DLC-Based Coatings Obtained by Low-Frequency Plasma-Enhanced Chemical Vapor Deposition (LFPECVD) in Cyclohexane, Principle and Examples
Abstract
:1. Introduction
2. Low-Frequency Plasma Enhanced Chemical Vapor Deposition Principle
2.1. Low Frequency and Radio-Frequency (RF) Discharges
2.2. Electrical Parameters Values
- The plasma potential Vp: near a surface, the plasma interacts with it. Because of their mass, the speed of electrons is much greater than that of ions. Thus, at the initial instant, the flow of electrons on a wall is much greater than that of ions. The plasma then acquires a concentration of positive residual charges due to the excess of positive ions; this is the plasma potential, Vp. It is therefore positive with respect to the walls and is often used as a reference.
- The floating potential Vf: it corresponds to the potential to which an isolated sample, immersed in a plasma, is fixed. It is due to the fact that this sample first charges negatively thanks to high mobility of electrons. The sample stabilizes at this potential Vf when the balance between electronic and ionic flux is achieved.
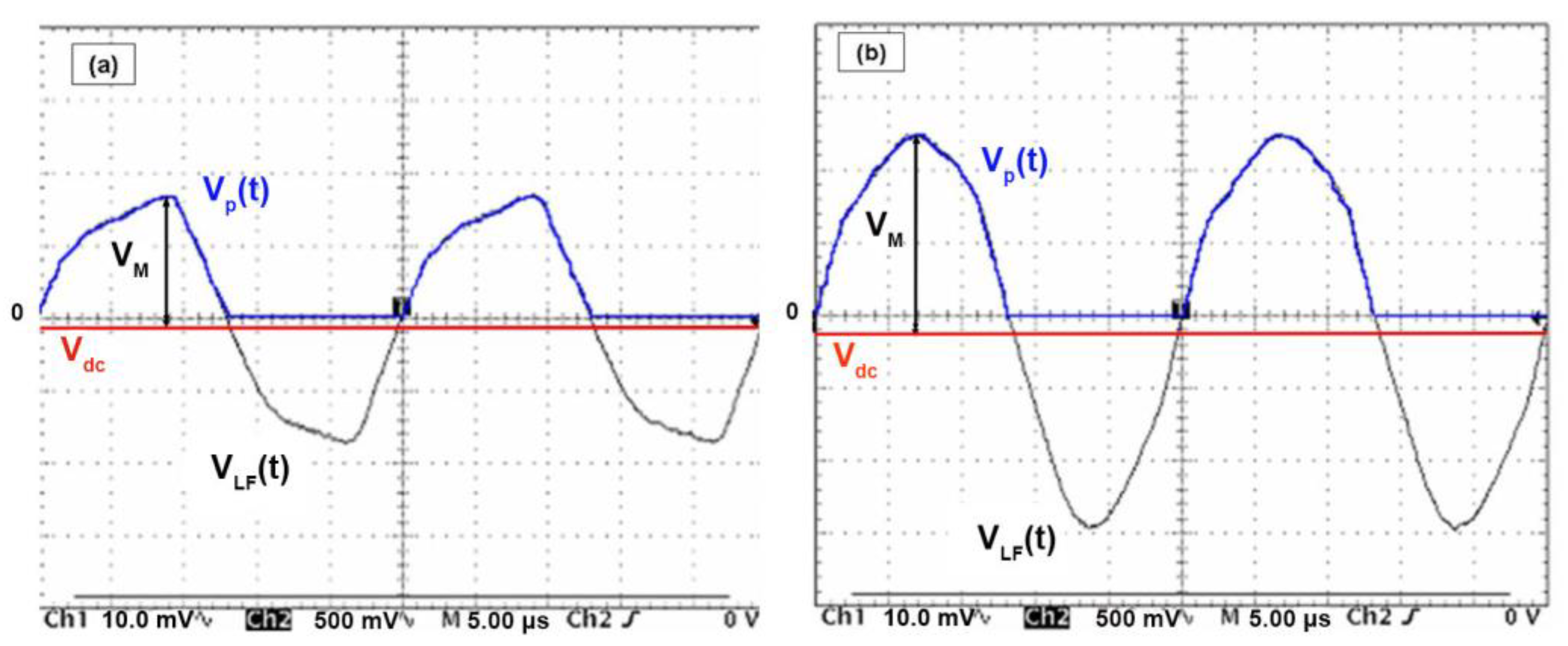
- The self-bias voltage, Vdc, appears on the active electrode with the introduction of a blocking capacitor between the generator and the cathode. During the first positive half-period of a cycle, electrons are attracted to the cathode, causing negative charges to build up due to the existence of the blocking capacity. An identical phenomenon is obtained, but in the opposite direction for the ions during the next negative half-period. However, due to the difference in mobilities of ions and electrons, this variation in the potential of the electrode is greater during positive alternation. Thus, after a few periods, a balance will be reached between the electronic and ionic currents and the voltage at the level of the cathode stabilizes at the continuous value Vdc. The value of this voltage depends in particular on the area ratio between the grounded electrode (or the reactor) and the active electrode. It is given by Turban [35] as follows:with, Aa: area of the anode; Ac: area of the cathode; : mean plasma potential; n: pressure-dependent