Friction and Wear Behaviors of Fe-19Cr-15Mn-0.66N Steel at High Temperature
Abstract
:1. Introduction
2. Experimental Details
3. Results
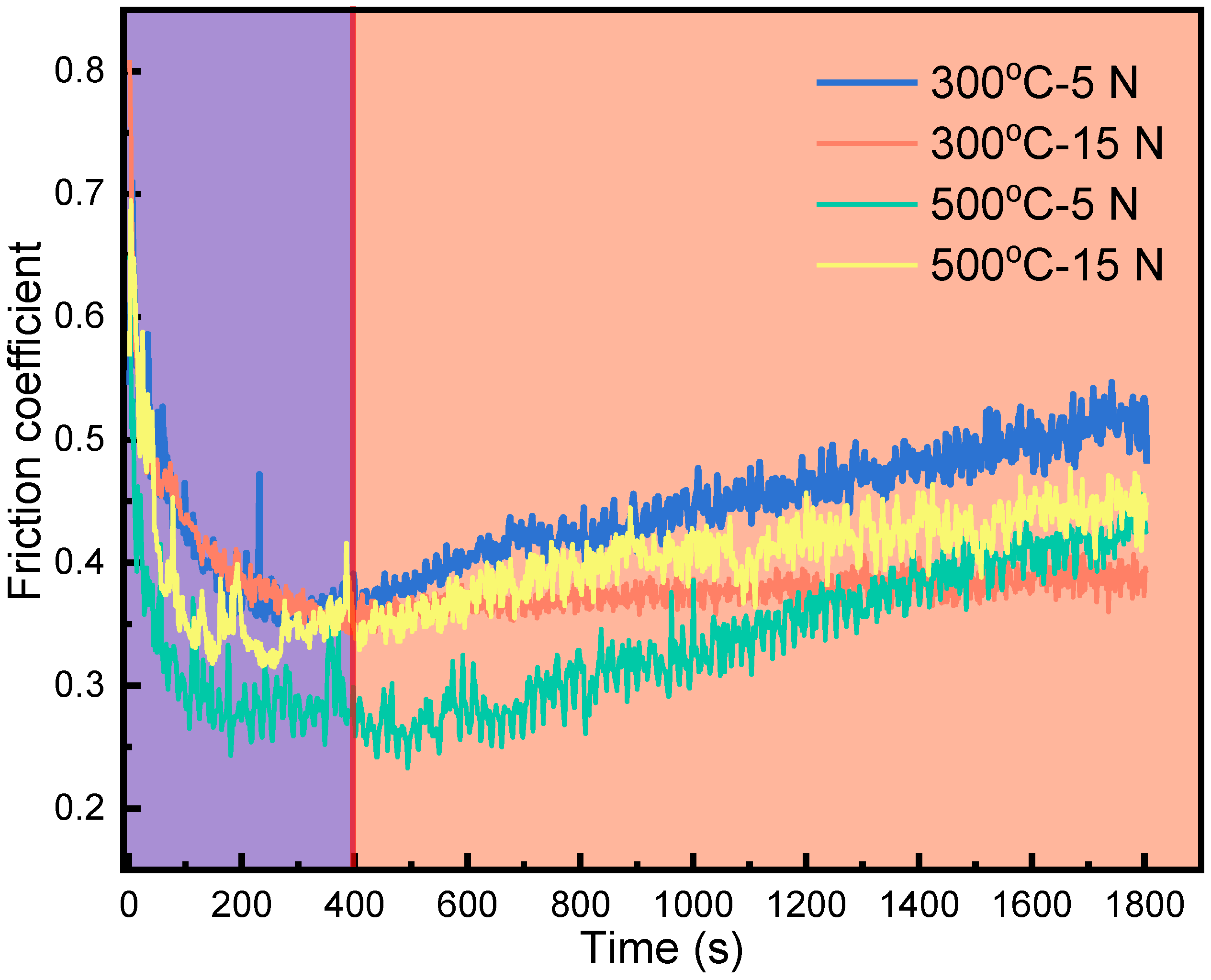
3.1. Friction Coefficient
