Design and Preparation of Nanoporous Cu/Ag Multilayer Films
Abstract
:1. Introduction
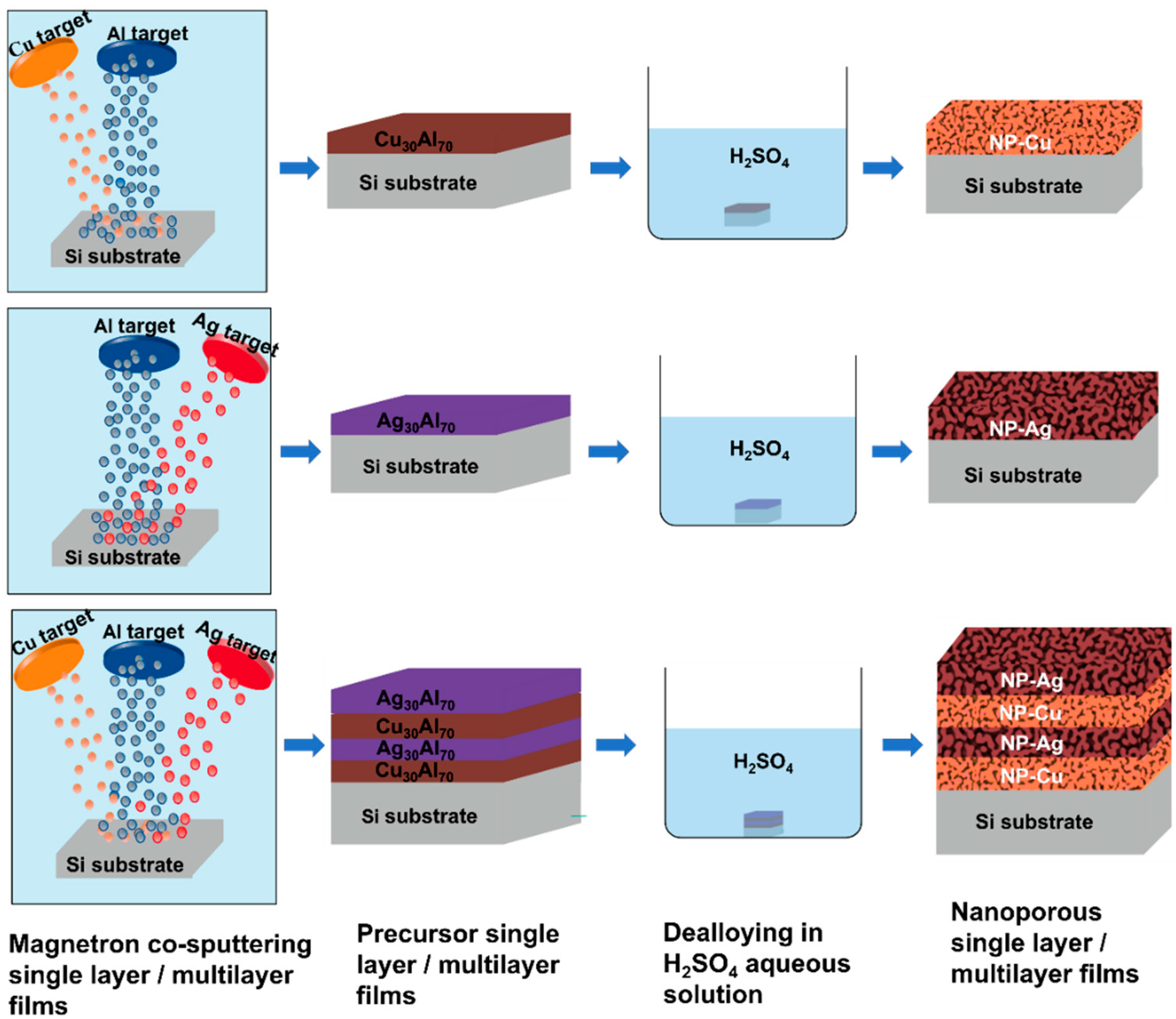
2. Materials and Methods
3. Results and Discussion
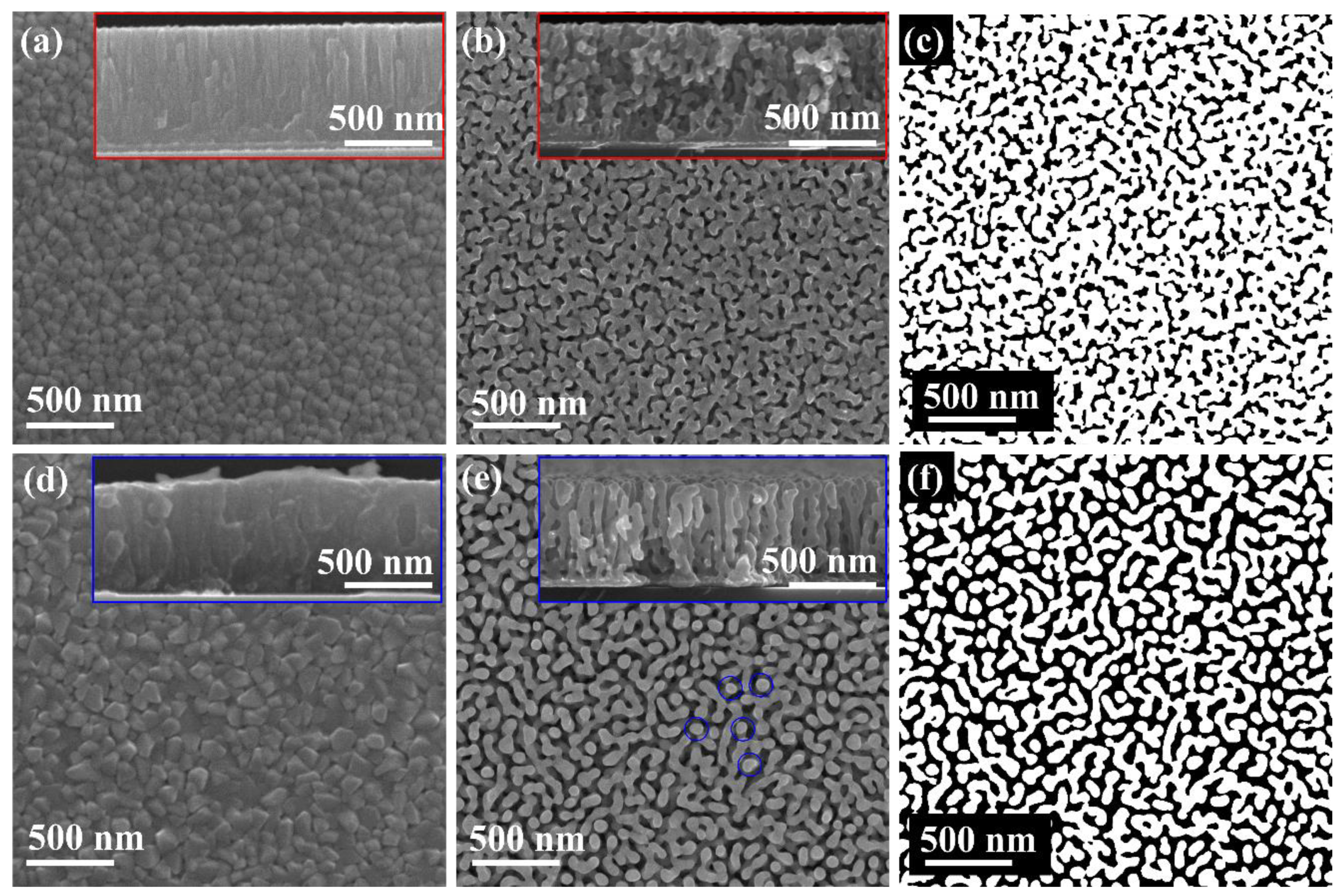
3.1. Preparation of Single Layer NP-Cu and NP-Ag Films
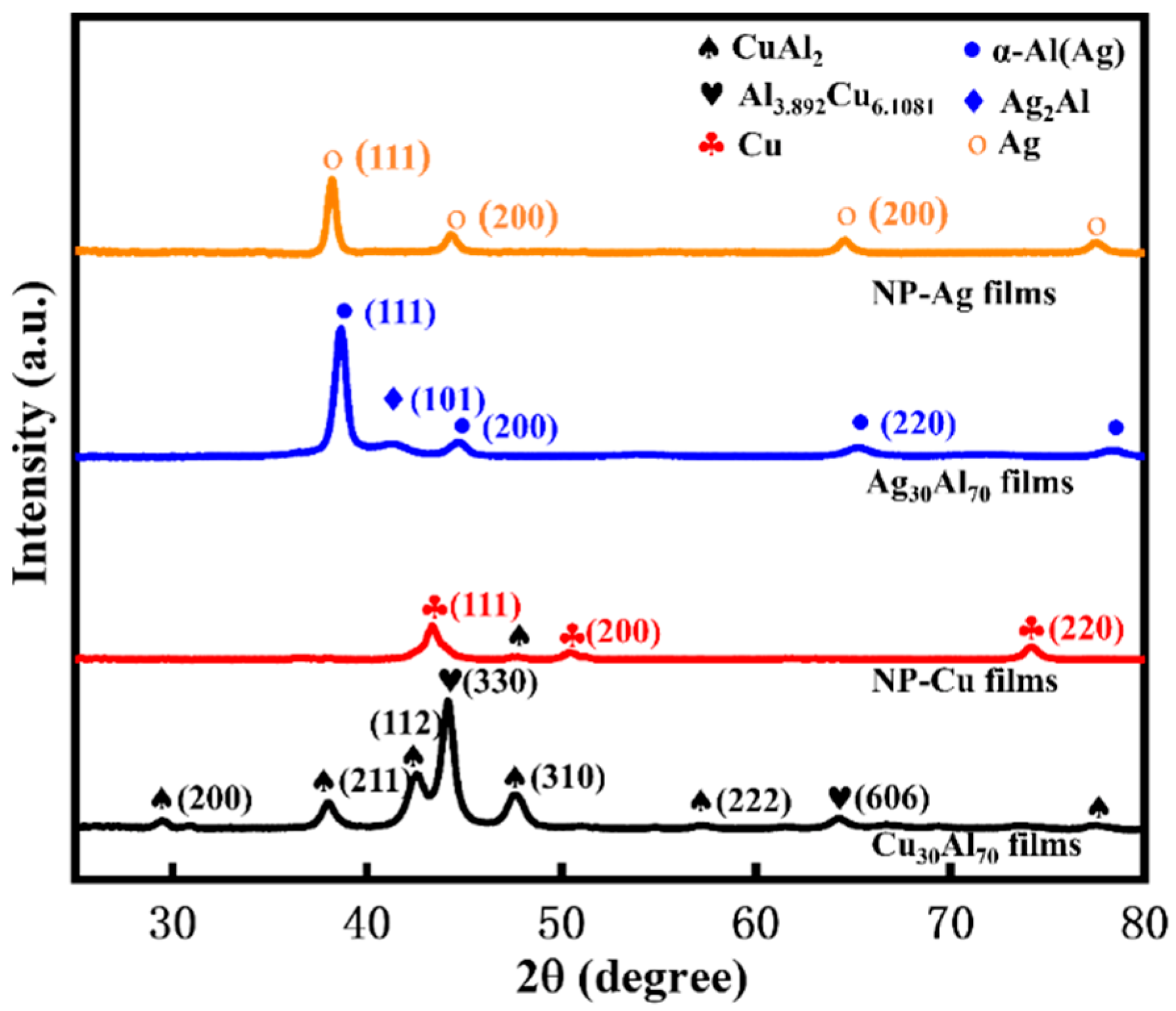
3.2. Phase Composition of Monolayer Precursor Films Cu30Al70 and Ag30Al70 and Monolayer NP-Cu and NP-Ag Films
3.3. Preparation of Nanoporous Cu/Ag Multilayer Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zinkle, S.J.; Was, G.S. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Allen, T.R.; Sridharan, K.; Tan, L.; Windes, W.E.; Cole, J.I.; Crawford, D.C.; Was, G.S. Materials challenges for generation IV nuclear energy systems. Nucl. Technol. 2017, 162, 342–357. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Snead, L.L. Designing radiation resistance in materials for fusion energy. Annu. Rev. Mater. Res. 2014, 44, 241–267. [Google Scholar] [CrossRef]
- Zhang, X.; Hattar, K.; Chen, Y.; Shao, L.; Li, J.; Sun, C.; Yu, K.; Li, N.; Taheri, M.L.; Wang, H.; et al. Radiation damage in nanostructured materials. Prog. Mater. Sci. 2018, 96, 217–321. [Google Scholar] [CrossRef]
- Osetsky, Y.N.; Stoller, R.E.; Matsukawa, Y. Dislocation–stacking fault tetrahedron interaction: What can we learn from atomic-scale modelling. J. Nucl. Mater. 2004, 329–333, 1228–1232. [Google Scholar] [CrossRef]
- Du, J.L.; Chen, H.Q.; Xu, C.; Fan, Y.; Qiu, Y.H.; Wang, H.; Fu, E.G. Stress of misfit dislocation at Fe/MgO interface drives the annihilation of radiation induced defects. Acta Mater. 2021, 210, 116798. [Google Scholar] [CrossRef]
- Valles, G.; Panizo-Laiz, M.; González, C.; Martin-Bragado, I.; González-Arrabal, R.; Gordillo, N.; Iglesias, R.; Guerrero, C.L.; Perlado, J.M.; Rivera, A. Influence of grain boundaries on the radiation-induced defects and hydrogen in nanostructured and coarse-grained tungsten. Acta Mater. 2017, 122, 277–286. [Google Scholar] [CrossRef]
