scholar] [CrossRef] [PubMed]
- Douterelo, I.; Sharpe, R.L.; Boxall, J.B. Influence of hydraulic regimes on bacterial community structure and composition in an experimental drinking water distribution system. Water Res. 2013, 47, 503–516. [Google Scholar] [CrossRef]
- Douterelo, I.; Jackson, M.; Solomon, C.; Boxall, J. Spatial and temporal analogies in microbial communities in natural drinking water biofilms. Sci. Total Environ. 2017, 581–582, 277–288. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, C.; Zhong, D.; Yuan, Y.; Shan, L.; Zhang, J. Effects of Pipe Materials on chlorine-resistant biofilm formation under long-term high chlorine level. Appl. Biochem. Biotechnol. 2014, 173, 1564–1578. [Google Scholar] [CrossRef]
- Ji, P.; Rhoads, W.J.; Edwards, M.A.; Pruden, A. Impact of water heater temperature setting and water use frequency on the building plumbing microbiome. ISME J. 2017, 11, 1318–1330. [Google Scholar] [CrossRef] [Green Version]
- Tsvetanova, Z. Quantification of the bacterial community of drinking water-associated biofilms under different flow velocities and changing chlorination regimes. Appl. Water Sci. 2020, 10, 3. [Google Scholar] [CrossRef] [Green Version]
- Górka, A.; Papciak, D.; Zamorska, J.; Antos, D. The Influence of biofilm on the effectiveness of ion exchange process. Ind. Eng. Chem. Res. 2008, 47, 7456–7464. [Google Scholar] [CrossRef]
- Boe-Hansen, R.; Martiny, A.C.; Arvin, E.; Albrechtsen, H.-J. Monitoring biofilm formation and activity in drinking water distribution networks under oligotrophic conditions. Water Sci. Technol. 2003, 47, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Harrington, G.W.; Xagoraraki, I.; Goel, R. Factors affecting bulk to total bacteria ratio in drinking water distribution systems. Water Res. 2008, 42, 3393–3404. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-J. Effects of diverse water pipe materials on bacterial communities and water quality in the annular reactor. J. Microbiol. Biotechnol. 2011, 21, 115–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamorska, J.; Papciak, D. Activity of nitrifying biofilm in the process of water treatment in diatomite bed. Environ. Prot. Eng. 2008, 34, 37–52. [Google Scholar]
- D’Onofrio, A.; Crawford, J.M.; Stewart, E.J.; Witt, K.; Gavrish, E.; Epstein, S.; Clardy, J.; Lewis, K. Siderophores from neighboring organisms promote the growth of uncultured bacteria. Chem. Biol. 2010, 17, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Van Nevel, S.; Koetzsch, S.; Proctor, C.R.; Besmer, M.D.; Prest, E.I.; Vrouwenvelder, J.S.; Knezev, A.; Boon, N.; Hammes, F. Flow cytometric bacterial cell counts challenge conventional heterotrophic plate counts for routine microbiological drinking water monitoring. Water Res. 2017, 113, 191–206. [Google Scholar] [CrossRef] [Green Version]
- Zamorska, J. Biological stability of water after the biofiltration process. J. Ecol. Eng. 2018, 19, 234–239. [Google Scholar] [CrossRef]
- Zamorska, J.; Papciak, D.; Zdeb, M. New method for evaluation of the microbiological quality of water. J. Civ. Eng. Environ. Archit. 2015, 62, 523–530. [Google Scholar] [CrossRef]