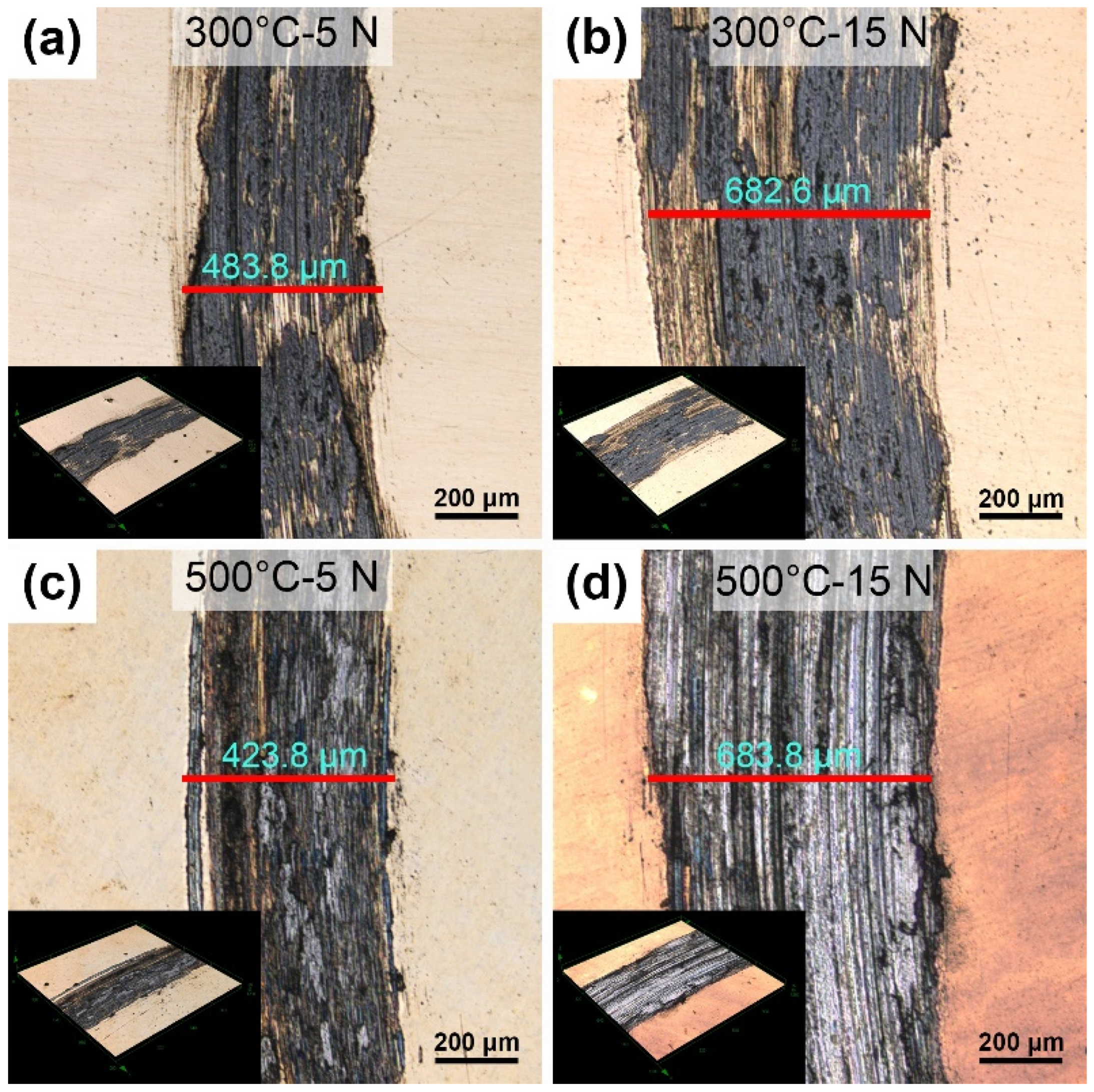
3.2. LSCM of the Wear Tracks
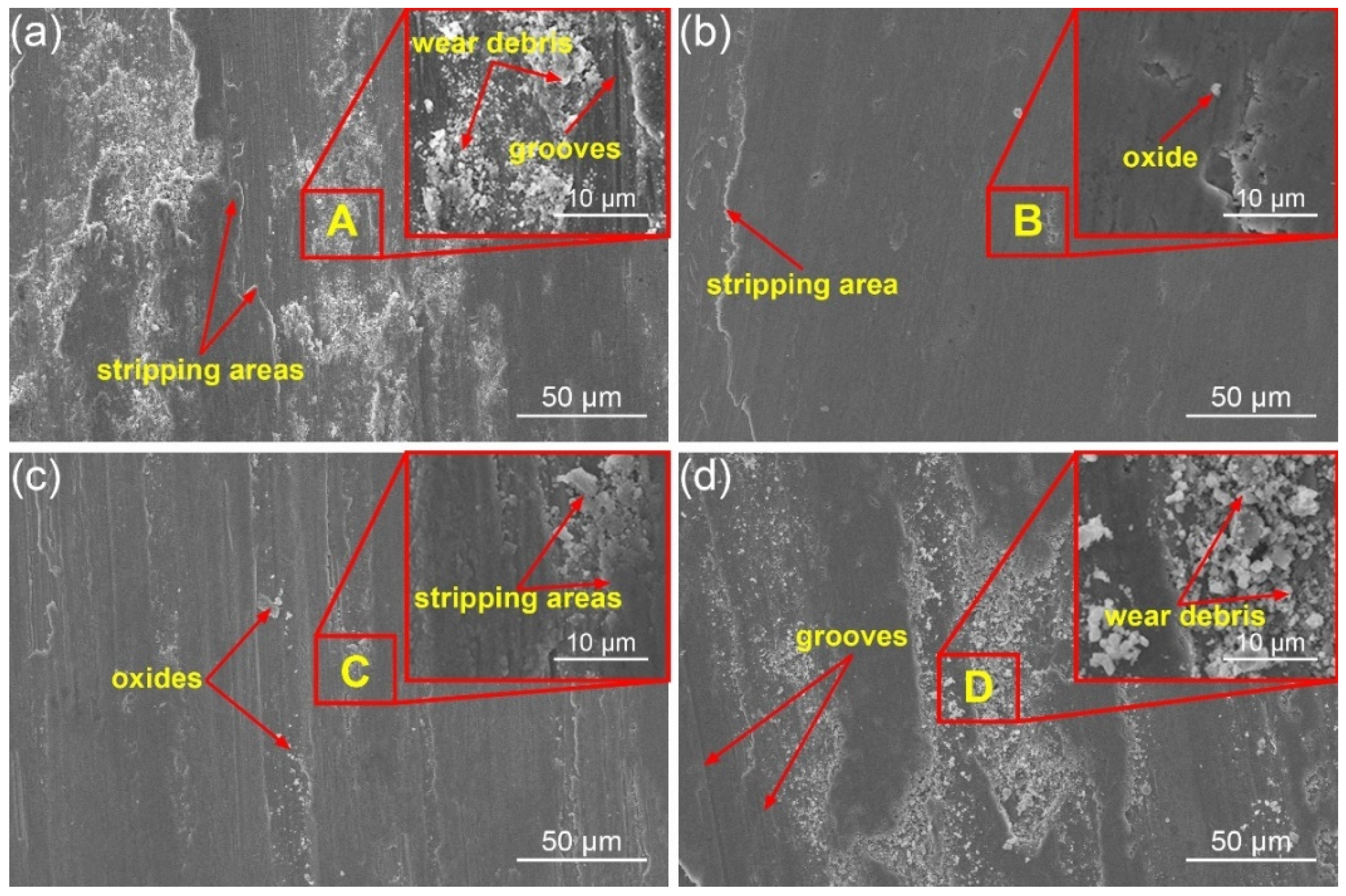
3.3. Morphologies of the Wear Tracks
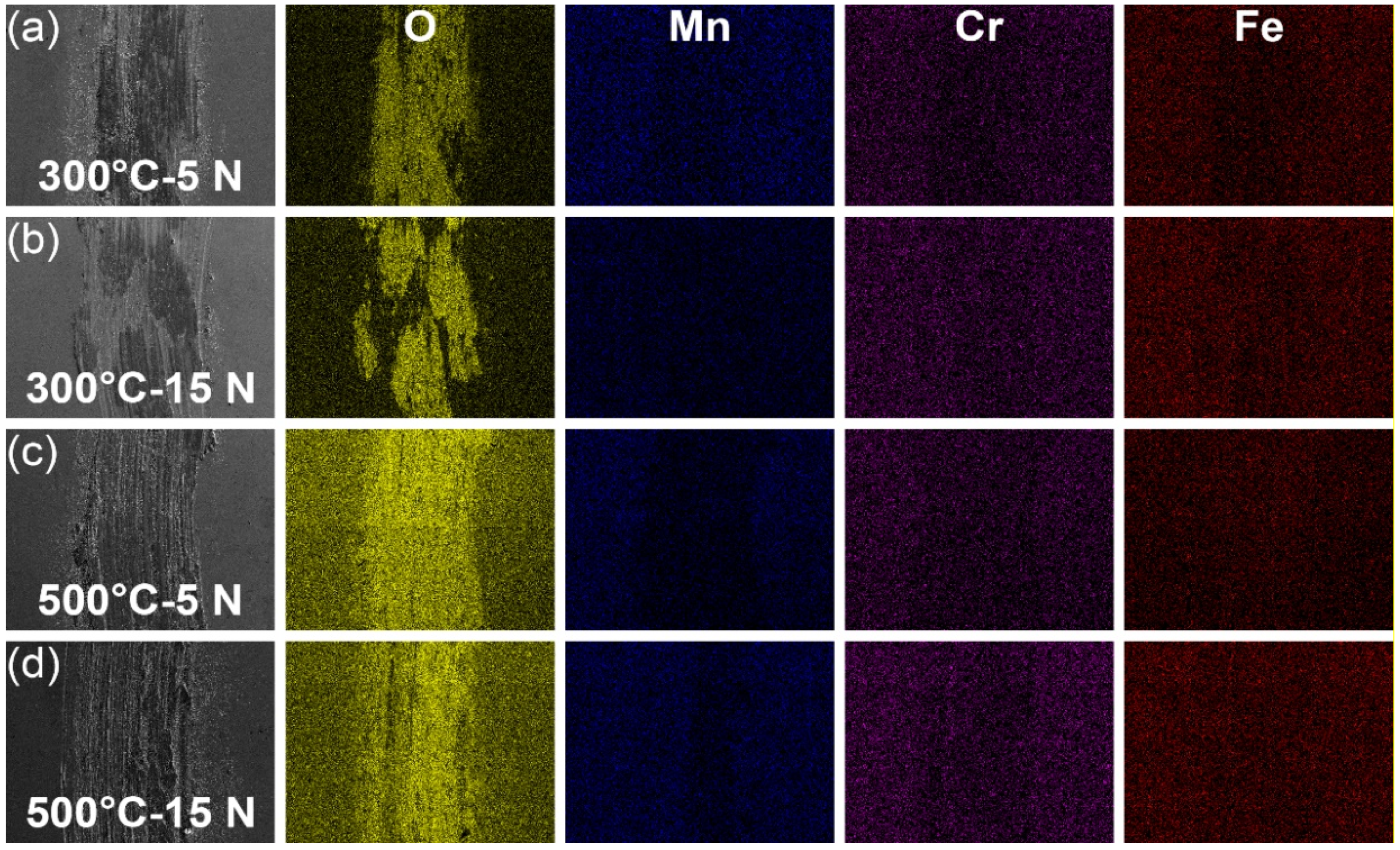
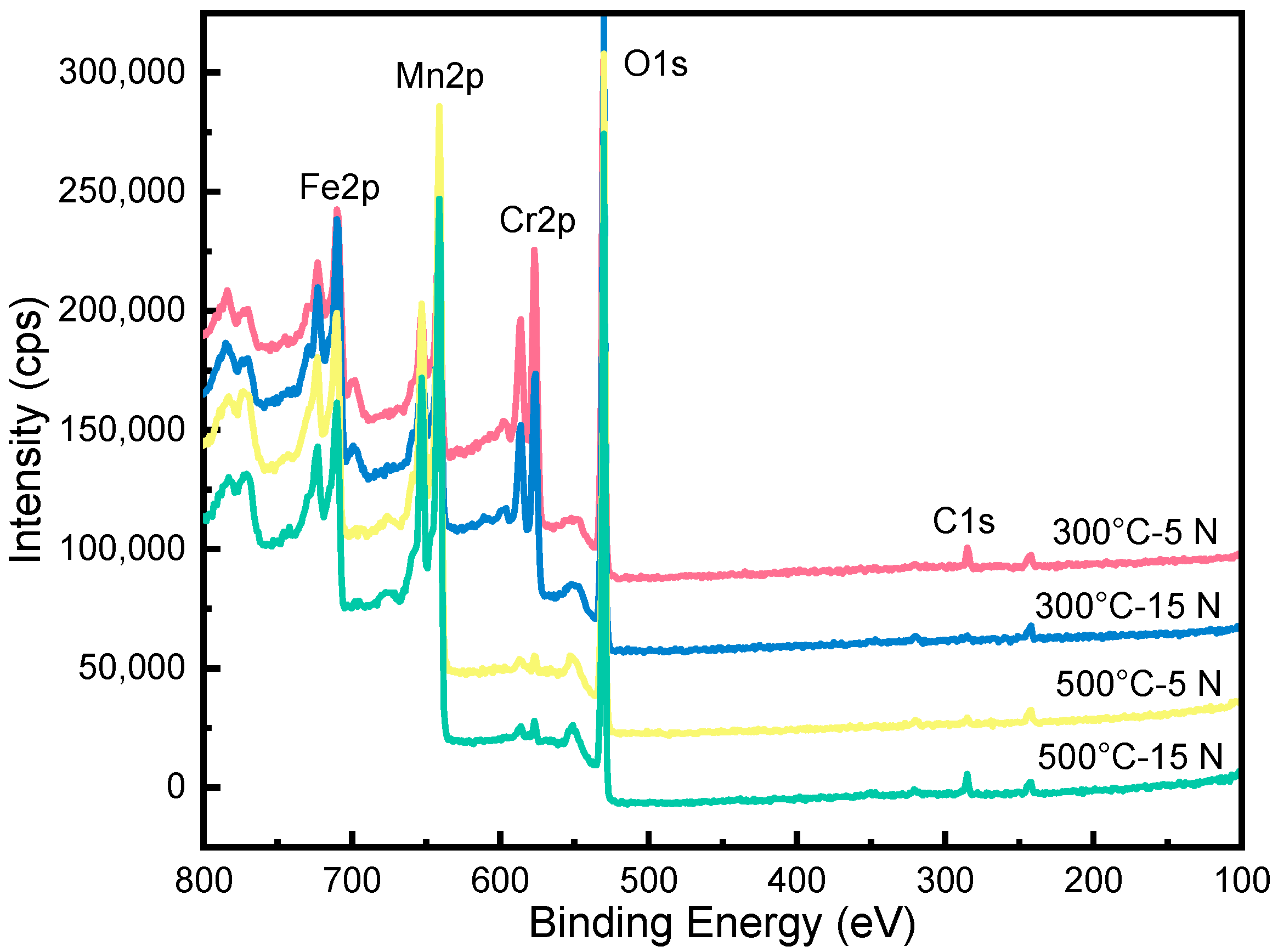