factor. The influence of the ratio of the areas of the active electrode to that of the mass, on the shapes of the plasma potential , and of the excitation signal V(t) (Figure 1) for direct and capacitive coupling is discussed by [36].
2.3. Example of Deposition
3. Examples of DLC Based Films Obtained by LFPECVD
3.1. Characterization Methods
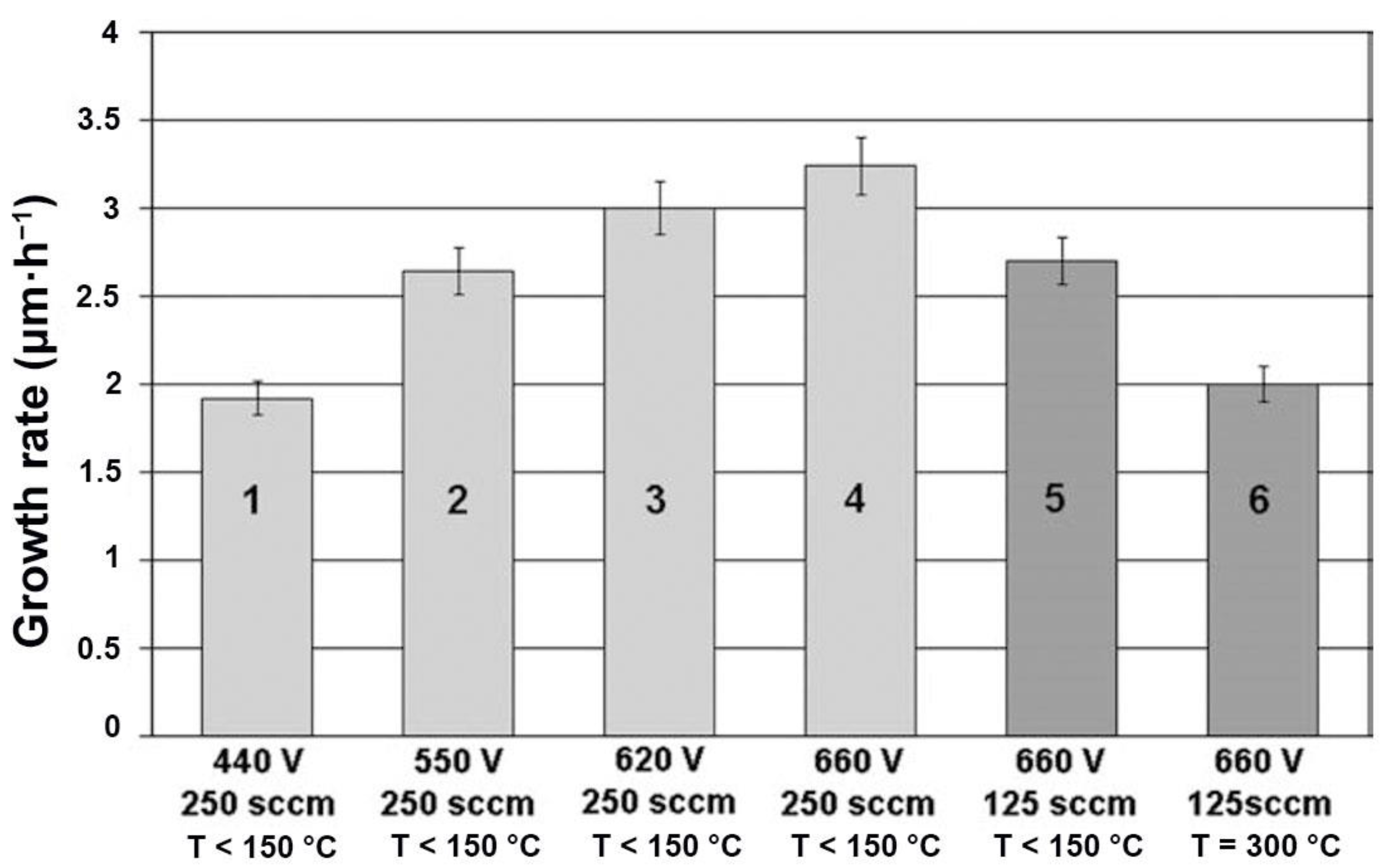
3.2. Deposition Rate
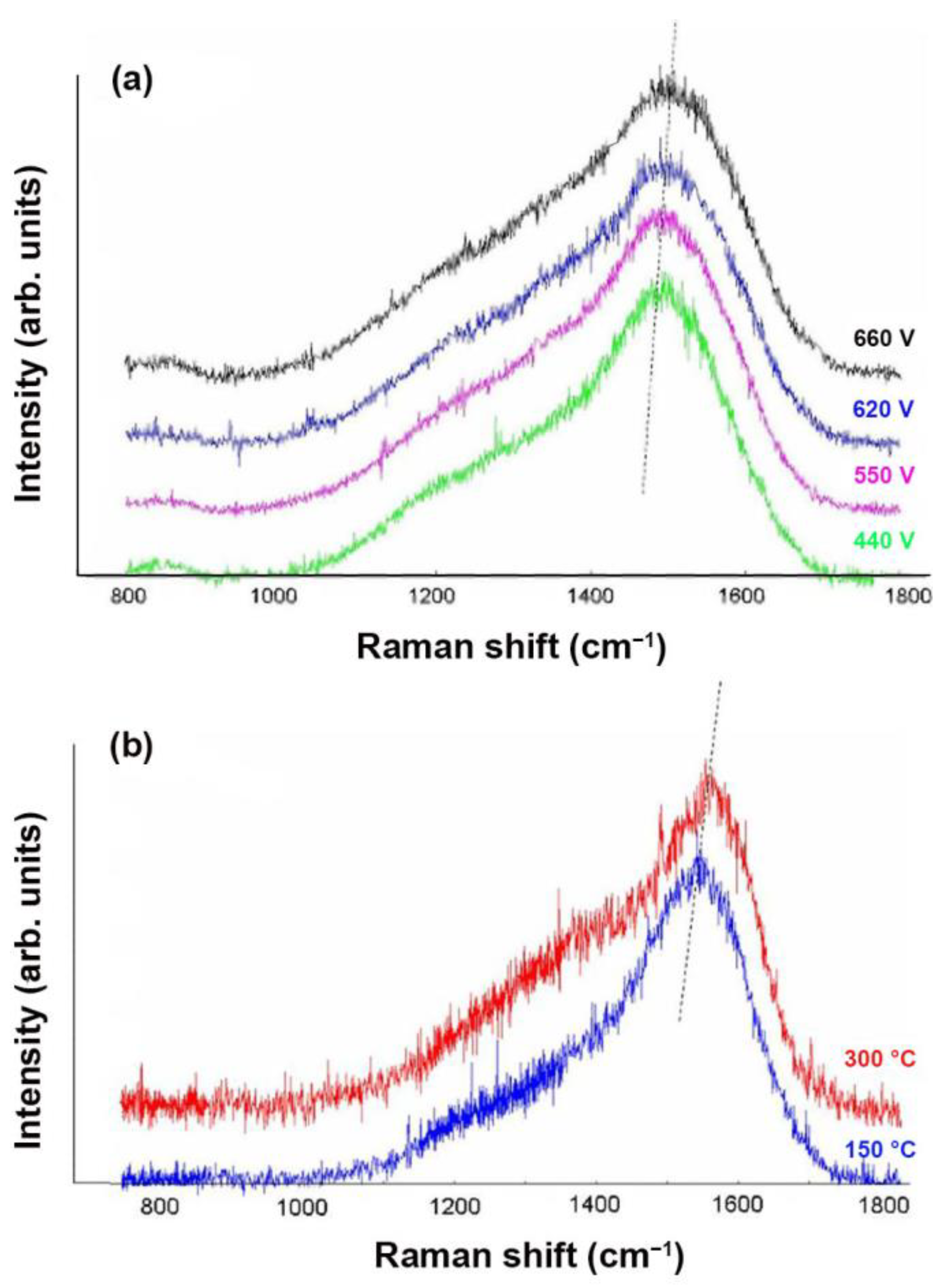
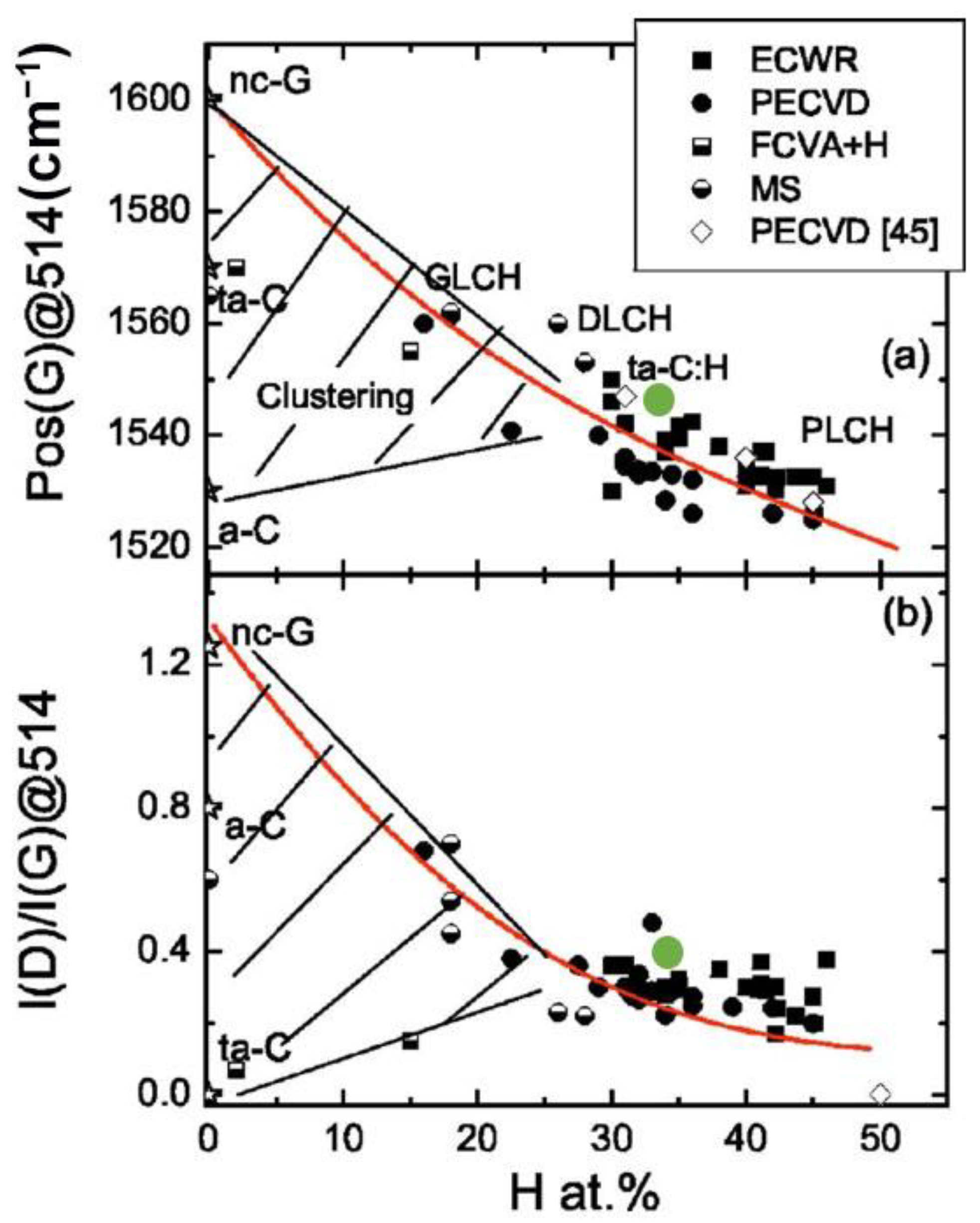
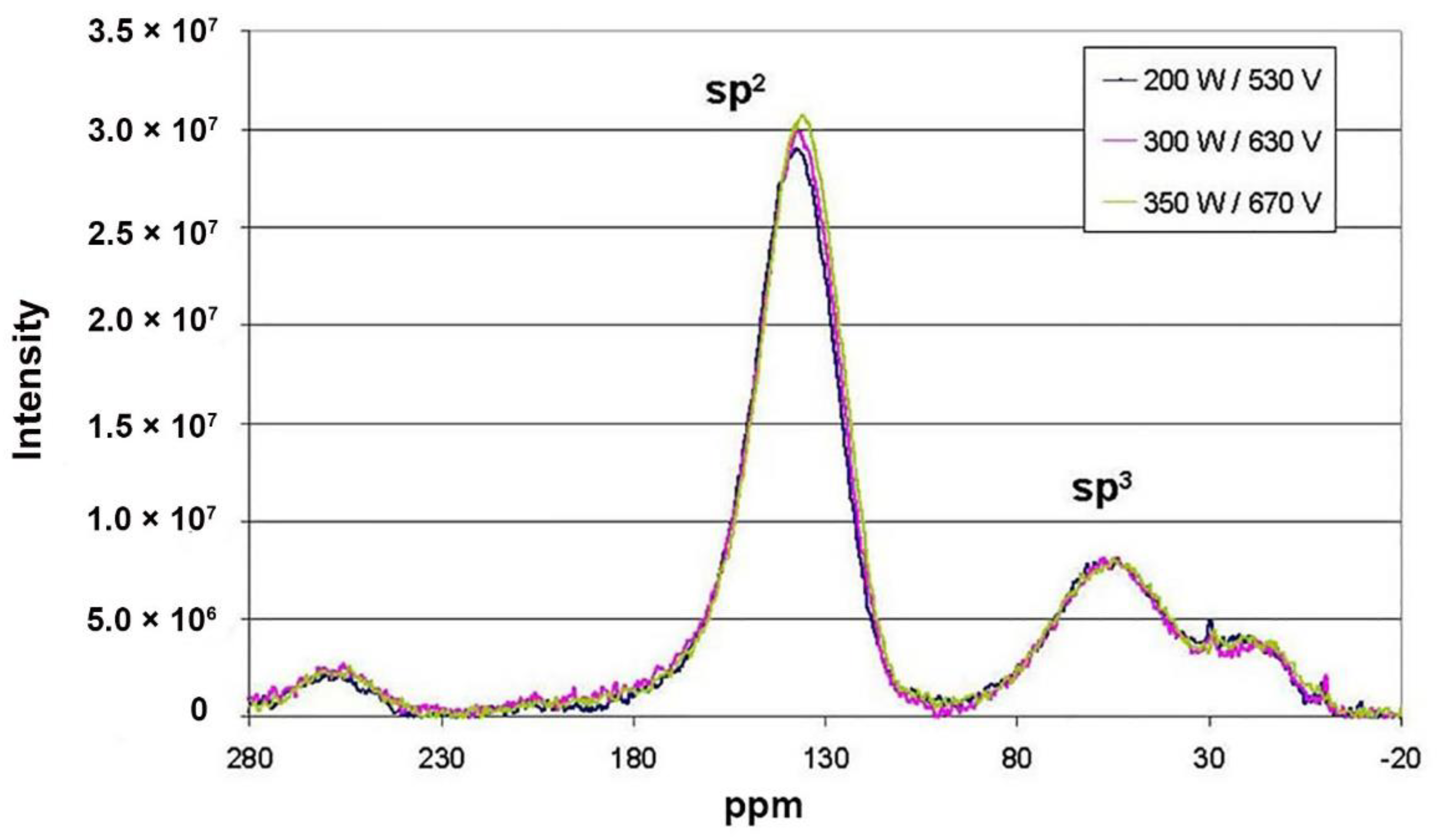
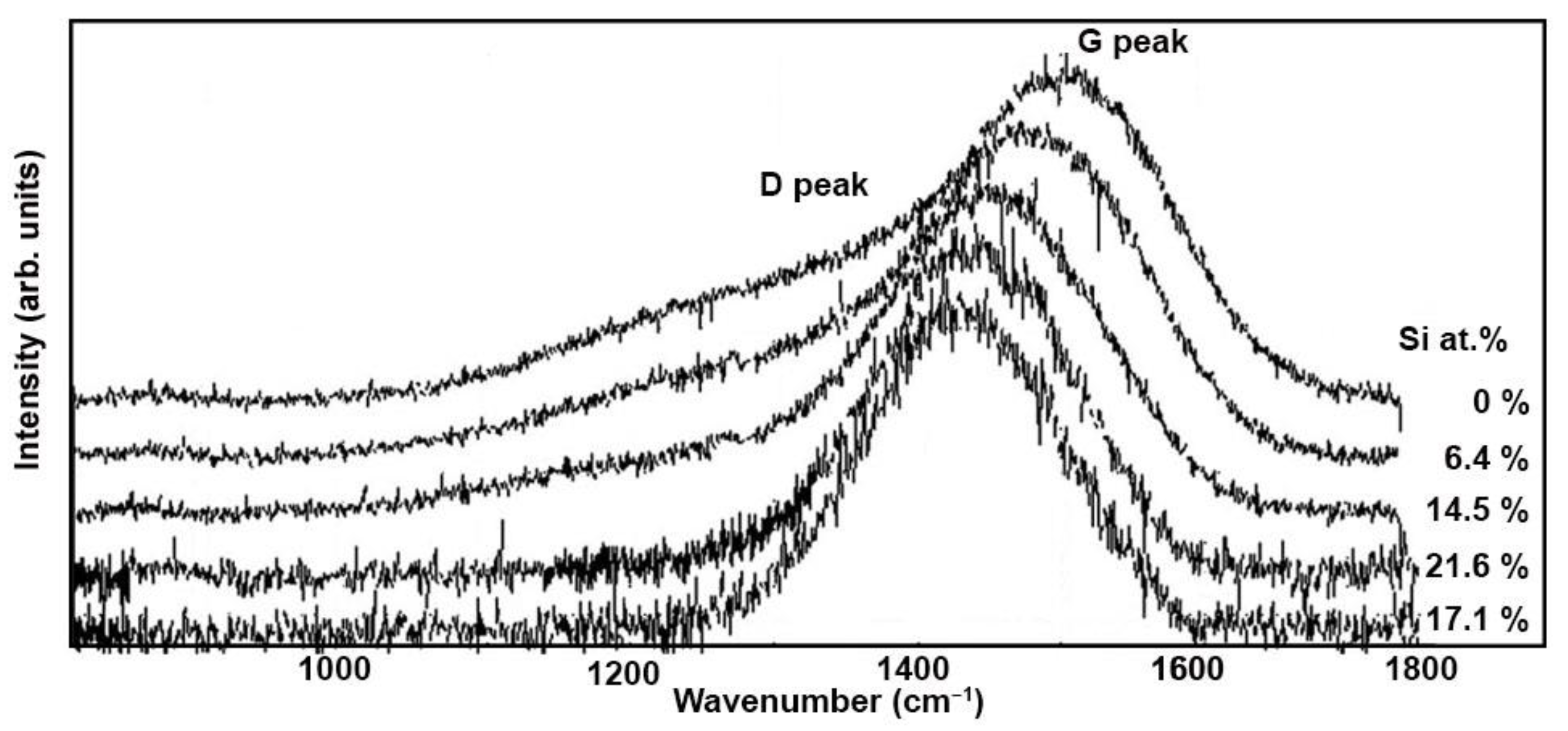
3.3. Structure of a-C:H Films
3.4. Mechanical Properties of a-C:H Films
3.5. Si Doped DLC
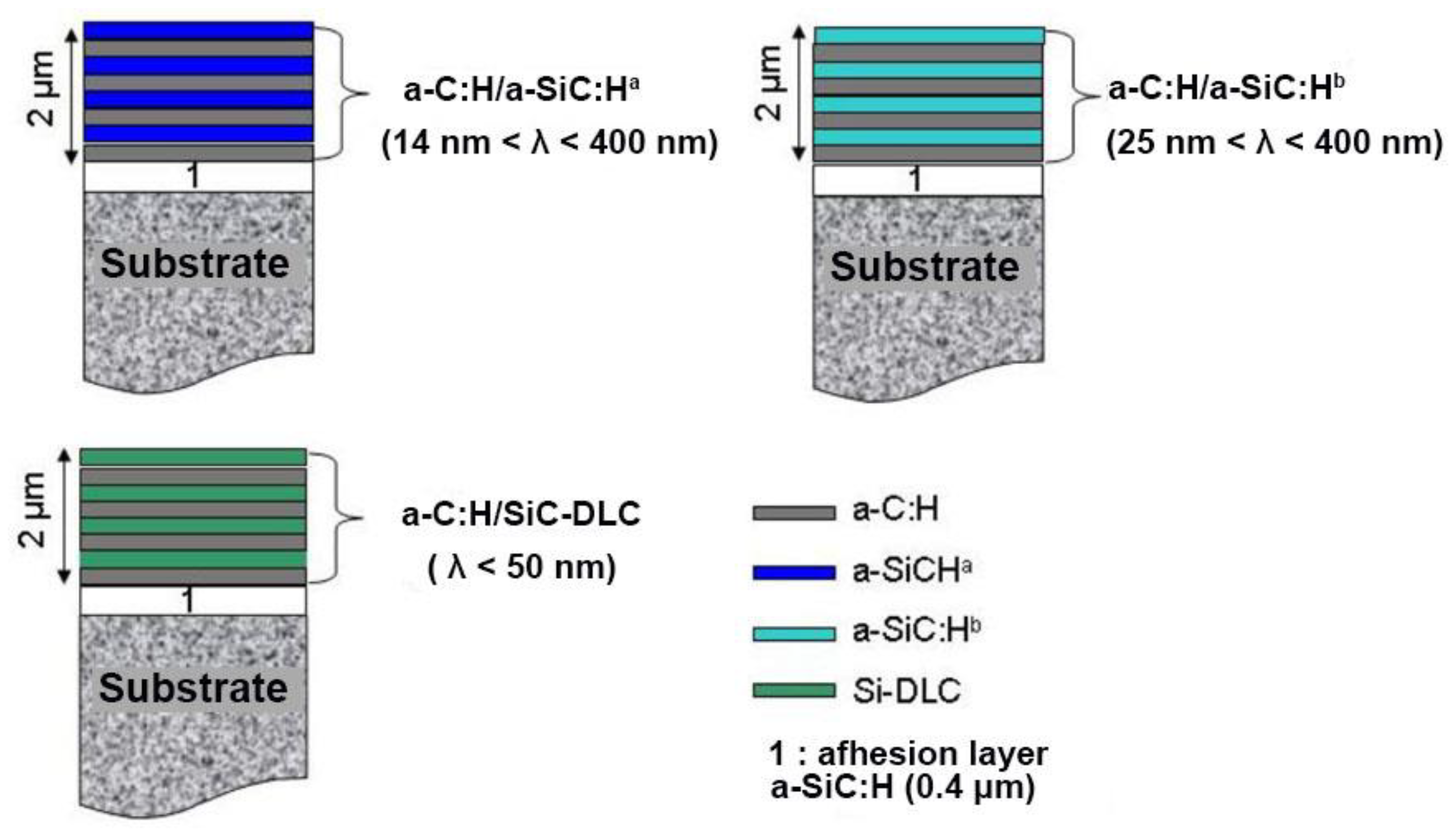
3.6. Multilayering Approach
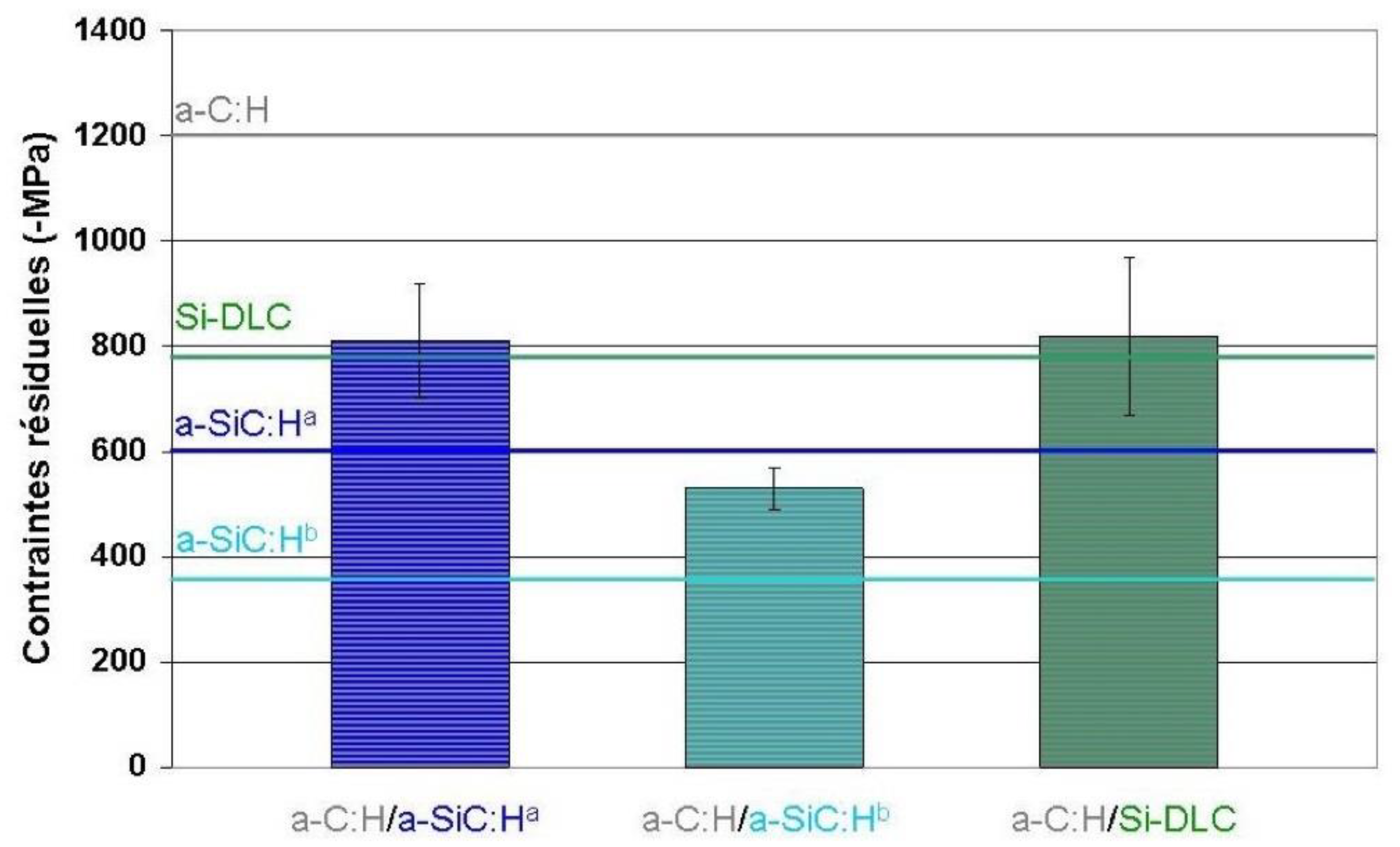
- an a-C:H/a-SiC:Ha system for which the two elementary layers have similar hardness and Young’s modulus but different residual stress values,
- an a-C:H/a-SiC:Hb system for which the two elementary layers have very different hardnesses, Young’s modulus and levels of residual stresses,
- an a-C:H/Si-DLC system for which the configuration is identical to that of the first stack, namely hardness and Young’s modulus similar for the two layers but with lower residual stress values for the Si-DLC layer. The difference arises, in this case, from the tribological properties of the Si-DLC layer (with R = 0.2), which are clearly superior to those of the two a-SiC:H layers.
3.6.1. Stress
3.6.2. Hardness and Young’s Modulus
- Whatever the nature of stacking a-C:H/a-SiC:Ha or a-C:H/a-SiC:Hb, evolutions of hardness and Young’s modulus as a function of the period are not significant. In particular, no increase in hardness could be observed even for the smallest period of 14 nm thick;
- For the two multilayer systems, hardness and Young’s modulus values were between those of the two monolayers constituting the stack and even appear very close to their average value. This appeared more clearly in the second case for which the properties of the two monolayers are very different. Each multilayer stack being composed of an identical proportion of a-C:H and a-SiC:H film, the obtained values therefore seem to respect the “law of mixtures”.