- Xiao, X.; Song, D.; Chu, H.; Xue, J.; Duan, H. Mechanical behaviors of irradiated FCC polycrystals with nanotwins. Int. J. Plast. 2015, 74, 110–126. [Google Scholar] [CrossRef]
- Du, J.L.; Wu, Z.M.; Fu, E.G.; Liang, Y.X.; Wang, X.J.; Wang, P.P.; Yu, K.Y.; Ding, X.D.; Li, M.M.; Kirk, M. Detwinning through migration of twin boundaries in nanotwinned Cu films under in situ ion irradiation. Sci. Technol. Adv. Mater. 2018, 19, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Liu, Y.; Fu, E.G.; Sun, C.; Yu, K.Y.; Song, M.; Li, J.; Wang, Y.Q.; Wang, H.; Zhang, X. Unusual size-dependent strengthening mechanisms in helium ion-irradiated immiscible coherent Cu/Co nanolayers. Acta Mater. 2015, 84, 393–404. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Fan, C.; Ding, J.; Xue, S.; Chen, Y.; Li, Q.; Wang, H.; Zhang, X. In situ heavy ion irradiation studies of nanopore shrinkage and enhanced radiation tolerance of nanoporous Au. Sci. Rep. 2017, 7, 39484. [Google Scholar] [CrossRef]
- Hsiung, L.L.; Fluss, M.J.; Tumey, S.J.; Choi, B.W.; Serruys, Y.; Willaime, F.; Kimura, A. Formation mechanism and the role of nanoparticles in Fe-Cr ODS steels developed for radiation tolerance. Phys. Rev. B 2010, 82, 184103. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, K.Y.; Liu, Y.; Shao, S.; Wang, H.; Kirk, M.A.; Wang, J.; Zhang, X. Damage-tolerant nanotwinned metals with nanovoids under radiation environments. Nat. Commun. 2015, 6, 7036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odette, G.R.; Alinger, M.J.; Wirth, B.D. Recent developments in irradiation-resistant steels. Annu. Rev. Mater. Res. 2008, 38, 471–503. [Google Scholar] [CrossRef]