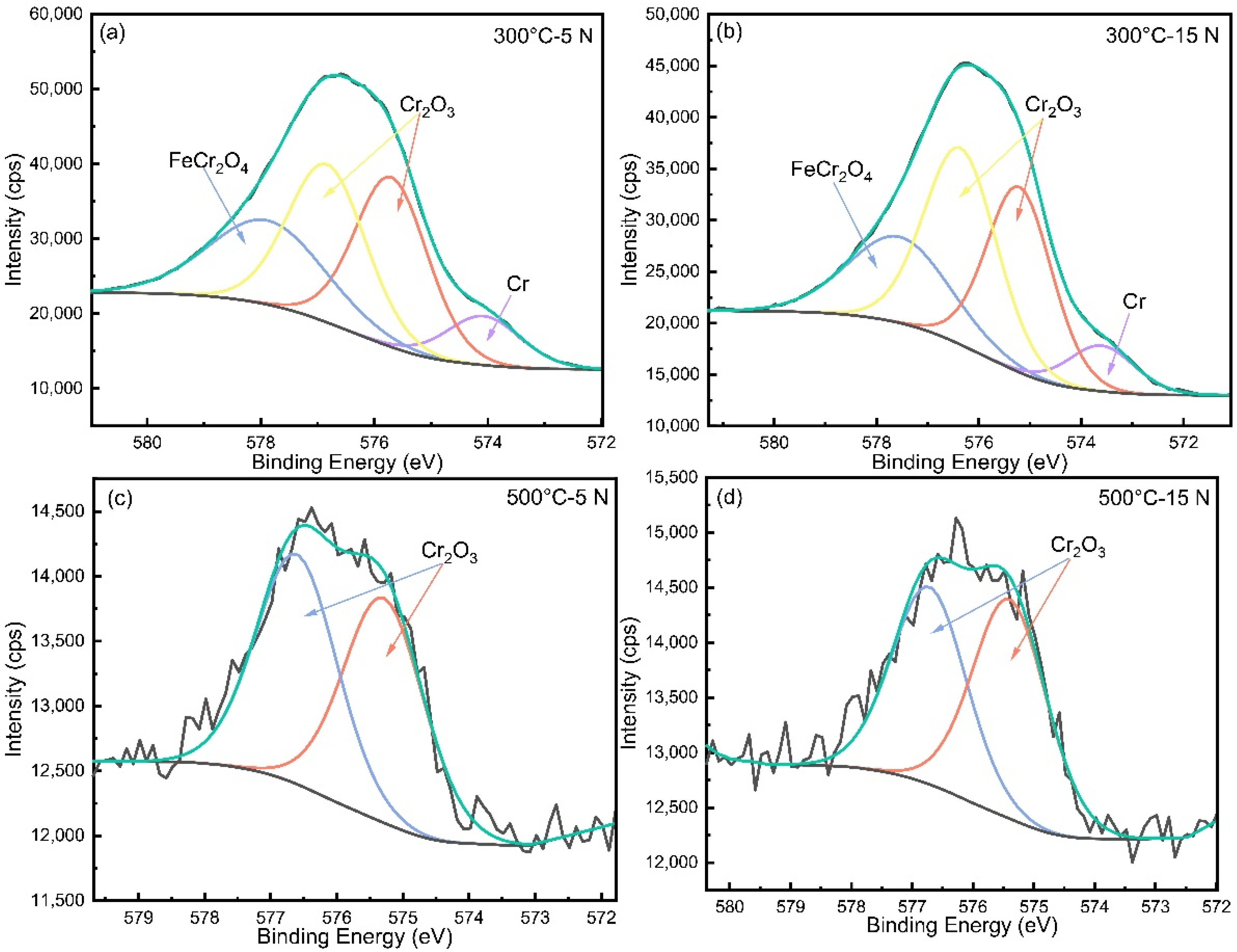
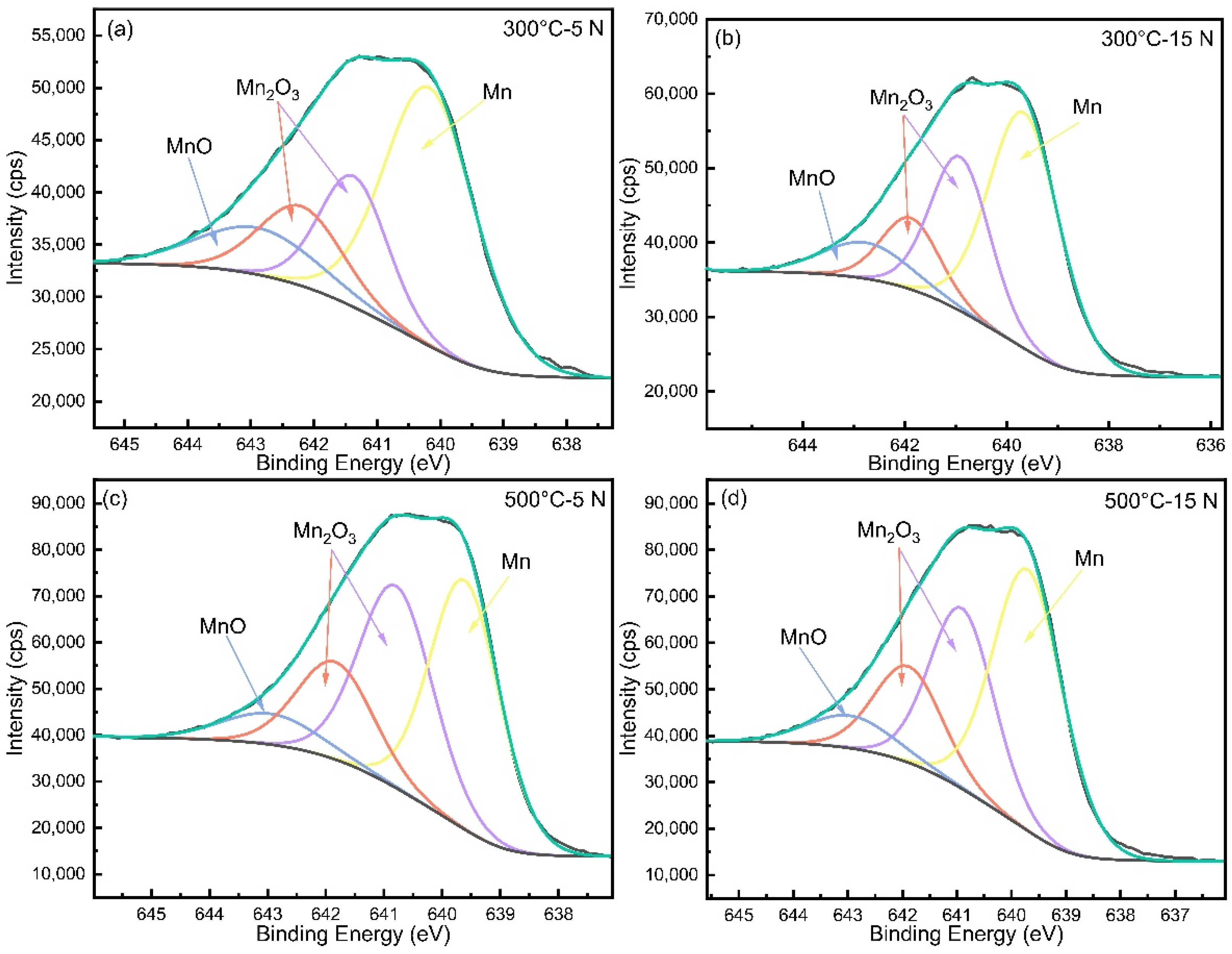
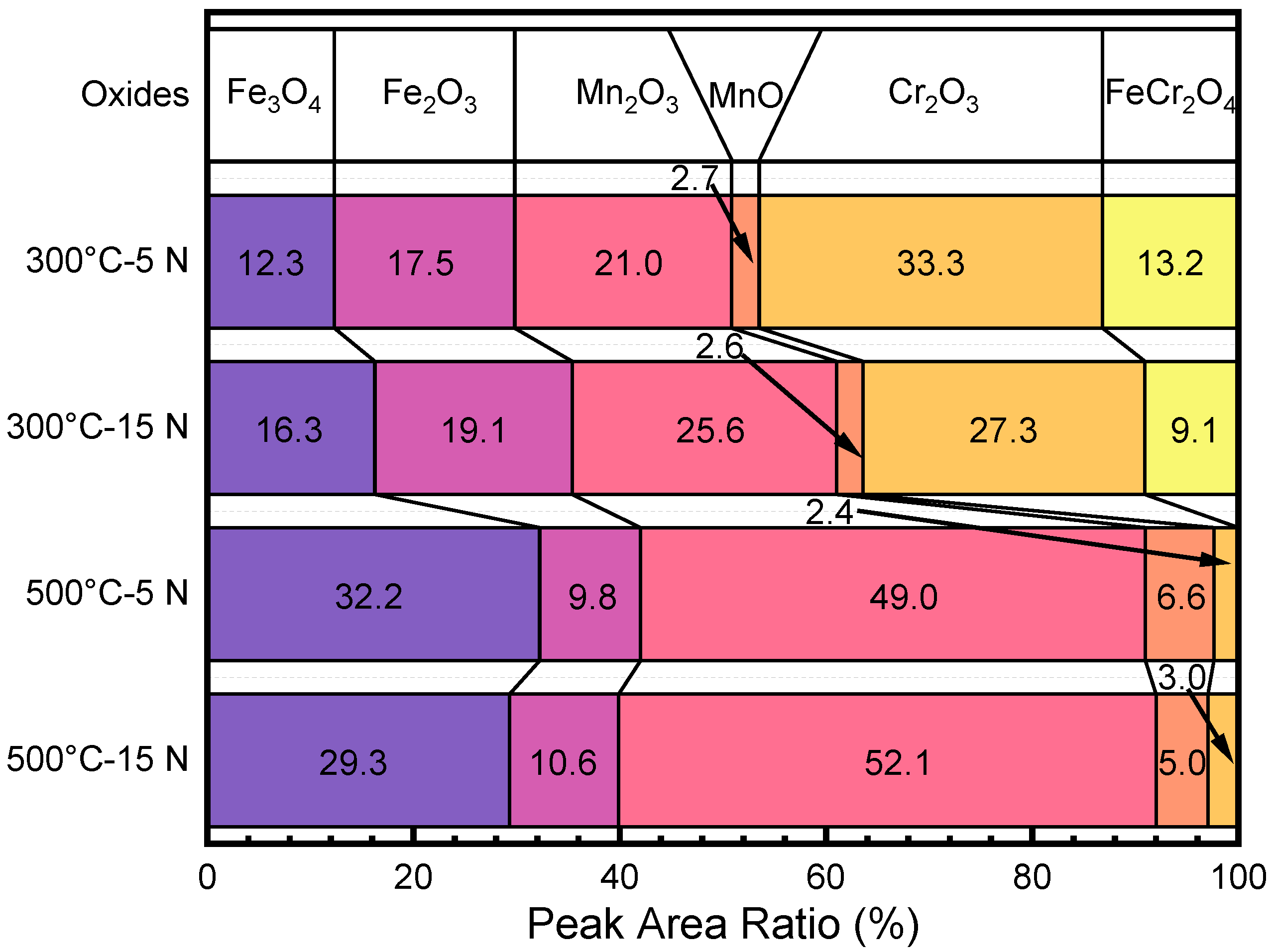
3.4. Microstructure Characteristics of the Wear Tracks
4. Discussion
5. Summary
- (1).
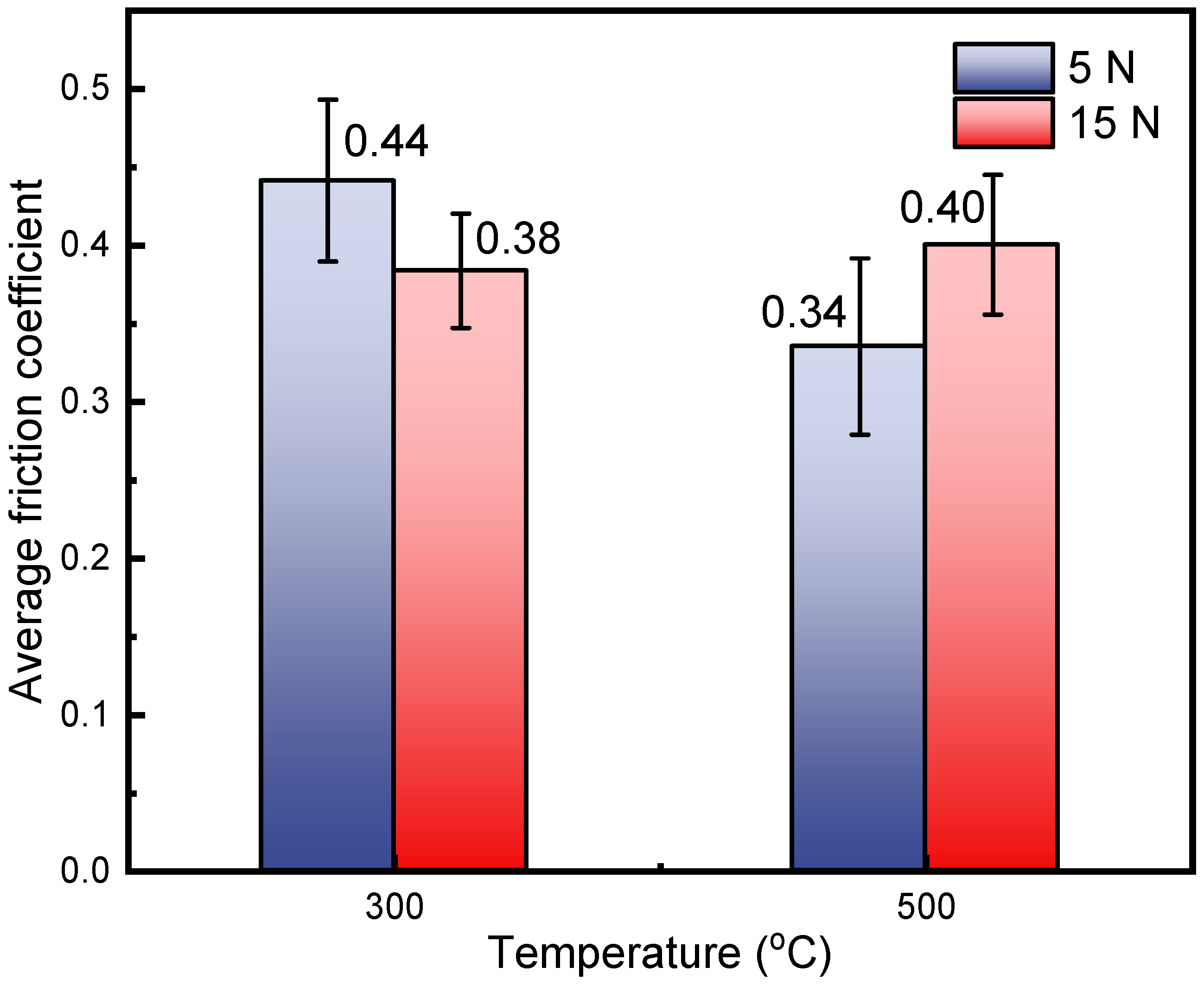
- The friction coefficient of HNSS decreased with time up to 400 s but rose up slowly or maintained a relatively stable trend as the time increased. The average friction coefficient decreased with increasing load at 300 °C, while it showed a reverse trend at 500 °C, which depended on the wear mechanism that reflects the competitive processes, including breakdown and reconsolidation of the oxide layer formed on wear tracks.