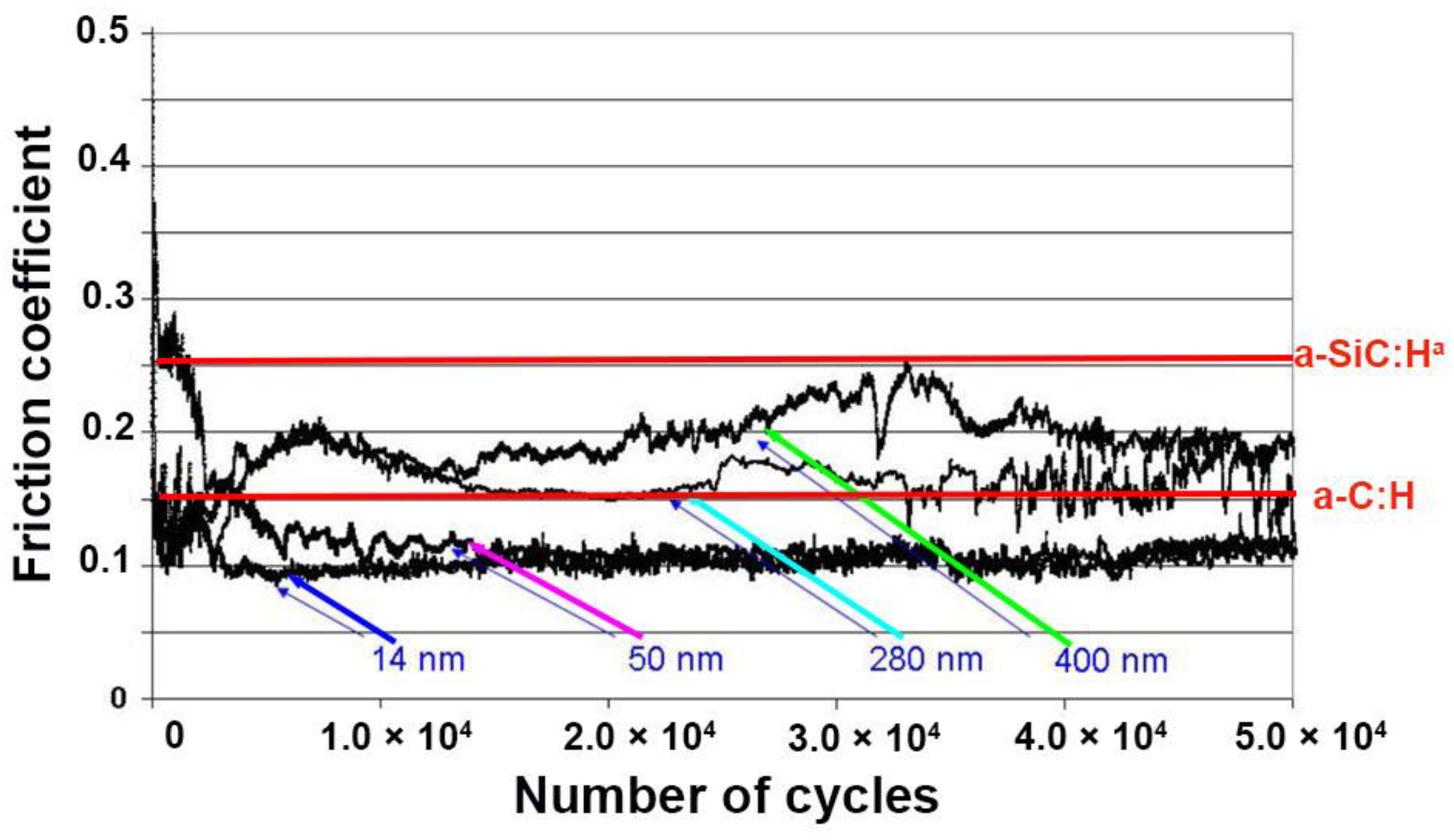
3.6.3. Friction Behavior-Influence of the Period in a-C:H/a-SiC:Ha Stack
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yee, M.J.; Mubarak, N.; Abdullah, E.; Khalid, M.; Walvekar, R.; Karri, R.R.; Nizamuddin, S.; Numan, A. Carbon nanomaterials źbased films for strain sensing application—A review. Nano-Struct. Nano-Objects 2019, 18, 100312. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Vo, D.-V.N.; Prakash, D.G.; Joseph, A.A.; Viswanathan, S.; Arun, J. Environmental applications of carbon-based materials: A review. Environ. Chem. Lett. 2021, 19, 557–582. [Google Scholar] [CrossRef]
- Rathanasamy, R.; Sahoo, S.; Lee, J.H.; Das, A.K.; Somasundaram, M.; Palaniappan, S.K.; Sivaraj, S. Carbon-based Multi-layered Films for Electronic Application: A Review. J. Electron. Mater. 2021, 50, 1845–1892. [Google Scholar] [CrossRef]
- Sivakumar, E.; Senthilkumar, P.; Sreenivasan, M.; Krishna, R. Experimental investigation of H-DLC coated exhaust valve characteristics of a diesel engine. Mater. Today-Proc. 2020, 33, 675–681. [Google Scholar] [CrossRef]
- Ferhati, H.; Djeffal, F.; Boubiche, N.; le Normand, F. An efficient ITO-free transparent electrode based on diamond-like carbon with an engineered intermediate metallic thin-film. Sol. Energy 2020, 196, 327–335. [Google Scholar] [CrossRef]
- Rao, X.; Yang, J.; Chen, Z.; Yuan, Y.; Chen, Q.; Feng, X.; Qin, L.; Zhang, Y. Tuning C–C sp2/sp3 ratio of DLC films in FCVA system for biomedical application. Bioact. Mater. 2020, 5, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Lengaigne, J.; Sharifi, N.; Pugh, M.; Moreau, C.; Dolatabadi, A.; Martinu, L.; Klemberg-Sapieha, J.E. Durability of superhydrophobic duplex coating systems for aerospace applications. Surf. Coat. Technol. 2020, 401, 126249. [Google Scholar] [CrossRef]
- Schmellenmeier, H. Experimentelle Technik der Physik 1; Deutscher Verlag der Wissenschaften: Berlin, Germany, 1953; p. 49. [Google Scholar]
- Aisenberg, S.; Chabot, R. Ion-beam deposition of thin films of diamondlike carbon. J. Appl. Phys. 1971, 42, 2953–2958. [Google Scholar] [CrossRef]
- Holland, L.; Ojha, S. Deposition of hard and insulating carbonaceous films on an rf target in a butane plasma. Thin Solid Film. 1976, 38, L17–L19. [Google Scholar] [CrossRef]
- King, F. Datapoint thin film media. IEEE Trans. Magn. 1981, 17, 1376–1379. [Google Scholar] [CrossRef]
- Kano, M. DLC coating technology applied to sliding parts of automotive engine. New Diam. Front. Carbon Technol. 2006, 16, 201–210. [Google Scholar]
- Maurin-perrier, P.; Heau, C.; Riand, R.; Engelric, B. Nouvelles voies tribologiques pour l’industrie mécanique. Traitement Therm. 2005, 364, 41–44. [Google Scholar]
- Badiger, P.V.; Desai, V.; Ramesh, M. Performance of DLC coated tool during machining of MDN431 alloyed steel. Mater. Today-Proc. 2018, 5, 17360–17370. [Google Scholar] [CrossRef]
- Huang, L.; Yuan, J.; Li, C.; Hong, D. Microstructure, tribological and cutting performance of Ti-DLC/α-C: H multilayer film on cemented carbide. Surf. Coat. Technol. 2018, 353, 163–170. [Google Scholar] [CrossRef]
- Koszela, W.; Pawlus, P.; Reizer, R.; Liskiewicz, T. The combined effect of surface texturing and DLC coating on the functional properties of internal combustion engines. Tribol. Int. 2018, 127, 470–477. [Google Scholar] [CrossRef]
- Tinchev, S.; Nikolova, P.; Dyulgerska, J.; Danev, G.; Babeva, T. aC: H absorber layer for solar cells matched to solar spectrum. Ol. Energy Mater. Sol. Cells 2005, 86, 421–426. [Google Scholar] [CrossRef]
- Grill, A. Diamond-like carbon coatings as biocompatible materials—An overview. Diam. Relat. Mater. 2003, 12, 166–170. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.-Q.; Lin, S.-S.; Gao, D.; Shi, Q.; Wei, C.-B.; Dai, M.-J.; Su, Y.-F.; Xu, W.; Tang, P.; Li, H. Modulation of Si on microstructure and tribo-mechanical properties of hydrogen-free DLC films prepared by magnetron sputtering. Appl. Surf. Sci. 2020, 509, 145381. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Wei, B.; Wen, F.; Cao, H.; Pei, Y.T. Effect of sputtering pressure on the surface topography, structure, wettability and tribological performance of DLC films coated on rubber by magnetron sputtering. Surf. Coat. Technol. 2019, 365, 33–40. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Y.; Ma, X.; Zhang, D.; Wang, L.; Li, X. Tribocorrosion behaviors of multilayer PVD DLC coated 304L stainless steel in seawater. Diam. Relat. Mater. 2017, 79, 70–78. [Google Scholar] [CrossRef]
- Santiago, J.; Fernández-Martínez, I.; Kozák, T.; Capek, J.A.; Wennberg, J.; Molina-Aldareguia, V.; Bellido-González, R.; González-Arrabal, M.; Monclús, M.A. The influence of positive pulses on HiPIMS deposition of hard DLC coatings. Surf. Coat. Technol. 2019, 358, 43–49. [Google Scholar] [CrossRef]