- Sun, C.; Song, M.; Yu, K.Y.; Chen, Y.; Kirk, M.; Li, M.; Wang, H.; Zhang, X. In situ evidence of defect cluster absorption by grain boundaries in Kr ion irradiated nanocrystalline Ni. Metall. Mater. Trans. A 2013, 44, 1966–1974. [Google Scholar] [CrossRef]
- Yu, K.Y.; Liu, Y.; Sun, C.; Wang, H.; Shao, L.; Fu, E.G.; Zhang, X. Radiation damage in helium ion irradiated nanocrystalline Fe. J. Nucl. Mater. 2012, 425, 140–146. [Google Scholar] [CrossRef]
- Wu, Z.M.; Zhang, J.; Zhang, J.; Huang, J.C.; Fan, Y.; Yu, X.H.; Zhao, Y.B.; Zhu, J.L.; Jin, C.Q.; Wang, P.; et al. Nanocrystalline W-based alloys with ultrahigh hardness and exceptional irradiation tolerance. Nucl. Fusion 2019, 59, 106050. [Google Scholar] [CrossRef]
- Fu, E.G.; Carter, J.; Swadener, G.; Misra, A.; Shao, L.; Wang, H.; Zhang, X. Size dependent enhancement of helium ion irradiation tolerance in sputtered Cu/V nanolaminates. J. Nucl. Mater. 2009, 385, 629–632. [Google Scholar] [CrossRef]
- Fu, E.G.; Wang, H.; Carter, J.; Shao, L.; Wang, Y.Q.; Zhang, X. Fluence-dependent radiation damage in helium (He) ion-irradiated Cu/V multilayers. Philos. Mag. 2013, 93, 883–898. [Google Scholar] [CrossRef]
- Fu, E.G.; Misra, A.; Wang, H.; Shao, L.; Zhang, X. Interface enabled defects reduction in helium ion irradiated Cu/V nanolayers. J. Nucl. Mater. 2010, 407, 178–188. [Google Scholar] [CrossRef]
- Saenz-Trevizo, A.; Hodge, A.M. Nanomaterials by design: A review of nanoscale metallic multilayers. Nanotechnology 2020, 31, 292002. [Google Scholar] [CrossRef]
- Fu, E.G.; Li, N.; Misra, A.; Hoagland, R.G.; Wang, H.; Zhang, X. Mechanical properties of sputtered Cu/V and Al/Nb multilayer films. Mater. Sci. Eng. A 2008, 493, 283–287. [Google Scholar] [CrossRef]
