Effect of Low-Pressure Plasma Nitriding with Hollow Cathode Discharge on the Surface Microstructure of WC-Co Cermet
Abstract
1. Introduction
2. Experimental Procedure
2.1. Plasma Nitriding and HFCVD
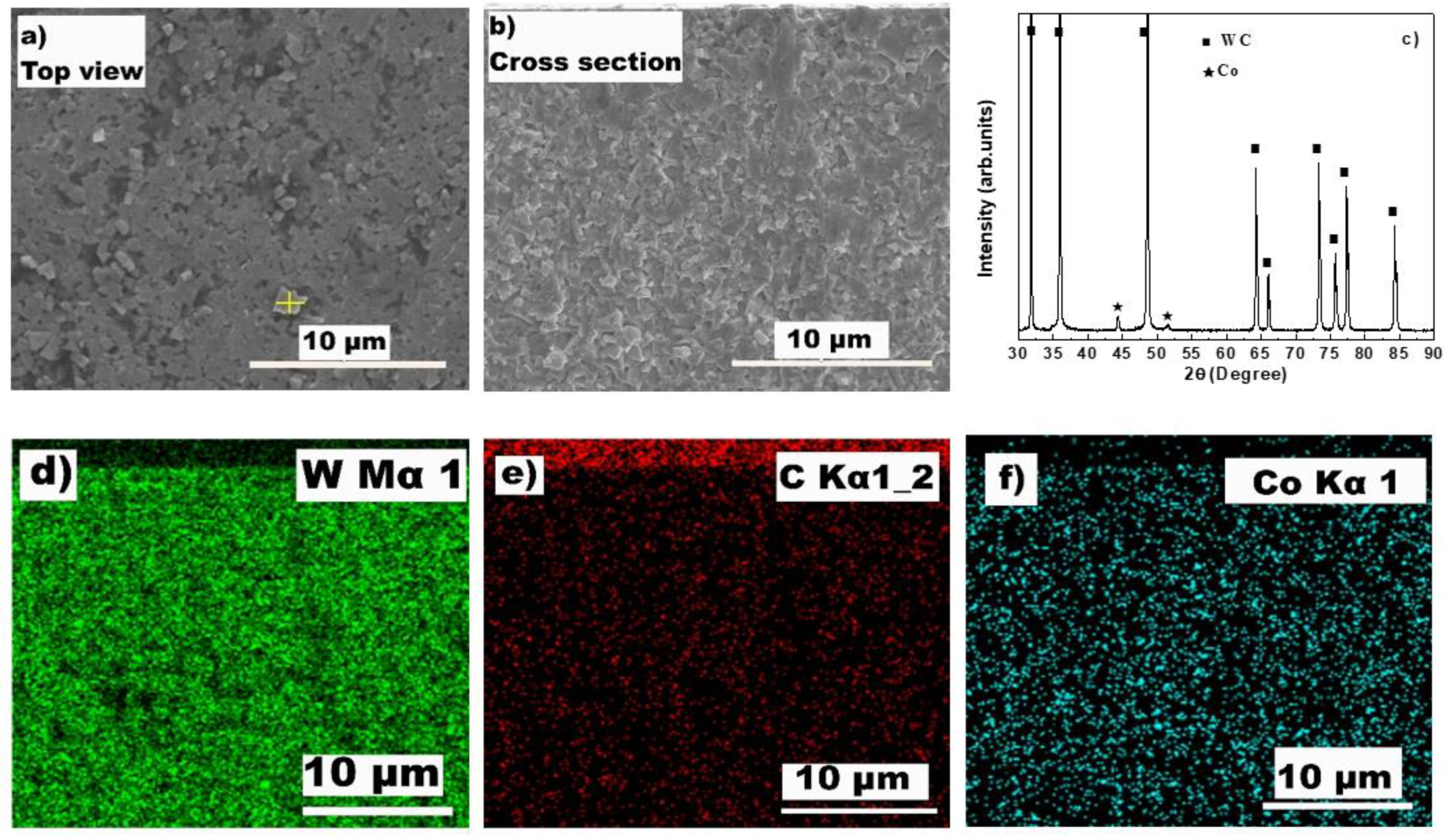
2.2. Measurement and Characterization
3. Results and Discussion
3.1. The Microstructure of the Surface Conversion Layer
3.2. Effect of the Conversion Layer on HFCVD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norgren, S.; García, J.; Blomqvist, A. Trends in the P/M hard metal industry. Int. J. Refract. Met. Hard Mater. 2015, 48, 31–45. [Google Scholar] [CrossRef]
- Langa, T.; Olubambi, P.; Shabalala, T.; Shongwe, M. Densification and structural transformation during spark plasma sintering of WC-Co-YSZ-cBN systems. Int. J. Refract. Met. Hard Mater. 2018, 72, 341–348. [Google Scholar] [CrossRef]
- Frykholm, R.; Ekroth, M.; Jansson, B.; OAndrén, H.; Ågren, J. Effect of cubic phase composition on gradient zone formation in cemented carbides. Int. J. Refract. Met. Hard Mater. 2001, 19, 527–538. [Google Scholar] [CrossRef]
- Tang, S.; Liu, D.; Li, P.; Jiang, L.; Liu, W.; Chen, Y.; Niu, Q. Microstructure and mechanical properties of functionally gradient cemented carbides fabricated by microwave heating nitriding sintering. Int. J. Refract. Met. Hard Mater. 2016, 58, 137–142. [Google Scholar] [CrossRef]
- Haubner, R.; Kalss, W. Diamond deposition on hardmetal substrates–Comparison of substrate pre-treatments and industrial applications. Int. J. Refract. Met. Hard Mater. 2010, 28, 475–483. [Google Scholar] [CrossRef]
- Ullram, S.; Haubner, R. Temperature pre-treatments of hardmetal substrates to reduce the cobalt content and improve diamond deposition. Diam. Relat. Mater. 2006, 15, 994–999. [Google Scholar] [CrossRef]
- Bobzin, K. High-performance coatings for cutting tools. CIRP J. Manuf. Sci. Technol. 2017, 18, 1–9. [Google Scholar] [CrossRef]