- Santiago, J.A.; Fernández-Martínez, I.; Sánchez-López, J.C.; Rojas, T.C.; Wennberg, A.; Bellido-González, V.; Molina-Aldareguia, J.M.; Monclús, M.A.; González-Arrabal, R. Tribomechanical properties of hard Cr-doped DLC coatings deposited by low-frequency HiPIMS. Surf. Coat. Technol. 2020, 382, 124899. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Wang, X. Structure characterization and antibacterial properties of Ag-DLC films fabricated by dual-targets HiPIMS. Surf. Coat. Technol. 2021, 410, 126967. [Google Scholar] [CrossRef]
- Bouabibsa, I.; Lamri, S.; Alhussein, A.; Minea, T.; Sanchette, F. Plasma investigations and deposition of Me-DLC (Me= Al, Ti or Nb) obtained by a magnetron sputtering-RFPECVD hybrid process. Surf. Coat. Technol. 2018, 354, 351–359. [Google Scholar] [CrossRef]
- Bouabibsa, I.; Lamri, S.; Sanchette, F. Structure, mechanical and tribological properties of Me-doped diamond-like carbon (DLC)(Me= Al, Ti, or Nb) hydrogenated amorphous carbon coatings. Coatings 2018, 8, 370. [Google Scholar] [CrossRef] [Green Version]
- Capote, G.; Mastrapa, G.; Trava-Airoldi, V. Influence of acetylene precursor diluted with argon on the microstructure and the mechanical and tribological properties of aC: H films deposited via the modified pulsed-DC PECVD method. Surf. Coat. Technol. 2015, 284, 145–152. [Google Scholar] [CrossRef]
- Capote, G.; Ramírez, M.; da Silva, P.; Lugo, D.; Trava-Airoldi, V. Improvement of the properties and the adherence of DLC coatings deposited using a modified pulsed-DC PECVD technique and an additional cathode. Surf. Coat. Technol. 2016, 308, 70–79. [Google Scholar] [CrossRef]
- Chang, S.-H.; Tang, T.-C.; Huang, K.-T.; Liu, C.-M. Investigation of the characteristics of DLC films on oxynitriding-treated ASP23 high speed steel by DC-pulsed PECVD process. Surf. Coat. Technol. 2015, 261, 331–336. [Google Scholar] [CrossRef]
- Chouquet, C.; Gavillet, J.; Ducros, C.; Sanchette, F. Effect of DLC surface texturing on friction and wear during lubricated sliding. Mater. Chem. Phys. 2010, 123, 367–371. [Google Scholar] [CrossRef]
- Chouquet, C.; Ducros, C.; Barrat, S.; Billard, A.; Sanchette, F. Mechanical properties of aC: H/Si-containing aC: H multilayered coatings grown by LF-PECVD. Surf. Coat. Technol. 2008, 203, 745–749. [Google Scholar] [CrossRef]
- Chouquet, C.; Gerbaud, G.; Bardet, M.; Barrat, S.; Billard, A.; Sanchette, F.; Ducros, C. Structural and mechanical properties of aC: H and Si doped aC: H thin films grown by LF-PECVD. Surf. Coat. Technol. 2010, 204, 1339–1346. [Google Scholar] [CrossRef]
- Lugo, D.; Silva, P.; Ramirez, M.; Pillaca, E.; Rodrigues, C.; Fukumasu, N.; Corat, E.; Tabacniks, M.; Trava-Airoldi, V. Characterization and tribologic study in high vacuum of hydrogenated DLC films deposited using pulsed DC PECVD system for space applications. Surf. Coat. Technol. 2017, 332, 135–141. [Google Scholar] [CrossRef]
- Turban, G. Les décharges luminescentes du type diode excites en radiofréquence. Modèles élémentaires et énergie d’impact des ions positifs, Interactions plasmas froids matériaux, Studies day Oléron 87. Unpublished work.
- Köhler, K.; Horne, D.; Coburn, J. Frequency dependence of ion bombardment of grounded surfaces in rf argon glow discharges in a planar system. J. Appl. Phys. 1985, 58, 3350–3355. [Google Scholar] [CrossRef]
- Stoney, G.G. The tension of metallic films deposited by electrolysis. Proc. R. Soc. Lond. A 1909, 82, 172–175. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Kersten, H.; Kroesen, G. On the temperature dependence of the deposition rate of amorphous, hydrogenated carbon films. J. Vac. Sci. Technol. A Vac. Surf. Film. 1990, 8, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Beeman, D.; Silverman, J.; Lynds, R.; Anderson, M.J.P.R.B. Modeling studies of amorphous carbon. J. Phys. Rev. B 1984, 30, 870. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. J. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef] [Green Version]
- Sanchette, F.; El Garah, M.; Achache, S.; Schuster, F.; Chouquet, C.; Ducros, C.; Billard, A. University of Technology of Troyes, Troyes, France. Unpuplished work. 2021. [Google Scholar]
- Ferrari, A.C. Non-destructive characterisation of carbon films. In Tribology of Diamond-Like Carbon Films; Donnet, C., Erdemir, A., Eds.; Springer: Boston, MA, USA, 2008; pp. 25–82. [Google Scholar]
- Lejeune, M.; Benlahsen, M.; Bouzerar, R. Stress and structural relaxation in amorphous hydrogenated carbon films. Appl. Phys. Let. 2004, 84, 344–346. [Google Scholar] [CrossRef]
- Casiraghi, C.; Ferrari, A.; Robertson, J. Raman spectroscopy of hydrogenated amorphous carbons. J. Phys. Rev. B 2005, 72, 085401. [Google Scholar] [CrossRef] [Green Version]
- Tomasella, E.; Thomas, L.; Meunier, C.; Nadal, M.; Mikhailov, S. Coupled effects of bombarding ions energy on the microstructure and stress level of RFPECVD aC: H films: Correlation with Raman spectroscopy. Surf. Coat. Technol. 2003, 174, 360–364. [Google Scholar] [CrossRef]
- Butt, M.; Khaleeq-ur-Rahman, M.; Ali, D.; Akmal, A.; Naseem, S. Deposition and characterization of multilayer DLC: Mo thin films grown on silicon substrate by off-axis pulsed laser deposition technique. Appl. Surf. Sci. 2015, 331, 407–414. [Google Scholar] [CrossRef]