- Wang, P.P.; Wang, X.J.; Du, J.L.; Ren, F.; Zhang, Y.; Zhang, X.; Fu, E.G. The temperature and size effect on the electrical resistivity of Cu/V multilayer films. Acta Mater. 2017, 126, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.J.; Cai, X.; Mao, J.F.; Jin, C.Y. ZnS/Ag/ZnS nano-multilayer films for transparent electrodes in flat display application. Appl. Surf. Sci. 2001, 183, 103–110. [Google Scholar] [CrossRef]
- Mangin, S.; Ravelosona, D.; Katine, J.A.; Carey, M.J.; Terris, B.D.; Fullerton, E.E. Current-induced magnetization reversal in nanopillars with perpendicular anisotropy. Nat. Mater. 2006, 5, 210–215. [Google Scholar] [CrossRef]
- Chen, Y.X.; Fu, E.G.; Yu, K.Y.; Song, M.; Liu, Y.; Wang, Y.; Wang, H.Y.; Zhang, X. Enhanced radiation tolerance in immiscible Cu/Fe multilayers with coherent and incoherent layer interfaces. J. Mater. Res. 2015, 30, 1300–1309. [Google Scholar] [CrossRef]
- Wang, M.; Beyerlein, I.J.; Zhang, J.; Han, W.-Z. Defect-interface interactions in irradiated Cu/Ag nanocomposites. Acta Mater. 2018, 160, 211–223. [Google Scholar] [CrossRef]
- Ge, X.; Chen, L.; Zhang, L.; Wen, Y.; Hirata, A.; Chen, M. Nanoporous metal enhanced catalytic activities of amorphous molybdenum sulfide for high-efficiency hydrogen production. Adv Mater. 2014, 26, 3100–3104. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.J.; Wang, J.Q.; Liu, P.; Wang, Y.; Chen, M.W. Hierarchical nanoporous metal/metal-oxide composite by dealloying metallic glass for high-performance energy storage. Corros. Sci. 2015, 96, 196–202. [Google Scholar]
- Hu, Z.Y.; Wang, J.; Li, R.; Xu, C.; Liu, X.J.; Wang, Y.; Fu, E.G.; Lu, Z.P. Ion irradiation-enhanced raman scattering on nanoporous copper. Langmuir 2018, 34, 13041–13046. [Google Scholar] [CrossRef] [PubMed]