- Schleich, C.; Chan, S.; Pullerits, K.; Besmer, M.D.; Paul, C.J.; Rådström, P.; Keucken, A. Mapping dynamics of bacterial communities in a full-scale drinking water distribution system using flow cytometry. Water 2019, 11, 2137. [Google Scholar] [CrossRef] [Green Version]
- Gensberger, E.T.; Gössl, E.-M.; Antonielli, L.; Sessitsch, A.; Kostić, T. Effect of different heterotrophic plate count methods on the estimation of the composition of the culturable microbial community. PeerJ 2015, 3, e862. [Google Scholar] [CrossRef]
- Hammes, F.; Berney, M.; Wang, Y.; Vital, M.; Köster, O.; Egli, T. Flow-cytometric total bacterial cell counts as a descriptive microbiological parameter for drinking water treatment processes. Water Res. 2008, 42, 269–277. [Google Scholar] [CrossRef]
- Lautenschlager, K.; Hwang, C.; Liu, W.-T.; Boon, N.; Köster, O.; Vrouwenvelder, H.; Egli, T.; Hammes, F. A microbiology-based multi-parametric approach towards assessing biological stability in drinking water distribution networks. Water Res. 2013, 47, 3015–3025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besmer, M.D.; Sigrist, J.A.; Props, R.; Buysschaert, B.; Mao, G.; Boon, N.; Hammes, F. Laboratory-scale simulation and real-time tracking of a microbial contamination event and subsequent shock-chlorination in drinking water. Front. Microbiol. 2017, 8, 1900. [Google Scholar] [CrossRef] [PubMed]
- Zamorska, J.; Zdeb, M.; Papciak, D. Bacteriological quality of tap water. Inżynieria Ekol. 2016, 48, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Hammes, F.A.; Egli, T. New method for assimilable organic carbon determination using flow-cytometric enumeration and a natural microbial consortium as inoculum. Environ. Sci. Technol. 2005, 39, 3289–3294. [Google Scholar] [CrossRef]
- Gillespie, S.; Lipphaus, P.; Green, J.; Parsons, S.; Weir, P.; Juskowiak, K.; Jefferson, P.; Jarvis, P.; Nocker, A. Assessing microbiological water quality in drinking water distribution systems with disinfectant residual using flow cytometry. Water Res. 2014, 65, 224–234. [Google Scholar] [CrossRef]
- Liu, G.; Ling, F.Q.; Magic-Knezev, A.; Liu, W.T.; Verberk, J.Q.J.C.; Van Dijk, J.C. Quantification and identification of particle-associated bacteria in unchlo-rinated drinking water from three treatment plants by cultivation-independent methods. Water Res. 2013, 47, 3523–3533. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Braun, K.; Fabris, R.; Hoefel, D.; Morran, J.; Monis, P.; Drikas, M. Comparison of drinking water treatment process streams for optimal bacteriological water quality. Water Res. 2012, 46, 3934–3942. [Google Scholar] [CrossRef] [PubMed]
- Nescerecka, A.; Hammes, F.; Juhna, T. A pipeline for developing and testing staining protocols for flow cytometry, demonstrated with SYBR Green I and propidium iodide viability staining. J. Microbiol. Methods 2016, 131, 172–180. [Google Scholar] [CrossRef]
- Van der Wielen, P.W.J.J.; van der Kooij, D. Effect of water composition, distance and season on the adenosine triphosphate concentration in unchlo-rinated drinking water in the Netherlands. Water Res. 2010, 44, 4860–4867. [Google Scholar] [CrossRef] [PubMed]