- Najar, K.; Sheikh, N.; Butt, M.; Mushtaq, S.; Shah, M. Engineered synthetic diamond film as a protective layer for tribological and machining applications: A review. J. Bio-Tribo-Corros. 2019, 5, 59. [Google Scholar] [CrossRef]
- Sarangi, S.; Chattopadhyay, A.; Chattopadhyay, A. Effect of pretreatment methods and chamber pressure on morphology, quality and adhesion of HFCVD diamond coating on cemented carbide inserts. Appl. Surf. Sci. 2008, 254, 3721–3733. [Google Scholar] [CrossRef]
- Fu, R.; Kwok, S.; Chen, P.; Yang, P.; Ngai, R.; Tianc, X.; Chu, P. Surface modification of cemented carbide using plasma nitriding and metal ion implantation. Surf. Coat. Technol. 2005, 196, 150–154. [Google Scholar] [CrossRef]
- Thakur, D.; Ramamoorthy, B.; Vijayaraghavan, L. Influence of different post treatments on tungsten carbide–cobalt inserts. Mater. Lett. 2008, 62, 4403–4406. [Google Scholar] [CrossRef]
- Peng, W.; Hao, S.; Zhao, L.; Chen, J.; Li, W. Formation mechanism of graphite nanospheres in WC-Co system under high current pulsed electron beam irradiation. Mater. Lett. 2019, 244, 207–210. [Google Scholar] [CrossRef]
- Yi, M.; Deng, B.; Xiao, H.; Wei, Q.; Ma, L.; Zhou, K.; Luo, Y.; Li, L.; Zhang, L.; Yu, Z. Gaseous boronizing pretreatment for the deposition of nanocrystalline diamond films on cemented carbide substrates. Mater. Res. Express 2019, 6, 076404. [Google Scholar] [CrossRef]
- Campos, R.; Contin, A.; Trava-Airoldi, V.; Barquete, D.; Moro, J.; Corat, E. Influence of boriding process in adhesion of CVD diamond films on tungsten carbide substrates. Mater. Res. 2015, 18, 925–930. [Google Scholar] [CrossRef][Green Version]
- Gyawali, G.; Joshi, B.; Tripathi, K.; Lee, S. Effect of ultrasonic nanocrystal surface modification on properties of electrodeposited Ni and Ni-SiC composite coatings. JMEPEG 2017, 26, 4462–4469. [Google Scholar] [CrossRef]