- Cooper, C.V.; Wang, R.; Yoon, H.K.; Taher, M.A. The microstructure and wear behavior of Cr-and W-DLC coatings sputter-deposited onto AISI 52100 substrates as elucidated using focused-ion-beam SEM. MRS Online Proc. Libr. 2021, 697, 36. [Google Scholar] [CrossRef]
- Mutafov, P.; Lanigan, J.; Neville, A.; Cavaleiro, A.; Polcar, T. DLC-W coatings tested in combustion engine—Frictional and wear analysis. Surf. Coat. Technol. 2014, 260, 284–289. [Google Scholar] [CrossRef]
- Dimigen, H.; Hübsch, H.; Memming, R. Tribological and electrical properties of metal-containing hydrogenated carbon films. Appl. Phys. Let. 1987, 50, 1056–1058. [Google Scholar] [CrossRef]
- Donnet, C. Recent progress on the tribology of doped diamond-like and carbon alloy coatings: A review. Surf. Coat. Technol. 1998, 100, 180–186. [Google Scholar] [CrossRef]
- Lee, K.-R.; Eun, K.Y.; Kim, K.-M.; Choi, K.-C. Application of diamond-like carbon films for anti-abrasion and low friction properties of VCR head drums. Surf. Coat. Technol. 1995, 76, 786–790. [Google Scholar] [CrossRef]
- Roth, D.; Rau, B.; Roth, S.; Mai, J.; Dittrich, K.-H. Large area and three-dimensional deposition of diamond-like carbon films for industrial applications. Surf. Coat. Technol. 1995, 74, 637–641. [Google Scholar] [CrossRef]
- Voevodin, A.; Prasad, S.; Zabinski, J. Nanocrystalline carbide/amorphous carbon composites. J. Appl. Phys. 1997, 82, 855–858. [Google Scholar] [CrossRef]
- Cui, J.; Qiang, L.; Zhang, B.; Ling, X.; Yang, T.; Zhang, J. Mechanical and tribological properties of Ti-DLC films with different Ti content by magnetron sputtering technique. Appl. Surf. Sci. 2012, 258, 5025–5030. [Google Scholar] [CrossRef]
- Jo, Y.J.; Zhang, T.F.; Son, M.J.; Kim, K.H. Synthesis and electrochemical properties of Ti-doped DLC films by a hybrid PVD/PECVD process. Appl. Surf. Sci. 2018, 433, 1184–1191. [Google Scholar] [CrossRef]
- Ma, G.; Gong, S.; Lin, G.; Zhang, L.; Sun, G. A study of structure and properties of Ti-doped DLC film by reactive magnetron sputtering with ion implantation. Appl. Surf. Sci. 2012, 258, 3045–3050. [Google Scholar] [CrossRef]
- Qiang, L.; Gao, K.; Zhang, L.; Wang, J.; Zhang, B.; Zhang, J. Further improving the mechanical and tribological properties of low content Ti-doped DLC film by W incorporating. Appl. Surf. Sci. 2015, 353, 522–529. [Google Scholar] [CrossRef]
- Yang, W.J.; Sekino, T.; Shim, K.B.; Niihara, K.; Auh, K.H. Deposition and microstructure of Ti-containing diamond-like carbon nanocomposite films. Thin Solid Films. 2005, 473, 252–258. [Google Scholar] [CrossRef]
- Amanov, A.; Watabe, T.; Tsuboi, R.; Sasaki, S. Fretting wear and fracture behaviors of Cr-doped and non-doped DLC films deposited on Ti–6al–4V alloy by unbalanced magnetron sputtering. Tribol. Int. 2013, 62, 49–57. [Google Scholar] [CrossRef]
- Dai, W.; Ke, P.; Wang, A. Microstructure and property evolution of Cr-DLC films with different Cr content deposited by a hybrid beam technique. Vacuum 2011, 85, 792–797. [Google Scholar] [CrossRef]
- Dai, W.; Zheng, H.; Wu, G.; Wang, A. Effect of bias voltage on growth property of Cr-DLC film prepared by linear ion beam deposition technique. CVacuum 2010, 85, 231–235. [Google Scholar] [CrossRef]
- Baba, K.; Hatada, R.; Tanaka, Y. Preparation and properties of W-containing diamond-like carbon films by magnetron plasma source ion implantation. Surf. Coat. Technol. 2007, 201, 8362–8365. [Google Scholar] [CrossRef]
- Mercer, C.; Evans, A.; Yao, N.; Allameh, S.; Cooper, C. Material removal on lubricated steel gears with W-DLC-coated surfaces. Surf. Coat. Technol. 2003, 173, 122–129. [Google Scholar] [CrossRef]
- Dai, W.; Ke, P.; Wang, A. Influence of bias voltage on microstructure and properties of Al-containing diamond-like carbon films deposited by a hybrid ion beam system. Surf. Coat. Technol. 2013, 229, 217–221. [Google Scholar] [CrossRef]
- Dai, W.; Wang, A. Deposition and properties of Al-containing diamond-like carbon films by a hybrid ion beam sources. J. Alloys Compd. 2011, 509, 4626–4631. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, L.; Xue, Q. The structure and tribological properties of aluminum/carbon nanocomposite thin films synthesized by reactive magnetron sputtering. Surf. Interface Anal. 2011, 43, 1057–1063. [Google Scholar] [CrossRef]
- Dai, W.; Wang, A.; Wang, Q. Microstructure and mechanical property of diamond-like carbon films with ductile copper incorporation. Surf. Coat. Technol. 2015, 272, 33–38. [Google Scholar] [CrossRef]
- Meškinis, Š.; Gudaitis, R.; Vasiliauskas, A.; Čiegis, A.; Šlapikas, K.; Tamulevičius, T.; Andrulevičius, M.; Tamulevičius, S. Piezoresistive properties of diamond like carbon films containing copper. Diam. Relat. Mater. 2015, 60, 20–25. [Google Scholar] [CrossRef]
- Yi, J.W.; Lee, Y.H.; Farouk, B. Effects of gas composition and rf power on properties of aC: H/SiC: H composite films grown by plasma-enhanced chemical vapor deposition. Thin Solid Film. 1998, 326, 154–159. [Google Scholar] [CrossRef]