- Saccani, G.; Bernasconi, M.; Antonelli, M. Optimization of low energy sonication treatment for granular activated carbon colonizing biomass assessment. Environ. Technol. 2014, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]



| Value | The Ultrasound Duration | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 s | 60 s | 75 s | ||||||||||
| ATP 1 | FC 2 | HPC 3 Mesophilic Bacteria | HPC 4 Psychrophilic Bacteria | ATP 1 | FC 2 | HPC 3 Mesophilic Bacteria | HPC 4 Psychrophilic Bacteria | ATP 1 | FC 2 | HPC 3 Mesophilic Bacteria | HPC 4 Psychrophilic Bacteria | |
| MIN | 9265 | 7,687,180 | 6200 | 1700 | 19,500 | 7,795,600 | 11,500 | 12,000 | 12,270 | 8,000,540 | 10,600 | 3200 |
| MAX | 14,888 | 10,950,105 | 10,300 | 3100 | 24,724 | 10,106,660 | 17,700 | 13,600 | 19,414 | 9,989,095 | 14,900 | 5700 |
| M | 12,328 | 9,168,305 | 8167 | 2400 | 22,303 | 9,067,320 | 14,600 | 12,800 | 16,428 | 8,873,200 | 12,750 | 4450 |
| Me | 12,830 | 8,867,630 | 8000 | 2400 | 22,685 | 9,299,700 | 14,600 | 12,800 | 17,601 | 8,629,964 | 12,750 | 4450 |
| SD | 2845 | 1,652,112 | 2055 | 990 | 2633 | 1,172,924 | 4384 | 1131 | 3714 | 1,016,347 | 3041 | 1768 |
| Value | PBS Solution | Ringer’s Solution | Swab + Ringer’s Solution | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATP 1 | FC 2 | HPC 3 Mesophilic Bacteria | HPC 4 Psychrophilic Bacteria | ATP 1 | FC 2 | HPC 3 Mesophilic Bacteria | HPC 4 Psychrophilic Bacteria | ATP 1 | FC 2 | HPC 3 Mesophilic Bacteria | HPC 4 Psychrophilic Bacteria | |
| MIN | 9490 | 3,318,150 | 8100 | 9100 | 18,709 | 7,192,950 | 10,000 | 20,000 | 20,666 | 6,607,820 | 950 | 1680 |
| MAX | 13,100 | 6,499,650 | 9800 | 10,600 | 25,835 | 9,558,085 | 14,000 | 24,500 | 25,403 | 8,123,380 | 1500 | 2170 |
| M | 11,620 | 5,115,830 | 9100 | 9750 | 22,205 | 8,270,675 | 11,500 | 22,000 | 22,650 | 7,300,500 | 1150 | 1950 |
| Me | 12,269 | 5,529,690 | 9400 | 9550 | 22,070 | 8,060,990 | 10,500 | 21,500 | 21,880 | 7,170,300 | 1000 | 2000 |
| SD | 1891 | 1,630,627 | 889 | 770 | 3565 | 1,196,429 | 2179 | 2291 | 2461 | 766,123 | 304 | 249 |
| Parameters | Sterile Coupon | Coupon with Biofilm |
|---|---|---|
| Fractal dimension D ± standard error (-) | 1.23 ± 0.016 | 1.18 ± 0.015 |
| Total height of the roughness profile Pt ± standard error (µm) | 83.6 ± 6.2 | 393.9 ± 23.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papciak, D.; Domoń, A.; Zdeb, M.; Skwarczyńska-Wojsa, A.; Konkol, J. Optimization of Quantitative Analysis of Biofilm Cell from Pipe Materials. Coatings 2021, 11, 1286. https://doi.org/10.3390/coatings11111286
Papciak D, Domoń A, Zdeb M, Skwarczyńska-Wojsa A, Konkol J. Optimization of Quantitative Analysis of Biofilm Cell from Pipe Materials. Coatings. 2021; 11(11):1286. https://doi.org/10.3390/coatings11111286
Chicago/Turabian StylePapciak, Dorota, Andżelika Domoń, Monika Zdeb, Agata Skwarczyńska-Wojsa, and Janusz Konkol. 2021. "Optimization of Quantitative Analysis of Biofilm Cell from Pipe Materials" Coatings 11, no. 11: 1286. https://doi.org/10.3390/coatings11111286
APA StylePapciak, D., Domoń, A., Zdeb, M., Skwarczyńska-Wojsa, A., & Konkol, J. (2021). Optimization of Quantitative Analysis of Biofilm Cell from Pipe Materials. Coatings, 11(11), 1286. https://doi.org/10.3390/coatings11111286