- An, S.S.; Zhang, S.C.; Liu, W.B.; Fang, H.; Zhang, M.L.; Yu, Y. Dealloying behavior of Mn-30Cu alloy in acetic acid solution. Corros. Sci. 2013, 75, 256–261. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Zhang, X. A review on the radiation response of nanoporous metallic materials. JOM 2018, 70, 2753–2764. [Google Scholar] [CrossRef]
- Fu, E.G.; Caro, M.; Zepeda-Ruiz, L.A.; Wang, Y.Q.; Baldwin, K.; Bringa, E.; Nastasi, M.; Caro, A. Surface effects on the radiation response of nanoporous Au foams. Appl. Phys. Lett. 2012, 101, 191607. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Bufford, D.; Chen, Y.; Kirk, M.A.; Wang, Y.Q.; Li, M.; Wang, H.; Maloy, S.A.; Zhang, X. In situ study of defect migration kinetics in nanoporous Ag with enhanced radiation tolerance. Sci. Rep. 2014, 4, 3737. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-Z.; Zeng, W.-Y.; Cheng, I.C. Synthesis and characterization of nanoporous copper thin films by magnetron sputtering and subsequent dealloying. Thin Solid Film 2020, 699, 137913. [Google Scholar] [CrossRef]
- Detsi, E.; Vuković, Z.; Punzhin, S.; Bronsveld, P.M.; Onck, P.R.; Hosson, J.T.M.D. Fine-tuning the feature size of nanoporous silver. CrystEngComm 2012, 14, 5402–5406. [Google Scholar] [CrossRef]
- Chauvin, A.; Heu, W.T.C.; Tessier, P.-Y.; El Mel, A.-A. Impact of the morphology and composition on the dealloying process of co-sputtered silver-aluminum alloy thin films. Phys. Status Solidi (b) 2016, 253, 2167–2174. [Google Scholar] [CrossRef]
- Stuckner, J.; Frei, K.; McCue, I.; Demkowicz, M.J.; Murayama, M. AQUAMI: An open source Python package and GUI for the automatic quantitative analysis of morphologically complex multiphase materials. Comput. Mater. Sci. 2017, 139, 320–329. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Z.; Zhao, C.; Wang, W.; Zhang, Z. Influence of alloy composition and dealloying solution on the formation and microstructure of monolithic nanoporous silver through chemical dealloying of Al-Ag alloys. J. Phys. Chem. C 2009, 113, 13139–13150. [Google Scholar] [CrossRef]