- Bendavid, A.; Martin, P.; Comte, C.; Preston, E.; Haq, A.; Ismail, F.M.; Singh, R. The mechanical and biocompatibility properties of DLC-Si films prepared by pulsed DC plasma activated chemical vapor deposition. Diam. Relat. Mater. 2007, 16, 1616–1622. [Google Scholar] [CrossRef]
- Papakonstantinou, P.; Zhao, J.; Lemoine, P.; McAdams, E.; McLaughlin, J. The effects of Si incorporation on the electrochemical and nanomechanical properties of DLC thin films. Diam. Relat. Mater. 2002, 11, 1074–1080. [Google Scholar] [CrossRef]
- Ducros, C.; Sanchette, F. Multilayered and nanolayered hard nitride thin films deposited by cathodic arc evaporation. Part 2: Mechanical properties and cutting performances. Surf. Coat. Technol. 2006, 201, 1045–1052. [Google Scholar] [CrossRef]
- Martínez, E.; Romero, J.; Lousa, A.; Esteve, J. Mechanical strengthening in nanometric CrN/Cr multilayers measured by nanoindentation. J. Phys. D Appl. Phys. 2002, 35, 1880. [Google Scholar] [CrossRef]
- Logothetidis, S.; Kassavetis, S.; Charitidis, C.; Panayiotatos, Y.; Laskarakis, A. Nanoindentation studies of multilayer amorphous carbon films. Carbon 2004, 42, 1133–1136. [Google Scholar] [CrossRef]

| Sample | Raman | ERDA | |||
|---|---|---|---|---|---|
| λ = 514 nm | λ = 633 nm | Total H Content (±2 at.%) | |||
| G Position (cm−1) | ID/IG | G Position (cm−1) | ID/IG | ||
| 1 | 1542 | 0.41 | 1507 | 0.60 | 33 |
| 2 | 1543 | 0.36 | 1511 | 0.62 | - |
| 3 | 1564 | 0.42 | 1519 | 0.64 | - |
| 4 | - | - | 1521 | 0.70 | 33 |
| 5 | 1547 | 0.44 | - | - | 25 |
| 6 | 1567 | 0.73 | - | - | 26 |
| Deposition Conditions | Raman Parameters | |||||
|---|---|---|---|---|---|---|
| Total Flow Rate (sccm) | Bias Voltage (V) | Ts (°C) | λ = 514 nm | λ = 633 nm | ||
| G Band Position (cm−1) | ID/IG | G Band Position (cm−1) | ID/IG | |||
| 250 | 440 | 120 | 1542 | 0.41 | 1507 | 0.6 |
| 250 | 550 | 130 | 1543 | 0.36 | 1511 | 0.62 |
| 250 | 620 | 140 | 1546 | 0.42 | 1519 | 0.64 |
| 250 | 660 | 150 | - | - | 1521 | 0.7 |
| 125 | 660 | 150 | 1547 | 0.44 | - | - |
| 125 | 660 | 300 | 1567 | 0.73 | - | - |
| Pressure (Pa) | Total Flow Rate (sccm) | C6H12/(C6H12+H2) | Bias Voltage (V) | Ts (°C) | Hardness (GPa) | Young Modulus (GPa) |
|---|---|---|---|---|---|---|
| 4 | 125 | 0.8 | 660 | 150 | 21 ± 2 | 160 ± 9 |
| 300 | 26 ± 2 | 190 ± 10 |
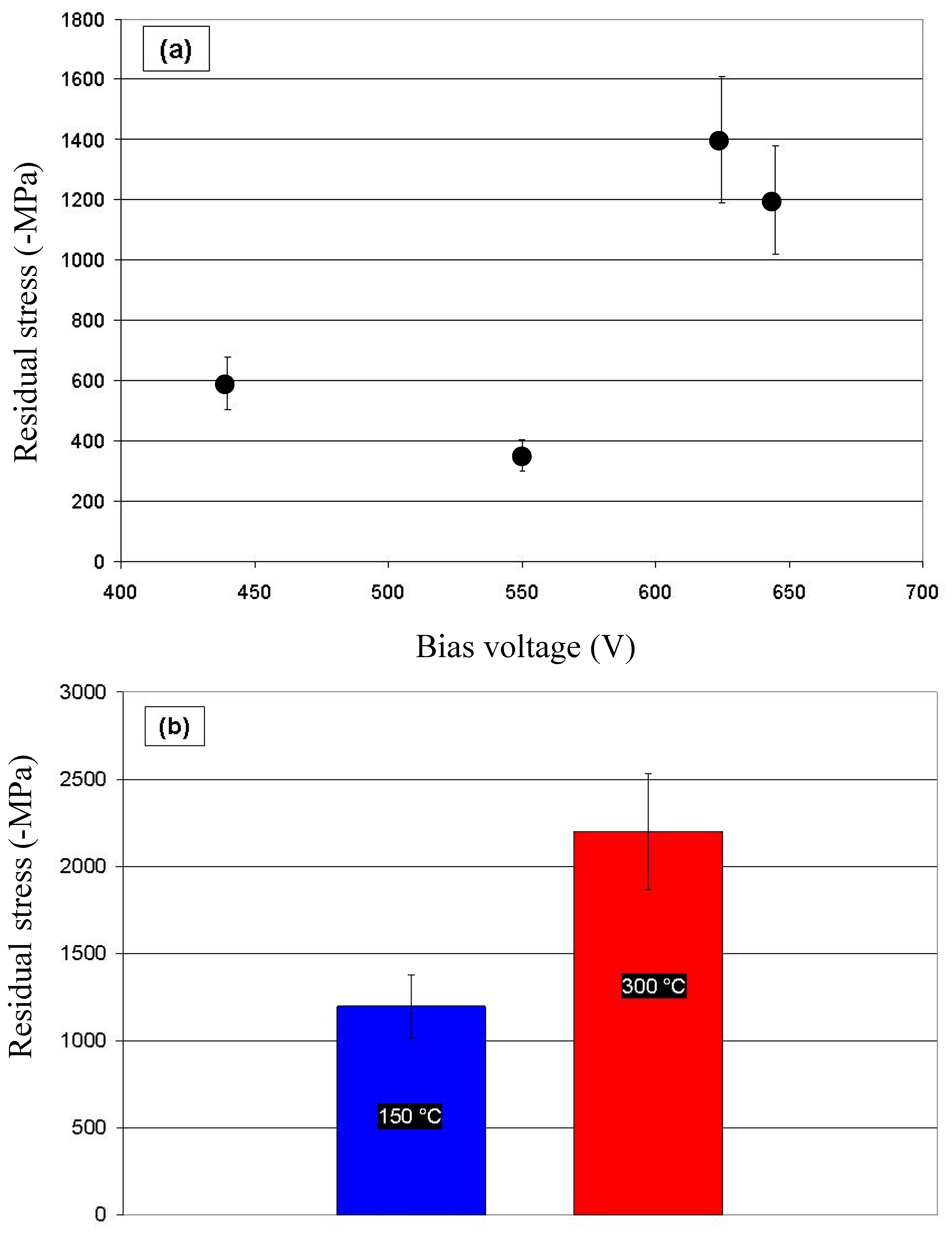
| R | Composition (at.%) | Residual Stress (-MPa) | Hardness (GPa) | Young’s Modulus (GPa) | |||
|---|---|---|---|---|---|---|---|
| C | O | N | Si | ||||
| 0 | 89.3 | 9.8 | 0.9 | 0 | 1200 ± 150 | 21.5 ± 2 | 160 ± 8 |
| 0.2 | 81.6 | 11.5 | 0.6 | 6.4 | 780 ± 70 | 19.5 ± 2 | 142 ± 9 |
| 0.5 | 73.1 | 11.8 | 0.6 | 14.5 | 450 ± 90 | 17.5 ± 1.5 | 141 ± 9 |
| 0.8 | 65.0 | 12.8 | 0.6 | 21.6 | 350 ± 50 | 18.5 ± 2 | 143 ± 11 |
| 1 | 68.6 | 14.1 | 0.2 | 17.1 | 400 ± 40 | 18.5 ± 1 | 144 ± 6 |
| a-C:H | a-SiC:Ha | a-SiC:Hb | Si-DLC | |
|---|---|---|---|---|
| Gas mixture (% of Each Gas) | C6H12 (80) H2 (20) | TMS (50) Ar (50) | TMS (100) | TMS (20) C6H12 (70) H2 (10) |
| Bias voltage (V) | 660 | 660 | 400 | 660 |
| Hardness (GPa) | 21 ± 2 | 20 ± 1.5 | 7 ± 1 | 20 ± 2 |
| Young’s modulus (GPa) | 160 ± 9 | 165 ± 8 | 53 ± 4 | 142 ± 9 |
| Residual stress (MPa) | −1200 | −600 | −360 | −780 |
| Monolayer Films (2 µm) | Multilayer Films (2 µm) Period 50 nm | ||||||
|---|---|---|---|---|---|---|---|
| a-C:H | a-SiC:Ha | a-SiC:Hb | Si-DLC (R = 0.2) | a-C:H/ a-SiC:Ha | a-C:H/ a-SiC:Hb | a-C:H/ Si-DLC | |
| Hardness (GPa) | 21 ± 2 | 20 ± 1.5 | 7 ± 1 | 20 ± 2 | 20 ± 2 | 15 ± 1 | 21 ± 2 |
| Young’s modulus (GPa) | 160 ± 9 | 165 ± 8 | 53 ± 4 | 142 ± 9 | 158 ± 8 | 116 ± 5 | 150 ± 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchette, F.; El Garah, M.; Achache, S.; Schuster, F.; Chouquet, C.; Ducros, C.; Billard, A. DLC-Based Coatings Obtained by Low-Frequency Plasma-Enhanced Chemical Vapor Deposition (LFPECVD) in Cyclohexane, Principle and Examples. Coatings 2021, 11, 1225. https://doi.org/10.3390/coatings11101225
Sanchette F, El Garah M, Achache S, Schuster F, Chouquet C, Ducros C, Billard A. DLC-Based Coatings Obtained by Low-Frequency Plasma-Enhanced Chemical Vapor Deposition (LFPECVD) in Cyclohexane, Principle and Examples. Coatings. 2021; 11(10):1225. https://doi.org/10.3390/coatings11101225
Chicago/Turabian StyleSanchette, Frederic, Mohamed El Garah, Sofiane Achache, Frederic Schuster, Caroline Chouquet, Cédric Ducros, and Alain Billard. 2021. "DLC-Based Coatings Obtained by Low-Frequency Plasma-Enhanced Chemical Vapor Deposition (LFPECVD) in Cyclohexane, Principle and Examples" Coatings 11, no. 10: 1225. https://doi.org/10.3390/coatings11101225
APA StyleSanchette, F., El Garah, M., Achache, S., Schuster, F., Chouquet, C., Ducros, C., & Billard, A. (2021). DLC-Based Coatings Obtained by Low-Frequency Plasma-Enhanced Chemical Vapor Deposition (LFPECVD) in Cyclohexane, Principle and Examples. Coatings, 11(10), 1225. https://doi.org/10.3390/coatings11101225