- Lanzutti, A.; Lekka, M.; Leitenburg, C.; Fedrizzi, L. Effect of pulse current on wear behavior of Ni matrix micro-and nano-SiC composite coatings at room and elevated temperature. Tribol. Int. 2019, 132, 50–61. [Google Scholar] [CrossRef]
- Sataev, M.; Koshkarbaeva, S.; Perni, S.; Nauryzova, S.; Prokopovich, P. A galvanic-chemical method for preparing diamond containing coatings. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 384–389. [Google Scholar] [CrossRef][Green Version]
- Qiu, W.; Liu, Z.; He, X.; Zeng, D.; Mai, Y. Improved interfacial adhesion between diamond film and copper substrate using a Cu (Cr)–diamond composite interlayer. Mater. Lett. 2012, 81, 155–157. [Google Scholar] [CrossRef]
- Polini, R.; Barletta, M.; Rubino, G.; Vesco, S. Recent advances in the deposition of diamond coatings on co-cemented tungsten carbides. Adv. Mater. Sci. Eng. 2012, 2012, 151629. [Google Scholar] [CrossRef]
- Polini, R.; Casadei, F.; D’Antonio, P.; Traversa, E. Dry turning of alumina/aluminum composites with CVD diamond coated Co-cemented tungsten carbide tools. Surf. Coat. Technol. 2003, 166, 127–134. [Google Scholar] [CrossRef]
- Li, T.; Lou, Q.; Don, J.; We, Y.; Liu, J. Modified surface morphology in surface ablation of cobalt-cemented tungsten carbide with pulsed UV laser radiation. Appl. Surf. Sci. 2001, 172, 331–344. [Google Scholar] [CrossRef]
- Barletta, M.; Rubino, G.; Valle, R.; Polini, R. Chemical vapor deposition of highly adherent diamond coatings onto co-cemented tungsten carbides irradiated by high power diode laser. ACS Appl. Mater. Interfaces 2012, 4, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhu, Z.; Su, D. Sliding wear of nitrided and duplex coated H13 steel against aluminium alloy. Tribol. Int. 2019, 129, 232–238. [Google Scholar] [CrossRef]
- Ichimura, S.; Takashima, S.; Tsuru, I.; Ohkubo, D.; Matsuo, H.; Goto, M. Application and evaluation of nitriding treatment using active screen plasma. Surf. Coat. Technol. 2019, 374, 210–221. [Google Scholar] [CrossRef]