- (2).
- At 300 °C, the friction coefficient curve of HNSS with 15 N applied showed a stable friction state after 400 s due to the formation of a denser oxide layer consisting of Cr2O3, Fe2O3, and Mn2O3 and increased hardness caused by work hardening, showing the wear mechanisms of oxidation wear and adhesive wear.
- (3).
- Under the conditions of 500 °C–5 N, softening at high temperature and the production of large amounts of Mn2O3, which has a lubricating effect, minimized the friction of the sample, showing the lowest friction coefficient with a value of 0.34. In addition, the increased temperature also weakened the work hardening, resulting in severe abrasive wear at 500 °C–15 N.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.R.; Sun, S.C.; Sun, G.X.; Han, S.; Jiang, Z.H.; Lian, J.S. Nanoindentation creep deformation behaviour of high nitrogen nickel-free austenitic stainless steel. Mater. Sci. Technol. 2019, 35, 1592–1599. [Google Scholar] [CrossRef]
- Li, J.G.; Li, H.; Peng, W.; Xiang, T.; Xu, Z.Y.; Yang, J.C. Effect of simulated welding thermal cycles on microstructure and mechanical properties of coarse-grain heat-affected zone of high nitrogen austenitic stainless steel. Mater. Charact. 2019, 149, 206–217. [Google Scholar] [CrossRef]
- Vats, V.; Baskaran, T.; Arya, S.B. Tribo-corrosion study of nickel-free, high nitrogen and high manganese austenitic stainless steel. Tribol. Int. 2018, 119, 659–666. [Google Scholar] [CrossRef]
- Qiao, Y.X.; Wang, X.Y.; Chen, J.; Yang, L.L.; Wang, X.J.; Zhou, H.L.; Zou, J.S. Electrochemical behavior and passive film composition of a high-nitrogen nickel-free austenitic stainless steel. Arab. J. Sci. Eng. 2021, 1–8. [Google Scholar] [CrossRef]
- Qiao, Y.X.; Sheng, S.L.; Zhang, L.M.; Chen, J.; Yang, L.L.; Zhou, H.L.; Wang, Y.X.; Li, H.B.; Zheng, Z.B. Friction and wear behaviors of a high nitrogen austenitic stainless steel Fe-19Cr-15Mn-0.66N. J. Min. Metall. Sect. B Metall. 2021, 57, 285–293. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, Y.; Dong, J.; Yang, K. The microstructure and tribological behavior of a pre-cold-deformed 0.90 % nitrogen containing stainless steel. Mater. Werkst. 2018, 49, 1439–1448. [Google Scholar] [CrossRef]
- Lin, H.; Yang, M.S.; Shu, B.P. Fretting wear behaviour of high-nitrogen stainless bearing steel under lubrication condition. J. Iron Steel Res. Int. 2020, 27, 849–866. [Google Scholar] [CrossRef]
- Chen, Z.X.; Hu, H.X.; Guo, X.M.; Zheng, Y.G. Effect of groove depth on the slurry erosion of V-shaped grooved surfaces. Wear 2021, 488–489, 204133. [Google Scholar] [CrossRef]
- Qiao, Y.X.; Tian, Z.H.; Cai, X.; Chen, J.; Wang, Y.X.; Song, Q.N.; Li, H.B. Cavitation erosion behaviors of a nickel-free high-nitrogen stainless steel. Tribol. Lett. 2019, 67, 1. [Google Scholar] [CrossRef]
- Qiao, Y.X.; Chen, J.; Zhou, H.L.; Wang, Y.X.; Song, Q.N.; Li, H.B.; Zheng, Z.B. Effect of solution treatment on cavitation erosion behavior of high-nitrogen austenitic stainless steel. Wear 2019, 424–425, 70–77. [Google Scholar] [CrossRef]
- Qiao, Y.X.; Wang, X.Y.; Yang, L.L.; Wang, X.J.; Chen, J.; Wang, Z.B.; Zhou, H.L.; Zou, J.S.; Wang, F.H. Effect of aging treatment on microstructure and corrosion behavior of a Fe-18Cr-15Mn-0.66N stainless steel. J. Mater. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.X.; Ren, Y.B.; Dong, J.H.; Yang, K. Effect of cold deformation on corrosion fatigue behavior of nickel-free high nitrogen austenitic stainless steel for coronary stent application. J. Mater. Sci. Technol. 2018, 34, 660–665. [Google Scholar] [CrossRef]
- Korashy, A.; Attia, H.; Thomson, V.; Oskooei, S. Fretting wear behavior of cobalt—Based superalloys at high temperature—A comparative study. Tribol. Int. 2020, 145, 106155. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, S.Y.; Wang, X.Y.; Sui, X.D.; Zhang, S.T.; Hao, J.Y.; Zhao, T. High temperature tribological behavior of polymer-derived Ta4HfC5 nanoceramics. Tribol. Int. 2021, 156, 106859. [Google Scholar] [CrossRef]
- Xue, Y.W.; Wu, C.H.; Shi, X.L.; Zhang, K.P.; Huang, Q.P. High temperature tribological behavior of textured CSS-42L bearing steel filled with Sn-Ag-Cu-Ti3C2. Tribol. Int. 2021, 164, 107205. [Google Scholar] [CrossRef]
- Pauschitz, A.; Roy, M.; Franek, F. Mechanisms of sliding wear of metals and alloys at elevated temperatures. Tribol. Int. 2008, 41, 584–602. [Google Scholar] [CrossRef]
- Stott, F.H. High-temperature sliding wear of metals. Tribol. Int. 2002, 35, 489–495. [Google Scholar] [CrossRef]
- Eder, S.J.; Grutzmacher, P.G.; Ripoll, M.R.; Dini, D.; Gachot, C. Effect of temperature on the deformation behavior of copper nickel alloys under sliding. Materials 2020, 14, 60. [Google Scholar] [CrossRef]
- Torres, H.; Varga, M.; Ripoll, M.R. High temperature hardness of steels and iron-based alloys. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2016, 671, 170–181. [Google Scholar] [CrossRef]
- Li, L.; He, K.; Sun, S.Y.; Yang, W.Z.; Yue, Z.F.; Wan, H. High-temperature friction and wear features of nickel-based single crystal superalloy. Tribol. Lett. 2020, 68, 26. [Google Scholar] [CrossRef]