- Thompson, C.V. Structure evolution during processing of polycrystalline films. Annu. Rev. Mater. Sci. 2000, 30, 159–190. [Google Scholar] [CrossRef]
- Thornton, J.A. High rate thick film growth. Annu. Rev. Mater. Sci. 1977, 7, 239–260. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Wang, P.P.; Fu, E.G.; Wang, X.J.; Yan, X.Q.; Xu, P.; Wu, Z.M.; Zhao, Y.B.; Liang, Y.X. Bilayer nanoporous copper films with various morphology features synthesized by one-step dealloying. J. Alloys Compd. 2018, 754, 26–31. [Google Scholar] [CrossRef]
- Yamauchi, I.; Kajiwara, T.; Mase, T.; Saraoka, M. Formation of highly saturated Al–Ag precursor by rapid solidification for skeletal silver synthesis. J. Alloys Compd. 2002, 336, 206–212. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, S.; Wu, Z.; Fan, Y.; Chen, H.; Meng, Q.; Yan, Y.; Hu, Z.; Wang, J.; Fu, E. Design and Preparation of Nanoporous Cu/Ag Multilayer Films. Coatings 2021, 11, 1187. https://doi.org/10.3390/coatings11101187
Wang Y, Zhang S, Wu Z, Fan Y, Chen H, Meng Q, Yan Y, Hu Z, Wang J, Fu E. Design and Preparation of Nanoporous Cu/Ag Multilayer Films. Coatings. 2021; 11(10):1187. https://doi.org/10.3390/coatings11101187
Chicago/Turabian StyleWang, Yufei, Shuangle Zhang, Zefeng Wu, Yong Fan, Huaqiang Chen, Qingning Meng, Yang Yan, Zhaoyi Hu, Jing Wang, and Engang Fu. 2021. "Design and Preparation of Nanoporous Cu/Ag Multilayer Films" Coatings 11, no. 10: 1187. https://doi.org/10.3390/coatings11101187
APA StyleWang, Y., Zhang, S., Wu, Z., Fan, Y., Chen, H., Meng, Q., Yan, Y., Hu, Z., Wang, J., & Fu, E. (2021). Design and Preparation of Nanoporous Cu/Ag Multilayer Films. Coatings, 11(10), 1187. https://doi.org/10.3390/coatings11101187