- Fernandes, C.; Senos, A. Cemented carbide phase diagrams: A review. Int. J. Refract. Met. Hard Mater. 2011, 29, 405–418. [Google Scholar] [CrossRef]
- Dubrovinskaia, N.; Dubrovinsky, L.; Saxena, S.; Selleby, M.; Sundman, B. Thermal expansion and compressibility of Co6W6C. J. Alloys Comp. 1999, 285, 242–245. [Google Scholar] [CrossRef]
- Polini, R.; Marcheselli, G.; Traversa, E. Nucleation and growth of diamond films on Ni-cemented tungsten carbide: Effects of substrate pretreatments. J. Am. Ceram. Soc. 1994, 77, 2043–2048. [Google Scholar] [CrossRef]
- Sakai, K.; Suzuki, Y.; Inoue, H.; Utino, K.; Horikoshi, Y. Tool life improvement of coated carbide tools treated by novel nitriding technique. Key Eng. Mater. 2009, 407, 24–27. [Google Scholar] [CrossRef]
- Sato, T.; Hosokawa, Y.; Ito, S.; Akashi, K. Plasma carbonitriding of cemented carbide substrate as an effective pretreatment process for diamond CVD. Surf. Coat. Technol. 1999, 112, 189–193. [Google Scholar] [CrossRef]
- Hamzaoglu, E.; Yilmaz, S.; Gulmez, T. Effect of plasma nitriding on the performance of WC-Co cutting tools. JMEPEG 2011, 20, 405–408. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, S.; Shen, B.; Sun, F. CVD diamond coated drawing dies: A review. Mater. Manufact. Process. 2021, 36, 381–408. [Google Scholar] [CrossRef]
- Anderson, T.; Hobart, K.; Tadjer, M.; Koehler, A.; Imhoff, E.; Hite, J.; Feygelson, T.; Pate, B.; Eddy, C.; Kub, F. Nanocrystalline Diamond Integration with III-Nitride HEMTs. ECS J. Solid State Sci. Technol. 2017, 6, Q3036–Q3039. [Google Scholar] [CrossRef]
- Ramasubramanian, K.; Arunachalam, N.; Rao, M. Investigation on tribological behaviour of boron doped diamond coated cemented tungsten carbide for cutting tool applications. Surf. Coat. Technol. 2017, 332, 332–340. [Google Scholar] [CrossRef]
- Najara, K.; Sheikha, N.; Shah, M. Enhancement in tribological and mechanical properties of cemented tungsten carbide substrates using CVD-diamond coatings. Tribol. Ind. 2017, 39, 20–30. [Google Scholar]