- Pei, Y.C.; Xia, D.X.; Wang, S.R.; Cong, L.; Wang, X.L.; Wang, D.Y. Effects of temperature on the tribological properties of NM600 under sliding wear. Materials 2019, 12, 4009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, H.; Ramakrishnan, V.; Albert, S.K.; Meikandamurthy, C.; Tata, B.V.R.; Bhaduri, A.K. High temperature wear and friction behaviour of 15Cr–15Ni–2Mo titanium-modified austenitic stainless steel in liquid sodium. Wear 2010, 270, 1–4. [Google Scholar] [CrossRef]
- Wang, L.; Tieu, A.K.; Wang, J.; Sang, P.T.; Xia, C.Y.; Zhu, H.T.; Deng, G.Y. High load capability, sticking scale inhabitation and promising lubrication of sodium carbonate coating for steel/steel contact at high temperature. Tribol. Int. 2021, 153, 106594. [Google Scholar] [CrossRef]
- Cheng, X.W.; Jiang, Z.Y.; Kosasih, B.; Wu, H.; Luo, S.Z.; Jiang, L.Z. Influence of Cr-Rich oxide scale on sliding wear mechanism of ferritic stainless steel at high temperature. Tribol. Lett. 2016, 63, 28. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.J.; Wang, Y.X.; Liu, H.; Wang, J.Q.; Yan, F.Y. Interrelated effects of temperature and load on fretting behavior of SAF 2507 super duplex stainless steel. Tribol. Int. 2019, 136, 140–147. [Google Scholar] [CrossRef]
- Torres, H.; Varga, M.; Adam, K.; Ripoll, M.R. The role of load on wear mechanisms in high temperature sliding contacts. Wear 2016, 364–365, 73–83. [Google Scholar] [CrossRef]
- Stott, F.H.; Jordan, M.P. The effects of load and substrate hardness on the development and maintenance of wear-protective layers during sliding at elevated temperatures. Wear 2001, 250, 391–400. [Google Scholar] [CrossRef]
- Razali, M.K.; Kim, S.W.; Irani, M.; Kim, M.C.; Joun, M.S. Practical quantification of the effects of flow stress, friction, microstructural properties, and the tribological environment on macro- and micro-structure formation during hot forging. Tribol. Int. 2021, 164, 107226. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Joun, M. On critical surface strain during hot forging of lubricated aluminum alloy. Tribol. Int. 2020, 141, 105855. [Google Scholar] [CrossRef]
- Cao, Y.J.; Sun, J.Q.; Ma, F.; Chen, Y.Y.; Cheng, X.Z.; Gao, X.; Xie, K. Effect of the microstructure and residual stress on tribological behavior of induction hardened GCr15 steel. Tribol. Int. 2017, 115, 108–115. [Google Scholar] [CrossRef]
- Berns, H.; Koch, S. Influence of abrasive particles on wear mechanism and wear resistance in sliding abrasion tests at elevated temperatures. Wear 1999, 233–235, 424–430. [Google Scholar] [CrossRef]
- Kumar, S.S.S.; Raghu, T.; Bhattacharjee, P.P.; Rao, G.A.; Borah, U. Work hardening characteristics and microstructural evolution during hot deformation of a nickel superalloy at moderate strain rates. J. Alloy Compd. 2017, 709, 394–409. [Google Scholar] [CrossRef]
- Ball, A. On the Importance of work hardenning in the design of wear-resistant materials. Wear 1983, 91, 201–207. [Google Scholar] [CrossRef]
- Llewellyn, D.T. Work hardening effects in austenitic stainless steels. Mater. Sci. Technol. 2013, 13, 389–400. [Google Scholar] [CrossRef]
- Wang, D.P.; Zhang, H.T.; Guo, P.Y.; Sun, B.A.; Wang, Y.X. Nanoscale periodic distribution of energy dissipation at the shear band plane in a Zr-based metallic glass. Scr. Mater. 2021, 197, 4. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wang, S.H.; Hao, Y.; Pu, J.B.; Jiang, X.; Huang, L.F.; Wang, L.P. Friction and wear behavior of CrN coating on 316L stainless steel in liquid sodium at elevated temperature. Tribol. Int. 2020, 143, 106079. [Google Scholar] [CrossRef]
- Xu, Y.W.; Jing, H.Y.; Xu, L.Y.; Han, Y.D.; Zhao, L. Effect of overload on the oxidation behavior of CF8A austenitic stainless steel in a high-temperature water environment. Corros. Sci. 2020, 162, 108219. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Mills, P.; Sullivan, J.L. A study of the core level electrons in iron and its three oxides by means of X-ray photoelectron spectroscopy. J. Phys. D Appl. Phys. 1983, 16, 723–732. [Google Scholar] [CrossRef]
- Duhamel, C.; Sennour, M.; Georgi, F.; Guerre, C.; Chaumun, E.; Crépin, J.; Héripré, E.; Curières, I.D. Characterization of oxide scales formed on alloy 82 in nominal PWR Primary water at 340 °C and in hydrogenated steam at 400 °C. Corros. Sci. 2018, 131, 386–403. [Google Scholar] [CrossRef]
- Agostinelli, E.; Battistoni, C.; Fiorani, D.; Mattogno, G.; Nogues, M. An XPS study of the electronic structure of the ZnxCd1-xCr2X4 (X = S, Se) spinel system. J. Phys. Chem. Solids 1989, 50, 269–272. [Google Scholar] [CrossRef]
- Allen, G.C.; Tucker, P.M. Multiplet splitting of X-Ray photoelectron lines of chromium complexes. the effect of covalency on the 2p core level spin-orbit separation. Inorg. Chim. Acta 1976, 16, 41–45. [Google Scholar] [CrossRef]
- Wang, D.P.; Shen, J.W.; Chen, Z.; Chen, F.G.; Guo, P.Y.; Geng, Y.X.; Wang, Y.X. Relationship of corrosion behavior between single-phase equiatomic CoCrNi, CoCrNiFe, CoCrNiFeMn alloys and their constituents in NaCl solution. Acta Metall. Sin. (Engl. Lett.) 2021, 1–11. [Google Scholar] [CrossRef]
- Quinn, T.F.J.; Sullivan, J.L.; Rowson, D.M. Origins and development of oxidational ambient temperatures wear at low ambient temperatures. Wear 1984, 94, 175–191. [Google Scholar] [CrossRef]
- Stott, F.H. The role of oxidation in the wear of alloys. Tribol. Int. 1998, 31, 61–71. [Google Scholar] [CrossRef]
- Jiang, J.; Stott, F.H.; Stack, M.M. The role of triboparticulates in dry sliding wear. Tribol. Int. 1998, 31, 245–256. [Google Scholar] [CrossRef]
- Young, D.J. High Temperature Oxidation and Corrosion of Metals; Elsevier Ltd.: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Jin, X.Y.; Zhang, Y.F.; Chen, L.; Yu, J.H.; Xue, W.B. Preparation and tribological behaviors of DLC/spinel composite film on 304 stainless steel formed by cathodic plasma electrolytic oxidation. Surf. Coat. Technol. 2018, 338, 38–44. [Google Scholar] [CrossRef]
- Lai, Z.W.; Wu, J.L.; Wen, Y.H.; Li, N.; Wang, Z.C. Study of a new type austenitic stainless steel with better wear resistance. Tribology 2009, 29, 128–133. [Google Scholar]
- Song, Y.L.; Yu, C.; Miao, X.; Han, X.H.; Qian, D.S.; Chen, X. Tribological performance improvement of bearing steel GCr15 by an Alternating magnetic treatment. Acta Metall. Sin. (Engl. Lett.) 2017, 30, 957–964. [Google Scholar] [CrossRef]
- Choi, B.; Cho, I.S.; Jung, D.H.; Lee, M.G.; Jeon, Y. Friction and wear behavior of direct metal deposition on SUH3. Arch. Metall. Mater. 2019, 64, 841–844. [Google Scholar]
- Sourani, F.; Enayati, M.H.; Taghipour, M. High-temperature oxidation and wear behavior of (Fe,Cr)Al intermetallic compound and (Fe,Cr)Al-Al2O3 nanocomposites. J. Mater. Eng. Perform. 2021, 30, 3654–3669. [Google Scholar] [CrossRef]
- Song, Q.N.; Tong, Y.; Li, H.L.; Zhang, H.N.; Xu, N.; Zhang, G.Y.; Bao, Y.F.; Liu, W.; Liu, Z.G.; Qiao, Y.X. Corrosion and cavitation erosion resistance enhancement of cast Ni-Al bronze by laser surface melting. J. Iron Steel Res. Int. 2021. [Google Scholar] [CrossRef]
- Blau, P.J.; Brummett, T.M. High-temperature oxide regrowth on mechanically damaged surfaces. Tribol. Lett. 2008, 32, 153–157. [Google Scholar] [CrossRef]
- Jin, B.; Chen, G.Y.; Zhao, J.; He, Y.Y.; Huang, Y.Y.; Luo, J.B. Improvement of the lubrication properties of grease with Mn3O4/graphene (Mn3O4#G) nanocomposite additive. Friction 2021, 9, 1361–1377. [Google Scholar]
- Qu, J.; Blau, P.J.; Jolly, B.C. Tribological properties of stainless steels treated by colossal carbon supersaturation. Wear 2007, 263, 719–726. [Google Scholar] [CrossRef]
- Chandra-Ambhorn, S.; Chueaprakha, S.; Siripongsakul, T. High-temperature oxidation of the dissimilar weld between AISI 304L and Fe-15.6Cr-8.5Mn using Ar and Ar-4%N2 shielding gas. Anti-Corros. Method. Mater. 2019, 66, 210–214. [Google Scholar] [CrossRef]
- Li, L.L.; Wang, Z.B.; He, S.Y.; Zheng, Y.G. Correlation between depassivation and repassivation processes determined by single particle impingement: Its crucial role in the phenomenon of critical flow velocity for erosion-corrosion. J. Mater. Sci. Technol. 2021, 89, 158–166. [Google Scholar] [CrossRef]
- Heras, E.D.L.; Santamaría, D.G.; García-Luis, A.; Cabo, A.; Brizuela, M.; Ybarra, G.; Mingolo, N.; Brühl, S.; Corengia, P. Microstructure and wear behavior of DC-pulsed plasma nitrided AISI 316L austenitic stainless steel. Plasma Processe. Polym. 2007, 4, S741–S745. [Google Scholar] [CrossRef]
- Devaraju, A.; Perumal, A.E.; Alphonsa, J.; Kailas, S.V.; Venugopal, S. Sliding wear behavior of plasma nitrided austenitic stainless steel type AISI 316LN in the temperature range from 25 to 400 °C at 10−4 bar. Wear 2012, 288, 17–26. [Google Scholar] [CrossRef]
- Duarte, M.C.S.; Godoy, C.; Wilson, J.A.B. Analysis of sliding wear tests of plasma processed AISI 316L steel. Surf. Coat. Technol. 2014, 260, 316–325. [Google Scholar] [CrossRef]
- Wilson, S.; Alpas, A.T. Effect of temperature on the sliding wear performance of A1 alloys and A1 matrix composites. Wear 1996, 196, 270–278. [Google Scholar] [CrossRef]
- Pearson, S.R.; Shipway, P.H.; Abere, J.O.; Hewitt, R.A.A. The effect of temperature on wear and friction of a high strength steel in fretting. Wear 2013, 303, 622–631. [Google Scholar] [CrossRef]
- Mitchell, D.R.G.; Stott, F.H. The friction and wear of thin titanium nitride and silicon nitride coatings on stainless steel at temperatures to 500 °C. Surf. Coat. Technol. 1992, 50, 151–160. [Google Scholar] [CrossRef]

| C | Si | Mn | P | S | Cr | Mo | N | Fe |
|---|---|---|---|---|---|---|---|---|
| 0.044 | 0.24 | 15.80 | 0.017 | 0.005 | 18.40 | 2.19 | 0.66 | bal. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, S.; Zhou, H.; Wang, X.; Qiao, Y.; Yuan, H.; Chen, J.; Yang, L.; Wang, D.; Liu, Z.; Zou, J.; et al. Friction and Wear Behaviors of Fe-19Cr-15Mn-0.66N Steel at High Temperature. Coatings 2021, 11, 1285. https://doi.org/10.3390/coatings11111285
Sheng S, Zhou H, Wang X, Qiao Y, Yuan H, Chen J, Yang L, Wang D, Liu Z, Zou J, et al. Friction and Wear Behaviors of Fe-19Cr-15Mn-0.66N Steel at High Temperature. Coatings. 2021; 11(11):1285. https://doi.org/10.3390/coatings11111285
Chicago/Turabian StyleSheng, Shaolong, Huiling Zhou, Xiaojing Wang, Yanxin Qiao, Hongtao Yuan, Jian Chen, Lanlan Yang, Dongpeng Wang, Zhenguang Liu, Jiasheng Zou, and et al. 2021. "Friction and Wear Behaviors of Fe-19Cr-15Mn-0.66N Steel at High Temperature" Coatings 11, no. 11: 1285. https://doi.org/10.3390/coatings11111285