- Gupta, M.; Pandey, N.; Amir, S.M.; Pütter, S.; Mattauch, S. Synthesis, structure and magnetization of Co4N thin films. J. Magn. Magn. Mater. 2019, 489, 165376. [Google Scholar] [CrossRef]
- Balasubramanian, B.; Zhao, X.; Valloppilly, S.; Beniwal, S.; Skomski, R.; Sarella, A.; Jin, Y.; Li, X.; Xu, X.; Cao, H.; et al. Magnetism of new metastable cobalt-nitride compounds. Nanoscale 2018, 10, 13011–13021. [Google Scholar] [CrossRef]
- Sugumaran, A.; Shukla, K.; Khan, I.; Ehiasarian, A.; Hovsepian, P. Dry sliding wear mechanisms of HIPIMS plasma nitrided CoCrMo alloy for medical implant applications. Vacuum 2021, 185, 109994. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Wang, M.; Ma, Y.Q. Effects of nitriding temperature on the structure and magnetic properties of CoFe 2 alloy. J. Mater. Sci. Mater. Electron. 2018, 29, 20071–20080. [Google Scholar] [CrossRef]
- Liu, T.; Li, M.; Guo, L. Designing and facilely synthesizing a series of cobalt nitride (Co4N) nanocatalysts as non-enzymatic glucose sensors: A comparative study toward the influences of material structures on electrocatalytic activities. Talanta 2018, 181, 154–164. [Google Scholar] [CrossRef]
- Razzaq, R.; Li, C.; Usman, M.; Suzuki, K.; Zhang, S. A highly active and stable Co4N/γ-Al2O3 catalyst for CO and CO2 methanation to produce synthetic natural gas (SNG). Chem. Eng. J. 2015, 262, 1090–1098. [Google Scholar] [CrossRef]
- Peng, J.; Zeng, J.; Xiong, C.; Li, L. The effect of interlayer reactivity on the quality of diamond coating by HFCVD deposition. J. Alloys Comp. 2020, 835, 155035. [Google Scholar] [CrossRef]
- Peng, J.; Liao, J.; Yang, M.; Liao, J.; Bi, J. Effect of annealing treatment on the tribological performance of hydrogenated amorphous diamond coatings doped with nitrogen. Ceram. Int. 2021, 47, 13423–13431. [Google Scholar] [CrossRef]
- Hojman, E.; Akhvlediani, R.; Alagem, E.; Hoffman, A. Cobalt out-diffusion and carbon phase composition at the WC-10% Co/diamond film interface investigated by XPS, SEM, Raman and SIMS. Phys. Status Solidi A 2012, 209, 1726–1731. [Google Scholar] [CrossRef]
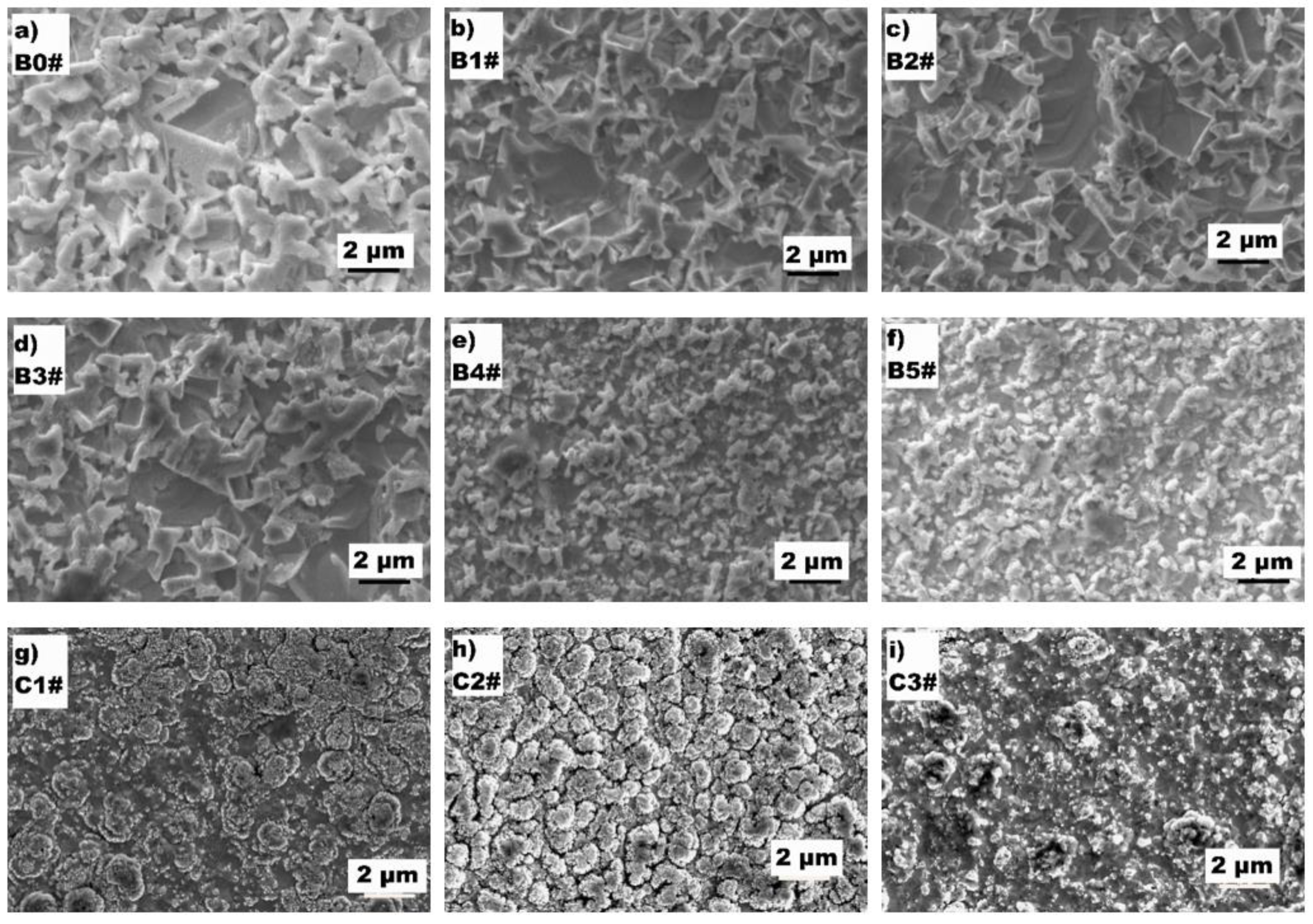
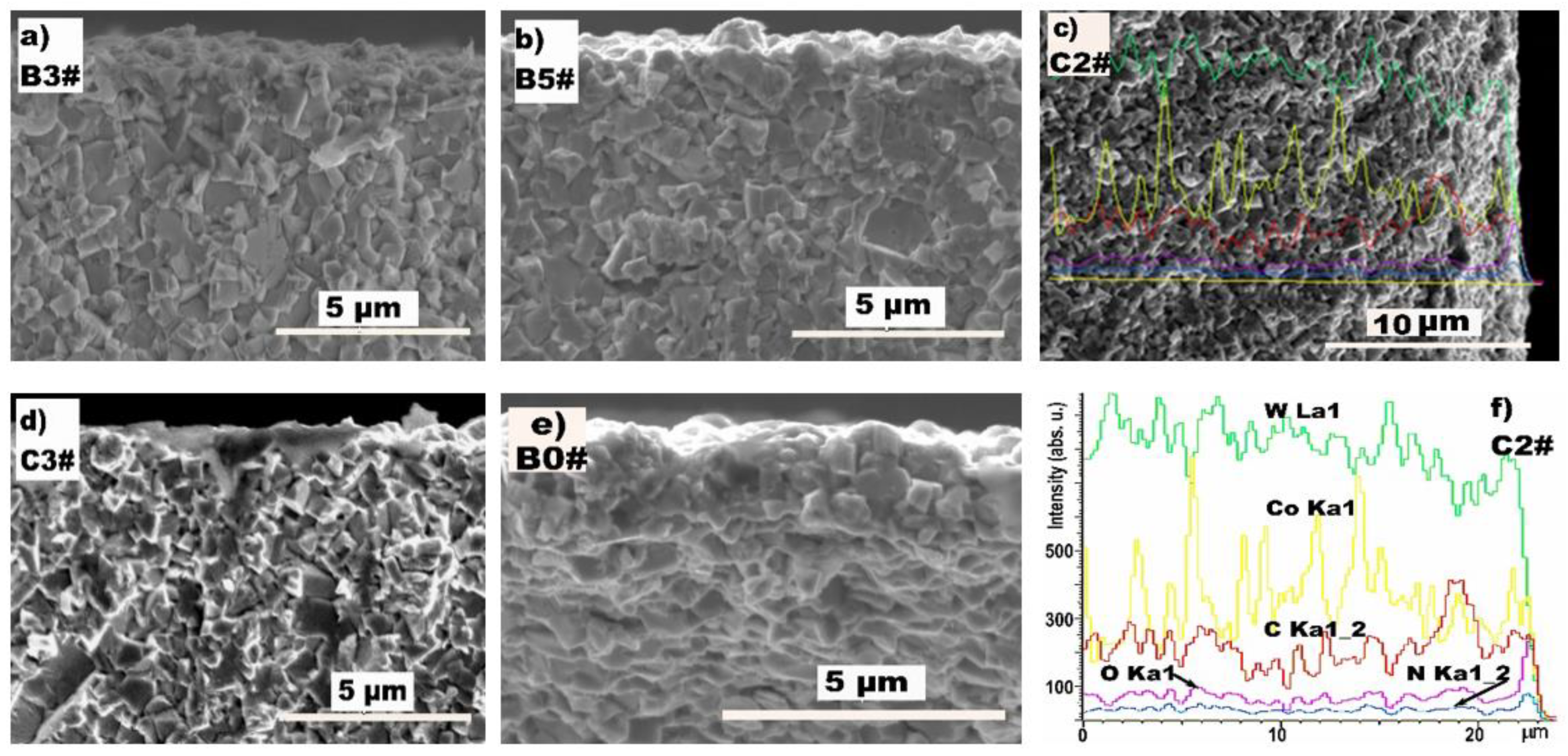


| Test Group | Work Gas | Pressure/Pa | Duration/h |
|---|---|---|---|
| B0# | - | - | - |
| B1# | N2:H2 = 1:3 | 3 | 2 |
| B2# | N2:H2 = 1:1 | 3 | 2 |
| B3# | N2:H2 = 3:1 | 3 | 2 |
| B4# | N2:H2 = 6:1 | 3 | 2 |
| B5# | Pure N2 | 3 | 2 |
| C1# | Pure N2 | 3 | 6 |
| C2# | Pure N2 | 5 | 6 |
| C3# | Pure N2 | 8 | 6 |
| B0# | B1# | B2# | B3# | B4# | B5# | C1# | C2# | C3# | |
|---|---|---|---|---|---|---|---|---|---|
| Co | 23.1 | 22.3 | 22.0 | 21.0 | 9.4 | 13.3 | 6.3 | 8.5 | 6.2 |
| N | -- (--) | -- (--) | -- (--) | -- (4.0) | -- (6.1) | 6.0 | 1.5 | 7.6 | 4.9 (2.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Xiao, Y.; Peng, Y.; Li, W.; Zeng, J. Effect of Low-Pressure Plasma Nitriding with Hollow Cathode Discharge on the Surface Microstructure of WC-Co Cermet. Coatings 2021, 11, 1149. https://doi.org/10.3390/coatings11101149
Peng J, Xiao Y, Peng Y, Li W, Zeng J. Effect of Low-Pressure Plasma Nitriding with Hollow Cathode Discharge on the Surface Microstructure of WC-Co Cermet. Coatings. 2021; 11(10):1149. https://doi.org/10.3390/coatings11101149
Chicago/Turabian StylePeng, Jihua, Yang Xiao, Yinglong Peng, Weiqiu Li, and Jiwei Zeng. 2021. "Effect of Low-Pressure Plasma Nitriding with Hollow Cathode Discharge on the Surface Microstructure of WC-Co Cermet" Coatings 11, no. 10: 1149. https://doi.org/10.3390/coatings11101149
APA StylePeng, J., Xiao, Y., Peng, Y., Li, W., & Zeng, J. (2021). Effect of Low-Pressure Plasma Nitriding with Hollow Cathode Discharge on the Surface Microstructure of WC-Co Cermet. Coatings, 11(10), 1149. https://doi.org/10.3390/coatings11101149






